Abstract
Background
The association between black race and worse outcomes in operable breast cancer reported in previous studies has been attributed to a higher incidence of more aggressive triple-negative disease, disparities in care, and comorbidities. We evaluated associations between black race and outcomes, by tumor hormone receptor and HER2 expression, in patients who were treated with contemporary adjuvant therapy.
Methods
The effect of black race on disease-free and overall survival was evaluated using Cox proportional hazards models adjusted for multiple covariates in a clinical trial population that was treated with anthracycline- and taxane-containing chemotherapy. Categorical variables were compared using the Fisher exact test. All P values are two-sided.
Results
Of 4817 eligible patients, 405 (8.4%) were black. Compared with nonblack patients, black patients had a higher rate of triple-negative disease (31.9% vs 17.2%; P < .001) and a higher body mass index (median: 31.7 vs 27.4 kg/m2; P < .001). Black race was statistically significantly associated with worse disease-free survival (5-year disease-free survival, black vs nonblack: 76.7% vs 84.5%; hazard ratio of recurrence or death = 1.58, 95% confidence interval = 1.19 to 2.10, P = .0015) and overall survival (5-year overall survival, black vs nonblack: 87.6% vs 91.9%; hazard ratio of death = 1.49, 95% confidence interval = 1.05 to 2.12, P = .025) in patients with hormone receptor–positive HER2-negative disease but not in patients with triple-negative or HER2-positive disease. In a model that included black race, hormone receptor–positive HER2-negative disease vs other subtypes, and their interaction, the interaction term was statistically significant for disease-free survival (P = .027) but not for overall survival (P = .086).
Conclusion
Factors other than disparities in care or aggressive disease contribute to increased recurrence in black women with hormone receptor–positive breast cancer.
CONTEXT AND CAVEATS
Prior knowledge
The increased disease recurrence and worse survival of black women with breast cancer have been attributed to various factors, including a higher incidence of more aggressive (ie, triple-negative) disease, disparities in care, and comorbidities.
Study design
A retrospective secondary analysis that compared the outcomes of black patients with those of nonblack patients in a large cohort of women with stage I–III breast cancer who participated in a randomized phase III trial that compared the efficacy of several taxane regimens.
Contribution
Among patients with stage I–III hormone receptor–positive breast cancer who were treated with standard chemohormonal therapy, black patients had worse disease-free and overall survival compared with nonblack patients.
Implication
Factors other than disparities in care or aggressive disease contribute to increased recurrence in black women with hormone receptor–positive breast cancer.
Limitations
The study was retrospective in nature, and the analysis was not prespecified in the original trial design. Black breast cancer patients differed from nonblack patients with respect to some characteristics. Data on adherence to endocrine therapy came from case reports.
From the Editors
Breast cancer is the most common cancer in women in the United States and the second leading cause of death (1). Black race is known to be associated with a worse prognosis in breast cancer (2) and in other hormone-dependent cancers, such as uterine and prostate cancers, but generally not in other cancer types (3,4). Multiple factors that may contribute to the worse outcomes for black women with breast cancer include their greater likelihood of having advanced-stage (5) or triple-negative (ie, tumors that lack expression of the estrogen receptor [ER], progesterone receptor [PR], and HER2/neu) (6) disease. Triple-negative disease is a surrogate for basal subtype of breast cancer, which has been associated with a poor prognosis (7). Other factors that are associated with poor prognosis in black women with breast cancer include poor adherence to chemotherapy (8) and endocrine therapy (9), increased number of comorbidities (10), and disparities in care (11–13). Black race has also been associated with worse outcomes in male breast cancer (14). Although breast cancer incidence and mortality have declined by approximately 35% in the United States since 1990, the mortality rates have declined less in black women, which has contributed to an approximately 35% higher breast cancer mortality rate for black women compared with other women (15). However, a widening racial gap has also been observed for women in the US Department of Defense health-care system, suggesting that factors other than disparities in care may be playing a role in contributing to inferior outcomes (16).
To disentangle the various factors that influence disease recurrence and survival of black women with breast cancer, we compared the outcomes of black patients with those of nonblack patients in a large cohort of women with stage I–III breast cancer who participated in a National Cancer Institute (NCI)–sponsored randomized phase III trial that compared the efficacy of several taxane regimens (trial E1199; http://Clinicaltrials.gov identifier: NCT00004125) (17).
Patients and Methods
Patient Selection and Treatment
Women who had operable adenocarcinoma of the breast with axillary lymph node metastases [tumor stage T1, T2, or T3; nodal stage N1 or N2, or high-risk node-negative disease (T2 or T3, N0) without distant metastases according to the American Joint Commission on Cancer, Fifth Edition (18)] were eligible for the randomized trial. Participants were required to have normal cardiac, renal, hepatic, and bone marrow function and good performance status. Other details regarding trial eligibility, the treatment administered, and the results have been previously reported (17). Briefly, patients received four cycles of doxorubicin and cyclophosphamide given every 3 weeks, followed by paclitaxel or docetaxel given every 3 weeks for four cycles or weekly for 12 cycles. Patients with hormone receptor–positive tumors (defined as ER and/or PR positive) were required to take tamoxifen (20 mg daily) for 5 years (or an aromatase inhibitor if there was a contraindication to tamoxifen therapy); the protocol was amended to allow switching from tamoxifen to an aromatase inhibitor after switching to an aromatase inhibitor was shown to be an effective alternative to tamoxifen alone (19). All patients received standardized care as stipulated by the trial. Information provided by the local institution was self-reported race and ethnicity at the time of registration in accordance with National Institute of Health guidelines used for NCI-sponsored trials (http://grants.nih.gov/grants/guide/notice-files/not-od-01-053.html).
Statistical Analysis
The disease-free and overall survival rates over time were estimated using Kaplan–Meier method, and the breast cancer–specific survival rates were estimated as 1 minus the cumulative incidence of breast cancer–specific death. Disease-free survival was defined as the time from randomization to disease recurrence, diagnosis of contralateral breast cancer, or death from any cause, whichever occurred first. Overall survival was defined as the time from randomization to death from any cause. Breast cancer–specific survival was defined as time from randomization to death from breast cancer. Patients whose cause of death was unknown but had breast cancer recurrence before death were also coded as death from breast cancer. Patients who died of other causes were censored at the date of death. Univariate and multivariable Cox proportional hazards models were used to estimate the unadjusted and adjusted hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). The covariates in the multivariable Cox proportional hazards models included race (black vs nonblack), body mass index (BMI) at registration (≥30 vs <30 kg/m2), age at registration (<50 vs ≥50 y), number of positive lymph nodes at registration (0 [referent] vs 1–3 vs ≥4), pathological tumor size at registration (≤2 vs >2 cm), most extensive surgical procedure at registration (breast-sparing procedure vs mastectomy), and hormone therapy on the study (tamoxifen alone vs aromatase inhibitor with or without tamoxifen [referent] vs no endocrine therapy or unknown). Hormone therapy was coded as a time-varying variable (patients started endocrine therapy at different time after registration in E1199), and all other variables were coded as time-fixed variables. The assumption of proportional hazards was checked by testing the statistical significance of interaction between covariates and time (in log scale) in Cox models. The proportional hazards assumption for all covariates was met for disease-free survival (based on P > .05 for the covariate-by-time interaction term in the Cox models, indicating that the HRs for the covariate do not change with time), whereas for overall survival and breast cancer–specific survival, the proportional hazards assumption for obesity only was not. Hence, sensitivity analysis was conducted by fitting separate Cox regression models for obese and nonobese patients. The results showed a slight change in hazard ratios for race but no change in the results of the statistical significance test (HR for race was somewhat higher for obese patients [overall survival: HR = 1.09; breast cancer–specific survival: HR = 1.22] and somewhat smaller for nonobese patients [overall survival: HR = 2.53; breast cancer–specific survival: HR = 2.78]). Overall, the model fit the data well. For the breast cancer–specific survival outcome, in addition to the cause-specific hazard analysis based on the multivariable Cox proportional hazard models, we also performed subdistribution hazards analysis for competing risks to adjust for comorbidities that compete with death from breast cancer. Patient characteristics were compared by the Fisher exact test for categorical variables and the two-sample Wilcoxon rank-sum test for continuous variables. Adverse events (defined by NCI Common Toxicity Criteria, version 2.0, available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf) were compared by the Fisher exact test. All P values are two-sided, and P values less than or equal to .05 were considered statistically significant. The median follow-up for surviving patients was 95 months (range = 0–119 months), at which time there were 1252 disease-free survival events, 904 deaths, and 704 breast cancer–specific survival events; the breast cancer–specific survival events included 577 patients who were coded by the treating institutions as having died of breast cancer (64% of all deaths) and 127 patients who had a breast cancer recurrence and whose deaths were coded by the treating institutions as due to an unknown cause (14% of all deaths). Other deaths included 119 patients who were coded as having died of other causes (13% of all deaths) and 81 patients who were coded as having died of an unknown cause and who did not have breast cancer recurrence (9% of all deaths). The median time from breast cancer recurrence to death was 15.2 for those coded as having died of breast cancer and 12.4 months for those coded as having died of an unknown cause after a breast cancer recurrence.
Results
Patient Characteristics
Of the 5052 patients enrolled on the study between October 1999 and January 2002, 4817 were included in this analysis, of whom 405 (8.4%) self-reported as being black or African American; 235 patients were not included in this analysis because they were ineligible for reasons described in the original report (n = 102) or were enrolled via the NCI Expanded Participation Project and had missing treatment and toxicity data (n = 133).
Table 1 presents the characteristics of black and nonblack patients included in this analysis. Compared with nonblack patients, black patients had statistically significantly higher rates of triple-negative disease (31.9% vs 17.2%; P < .001) and HER2-positive disease (24.7% vs 19.3%; P = .045) and larger tumors (P = .020) but less extensive nodal involvement (P < .001). Compared with nonblack patients, black patients were younger at trial enrollment (median age: 48 vs 51 years, P < .001), had a higher BMI (median BMI: 31.7 vs 27.4 kg/m2; P < .001), and were more likely to be obese (BMI ≥30 kg/m2: 57.8% vs 34.2%; P < .001). Black patients were more likely to have had breast-sparing surgery compared with nonblack patients (44.4% vs 38.8%, P = .025) but had similar rates of radiation therapy and comparable taxane treatment arm assignment. Black patients were more likely than nonblack patients to have received tamoxifen monotherapy, probably because of their younger age at diagnosis.
Table 1.
Patient characteristics*
| Characteristic | Black patients (N = 405) | Nonblack patients† (N = 4412) | P‡ |
| Age at trial enrollment in years, No. (%) | <.001 | ||
| <50 | 222 (54.8) | 1919 (43.5) | |
| ≥50 | 183 (45.2) | 2493 (56.5) | |
| Median (range) | 48 (26–78) | 51 (22–84) | |
| BMI in kg/m2, No. (%) | <.001 | ||
| <25 | 57 (14.1) | 1428 (32.4) | |
| 25–29.9 | 109 (26.9) | 1431 (32.4) | |
| ≥30 | 234 (57.8) | 1511 (34.2) | |
| Unknown | 5 (1.2) | 42 (1.0) | |
| Median (range) | 31.7 (16.4–74.4) | 27.4 (13.5–87.8) | |
| Tumor size in cm, No. (%) | .020 | ||
| ≤2 | 125 (30.9) | 1620 (36.7) | |
| >2 to ≤5 | 232 (57.3) | 2318 (52.5) | |
| >5 | 43 (10.6) | 429 (9.7) | |
| Unknown | 5 (1.2) | 45 (1.2) | |
| Median (range) | 2.5 (0.2–14.0) | 2.5 (0.1–17.0) | |
| Number of positive lymph nodes, No. (%) | <.001 | ||
| 0 | 58 (14.3) | 498 (11.3) | |
| 1–3 | 235 (58.0) | 2436 (55.2) | |
| 4–9 | 80 (19.8) | 1015 (23.0) | |
| ≥10 | 28 (6.9) | 437 (9.9) | |
| Unknown | 4 (1.0) | 26 (0.6) | |
| Median (range) | 2 (0–32) | 2 (0–41) | |
| ER and/or PR expression, No. (%) | <.001 | ||
| Positive | 223 (55.1) | 3219 (73.0) | |
| Negative | 174 (43.0) | 1130 (25.6) | |
| Unknown | 8 (2.0) | 63 (1.4) | |
| Her2/neu expression, No. (%) | .045 | ||
| Positive | 100 (24.7) | 852 (19.3) | |
| Negative | 281 (69.4) | 3090 (70.0) | |
| Unknown | 24 (5.9) | 470 (10.7) | |
| Triple-negative disease, No. (%) | 129 (31.9) | 757 (17.2) | <.001 |
| Most extensive surgical procedure, No. (%) | 180 (44.4) | 1712 (38.8) | .025 |
| Breast-sparing surgery | 222 (54.8) | 2674 (60.6) | |
| Mastectomy | 3 (0.7) | 26 (0.6) | |
| Unknown | |||
| Radiation therapy, No. (%) | .57 | ||
| Given | 233 (57.5) | 2471 (56.0) | |
| Not given | 172 (42.5) | 1941 (44.0) | |
| Treatment arm, No. (%) | .17 | ||
| Every 3-wk paclitaxel | 99 (24.4) | 1123 (25.5) | |
| Weekly paclitaxel | 104 (25.7) | 1092 (24.8) | |
| Every 3-wk docetaxel | 116 (28.6) | 1087 (24.6) | |
| Weekly docetaxel | 86 (21.2) | 1110 (25.2) | |
| Endocrine therapy given§, No. (%) | |||
| Tamoxifen alone | 85 (38.1) | 1045 (32.5) | .011|| |
| Tamoxifen and then aromatase inhibitor | 101 (45.3) | 1838 (57.1) | |
| Aromatase inhibitor alone | 9 (4.0) | 121 (3.8) | |
| None | 9 (4.0) | 67 (2.1) | |
| Unknown | 19 (8.5) | 148 (4.6) |
BMI = body mass index; ER = estrogen receptor; PR = progesterone receptor.
Among 4412 nonblack patients, 179 (4.1%) self-reported as being of Hispanic ethnicity and 148 (3.4%) self-reported as being of nonwhite race.
P values are two-sided and were calculated from Fisher exact test for categorical variables and from the two-sample Wilcoxon rank-sum test for continuous variables.
Among patients with ER- and/or PR-positive tumors.
In the test, the variable was treated as four-level categorical variable: tamoxifen alone, sequential tamoxifen followed by an aromatase inhibitor, aromatase inhibitor alone, and no endocrine therapy or unknown whether endocrine therapy was used.
Race and Survival Outcomes
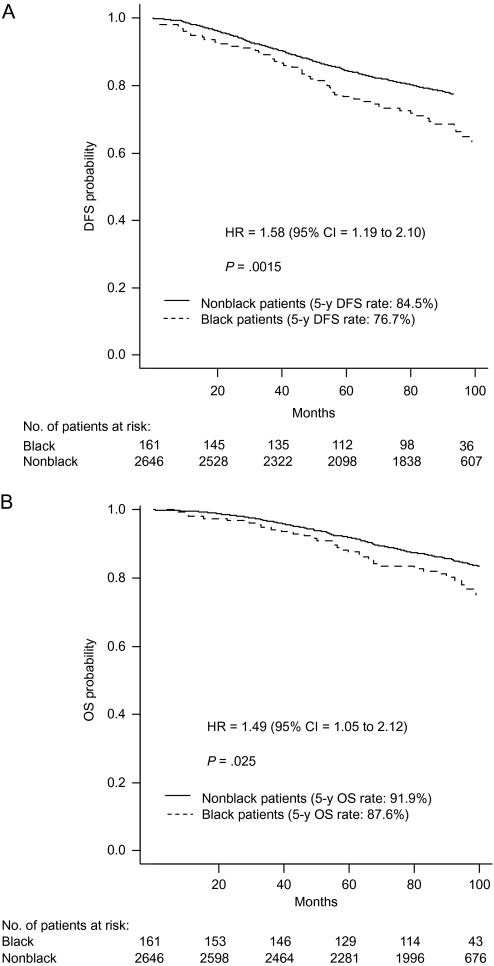
For the total study population, black race was statistically significantly associated with worse disease-free (HR of recurrence or death = 1.32, 95% CI = 1.10 to 1.58, P = .0033) and overall (HR of death = 1.30, 95% CI = 1.05 to 1.62, P = .017) survival. Among patients with hormone receptor–positive, HER2-negative, or unknown disease, black race was statistically significantly associated with worse disease-free (5-year disease-free survival, black vs nonblack: 76.7% vs 84.5%; HR of recurrence or death = 1.58, 95% CI = 1.19 to 2.10, P = .0015) and overall (5-year overall survival, black vs nonblack: 87.6% vs 91.9%; HR of death = 1.49, 95% CI = 1.05 to 2.12, P = .025) survival (Figure 1). However, there was no statistically significant association between race and either disease-free or overall survival among patients with triple-negative disease or among patients with HER2-positive disease (all Ps ≥ .30).
Figure 1.
Kaplan–Meier survival curves for breast cancer patients with hormone receptor–positive, HER2-negative or unknown disease. A) Disease-free survival (DFS). DFS rates at 60 months (5 years) were 76.7% (95% CI = 70.3% to 83.7%) for black patients and 84.5% (95% CI = 83.2% to 86.0%) for nonblack patients. The corresponding 100-month rates were 63.3% (95% CI = 55.2% to 72.5%) and 75.7% (95% CI = 73.9% to 77.5%), respectively. B) Overall survival (OS). OS rates at 60 months (5 years) were 87.6% (95% CI = 82.6% to 93.0%) for black patients and 91.9% (95% CI = 90.8% to 93.0%) for nonblack patients. Corresponding 100-month rates were 75.1% (95% CI = 67.6% to 83.4%) and 83.4% (95% CI = 81.8% to 85.0%), respectively. P values (two-sided) are from the log-rank test. CI = confidence interval; HR = hazard ratio.
To evaluate further whether the inferior overall survival of black patients was attributable specifically to breast cancer rather than to competing risks from comorbidities, we performed a post hoc cause-specific hazard analysis to examine the relationship between race and breast cancer–specific survival. For the total study population, black race was non-statistically significantly associated with worse breast cancer–specific survival (HR of death from breast cancer = 1.22, 95% CI = 0.95 to 1.53, P = .12). However, black race was statistically significantly associated with worse breast cancer–specific survival among patients with hormone receptor–positive, HER2-negative, or unknown disease (HR of death from breast cancer = 1.65, 95% CI = 1.11 to 2.46, P = .013) but not among patients with triple-negative disease (HR of death from breast cancer = 0.81, 95% CI = 0.51 to 1.28, P = .37) or those with HER2-positive disease (HR of death from breast cancer = 0.90, 95% CI = 0.51 to 1.59, P = .71). The results were nearly identical when we used subdistribution hazards analysis for competing risks (data not shown), which is another statistical method to adjust for competing risks (20). The results of these analyses suggest that the higher death rate among black patients with hormone receptor–positive and HER2-negative or unknown disease is due to higher breast cancer mortality and is not attributable to competing risks.
Multivariable Analysis in Hormone Receptor–Positive Disease
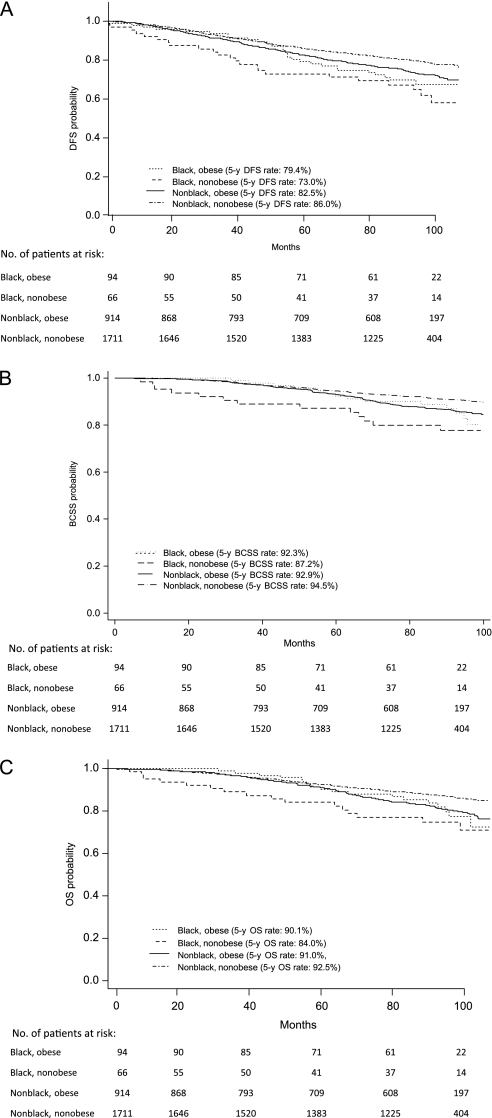
We further evaluated the statistically significant association between race and outcome in patients with hormone receptor–positive and HER2-negative or unknown disease by performing multivariable analyses that included potentially confounding covariates, including obesity (ie, BMI ≥ 30 kg/m2), which was more common among the black patients. Treatment arm was not included as a variable because there was no difference in the distribution of treatment arm assignment between black patients and nonblack patients (P = .91). We constructed four Cox proportional hazard models that included the following variables: 1) race alone, 2) obesity alone, 3) race and obesity, and 4) race, obesity, and their interaction (Table 2); results for these four models with inclusion of all covariates are shown in Supplementary Tables 1–3 (available online). Black race and obesity were independently and statistically significantly associated with worse disease-free, overall, and breast cancer–specific survival (models 1 and 2), and the associations with all three outcomes remained statistically significant when these variables were evaluated in the same model (model 3). In the model that included race, obesity, and their interaction (model 4), black race was statistically significantly associated with worse disease-free, overall, and breast cancer–specific survival in nonobese patients but not in obese patients. The Kaplan–Meier survival curves shown in Figure 2 demonstrate that black race was associated with inferior disease-free, breast cancer–specific, and overall survival outcomes only in patients who were not obese.
Table 2.
Estimated hazard ratios (HRs) and 95% confidence intervals (CIs) from multivariable models for disease-free, overall, and breast cancer–specific survival for black vs nonblack patients with hormone receptor–positive, HER2-negative or unknown disease*
| Model | Variable(s) | HR of breast cancer recurrence or death (95% CI) | HR of death (95% CI) | HR of death from breast cancer (95% CI) |
| 1 | Black race | 1.66 (1.25 to 2.21); P < .001 | 1.64 (1.15 to 2.33); P = .006 | 1.81 (1.23 to 2.68); P = .003 |
| 2 | Obesity (BMI ≥ 30 kg/m2) | 1.24 (1.08 to 1.49); P = .0032 | 1.40 (1.15 to 1.69); P < .001 | 1.45 (1.16 to 1.82); P = .0012 |
| 3 | Black race | 1.58 (1.18 to 2.11); P = .0021 | 1.51 (1.05 to 2.16); P = .025 | 1.65 (1.11 to 2.46); P = .013 |
| Obesity | 1.22 (1.04 to 1.44); P = .0141 | 1.35 (1.11 to 1.64); P = .003 | 1.39 (1.11 to 1.75); P = .005 | |
| 4 | Black race–obesity interaction: Black race vs other and BMI ≥30 kg/m2 | 1.19 (0.80 to 1.77); P = .3815 | 1.07 (0.65 to 1.74); P = .800 | 1.17 (0.68 to 2.01); P = .562 |
| Black race vs other and BMI <30 kg/m2 | 2.38 (1.57 to 3.60); P < .001 | 2.59 (1.56 to 4.32); P < .001 | 2.85 (1.61 to 5.02); P < .001 |
Other variables in these models include age (<50 vs ≥50 y), number of positive lymph nodes (0 [reference] vs 1–3 vs ≥4), tumor size (≤2 vs >2 cm), surgery (breast-sparing vs mastectomy), and type of hormone therapy used. Hormone therapy was coded as a time-varying variable, and all other variables were coded as time-fixed variables. All P values are two-sided. BMI = body mass index.
Figure 2.
Kaplan–Meier survival curves, by race and obesity, for breast cancer patients with hormone receptor–positive, HER2-negative or unknown disease. A) Disease-free survival (DFS). DFS rates at 60 months (5 years) were 79.4% (95% CI = 69.5% to 88.3%) for black obese patients, 73.0% (95% CI = 60.1% to 82.2%) for black nonobese patients, 82.5% (95% CI = 79.9% to 84.9%) for nonblack obese patients, and 86.0% (95% CI = 84.2% to 87.6%) for nonblack nonobese patients. The corresponding 100-month rates were 67.5% (95% CI = 56.0% to 76.6%), 58.1% (95% CI = 42.6% to 70.8%), 71.9% (95% CI = 68.6% to 75.0%), and 77.9% (95% CI = 75.6% to 80.0%), respectively. B) Breast cancer–specific survival (BCSS). BCSS rates at 60 months (5 years) were 92.3% (95% CI = 84.5% to 96.2%) for black obese patients, 87.2% (95% CI = 76.0% to 93.4%) for black nonobese patients, 92.9% (95% CI = 91.0% to 94.4%) for nonblack obese patients, and 94.5% (95% CI = 93.2% to 95.5%) for nonblack nonobese patients. The corresponding 100-month rates were 80.2% (95% CI = 67.8% to 88.2%), 77.6% (95% CI = 64.5% to 86.4%), 84.5% (95% CI = 81.6% to 86.9%), and 89.7% (95% CI = 87.9% to 91.2%), respectively. C) Overall survival (OS). OS rates at 60 months (5 years) were 90.1% (95% CI = 81.9% to 94.8%) for black obese patients, 84.0% (95% CI = 72.4% to 91.1%) for black nonobese patients, 91.0% (95% CI = 88.9% to 92.7%) for nonblack obese patients, and 92.5% (95% CI = 91.2% to 93.7%) for nonblack nonobese patients. The corresponding 100-month rates were 77.3% (95% CI = 65.1% to 85.7%), 71.1% (95% CI = 56.1% to 81.8%), 79.2% (95% CI = 76.1% to 82.0%), and 85.5% (95% CI = 83.9% to 87.6%), respectively. CI = confidence interval; HR = hazard ratio.
Because our breast cancer–specific survival analysis assumed that all patients who had a breast cancer recurrence but whose cause of death was coded as unknown had died of breast cancer (127 [18%] of 704 breast cancer–specific survival events), we performed a sensitivity analysis to examine whether the association between black race and worse breast cancer–specific survival would remain statistically significant if some of these patients had died of other causes. We therefore randomly attributed half of these 127 deaths to breast cancer and half to other causes and repeated the model 3 analysis 1000 times. In 40% of the 1000 repetitions, black race was associated with worse breast cancer–specific survival (P < .05; mean HR of death from breast cancer = 1.51, mean lower and upper limits of the 95% CIs were 0.98 and 2.33, respectively).
Interaction Analysis
We constructed additional models to further evaluate the interaction between race and other variables. In a model including race (black vs nonblack), disease subtype (hormone receptor positive and HER2 negative or unknown vs triple negative or HER2 positive), and their interaction, the interaction term was statistically significant for disease-free survival (P = .027) and breast cancer–specific survival (P = .0088) but not for overall survival (P = .086). In the disease-free survival model, estimated hazard ratios of recurrence or death for blacks vs nonblacks were 1.57 (95% CI = 1.18 to 2.08, P = .0019) for patients with hormone receptor–positive and HER2-negative or unknown disease and 1.03 (95% CI = 0.80 to 1.31, P = .84) for patients with other disease subtypes. In the overall survival model, the estimated hazard ratios of death for blacks vs nonblacks were 1.49 (95% CI = 1.05 to 2.11, P = .027) for patients with hormone receptor–positive and HER2-negative or unknown disease and 1.00 (95% CI = 0.75 to 1.33, P = .99) for patients with other disease subtypes. In the breast cancer–specific survival model, the estimated hazard ratios of death from breast cancer for blacks vs nonblacks were 1.67 (95% CI = 1.14 to 2.47, P = .0092) for patients with hormone receptor–positive and HER2-negative or unknown disease and 0.84 (95% CI = 0.59 to 1.18, P = .31) for patients with other disease subtypes.
Actual Delivery of Intended Chemotherapy and Endocrine Therapy
To examine whether inferior outcomes for black patients were due to disparities in the delivery of chemotherapy or endocrine therapy, we evaluated the actual therapy administered by race (Table 3). There were no statistically significant differences between black and nonblack patients in the proportion who received all four cycles of doxorubicin and cyclophosphamide (AC) or all cycles of taxane therapy. Black patients treated with docetaxel every 3 weeks were less likely than nonblack patients to require a dose reduction (18.9% vs 27.7%, P = .049), but there was otherwise no difference in the proportion who required taxane dose reduction in the other treatment arms. Information regarding adherence to endocrine therapy was missing for a considerable proportion of the patients. Among patients for whom this information was provided in the case report forms, there was no statistically significant difference in adherence to endocrine therapy between black and nonblack patients.
Table 3.
Chemotherapy and endocrine therapy adherence*
| Treatment administered | Black patients | Nonblack patients | P† |
| AC chemotherapy | |||
| No. of patients | 405 | 4412 | |
| Received all four cycles, No. (%) | 356 (87.9) | 3961 (89.8) | |
| Unknown, No. (%) | 6 (1.5) | 26 (0.6) | .48 |
| Every 3-wk paclitaxel | |||
| No. of patients | 99 | 1123 | .24 |
| Received all four cycles, No. (%) | 86 (86.9) | 1022 (91.0) | |
| Unknown, No. (%) | 2 (2.0) | 16 (1.4) | |
| No. of patients who received one or more cycles | 91 | 1063 | .75 |
| Dose reduced, No. (%) | 14 (15.4) | 148 (13.9) | |
| Unknown, No. (%) | 3 (3.3) | 42 (4.0) | |
| Weekly paclitaxel | |||
| No. of patients | 104 | 1092 | .25 |
| Received all 12 cycles, No. (%) | 84 (80.8) | 951 (87.1) | |
| Unknown, No. (%) | 5 (4.8) | 21 (1.9) | |
| No. of patients who received one or more cycles | 94 | 1034 | .78 |
| Dose reduced, No. (%) | 15 (16.0) | 184 (17.8) | |
| Unknown, No. (%) | 2 (2.1) | 32 (3.1) | |
| Every 3-wk docetaxel | |||
| No. of patients | 116 | 1087 | .48 |
| Received all four cycles, No. (%) | 91 (78.5) | 915 (84.2) | |
| Unknown, No. (%) | 6 (5.2) | 15 (1.4) | |
| No. of patients who received one or more cycles | 106 | 1031 | .049 |
| Dose reduced, No. (%) | 20 (18.9%) | 286 (27.7) | |
| Unknown, No. (%) | 3 (2.8%) | 40 (3.9) | |
| Weekly docetaxel | |||
| No. of patients | 86 | 1110 | .51 |
| Received all 12 cycles, No. (%) | 62 (72.1) | 828 (74.6) | |
| Unknown, No. (%) | 1 (1.2) | 20 (1.8) | |
| No. of patients who received one or more cycles | 80 | 1030 | .29 |
| Dose reduced, No. (%) | 16 (20.0) | 265 (25.7) | |
| Unknown, No. (%) | 2 (2.5) | 27 (2.6) | |
| Tamoxifen alone | |||
| No. with HR+ disease | |||
| Duration of tamoxifen use, No. (%) | 96 | 1160 | .67 |
| ≥5 y | 21 (21.9) | 210 (18.1) | |
| 3–5 y | 24 (25.0) | 314 (27.1) | |
| <3 y | 15 (15.6) | 188 (16.2) | |
| Unknown | 36 (37.5) | 448 (38.6) | |
| Sequential tamoxifen, aromatase inhibitor | |||
| No. with HR+ disease | |||
| Duration of tamoxifen use, No. (%) | .20 | ||
| ≥5 y | 101 | 1838 | |
| 3–5 y | 10 (9.9) | 141 (7.7) | |
| < 3 y | 33 (32.7) | 766 (41.7) | |
| Unknown | 41 (40.6) | 669 (36.4) | |
| Duration of aromatase inhibitor use, No. (%) | 17 (16.8) | 262 (14.3) | .57 |
| ≥3 y | 3 (3.0) | 42 (2.3) | |
| 1–3 y | 4 (4.0) | 122 (6.6) | |
| <1 y | 12 (11.9) | 233 (12.7) | |
| Unknown | 82 (81.2) | 1441 (78.4) |
AC = doxorubicin and cyclophosphamide; HR+ = hormone receptor positive.
P values are two-sided and calculated from the Fisher exact test.
Comparison of Adverse Events
We next examined whether the inferior outcomes for black patients were because they experienced more toxicity associated with therapy by evaluating adverse events associated with therapy by race. Black patients experienced less grade 4 neutropenia (31% vs 38%, P = .017) and more grade 2–4 neuropathy (25% vs 19%, P = .020) but had similar rates of grades 3 and 4 neuropathy and other adverse events compared with nonblack patients (Supplementary Table 4, available online). History of hypertension was not recorded at study entry. However, there was no statistically significant difference in the incidence of hypertension during adjuvant chemotherapy between black and nonblack patients (1.5% vs 9.8%, P = .16).
Discussion
We examined the association between black race and clinical outcomes in a cohort of 4817 women with stage I–III breast cancer who were enrolled in a clinical trial that included standard chemotherapy or chemotherapy plus endocrine therapy for hormone receptor–positive disease. Our results, similar to a previous report (6), revealed that the black patients were approximately twice as likely as the nonblack patients to have triple-negative disease, a breast cancer subtype that is associated with a higher risk of recurrence and death, which may contribute to the poorer outcomes for black patients with breast cancer. However, this is the first analysis to our knowledge to demonstrate worse outcomes in black women with hormone receptor–positive and HER2-negative disease but not in black women with other breast cancer subtypes.
Disparities in care and comorbidities are important factors that may contribute to inferior outcomes in black women with breast cancer. Previous population-based analyses (2) have demonstrated worse outcomes for black women with operable breast cancer, which have been attributed (21), in part, to disparities in care and the increase prevalence of comorbidities in black patients. However, all of the patients who participated in the E1199 trial received state-of-the-art medical care, suggesting that disparities in care were minimized. In addition, the eligibility criteria for the trial required patients to have normal cardiac, renal, hepatic, and bone marrow function, thereby excluding those with major comorbidities; also excluded were those with substantial cardiovascular disease, neuropathy, or poor performance status. However, information regarding lesser comorbidities that are known to be more common in black patients, such as hypertension and diabetes, was not collected, and their presence did not preclude trial participation.
Because eligibility for clinical trial participation introduces a selection bias, the effect of black race on clinical outcomes must be evaluated in the context of how care is delivered. For example, some evidence indicates that black breast cancer patients who are treated in community-based settings are more likely to discontinue adjuvant chemotherapy prematurely compared with nonblack patients (8). However, studies involving clinical trial populations have found that black patients had chemotherapy adherence and disease-free survival rates that were comparable to those for nonblack patients but still exhibited worse overall survival, suggesting that factors other than therapeutic adherence and cancer recurrence contributed to higher mortality rates in blacks (3,20,21). We have also found that black participants in a breast cancer clinical trial exhibited comparable adherence and toxicity compared with other trial participants (23). Although we cannot completely exclude the possibility that differences in the prevalence of comorbidities contributed to the poorer survival among the black women in this study, poorer survival should also have been evident across all breast cancer subtypes if it was a contributing factor. Moreover, the analysis of breast cancer–specific survival revealed that the higher death rates for black women were attributable, at least in part, to their higher breast cancer mortality rates.
To examine whether factors other than race contributed to the worse outcomes in black patients, we performed a multivariable analysis that evaluated associations between race and clinical outcomes after adjustment for other relevant prognostic factors. Because black patients had a statistically significantly higher median BMI and were more likely to be obese (defined as BMI ≥30 kg/m2) compared with nonblack patients, obesity was also included in the model. Obesity is known to be associated with more comorbidities and is also associated with higher breast cancer rates and worse outcomes for those who develop breast cancer (24). Our multivariable analyses suggest that race and obesity have independent adverse effects on survival: In multivariable models that evaluated the influence of race (excluding obesity as a variable) or obesity (excluding race as a variable) in patients with hormone receptor–positive and HER2-negative or unknown disease, each model demonstrated that obesity and black race were independently associated with inferior outcomes, including disease-free survival, breast cancer–specific survival, and overall survival. In a model that included race, obesity, and their interaction, there was a statistically significant interaction between black race and obesity, such that black race was associated with inferior outcomes only in nonobese patients. A similar finding was recently reported in a cohort of black woman diagnosed with breast cancer who were enrolled in the Women’s Contraceptive and Reproductive (CARE) Study (24). The results of our study and the CARE study suggest that black race confers additional risk of breast cancer recurrence and death but not in patients who are already at high risk because of obesity.
There are several plausible explanations for the associations we observed between race, obesity, and clinical outcomes. First, it is possible that the black patients were less adherent to endocrine therapy compared with nonblack patients. It is known that approximately 25% of women are not adherent to adjuvant tamoxifen or aromatase inhibitor therapy (19), and nonadherence may be more common in nonwhite patients (9). However, we found no evidence that adherence patterns differed between black and nonblack patients. Second, tamoxifen may be less extensively metabolized in blacks to its active metabolite endoxifen by the enzyme cytochrome 2D6 given the relatively high prevalence of certain cytochrome 2D6 alleles with reduced catalytic activity (eg, CYP2D6*2, CYP2D6*17) among blacks; however, it remains uncertain whether reduced tamoxifen metabolism is associated with worse clinical outcomes (25). Additional studies evaluating the association between race and outcomes in postmenopausal women treated with aromatase inhibitors may address this possibility. Third, obesity is associated with hyperinsulinemia, which has also been linked to poorer outcomes in women with operable breast cancer (26), possibly by contributing to the more aggressive behavior of endocrine-dependent breast cancer (27,28). Lastly, racial differences in the distribution of genotypically characterized breast cancer subtypes within the hormone receptor–positive subgroup examined (eg, luminal B vs luminal A disease) is another potential explanation that requires further study.
This study has several limitations. First, this was a retrospective analysis that was not prespecified in the original trial design. Second, although the multivariable models adjusted for clinical covariates, there were some differences in the characteristics of black breast cancer patients compared with nonblack patients that may have contributed to the observed differences. Third, we relied on information provided in the case report forms regarding adherence to endocrine therapy, which may not be as accurate as other more rigorous methods for monitoring adherence to orally administered drugs. Nevertheless, our results are consistent with the results of the CARE study and indicate that there is a complex relationship between black race, obesity, and clinical outcomes in breast cancer.
In conclusion, this analysis demonstrated that among patients with stage I–III hormone receptor–positive breast cancer who were treated with standard chemohormonal therapy, black patients had worse disease-free and overall survival compared with nonblack patients. Potential explanations include poorer adherence to endocrine therapy, differences in metabolism of antiestrogen therapy, and differences in other factors that contribute to antiestrogen therapy resistance. Additional studies are required to confirm these observations in other populations and to define contributing factors that could be targeted for therapeutic intervention. Studies that include ancestral genetic markers of race rather than self-reported race may provide additional insight into identifying a biological basis for these disparities.
Funding
Supported in part by grants from the Department of Health and Human Services and the National Institutes of Health (CA14958 to the Albert Einstein College of Medicine; CA23318 to the Eastern Cooperative Oncology Group (ECOG) statistical center; CA66636 to the ECOG data management center; CA21115 to the ECOG coordinating center and chairman's office; CA32012 to Southwest Oncology Group; CA11789 to Cancer and Leukemia Group B; CA25224 to North Central Cancer Treatment Group; CA49883 to the Indiana University School of Medicine; and CA16116 to Johns Hopkins Oncology Center).
Supplementary Material
Footnotes
V. Stearns has received grants or contracts from Abraxis, Merck, Novartis, and Pfizer and has received honoraria from AstraZeneca. E. A. Perez has received grants or contracts from Genentech, Sanofi Oncology, Novartis, and GlaxoSmithKline. G. W. Sledge is a paid board member of the Komen Foundation.
These data were presented in part at the San Antonio Breast Cancer Symposium, December 9–13, 2009 (San Antonio, TX).
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Newman LA, Mason J, Cote D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10,000 African-American and 40,000 White American patients with carcinoma of the breast. Cancer. 2002;94(11):2844–2854. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287(16):2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 5.Joslyn SA, West MM. Racial differences in breast carcinoma survival. Cancer. 2000;88(1):114–123. doi: 10.1002/(sici)1097-0142(20000101)88:1<114::aid-cncr16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 7.Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershman D, McBride R, Jacobson JS, et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639–6646. doi: 10.1200/JCO.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 9.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 10.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294(14):1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava A, Du XL. Racial and socioeconomic disparities in adjuvant chemotherapy for older women with lymph node-positive, operable breast cancer. Cancer. 2009;115(13):2999–3008. doi: 10.1002/cncr.24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider EC, Zaslavsky AM, Epstein AM. Racial disparities in the quality of care for enrollees in medicare managed care. JAMA. 2002;287(10):1288–1294. doi: 10.1001/jama.287.10.1288. [DOI] [PubMed] [Google Scholar]
- 13.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 14.Crew KD, Neugut AI, Wang X, et al. Racial disparities in treatment and survival of male breast cancer. J Clin Oncol. 2007;25(9):1089–1098. doi: 10.1200/JCO.2006.09.1710. [DOI] [PubMed] [Google Scholar]
- 15.Menashe I, Anderson WF, Jatoi I, Rosenberg PS. Underlying causes of the black-white racial disparity in breast cancer mortality: a population-based analysis. J Natl Cancer Inst. 2009;101(14):993–1000. doi: 10.1093/jnci/djp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer. 2003;98(5):894–899. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 17.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott-Conner CE, Christie DW. Cancer staging using the American Joint Committee on Cancer TNM System. J Am Coll Surg. 1995;181(2):182–188. [PubMed] [Google Scholar]
- 19.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23(3):619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 20.Fine JP Gray RJ. A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 21.Newman LA. Breast cancer in African-American women. Oncologist. 2005;10(1):1–14. doi: 10.1634/theoncologist.10-1-1. [DOI] [PubMed] [Google Scholar]
- 22.Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001;(30):36–43. doi: 10.1093/oxfordjournals.jncimonographs.a003458. [DOI] [PubMed] [Google Scholar]
- 23.Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009;27(13):2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, Ma H, Malone KE, et al. Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29(25):3358–3365. doi: 10.1200/JCO.2010.34.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briest S Stearns V. Tamoxifen metabolism and its effect on endocrine treatment of breast cancer. Clin Adv Hematol Oncol. 2009;7(3):185–192. [PubMed] [Google Scholar]
- 26.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20(1):42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 27.Oesterreich S, Zhang P, Guler RL, et al. Re-expression of estrogen receptor alpha in estrogen receptor alpha-negative MCF-7 cells restores both estrogen and insulin-like growth factor-mediated signaling and growth. Cancer Res. 2001;61(15):5771–5777. [PubMed] [Google Scholar]
- 28.Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin Cancer Res. 2001;7(12 Suppl):4429s–4435s. ; discussion 11s–12s. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.