Abstract
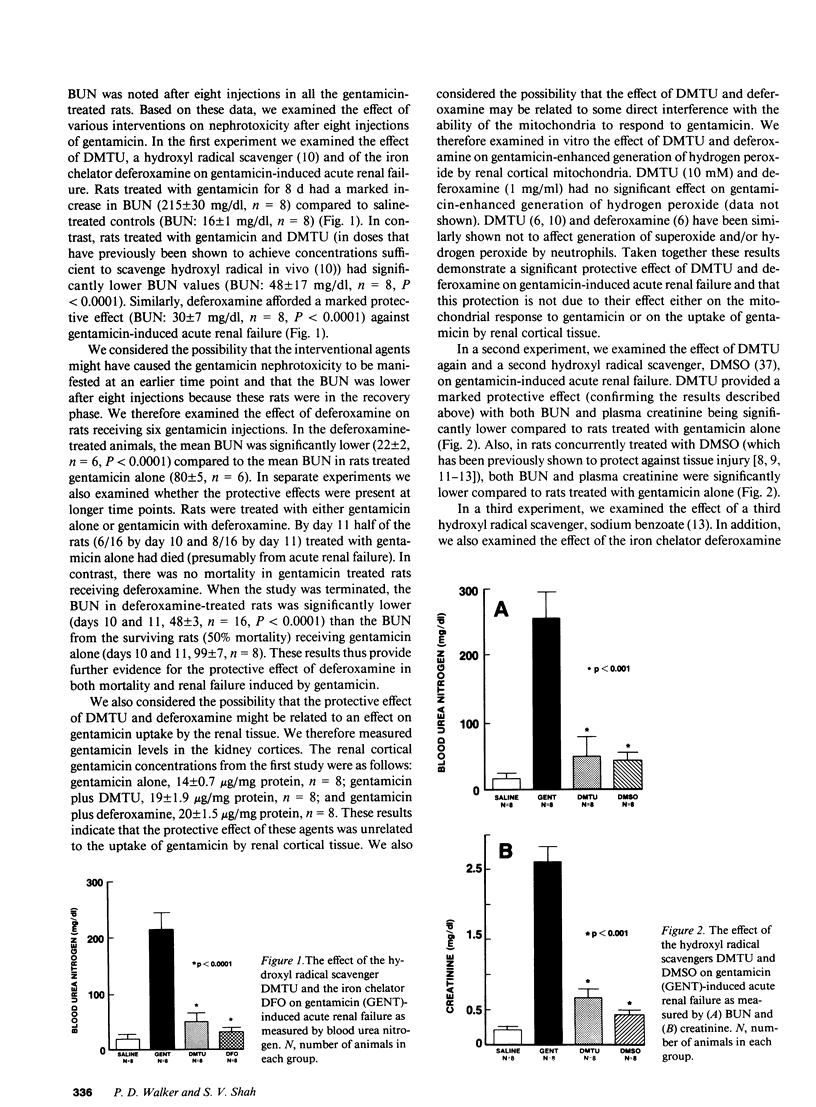
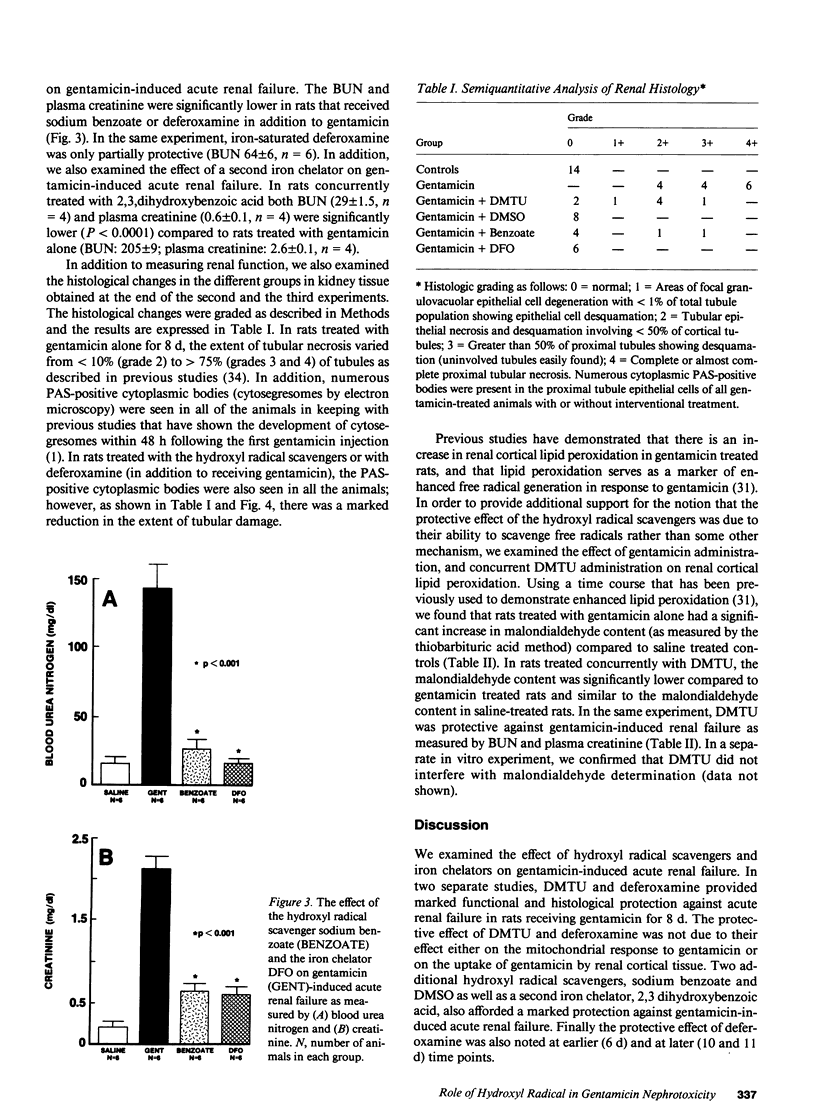
The protective effect of hydroxyl radical scavengers and iron chelators has strongly implicated the hydroxyl radical in several models of tissue injury. Based on in vitro studies showing gentamicin-enhanced generation of reactive oxygen metabolites in renal cortical mitochondria, we examined the effect of hydroxyl radical scavengers and iron chelators in gentamicin-induced acute renal failure. Rats treated with gentamicin (G) alone (100 mg/kg, s.c. x 8 d) developed advanced renal failure (BUN 215 +/- 30 mg/dl) compared to saline-treated controls (BUN 16 +/- 1 mg/dl, P less than 0.001). In contrast, rats treated with gentamicin and either dimethylthiourea (DMTU, an hydroxyl radical scavenger, 125 mg/kg, i.p. twice a day) or deferoxamine (DFO, an iron chelator, 20 mg/day by osmotic pump) had significantly lower BUN (G + DMTU 48.8 +/- 8 mg/dl, P less than 0.001, n = 8; G + DFO 30 +/- 7 mg/dl, P less than 0.001, n = 8). In separate experiments, treatment with two other hydroxyl radical scavengers (dimethyl sulfoxide or sodium benzoate) and a second iron chelator (2,3,dihydroxybenzoic acid) had a similar protective effect on renal function (as measured by both BUN and creatinine). In addition, histological evidence of damage was markedly reduced by the interventional agents. Finally, concurrent treatment with DMTU prevented the gentamicin induced increase in renal cortical malondialdehyde content (G: 4.4 +/- 0.2 nmol/mg; G + DMTU: 3.1 +/- 0.2 nmol/mg, P less than 0.0001, n = 8) suggesting that the protective effect of DMTU was related to free radical mechanisms rather than to some other effect. Taken together, these data strongly support a role for hydroxyl radical or a similar oxidant in gentamicin-induced acute renal failure.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., Beindorff C. M., Koster J. F. Superoxide-dependent and -independent mechanisms of iron mobilization from ferritin by xanthine oxidase. Implications for oxygen-free-radical-induced tissue destruction during ischaemia and inflammation. Biochem J. 1986 Oct 1;239(1):169–173. doi: 10.1042/bj2390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Martino E., Stoppani A. O. Evaluation of the horseradish peroxidase-scopoletin method for the measurement of hydrogen peroxide formation in biological systems. Anal Biochem. 1977 May 15;80(1):145–158. doi: 10.1016/0003-2697(77)90634-0. [DOI] [PubMed] [Google Scholar]
- Bowern N., Ramshaw I. A., Clark I. A., Doherty P. C. Inhibition of autoimmune neuropathological process by treatment with an iron-chelating agent. J Exp Med. 1984 Nov 1;160(5):1532–1543. doi: 10.1084/jem.160.5.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshow J. H., Davies K. J. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986 Mar 5;261(7):3068–3074. [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Polymorphonuclear leukocyte-mediated cell and tissue injury: oxygen metabolites and their relations to human disease. Hum Pathol. 1985 Oct;16(10):973–978. doi: 10.1016/s0046-8177(85)80273-2. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fligiel S. E., Ward P. A., Johnson K. J., Till G. O. Evidence for a role of hydroxyl radical in immune-complex-induced vasculitis. Am J Pathol. 1984 Jun;115(3):375–382. [PMC free article] [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano J. H., Grady R. W., Cerami A. The identification of 2, 3-dihydroxybenzoic acid as a potentially useful iron-chelating drug. J Pharmacol Exp Ther. 1974 Sep;190(3):570–575. [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of 'catalytic' iron and anti-oxidant activity in extracellular fluids. Biochem J. 1982 Sep 15;206(3):605–609. doi: 10.1042/bj2060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe S., Rowley D. A., Halliwell B. Reactions of ferrioxamine and desferrioxamine with the hydroxyl radical. Chem Biol Interact. 1982 Jul 15;41(1):75–81. doi: 10.1016/0009-2797(82)90018-7. [DOI] [PubMed] [Google Scholar]
- Houghton D. C., Plamp C. E., 3rd, DeFehr J. M., Bennett W. M., Porter G., Gilbert D. Gentamicin and tobramycin nephrotoxicity. A morphologic and functional comparison in the rat. Am J Pathol. 1978 Oct;93(1):137–152. [PMC free article] [PubMed] [Google Scholar]
- Humes H. D., Sastrasinh M., Weinberg J. M. Calcium is a competitive inhibitor of gentamicin-renal membrane binding interactions and dietary calcium supplementation protects against gentamicin nephrotoxicity. J Clin Invest. 1984 Jan;73(1):134–147. doi: 10.1172/JCI111184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A., Kunkel R. G., Wilson B. S. Mediation of IgA induced lung injury in the rat. Role of macrophages and reactive oxygen products. Lab Invest. 1986 May;54(5):499–506. [PubMed] [Google Scholar]
- Johnson W. T., Evans G. W. Effects of the interrelationship between dietary protein and minerals on tissue content of trace metals in streptozotocin-diabetic rats. J Nutr. 1984 Jan;114(1):180–190. doi: 10.1093/jn/114.1.180. [DOI] [PubMed] [Google Scholar]
- Komiyama T., Kikuchi T., Sugiura Y. Generation of hydroxyl radical by anticancer quinone drugs, carbazilquinone, mitomycin C, aclacinomycin A and adriamycin, in the presence of NADPH-cytochrome P-450 reductase. Biochem Pharmacol. 1982 Nov 15;31(22):3651–3656. doi: 10.1016/0006-2952(82)90590-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lotan D., Kaplan B. S., Fong J. S., Goodyer P. R., de Chadarevian J. P. Reduction of protein excretion by dimethyl sulfoxide in rats with passive Heymann nephritis. Kidney Int. 1984 May;25(5):778–788. doi: 10.1038/ki.1984.90. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd Nitrofurantoin: evidence for the oxidant injury of lung parenchymal cells. Am Rev Respir Dis. 1983 Apr;127(4):482–486. doi: 10.1164/arrd.1983.127.4.482. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor W. A. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- Ramsammy L. S., Josepovitz C., Ling K. Y., Lane B. P., Kaloyanides G. J. Effects of diphenyl-phenylenediamine on gentamicin-induced lipid peroxidation and toxicity in rat renal cortex. J Pharmacol Exp Ther. 1986 Jul;238(1):83–88. [PubMed] [Google Scholar]
- Ramsammy L. S., Josepovitz C., Ling K. Y., Lane B. P., Kaloyanides G. J. Failure of inhibition of lipid peroxidation by vitamin E to protect against gentamicin nephrotoxicity in the rat. Biochem Pharmacol. 1987 Jul 1;36(13):2125–2132. doi: 10.1016/0006-2952(87)90140-7. [DOI] [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Kunkel R. G., Wiggins R. C. Role of oxygen radicals in phorbol myristate acetate-induced glomerular injury. Kidney Int. 1985 Mar;27(3):503–511. doi: 10.1038/ki.1985.39. [DOI] [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Wiggins R. C., Kunkel R. G., Ward P. A. Evidence for the role of oxygen radicals in acute nephrotoxic nephritis. Lab Invest. 1984 Oct;51(4):396–403. [PubMed] [Google Scholar]
- Rehan A., Wiggins R. C., Kunkel R. G., Till G. O., Johnson K. J. Glomerular injury and proteinuria in rats after intrarenal injection of cobra venom factor. Evidence for the role of neutrophil-derived oxygen free radicals. Am J Pathol. 1986 Apr;123(1):57–66. [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. V., Baricos W. H., Basci A. Degradation of human glomerular basement membrane by stimulated neutrophils. Activation of a metalloproteinase(s) by reactive oxygen metabolites. J Clin Invest. 1987 Jan;79(1):25–31. doi: 10.1172/JCI112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. V., Cruz F. C., Baricos W. H. NADPH-induced chemiluminescence and lipid peroxidation in kidney microsomes. Kidney Int. 1983 May;23(5):691–698. doi: 10.1038/ki.1983.80. [DOI] [PubMed] [Google Scholar]
- Shah S. V. Effect of enzymatically generated reactive oxygen metabolites on the cyclic nucleotide content in isolated rat glomeruli. J Clin Invest. 1984 Aug;74(2):393–401. doi: 10.1172/JCI111434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Van Otto B., Smith A. L. A rapid chemical assay for gentamicin. N Engl J Med. 1972 Mar 16;286(11):583–586. doi: 10.1056/NEJM197203162861106. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Vanbenthuysen K. M., Shasby D. M., McMurtry I. F., Repine J. E. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis. 1982 Nov;126(5):802–806. doi: 10.1164/arrd.1982.126.5.802. [DOI] [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]
- Till G. O., Hatherill J. R., Tourtellotte W. W., Lutz M. J., Ward P. A. Lipid peroxidation and acute lung injury after thermal trauma to skin. Evidence of a role for hydroxyl radical. Am J Pathol. 1985 Jun;119(3):376–384. [PMC free article] [PubMed] [Google Scholar]
- Ulvik R. J. Relevance of ferritin-binding sites on isolated mitochondria to the mobilization of iron from ferritin. Biochim Biophys Acta. 1982 Mar 15;715(1):42–51. doi: 10.1016/0304-4165(82)90047-2. [DOI] [PubMed] [Google Scholar]
- Ulvik R., Romslo I. Studies on the mobilization of iron from ferritin by isolated rat liver mitochondria. Biochim Biophys Acta. 1979 Dec 3;588(2):256–271. doi: 10.1016/0304-4165(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Till G. O., Kunkel R. G., Ryan U. S., Ward P. A. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985 Dec;53(6):656–663. [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Hatherill J. R., Annesley T. M., Kunkel R. G. Systemic complement activation, lung injury, and products of lipid peroxidation. J Clin Invest. 1985 Aug;76(2):517–527. doi: 10.1172/JCI112001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]




