Abstract
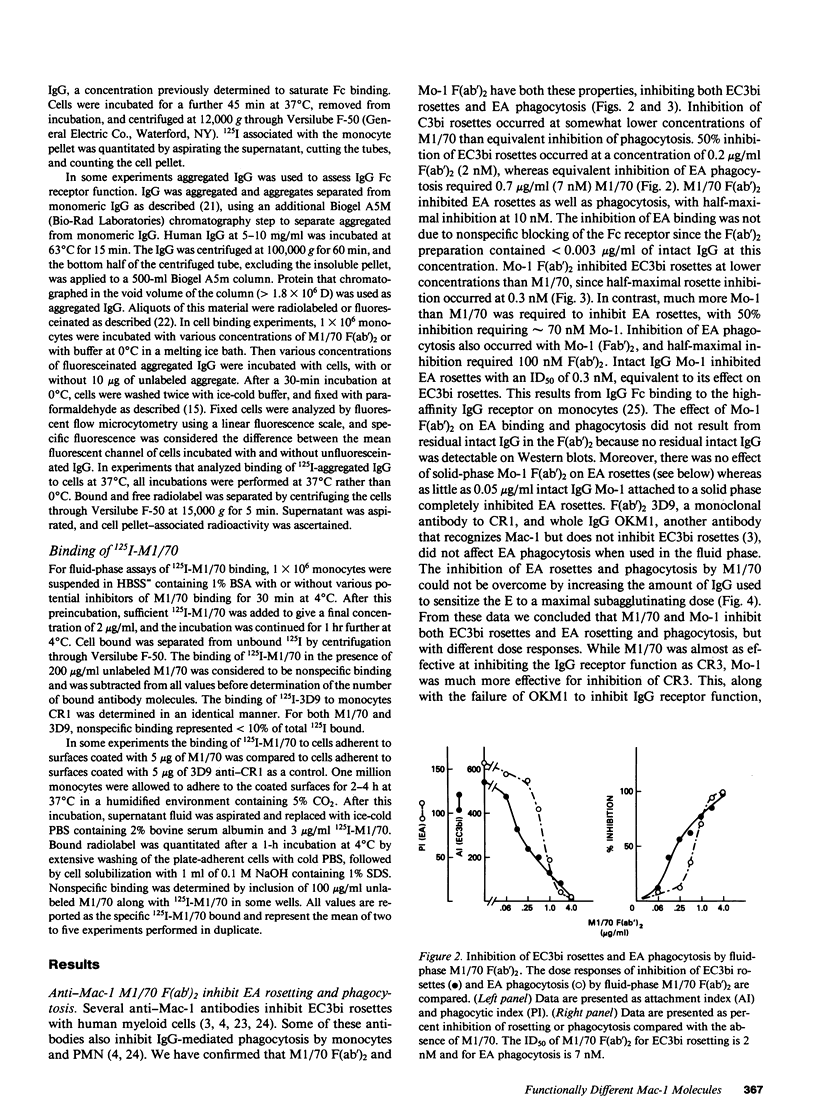
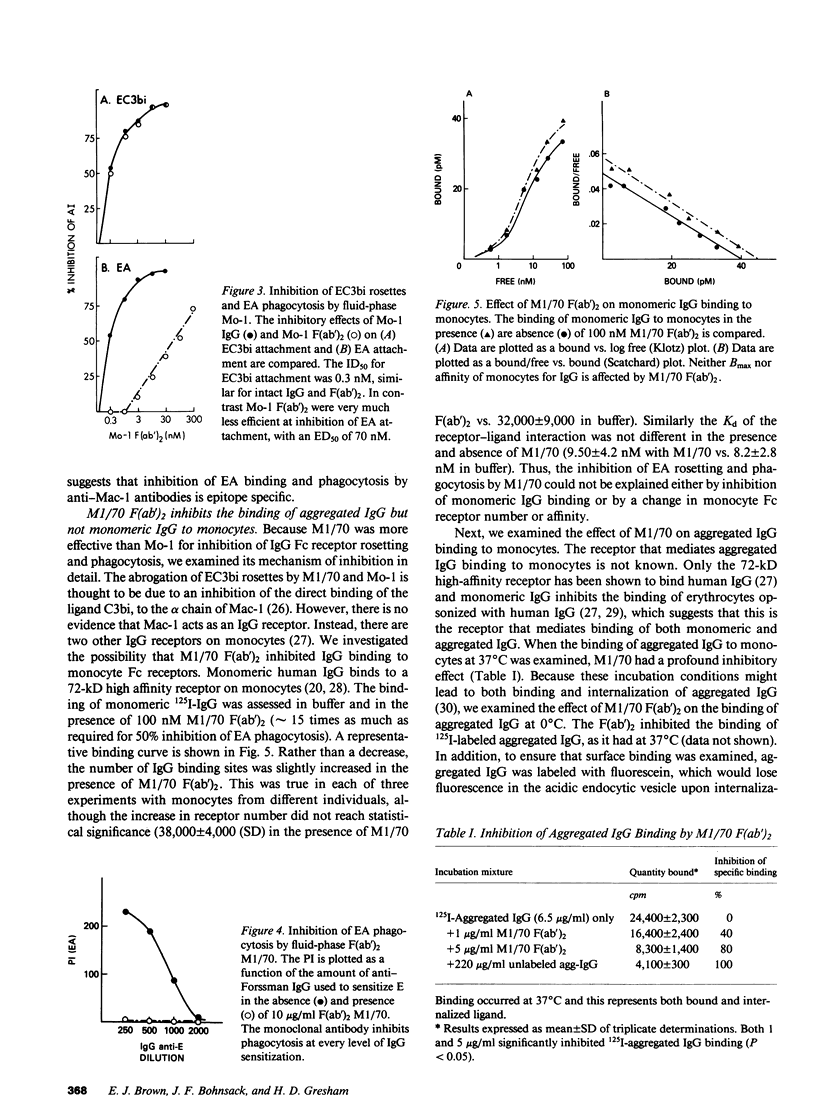
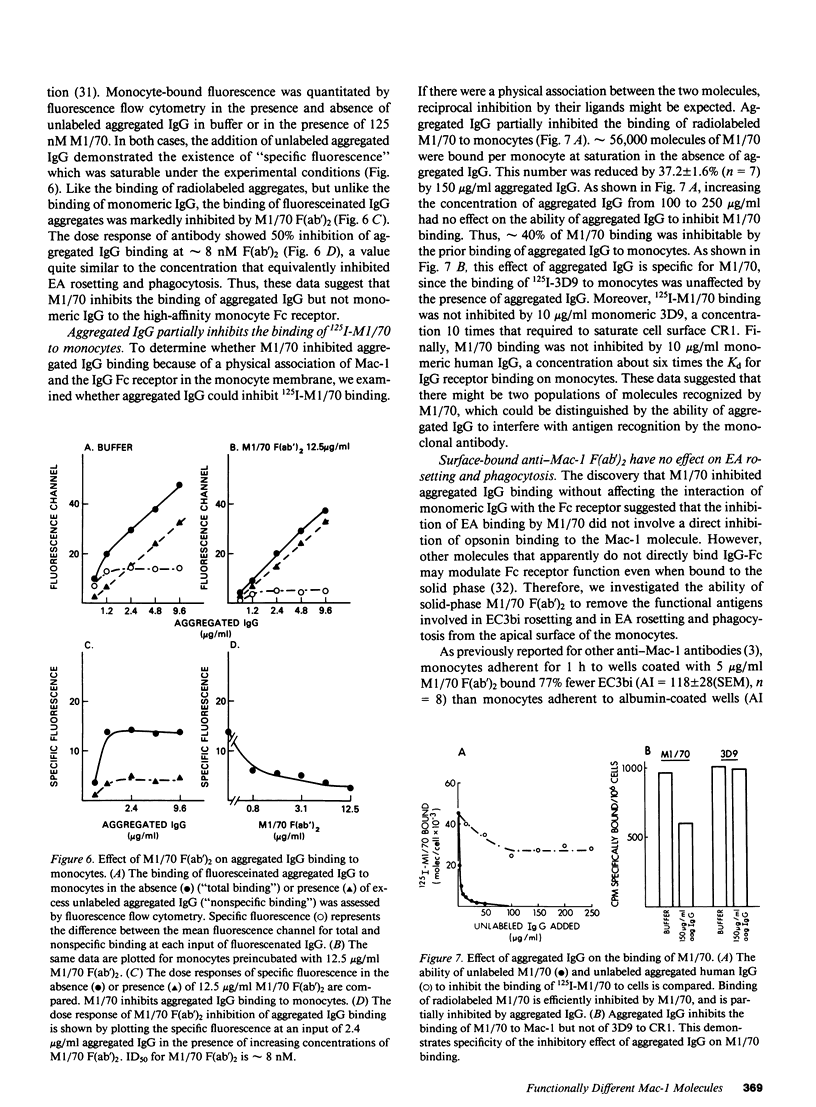
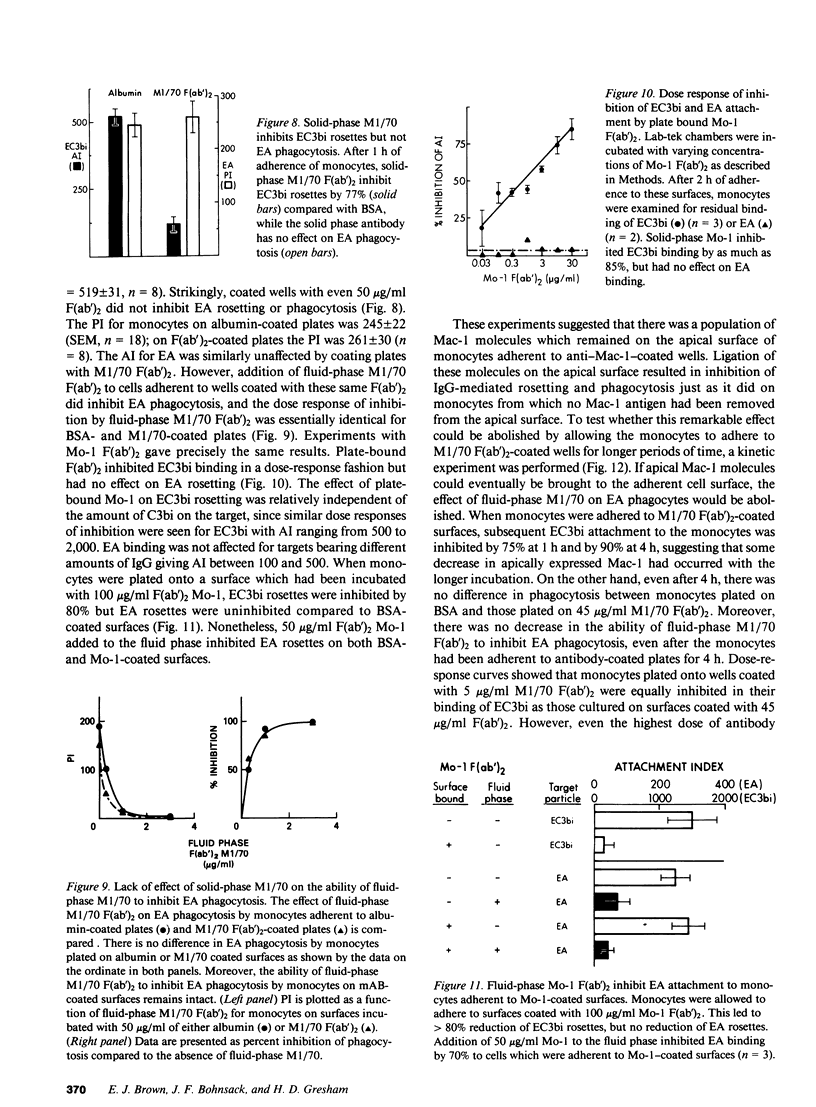
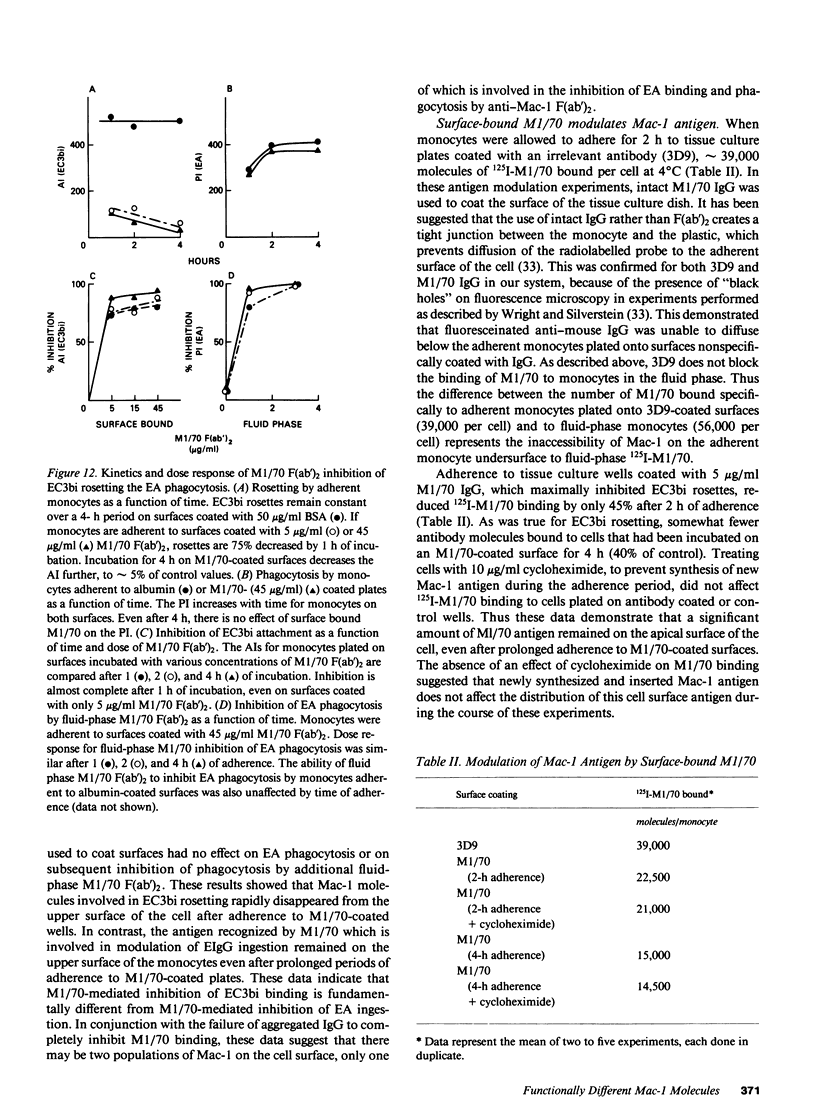
We have investigated the effects of the monoclonal antibodies against the cell surface molecule Mac-1 on C3bi-mediated rosetting and IgG-mediated rosetting and phagocytosis by human peripheral blood monocytes. Highly purified M1/70 F(ab')2, used in the fluid phase, inhibited both monocyte functions. Half-maximal C3bi rosette inhibition occurred at a concentration of 2 nM F(ab')2 M1/70. An equivalent decrease in IgG-mediated rosetting required 10 nM M1/70 F(ab')2, and 50% inhibition of IgG-mediated phagocytosis required 7 nM antibody. Mo-1 F(ab')2 inhibited EC3bi binding with an ID50 of 0.3 nM, whereas 50% decrease in IgG-mediated rosetting required 70 nM of this antibody. OKM1 did not inhibit rosettes of sheep erythrocytes opsonized with IgG antibody (EA) at all. F(ab')2 M1/70 did not affect the binding of monomeric human IgG to monocytes, but did substantially decrease the binding of IgG aggregates. Half-maximal inhibition of aggregated IgG binding at 0 degrees C occurred at 8 nM F(ab')2 M1/70, very close to the concentration that caused equivalent inhibition of IgG-mediated phagocytosis. Aggregated IgG inhibited the binding of radiolabeled M1/70 to monocytes by approximately 40%, suggesting that some, but not all Mac-1 molecules were associated with IgG receptors under these conditions. When cells were allowed to adhere to surfaces coated with M1/70 or Mo-1 F(ab')2, C3bi-mediated rosetting was inhibited, but IgG mediated-phagocytosis was unaffected. Moreover, the dose response of inhibition of phagocytosis by fluid-phase F(ab')2, of anti-Mac-1 monoclonals was similar on monocytes adherent to albumin-coated and antibody-coated surfaces. Kinetic experiments showed that even prolonged incubation of monocytes on M1/70 coated surfaces did not lead to inhibition of EA binding nor did these incubations alter the dose response for inhibition of EA binding by fluid-phase M1/70 F(ab')2. This suggested that not all molecules recognized by M1/70 are freely mobile in the plasma membrane. Indeed, only approximately 60% of 125I-M1/70-biding sites were lost even after 4 h when monocytes were adherent to M1/70-coated surfaces. We conclude that some anti-Mac-1 antibodies can inhibit EA binding because of their epitope specificity, independent of any direct interaction with monocyte Fc receptors. This interference with IgG-Fc receptor-mediated binding and ingestion apparently occurs because of antibody binding to a subpopulation of Mac-1 molecules which are associated with IgG Fc receptors and remain on the apical membrane of monocytes adherent to anti-Mac-1-coated surfaces. We suggest that there may be two functionally distinct molecules on human monocytes recognized by M1/70 and Mo-1 that can be distinguished by their mobility in the plane of the monocyte membrane. The more mobile form of Mac-1 is involved in C3bi rosettes, and does not affect IgG-mediated phagocytosis. The other antigen recognized by M1/70 does not diffuse within the plane of the membrane; ligation of the latter molecule by antibody is associated with inhibition of IgG-mediated phagocytosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L. Isolation of the receptor for IgG from a human monocyte cell line (U937) and from human peripheral blood monocytes. J Exp Med. 1982 Dec 1;156(6):1794–1806. doi: 10.1084/jem.156.6.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault K. A., Springer T. A. Cross-reaction of a rat-anti-mouse phagocyte-specific monoclonal antibody (anti-Mac-1) with human monocytes and natural killer cells. J Immunol. 1981 Jan;126(1):359–364. [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack J. F., Kleinman H. K., Takahashi T., O'Shea J. J., Brown E. J. Connective tissue proteins and phagocytic cell function. Laminin enhances complement and Fc-mediated phagocytosis by cultured human macrophages. J Exp Med. 1985 May 1;161(5):912–923. doi: 10.1084/jem.161.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack J. F., O'Shea J. J., Takahashi T., Brown E. J. Fibronectin-enhanced phagocytosis of an alternative pathway activator by human culture-derived macrophages is mediated by the C4b/C3b complement receptor (CR1). J Immunol. 1985 Oct;135(4):2680–2686. [PubMed] [Google Scholar]
- Brown E. J., Bekisz J. Neoantigens appear in human IgG upon antigen binding: detection by antibodies that react specifically with antigen-bound IgG. J Immunol. 1984 Mar;132(3):1346–1352. [PubMed] [Google Scholar]
- Carter S. D., Leslie R. G., Reeves W. G. Human monocyte binding of homologous monomer and complexed IgG. Immunology. 1982 Aug;46(4):793–800. [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis Y. I., Elson E. L. Inhibition of the mobility of mouse lymphocyte surface immunoglobulins by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1072–1076. doi: 10.1073/pnas.78.2.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Eskeland T., Seljelid R. Difference in the effect of immobilized ligands on the Fc and C3 receptors of mouse peritoneal macrophages in vitro. Scand J Immunol. 1978;7(1):19–24. doi: 10.1111/j.1365-3083.1978.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Kurlander R. J., Batker J. The binding of human immunoglobulin G1 monomer and small, covalently cross-linked polymers of immunoglobulin G1 to human peripheral blood monocytes and polymorphonuclear leukocytes. J Clin Invest. 1982 Jan;69(1):1–8. doi: 10.1172/JCI110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurlander R. J. Blockade of Fc receptor-mediated binding to U-937 cells by murine monoclonal antibodies directed against a variety of surface antigens. J Immunol. 1983 Jul;131(1):140–147. [PubMed] [Google Scholar]
- Kurlander R. J. Reversible and irreversible loss of Fc receptor function of human monocytes as a consequence of interaction with immunoglobulin G. J Clin Invest. 1980 Oct;66(4):773–781. doi: 10.1172/JCI109915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie R. G. Macrophage handling of soluble immune complexes. Ingestion and digestion of surface-bound complexes at 4, 20 and 37 degrees C. Eur J Immunol. 1980 May;10(5):323–333. doi: 10.1002/eji.1830100503. [DOI] [PubMed] [Google Scholar]
- Looney R. J., Abraham G. N., Anderson C. L. Human monocytes and U937 cells bear two distinct Fc receptors for IgG. J Immunol. 1986 Mar 1;136(5):1641–1647. [PubMed] [Google Scholar]
- Madsen L. H., Rodkey L. S. A method for preparing IgG F(ab')2 fragments using small amounts of serum. J Immunol Methods. 1976;9(3-4):355–361. doi: 10.1016/0022-1759(76)90210-6. [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H., Ukkonen P. Internalization and rapid recycling of macrophage Fc receptors tagged with monovalent antireceptor antibody: possible role of a prelysosomal compartment. J Cell Biol. 1984 Apr;98(4):1163–1169. doi: 10.1083/jcb.98.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Bell G. I., Silverstein S. C. Fc receptor modulation in mononuclear phagocytes maintained on immobilized immune complexes occurs by diffusion of the receptor molecule. J Exp Med. 1983 Jun 1;157(6):2121–2139. doi: 10.1084/jem.157.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Silverstein S. C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979 Sep 19;150(3):607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl J., Unkeless J. C., Pieczonka M. M., Silverstein S. C. Modulation of Fc receptors of mononuclear phagocytes by immobilized antigen-antibody complexes. Quantitative analysis of the relationship between ligand number and Fc receptor response. J Exp Med. 1983 Jun 1;157(6):1746–1757. doi: 10.1084/jem.157.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef W. M., De Alarcon P., Bale J. F., Clark R. A. Aberrant activation and regulation of the oxidative burst in neutrophils with Mol glycoprotein deficiency. J Immunol. 1986 Jul 15;137(2):636–642. [PubMed] [Google Scholar]
- O'Shea J. J., Brown E. J., Seligmann B. E., Metcalf J. A., Frank M. M., Gallin J. I. Evidence for distinct intracellular pools of receptors for C3b and C3bi in human neutrophils. J Immunol. 1985 Apr;134(4):2580–2587. [PubMed] [Google Scholar]
- Parham P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J Immunol. 1983 Dec;131(6):2895–2902. [PubMed] [Google Scholar]
- Peters R. Translational diffusion in the plasma membrane of single cells as studied by fluorescence microphotolysis. Cell Biol Int Rep. 1981 Aug;5(8):733–760. doi: 10.1016/0309-1651(81)90231-9. [DOI] [PubMed] [Google Scholar]
- Pommier C. G., Inada S., Fries L. F., Takahashi T., Frank M. M., Brown E. J. Plasma fibronectin enhances phagocytosis of opsonized particles by human peripheral blood monocytes. J Exp Med. 1983 Jun 1;157(6):1844–1854. doi: 10.1084/jem.157.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier C. G., O'Shea J., Chused T., Takahashi T., Ochoa M., Nutman T. B., Bianco C., Brown E. J. Differentiation stimuli induce receptors for plasma fibronectin on the human myelomonocytic cell line HL-60. Blood. 1984 Oct;64(4):858–866. [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Ross G. D., Newman S. L., Lambris J. D., Devery-Pocius J. E., Cain J. A., Lachmann P. J. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983 Aug 1;158(2):334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Steplewski Z., Lubeck M. D., Koprowski H. Human macrophages armed with murine immunoglobulin G2a antibodies to tumors destroy human cancer cells. Science. 1983 Aug 26;221(4613):865–867. doi: 10.1126/science.6879183. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Mouse peritoneal macrophages plated on mannan- and horseradish peroxidase-coated substrates lose the ability to phagocytose by their Fc receptors. J Immunol. 1985 Jun;134(6):3712–3717. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Phagocytosing macrophages exclude proteins from the zones of contact with opsonized targets. Nature. 1984 May 24;309(5966):359–361. doi: 10.1038/309359a0. [DOI] [PubMed] [Google Scholar]