Abstract
Background
22q11 Deletion Syndrome is a genetic syndrome associated with an increased risk for developing schizophrenia. Brain abnormalities have been reported in 22q11 Deletion Syndrome, but little is known about whether differences in brain structure underlie the psychotic disorders associated with this syndrome. In the current study, we used magnetic resonance imaging to characterize the structural brain abnormalities found in adults who have both 22q11 Deletion Syndrome and schizophrenia.
Methods
Magnetic resonance imaging brain scans of 14 adults (7 male, 7 female) with 22q11 Deletion Syndrome and schizophrenia and 14 age- and gender-matched healthy volunteers were analyzed to derive measures of gray matter, white matter, and cerebrospinal fluid. Differences between the two groups were tested using student t tests.
Results
22q11 Deletion Syndrome and schizophrenia subjects had significantly smaller total gray matter volume (t = 2.88, p < .01) and larger lateral ventricles (t = 4.08, p < .001) than healthy controls. Gray matter deficits were most prominent in the frontal and temporal lobes. Total white matter volumes did not differ between the two groups.
Conclusions
Findings from this 22q11 Deletion Syndrome and schizophrenia study are similar to those reported in other patients with schizophrenia, but only partially consistent with those reported in nonpsychotic children with 22q11 Deletion Syndrome. 22q11 Deletion Syndrome may provide a valuable genetic neurodevelop-mental model for investigating the relationship between abnormalities in brain development and the expression of schizophrenia.
Keywords: 22q11 Deletion Syndrome (22qDS), velocardiofacial syndrome, schizophrenia, magnetic resonance imaging (MRI), gray matter, cerebrospinal fluid (CSF) volumes
Introduction
22q11 Deletion Syndrome (22qDS), also known as velocardiofacial syndrome, DiGeorge syndrome, and conotruncal anomaly face syndrome, is a genetic syndrome associated with interstitial deletions at chromosome 22q11.2 (Cohen et al 1999; Thomas and Graham Jr. 1997). 22qDS is characterized by a variable phenotype that includes palatal abnormalities, characteristic facial features, congenital heart and other birth defects, and learning disabilities (Bassett and Chow 1999). Structural abnormalities of the brain have also been associated with the syndrome (Beemer et al 1986; Bingham et al 1997; Chow et al 1999; Mitnick et al 1994). Over 25% of adult patients with 22qDS develop psychotic disorders, predominantly schizophrenia (Murphy et al 1999). Chromosome 22q11.2 deletions have also been reported in up to 2% of patients with schizophrenia (Karayiorgou et al 1995), suggesting that 22qDS likely represents a genetic subtype of schizophrenia (Bassett and Chow 1999; Bassett et al 2000; Bassett et al 1998; Cowan and Kandel 2001). Investigation of brain structure in this more homogeneous subtype of schizophrenia may help elucidate the neurobiological mechanisms that underlie the expression of schizophrenia.
Previous neuroimaging studies of 22qDS have mainly involved children who do not have psychotic illnesses. Reported abnormalities include minor midline defects, focal white matter foci visible on T2-weighted magnetic resonance imaging (MRI) images, sulcal and ventricular enlargement, hypoplastic corpus callosum, small posterior fossa, and hypoplasia of cerebellar vermis (Altman et al 1995; Beemer et al 1986; Bingham et al 1997; Haapanen and Somer 1993; Lynch et al 1995; McDonald-McGinn et al 1995; Mitnick et al 1994). A quantitative MRI study by Eliez et al (2000) reported smaller total brain volumes, especially white matter volumes, greater corrected frontal lobe volume, and greater ventricle-to-brain volume ratios in 15 children and adolescents with 22qDS when compared with age- and gender-matched healthy controls. A similar study of 10 22qDS children and adolescents also found greater corrected frontal lobe and smaller nonfrontal lobar volumes than in age- and gender-matched healthy controls (Kates et al 2001).
In schizophrenia, the most consistent structural neuro-imaging findings are larger lateral and third ventricles and smaller gray matter volumes (Gur et al 2000a; Gur et al 2000b; Harvey et al 1993; Lim et al 1996; Zipursky et al 1998; Zipursky et al 1992; Zipursky et al 1997). White matter volumes in patients with schizophrenia tend not to be different from controls (Lim et al 1996; Zipursky et al 1998; Zipursky et al 1992). Smaller intracranial volumes in patients with schizophrenia have also been described, although a recent meta-analysis suggests that this effect is relatively small (Ward et al 1996). Ventricular and gray matter findings are present at the time of the first episode and current evidence suggests that the degree to which these findings deviate from healthy controls is not related to the duration of illness (Marsh et al 1994; Zipursky et al 1988). Midline defects such as cavum vergae or cavum septum pellucidum (Kwon et al 1998; Nopoulos et al 1998) and cerebellar changes (Andreasen et al 1994; Levitt et al 1999) have also been associated with schizophrenia.
There are few imaging studies of 22qDS individuals with a psychotic disorder. Greater mean ventricular volumes were reported in three adolescents with 22qDS and childhood-onset schizophrenia when compared with 64 healthy control children (Usiskin et al 1999), and there was a case report of observable midline defects and cerebral atrophy in an adult patient with 22qDS and schizophrenia (Vataja and Elomaa 1998). We have previously reported qualitative MRI findings in 11 adults with 22qDS and schizophrenia (22qDS-SZ) (Chow et al 1999) that included a high prevalence of white matter bright foci, cavum vergae/cavum septum pellucidum, sulcal and ventricular enlargement, and cerebellar atrophy. To our knowledge, no studies have described quantitative MRI findings in adult patients who have both 22qDS and schizophrenia.
Our current study examines the quantitative brain findings in adults with 22qDS-SZ using MRI. We predicted that patients with 22qDS-SZ would have quantitative differences in brain structure similar to those described in other patients with schizophrenia. To this end, we hypothesized that adults with 22qDS-SZ would have smaller gray matter volumes and larger ventricular volumes than age-and gender-matched healthy volunteers.
Methods and Materials
Subjects
Fourteen subjects (7 male, 7 female) who met clinical criteria for 22qDS and suffered from schizophrenia or schizoaffective disorder were studied. All had evidence of chromosome 22q11.2 deletions confirmed by fluorescence-in situ-hybridization testing using the N25 (Oncor Inc, Gaithersburg, MD) or the TUPLE1 probe (Vysis Inc, Downers Grove, IL). DSM-IV diagnoses were determined by research psychiatrists using the Structured Clinical Interview for DSM-IV (SCID-IV) (First et al 1995): 11 subjects met criteria for schizophrenia and three met criteria for schizoaffective disorder. The qualitative MRI results of 11 of the 22qDS-SZ subjects in this study have been reported previously (Chow et al 1999). The mean age for the 14 subjects with 22qDS-SZ was 27.5 (SD 6.4) years. The mean age at onset of psychosis was 21.0 (SD 2.1) years. The Silverstein method (Silverstein 1982) was used to estimate IQ of the 22qDS-SZ subjects with the vocabulary and block design subtests from the Weschler Adult Intelligence Scale, Revised (WAIS-R), (Wechsler 1981) yielding a group mean IQ of 71.3 (SD 10.0).
Fourteen normal control subjects who matched the 22qDS-SZ subjects in gender, and age to within 2 years (mean age for controls = 28.2 years, SD = 6.6 years) were used for age- and gender-matched statistical comparison of brain volumes. These control subjects were selected from a larger group of 47 community volunteers who had previously served as normal controls for a MRI study of first-episode schizophrenia (Zipursky et al 1998). All control subjects were interviewed either using the nonpatient version of the Structured Clinical Interview for DSM-III-R (Spitzer et al 1990) or for DSM-IV (First et al 1998) to exclude those with a history of any psychiatric disorder or any lifetime history of substance abuse or dependence. Control subjects were also excluded if they had a history of any neurologic or medical history that would affect brain structure. IQ, estimated using the National Assessment Reading Test (NART) (Blair and Spreen 1989), was available for 13 of the 14 age- and gender-matched control subjects: mean was 116.2 (SD 6.7). This research project was approved by the Research Ethics Board of the Center for Addiction and Mental Health and University of Toronto. Written informed consent to participate in this study was obtained for all 22qDS-SZ and control subjects.
MRI Protocol and Image Processing
All subjects were scanned on a GE Signa 1.5 Tesla MRI scanner (General Electric Medical Systems, Milwaukee) using a coronal 3-dimensional radio-frequency spoiled-gradient recalled echo (SPGR) sequence which yielded 124 contiguous images of 1.5 mm thickness. Images were processed using previously described BrainImage v. 2.29 software (Reiss et al 1995) running on an Apple PowerMac platform to yield whole brain volumes in cubic centimeters (cc) for gray matter, white matter, cerebrospinal fluid (CSF) and lateral ventricles. Images from the 14 22qDS-SZ subjects and the 14 matched community volunteers were processed by one rater. This rater, together with other raters with whom he had established acceptable inter-rater reliability (intraclass correlation coefficients= .99, .98, and .99, respectively, for gray matter, white matter, and CSF) processed the images from the remaining 34 of the total 47 community volunteer studied. All raters were blind to group membership of the subjects. As part of the processing, all brains were oriented in Talairach space, which facilitated the determination of gray matter and white matter volumes in each of the cerebral lobes (Reiss et al 1998; Talairach and Tournoux 1988). Motion artifact precluded tissue segmentation in one of the 22qDS-SZ subjects. Therefore, only ventricular volume was calculated for this subject and her matched control.
Data Analysis
Brain volumes were compared between the two subject groups using student t tests. All statistical tests were two-tailed and were considered significant at p < .05. The primary hypotheses being tested were that patients would have smaller total gray matter volumes and larger ventricle volumes than healthy volunteers. Group differences were tested using volumes uncorrected for total intracranial volume (ICV) as it can be argued that in disorders where ICV is reduced, removing the effect of ICV from individual brain measures may have the result of removing the very effect that is being investigated. When the brain measures from the 22qDS subjects were pooled with those of their matched healthy volunteers, volumes for total gray matter, total white matter, and total CSF were highly correlated with volume of total intracranial contents, ICV (gray matter + white matter + CSF) (r = .89, r = .78, r = .45, respectively). Therefore, corrected values for all brain measures were also calculated, using linear regression to remove the variance accounted for by ICV, and compared between the two groups.
To determine whether differences in brain volumes were restricted to particular brain areas, volumes for gray matter, white matter, and CSF were calculated for each lobe and compared between the two subject groups. As the sample size was limited, these analyses were considered to be exploratory.
To explore the effects of age on brain volumes in each group, we plotted the gray matter, white matter, CSF, and ventricular volumes (as a percentage of ICV) against age for the subjects in each group. To ascertain whether age affected gray matter differentially as a function of group after taking other factors into account, we performed a hierarchical multiple regression analysis. In this analysis, total gray matter volume served as the dependent variable, while independent predictor variables were entered in the following order: ICV, age, and diagnosis. In step 4, an interaction term was created by dummy coding diagnosis and multiplying it by age. This exploratory analysis was carried out for total gray matter volume and for gray matter volume in each of the lobes.
Results
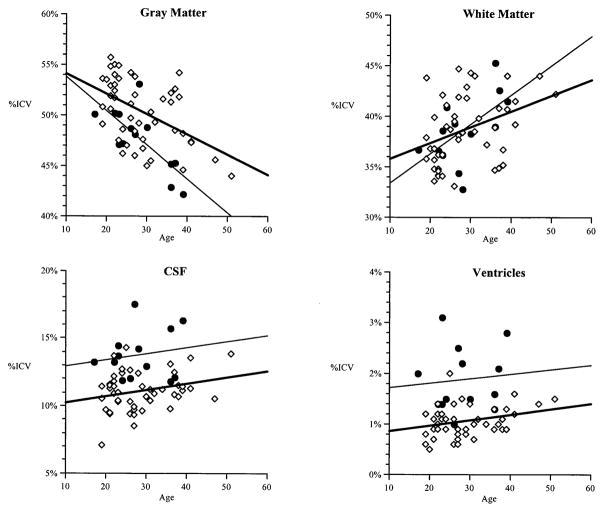
Gray matter, white matter, and CSF volumes for each hemisphere and for each cerebral lobe are listed in Table 1. Figure 1 shows total volumes for gray matter, white matter, ventricles, and CSF for the 13 22qDS-SZ subjects. The gray matter, white matter, ventricles, and CSF volumes for the 47 community volunteers are also shown in Figure 1 to enhance graphical presentation of data.
Table 1.
Brain Volumes of 13 22q Deletion Syndrome and Schizophrenia (22qDS-SZ) and 13 Age and Gender Matched Control Subjects, Uncorrected for Total Intracranial Volume
| Mean (SD)
|
||||
|---|---|---|---|---|
| Left Hemisphere
|
Right Hemisphere
|
|||
| 22qDS-SZ Subjects | Controls | 22qDS-SZ Subjects | Controls | |
| Total GM | 304.3 (35.4)c | 362.1 (51.3) | 307.8 (35.3)b | 343.3 (44.9) |
| WM | 247.1 (30.2) | 250.2 (38.8) | 249.3 (31.2) | 265.2 (50.1) |
| CSF | 88.9 (14.0) | 82.2 (16.9) | 88.0 (15.2)b | 75.3 (12.4) |
| Lateral Ventriclesa | 12.7 (4.9)c | 7.4 (3.3) | 12.1 (4.8)d | 5.7 (2.1) |
| Frontal GM | 92.6 (12.9)c | 110.1 (18.7) | 87.4 (15.8)b | 102.5 (13.3) |
| WM | 90.5 (12.2) | 87.0 (15.9) | 104.0 (16.9) | 97.8 (20.8) |
| CSF | 29.2 (5.2) | 26.9 (5.6) | 28.0 (5.9) | 24.9 (4.5) |
| Temporal GM | 61.7 (6.8)c | 75.0 (11.3) | 61.7 (6.2)b | 70.1 (10.2) |
| WM | 33.5 (5.0) | 34.0 (4.7) | 37.1 (6.2) | 40.7 (9.9) |
| CSF | 15.0 (2.7) | 13.6 (2.4) | 13.8 (2.6) | 12.2 (1.8) |
| Parietal GM | 52.1 (6.2)c | 62.4 (7.8) | 55.5 (6.6) | 59.0 (7.2) |
| WM | 50.1 (6.1) | 52.6 (8.8) | 48.5 (4.4)b | 55.1 (9.3) |
| CSF | 15.4 (2.5) | 14.6 (3.7) | 15.5 (3.0) | 13.1 (3.0) |
| Occipital GM | 30.4 (4.2)b | 34.0 (3.6) | 33.4 (4.9) | 32.4 (3.9) |
| WM | 22.9 (3.9) | 23.5 (5.6) | 15.3 (2.8)b | 20.7 (4.5) |
| CSF | 9.9 (2.0) | 9.0 (2.4) | 10.6 (1.9)c | 8.3 (1.8) |
Volumes are presented in cc; CSF, cerebrospinal fluid; GM, gray matter; WM, white matter.
N = 14 in each group.
p < .05.
p < .01.
p < .001.
Figure 1.
Gray matter, white matter, cerebro-spinal fluid (CSF), and ventricular volumes for 13 (14 for ventricular volumes) subjects with 22q11 Deletion Syndrome and schizophrenia (22qDS-SZ) (black circles) and 47 normal controls (open squares) by age at magnetic resonance imaging scan. All volumes are expressed as a percentage of intracranial volume (ICV). Regression lines are represented by solid bold lines for the healthy controls, and thin nonbolded lines for the 22qDS-SZ gray matter volumes.
Total Intracranial Volumes (ICV)
Subjects with 22qDS-SZ had brains which were on average 82 mL (6%) smaller than controls, but this difference was not statistically significant (mean ICV 1285.5 (SD 109.7) cc versus 1367.7 (SD 160) cc, t = 1.49, p = .15).
Gray Matter Volumes
Total gray matter volumes were significantly smaller in the 22qDS-SZ group than controls (mean = 612.2 [SD 68.5] cc vs. 705.5 (SD 94.7) cc, t = 2.88, p < .01). This finding persisted after correction for ICV (t = 3.16, p < .01). Gray matter volumes uncorrected for ICV were significantly smaller in the 22qDS-SZ group in right and left frontal lobes (t = 2.63, p < .05 and t = 2.78, p < .01), right and left temporal lobes (t = 2.56, p < .05 and t = 3.66, p < .01), left parietal lobe (t = 3.71, p < .01), and left occipital lobes (t = 2.32, p < .05). After correction for ICV, all differences remained significant except for those in the left occipital lobe.
White Matter Volumes
Total white matter volumes did not differ significantly between the two groups (mean = 496.4 [SD 60.4] cc vs. 515.4 [SD 88.5] cc, t = 0.64, p = .53); however, white matter volumes were smaller in the right parietal and occipital lobes in the 22qDS-SZ group (t = 2.30, p < .05 and t = 3.70, p < .01). After correction for ICV, the 22qDS-SZ group showed significant white matter deficits in the right frontal (t = 2.44, p < .05) and left frontal (t = 2.33, p < .05) as well as right occipital lobes (t = 3.29, p < .01).
Cerebrospinal Fluid Volumes and Lateral Ventricles
Total CSF volumes were greater in the 22qDS-SZ group (mean = 176.9 [SD 27.1] cc vs. 157.4 [SD 28.5] cc, t = 1.78, p = .09), reaching statistical significance only after controlling for ICV (t = 3.04, p < .01). Sulcal volumes were significantly greater in the right occipital lobe before correction for ICV (t = 3.13, p < .01), and after ICV correction in the right and left frontal lobes (t = 2.29, p < .05 and t = 2.06, p < .05), right and left temporal lobes (t = 2.91, p < .01 and t = 3.00, p < .01), right parietal lobe (t = 2.6, p < .05), and right occipital lobe (t = 3.65, p < .01).
Lateral ventricle volumes were much larger in the 22qDS-SZ group bilaterally, both before (t = 4.08, p < .001) and after correction for ICV (t = 4.37, p < .001).
Age Effects
There was a stronger correlation between smaller total gray matter volumes and older age in the 22qDS-SZ group (r = − .68) than in the control group (r = − .31) (Figure 1). The multiple regression analysis showed that, as expected, ICV was highly correlated with overall gray matter volumes (R2 = .79, p < .001). Age accounted for 4% of the variance (R2 = .04, p < .05) and diagnostic group for 7% of the variance (R2 = .073, p < .001). Age-by-diagnosis interaction was not significant for the total gray matter measure, and on regional analysis, there was a significant age-by-diagnosis interaction only for right occipital gray matter volumes (R2 = .143, F[4,21] = 6.21, p = .02).
Discussion
This is the first study to demonstrate that adult subjects with 22qDS and schizophrenia differ from normal controls on quantitative measures of brain tissue and fluid volumes. Subjects with 22qDS-SZ had strikingly larger lateral ventricles together with smaller gray matter volumes. The deficits in gray matter volume were most prominent in the frontal and temporal lobes. These findings are consistent with findings in other patients with schizophrenia (Gur et al 2000a; Gur et al 2000b; Harvey et al 1993; Lim et al 1996; Sullivan et al 1998; Zipursky et al 1992). These results raise the possibility that genetic abnormalities underlying 22qDS may provide clues to the pathogenesis of the brain abnormalities associated with schizophrenia.
Patients with schizophrenia show substantial variability in measures of ventricular and gray matter volumes as well as considerable overlap with normal controls. It has been tempting in the past to explain this variability as being the result of the etiologic heterogeneity of schizophrenia; however, all subjects with schizophrenia in this study had confirmed chromosome 22q11.2 deletions. It could be that the variability in brain structures observed reflects variability in the size and precise location of the deletions, although there are no reported correlations between the extent of the 22q11.2 deletion and the severity or spectrum of the 22qDS phenotype (Demczuk and Aurias 1995; Lindsay et al 1995). Alternatively, patients with similar genetic deletions and a shared etiology for their schizophrenia may still demonstrate substantial variability in measures of brain tissue and fluid volumes as a result of heterogeneity in the expression of the genetic abnormality. This is consistent with the recent report by Pearlson et al (1998) of substantial variability in brain measures in individuals with Down’s syndrome and the general variability of the 22qDS phenotype (Bassett and Chow 1999; Cohen et al 1999; Demczuk and Aurias 1995; Lindsay et al 1995).
Our findings of increased ventricular volume and decreased total gray matter volume are consistent with a study of children and adolescents with 22qDS by Eliez et al (2000). Both the current study and the Eliez et al (2000) study noted decreased left parietal gray matter volume. A recent study by Kates et al (2001) also found decreased left parietal lobe volume in 22qDS children, but the deficit was in white matter. There are several other findings reported in studies of children with 22qDS that were not detected in the current study of adults with 22qDS: decreased total brain volume, decreased total white matter volume, increased adjusted frontal lobe volume, and normal temporal lobe volume (Eliez et al 2000; Kates et al 2001). These differences are unlikely to be due to the image processing techniques, as all three studies used the same image processing software (Kates et al 1999). A number of factors likely account for these differences.
The current study, the Kates et al study (Kates et al 2001), and the Eliez et al study (Eliez et al 2000) involved small samples: 14, 10, and 15 22qDS subjects, respectively. The varying difference in the decreases in total brain volume observed 6% in the current study, 8.5% in the Kates et al (2001) study (both not statistically different), and 11% in the Eliez et al (2000) study (statistically significant) may therefore reflect sampling errors commonly associated with small sample sizes.
As in the general population, children with 22qDS may have a different pattern of volumetric brain measures than adults with the syndrome. Cross-sectional studies of healthy subjects indicate that cortical gray matter volumes decrease from childhood to adulthood, especially in the frontal lobes, and white matter volumes increase with age (Jernigan and Tallal 1990; Pfefferbaum et al 1994; Sowell et al 1999). A longitudinal MRI study confirmed significant decreases in total cortical, frontal, and parietal gray matter in normal adolescents over a 4-year period (Rapoport et al 1999). Gray matter volume decreases may therefore only become apparent during or after adolescence. Similarly, white matter deficits that were due to delayed neurodevelopment in 22qDS may disappear by adulthood.
All 22qDS subjects in the current study suffered from schizophrenia or schizoaffective disorder. The psychiatric status of children and adolescents with 22qDS in the two previous studies (Eliez et al 2000; Kates et al 2001) was unspecified. It is unlikely, however, that at an average age of 10.5 years (Eliez et al 2000) or 10.1 years (Kates et al 2001), any of the subjects had schizophrenia. A recent study of childhood onset schizophrenia suggests that total cortical gray matter reduction over time may be greater in schizophrenia than controls, and temporal lobe gray matter volume reduction over time may be associated with schizophrenia (Rapoport et al 1999). Cross-sectional data in the current study of 22qDS adults (Figure 1) suggested that gray matter volumes might be declining more rapidly with age in the patient group relative to the control group. Even though a greater age-dependent decline in total gray matter volume in 22qDS-SZ individuals relative to controls was not confirmed in a regression analysis, there was a significant age effect on regional (right occipital) gray matter volume reduction in 22qDS-SZ. Interestingly, an age-related decline in temporal gray matter volumes was recently reported in a cross-sectional study of 22qDS children (Eliez et al 2001). Taken together, these two findings may provide some supportive evidence of an abnormal or deteriorating neurodevelopmental course in 22qDS, which may or may not be modified by the expression of schizophrenia. Only a longitudinal investigation of individuals with 22qDS including repeated MRI assessments from childhood to adulthood could properly address the issue as to whether the degree and pattern of reductions in gray matter volumes are different in 22qDS subjects who eventually develop schizophrenia than in those who do not.
While we have described significant quantitative differences in ventricular and gray matter volumes in patients with 22qDS and schizophrenia in the current study, it would be very important to know whether those patients with 22qDS who do not go on to develop schizophrenia differ from this group in measures of brain structure. This would be critical in establishing a link between the type and magnitude of the brain abnormalities and the development of schizophrenia. Patients with 22qDS are believed to have a 25% risk of developing schizophrenia (Murphy et al 1999). If structural brain abnormalities are more common in those who develop schizophrenia, then qualitative and quantitative evaluation of MRI brain scans may have potential value in assisting in the identification of individuals with 22qDS who are most likely to develop schizophrenia. This could be of significant clinical importance in developing strategies for the possible prevention or early treatment of schizophrenia in individuals with 22qDS.
The current study has several limitations: 1) As mentioned above, the sample size for the 22qDS-SZ subjects and matched controls was small, and some true differences may therefore have been missed due to low power; however, the fact that several significant differences were observed indicates that effect sizes for brain structural abnormalities in this subtype of schizophrenia are relatively large. 2) Although other studies have noted changes in the size of cerebellum and corpus callosum in children with 22qDS (Altman et al 1995; Eliez et al 2000; Mitnick et al 1994; Usiskin et al 1999), these structures were not assessed in the present study. 3) We did not control for differences in IQ between the two groups in this study. It could be argued that some of the findings may be due to the lower IQ of the 22qDS-SZ subjects. IQ appears to correlate with total brain volumes in adults (Pennington et al 2000); however, total brain volumes did not differ significantly between the two groups in the current study, and our findings changed little after correction for total brain volumes. Also, lower intellectual functioning is a common feature of 22qDS (Bassett et al 1998; Golding-Kushner et al 1985; Swillen et al 1997); therefore, controlling for IQ may remove the precise effects that are of interest. 4) Findings in the current study may not apply to adults with 22qDS who do not have a psychotic disorder. Such individuals would make an interesting comparison group in future studies to help determine which findings are related to schizophrenia and which to 22qDS.
In summary, this quantitative MRI study of adults with 22qDS-SZ demonstrated differences in brain structures relative to normal controls that are consistent with previous findings in schizophrenia. This work suggests that 22qDS-SZ is associated with a number of brain structural abnormalities, which can be identified using quantitative methods. As only some individuals with 22qDS develop schizophrenia, it may be that particular brain differences underlie the development of schizophrenia in those affected patients. The results also suggest that 22qDS may provide a valuable model for investigating the relationship between genetically determined abnormalities in brain development and the expression of schizophrenia.
Acknowledgments
This study was supported by funding from the National Alliance for Research on Schizophrenia and Depression, Ontario Mental Health Foundation, Scottish Rite Schizophrenia Research Program, Clarke Institute Foundation Research Fund, and the Medical Research Council of Canada. The authors would like to thank Dr. Bruce Christensen for statistical advice, Laura Scutt for her help during MRI scanning, and Jeff Logan, Rae Dolman, and Edmond Chong for their help in processing MRI data.
References
- Altman DH, Altman NR, Mitnick RJ, Shprintzen RJ. Further delineation of brain anomalies in velo-cardio-facial syndrome. Am J Med Genet. 1995;60:174–175. doi: 10.1002/ajmg.1320600218. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V, O’Leary DS, et al. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. J Am Med Assoc. 1994;272:1763–1769. [PubMed] [Google Scholar]
- Bassett AS, Chow EWC. 22q11 Deletion Syndrome: A genetic subtype of schizophrenia. Biol Psychiatry. 1999;46:882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, Weksberg R. Chromosomal abnormalities and schizophrenia. Am J Med Genet. 2000;97:45–51. doi: 10.1002/(sici)1096-8628(200021)97:1<45::aid-ajmg6>3.0.co;2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Hodgkinson K, Chow EWC, Correia S, Scutt LE, Weksberg R. 22q11 Deletion Syndrome in adults with schizophrenia. Am J Med Genet (Neuropsychiatric Genet) 1998;81:328–337. [PMC free article] [PubMed] [Google Scholar]
- Beemer FA, de Nef JJ, Delleman JW, Bleeker-Wagemakers EM, Shprintzen RJ. Additional eye findings in a girl with the velo-cardio-facial syndrome (letter) Am J Med Genet. 1986;24:541–542. doi: 10.1002/ajmg.1320240319. [DOI] [PubMed] [Google Scholar]
- Bingham PM, Zimmerman RA, McDonald-McGinn D, Driscoll D, Emanuel BS, Zackai E. Enlarged sylvian fissures in infants with interstitial deletion of chromosome 22q11. Am J Med Genet. 1997;74:538–543. [PubMed] [Google Scholar]
- Blair J, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- Chow EWC, Mikulis DJ, Zipursky RB, Scutt LE, Weksberg R, Bassett AS. Qualitative MRI findings in adults with 22q11 Deletion Syndrome and schizophrenia. Biol Psychiatry. 1999;46:1436–1442. doi: 10.1016/s0006-3223(99)00150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Chow EWC, Weksberg R, Bassett AS. The phenotype of adults with the 22q11 Deletion Syndrome: A review. Am J Med Genet. 1999;86:359–365. doi: 10.1002/(sici)1096-8628(19991008)86:4<359::aid-ajmg10>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan WM, Kandel ER. Prospects for neurology and psychiatry. J Am Med Assoc. 2001;285:594–600. doi: 10.1001/jama.285.5.594. [DOI] [PubMed] [Google Scholar]
- Demczuk S, Aurias A. DiGeorge syndrome and related syndromes associated with 22q11.2 deletions: A review. Ann Genet. 1995;38:59–76. [PubMed] [Google Scholar]
- Eliez S, Blasey CM, Schmitt EJ, White CD, Hu D, Reiss AL. Velocardiofacial syndrome Are structural changes in the temporal and mesial temporal regions related to schizophrenia? Am J Psychiatry. 2001;158:447–453. doi: 10.1176/appi.ajp.158.3.447. [DOI] [PubMed] [Google Scholar]
- Eliez S, Schmitt JE, White CD, Reiss AL. Children and adolescents with velocardiofacial syndrome: A volumetric MRI study. Am J Psychiatry. 2000;157:409–415. doi: 10.1176/appi.ajp.157.3.409. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders -Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders -Non-patient Edition, Version 2.0. New York: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- Golding-Kushner KJ, Weller G, Shprintzen RJ. Velo-cardio-facial syndrome: Language and psychological profiles. J Craniofac Genetics Dev Biol. 1985;5:259–266. [PubMed] [Google Scholar]
- Gur R, Cowell P, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, et al. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000a;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Gur R, Turetsky B, Cowell P, Finkelman C, Maany V, Grossman RI, et al. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000b;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Haapanen ML, Somer M. Velocardiofacial syndrome: Analysis of phoniatric and other clinical findings. Folia Phoniatr. 1993;45:239–246. doi: 10.1159/000266268. [DOI] [PubMed] [Google Scholar]
- Harvey I, Ron M, Du Bouley G, Wicks D, Lewis S, Murray R. Reduction of cortical volume in schizophrenia on magnetic resonance imaging. Psychol Med. 1993;23:591–604. doi: 10.1017/s003329170002537x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Tallal P. Late childhood changes in brain morphology observable with MRI. Dev Med Child Neurol. 1990;32:379–385. doi: 10.1111/j.1469-8749.1990.tb16956.x. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates W, Warsofsky I, Patwardhan A, Abrams MT, Liu AM, Naidu S, et al. Automated Talairach atlas-based parcellation and measurement of cerebral lobes in children. Psychiatry Res. 1999;91:11–30. doi: 10.1016/s0925-4927(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Jabs EW, Rutberg J, Murphy AM, Grados M, et al. Regional cortical white matter reductions in velocardiofacial syndrome: A volumetric MRI analysis. Biol Psychiatry. 2001;49:677–684. doi: 10.1016/s0006-3223(00)01002-7. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Shenton ME, Hirayasu Y, Salisbury D, Fischer IA, Dickey CC, et al. MRI study of cavum septi pellucidi in schizophrenia, affective disorder, and schizotypal personality disorder. Am J Psychiatry. 1998;155:509–515. doi: 10.1176/ajp.155.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J, McCarley R, Nestor P, Petrescu C, Donnino R, Hirayasu Y, et al. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: Clinical and cognitive correlates. Am J Psychiatry. 1999;156:1105–1107. doi: 10.1176/ajp.156.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K, Sullivan E, Zipursky R, Pfefferbaum A. Cortical gray matter volume deficits in schizophrenia: A replication. Schizophr Res. 1996;20:157–164. doi: 10.1016/0920-9964(95)00081-x. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Greenberg F, Shaffer LG, Shapira SK, Scambler PJ, Baldini A. Submicroscopic deletions at 22q11.2: Variability of the clinical picture and delineation of a commonly deleted region. Am J Medical Genet. 1995;56:191–197. doi: 10.1002/ajmg.1320560216. [DOI] [PubMed] [Google Scholar]
- Lynch DR, McDonald-McGinn DM, Zackai EH, Emanuel BS, Driscoll DA, Whitaker LA, et al. Cerebellar atrophy in a patient with velocardiofacial syndrome. J Med Genet. 1995;32:561–563. doi: 10.1136/jmg.32.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L, Suddath R, Higgins N, Weinberger D. Medial temporal lobe structures in schizophrenia: Relationship of size to duration of illness. Schizophr Res. 1994;11:225–238. doi: 10.1016/0920-9964(94)90016-7. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Driscoll DA, Bason L, Christensen K, Lynch D, Sullivan K, et al. Autosomal dominant “Opitz” GBBB syndrome due to a 22q11.2 deletion. Am J Med Genet. 1995;59:103–113. doi: 10.1002/ajmg.1320590122. [DOI] [PubMed] [Google Scholar]
- Mitnick RJ, Bello JA, Shprintzen RJ. Brain anomalies in velo-cardio-facial syndrome. Am J Med Genet (Neuropsychiatric Genet) 1994;54:100–106. doi: 10.1002/ajmg.1320540204. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Giedd J, Andreasen N, Rapoport J. Frequency and severity of enlarged cavum septi pellucidi in childhood-onset schizophrenia. Am J Psychiatry. 1998;155:1074–1079. doi: 10.1176/ajp.155.8.1074. [DOI] [PubMed] [Google Scholar]
- Pearlson G, Breiter S, Aylward E, Warren AC, Grygorcewicz M, Frangou S, et al. MRI brain changes in subjects with Down syndrome with and without dementia. Dev Med Child Neurol. 1998;40:326–334. [PubMed] [Google Scholar]
- Pennington B, Filipek P, Lefly D, Chhabildas N, Kennedy DN, Simon JH, et al. A twin MRI study of size variations in human brain. J Cogn Neurosci. 2000;12:223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Hennessey J, Subramanium B, Rubin M, Beach L. Brain Image. Baltimore: Kennedy Krieger Institute Neuroimaging Center; 1995. [Google Scholar]
- Reiss AL, Hennessey JG, Rubin M, Beach L, Abrams MT, Warsofsky IS, et al. Reliability and validity of an algorithm for fuzzy tissue segmentation of MRI. J Comput Assist Tomogr. 1998;22:471–479. doi: 10.1097/00004728-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Silverstein AB. Two- and four- subtest short forms of the Wechsler Adult Intelligence Scale Revised. J Consult Clin Psychol. 1982;50:415–418. [Google Scholar]
- Sowell E, Thompson P, Holmes C, Batth R, Jernigan T, Toga A. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. Structured Clinical Interview for DSM-III-R. Washington, DC: American Psychiatric Press; 1990. Non-patient Edition. [Google Scholar]
- Sullivan E, Lim K, Mathalon D, Marsh L, Beal DM, Harris D, et al. A profile of cortical gray matter volume deficits characteristic of schizophrenia. Cereb Cortex. 1998;8:117–124. doi: 10.1093/cercor/8.2.117. [DOI] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, et al. Intelligence and psychosocial adjustment in velocardiofacial syndrome: A study of 37 children and adolescents with VCFS. J Med Genet. 1997;34:453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- Thomas JA, Graham JM., Jr Chromosome 22q11 deletion syndrome: An update and review for the primary pediatrician. Clin Pediatr. 1997;36:253–266. doi: 10.1177/000992289703600502. [DOI] [PubMed] [Google Scholar]
- Usiskin SI, Nicolson R, Krasnewich DM, Yan W, Lenane M, Wudarsky M, et al. Velocardiofacial syndrome in childhood-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 1999;38:1536–1543. doi: 10.1097/00004583-199912000-00015. [DOI] [PubMed] [Google Scholar]
- Vataja R, Elomaa E. Midline brain anomalies and schizophrenia in people with CATCH 22 syndrome. Br Journal Psychiatry. 1998;172:518–520. doi: 10.1192/bjp.172.6.518. [DOI] [PubMed] [Google Scholar]
- Ward K, Friedman L, Wise A, Schulz S. Meta-analysis of brain and cranial size in schizophrenia. Schizophr Res. 1996;22:197–213. doi: 10.1016/s0920-9964(96)00076-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation. Harcourt Brace Jovanovich; 1981. [Google Scholar]
- Zipursky R, Lim K, Rosenbloom M, Pfefferbaum A. Differing effects of duration of illness on CT findings in alcoholism and schizophrenia. Psychopharmacol Bull. 1988;24:495–500. [PubMed] [Google Scholar]
- Zipursky RB, Lambe EK, Kapur S, Mikulis DJ. Cerebral gray matter volume deficits in first episode psychosis. Arch Gen Psychiatry. 1998;55:540–546. doi: 10.1001/archpsyc.55.6.540. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry. 1992;49:195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Seeman MV, Bury A, Langevin R, Wortzman G, Katz R. Deficits in gray matter volume are present in schizophrenia but not bipolar disorder. Schizophren Res. 1997;26:85–92. doi: 10.1016/s0920-9964(97)00042-x. [DOI] [PubMed] [Google Scholar]