Abstract
Gallstone disease (GD) is a chronic recurrent hepatobiliary disease, the basis for which is the impaired metabolism of cholesterol, bilirubin and bile acids, which is characterized by the formation of gallstones in the hepatic bile duct, common bile duct, or gallbladder. GD is one of the most prevalent gastrointestinal diseases with a substantial burden to health care systems. GD can result in serious outcomes, such as acute gallstone pancreatitis and gallbladder cancer. The epidemiology, pathogenesis and treatment of GD are discussed in this review. The prevalence of GD varies widely by region. The prevalence of gallstone disease has increased in recent years. This is connected with a change in lifestyle: reduction of motor activity, reduction of the physical load and changes to diets. One of the important benefits of early screening for gallstone disease is that ultrasonography can detect asymptomatic cases, which results in early treatment and the prevention of serious outcomes. The pathogenesis of GD is suggested to be multifactorial and probably develops from complex interactions between many genetic and environmental factors. It suggests that corticosteroids and oral contraceptives, which contain hormones related to steroid hormones, may be regarded as a model system of cholelithiasis development in man. The achievement in the study of the physiology of bile formation and the pathogenesis of GD has allowed expanding indications for therapeutic treatment of GD.
Keywords: Gallstone disease: Epidemiology, Pathogenesis of cholesterol stones, Treatment
INTRODUCTION
Gallstone disease (GD) (cholelithiasis) is one of the most prevalent gastrointestinal diseases, with a substantial burden to health care systems[1]. Gallstones (GS) may form because of many different disorders[2]. GD is a chronic recurrent hepatobiliary disease, the basis for which is the impaired metabolism of cholesterol, bilirubin and bile acids, which is characterized by the formation of gallstones in the hepatic bile duct, common bile duct, or gallbladder[3]. GD and cardiovascular disease, common diseases worldwide, are strongly associated and have considerable economical impact[4-6]. Among gastroenterological diseases, GD is one of the world’s most expensive medical conditions[7]. In the United States, there are more than 500 000 cholecystectomies, the total cost of which exceeds 5 billion dollars[8]. GS are considered avoidable causes of death[9].
EPIDEMIOLOGY
GD is a common disorder all over the world[10]. The prevalence of GD varies widely by region. In Western countries, the prevalence of gallstone disease reportedly ranges from approximately 7.9% in men to 16.6% in women[11]. In Asians, it ranges from approximately 3% to 15%, is nearly non-existent (less than 5%) in Africans[12,13], and ranges from 4.21% to 11% in China[14]. The prevalence of gallstone disease is also high in some ethnic groups, e.g., 73% in Pima Indian women; 29.5% and 64.1% of American Indian men and women, respectively; and 8.9% and 26.7% of Mexican American men and women, respectively[11,15,16]. With an overall prevalence of 10%-20%, GD represents one of the most frequent and economically relevant health problems of industrialized countries[17]. There is a steady-state trend for higher GD morbidity, which is associated with the improved diagnosis of the disease. One of the important benefits of early screening for gallstone disease is that ultrasonography can detect asymptomatic cases, which results in early treatment and the prevention of serious outcomes[1,18]. The reference standard to detect GS was represented, not only by the ultrasonographic scan of the gallbladder, but also by the direct examination of the explanted liver[2].
The Hispanic and indigenous populations of the United States show particularly high morbidity rates[19,20]. Epidemiological survey data in the United States suggest that approximately 20 million Americans suffer from GD. At the same time, GD is, on the contrary, less characteristic for the peoples of southeast Asia, Africa and the far north[21].
In Russia, the prevalence of GD among the examinees ranges from 3% to 12%. The prevalence of gallbladder and biliary tract diseases among the digestive ones is 15.8% in Russian adults, while this index is as high as 22% in Moscow.
ETIOLOGY OF GALLSTONE DISEASE
GD is a multifactorial disease. In the general population, one of the main risk factors for developing GD is gender: gallstones are more common in women than in men. Other factors are age, genes and race. Additional factors are obesity, rapid weight loss, glucose intolerance, insulin resistance, high dietary glycemic load, alcohol use, diabetes mellitus, hypertriglyceridemia, drugs and pregnancy[2]. Four major groups of factors that contribute to the formation of cholesterol gallstones to some degree may be identified[22,23]: (1) those that contribute to cholesterol supersaturation of bile; (2) those that contribute to cholesterol precipitation and crystallization core formation; (3) those that result in impairment of basic gallbladder functions (contraction, absorption, secretion, etc); and (4) those that lead to impairment of the enterohepatic circulation of bile acids.
Factors that contribute to bile cholesterol supersaturation
Age: Gallstone detection rates increase with age, which makes it possible to consider it one of the risk factors for GD[24]. No significant differences have been found in the frequency of gallstone formations in childhood and adolescence. Cholelithiasis in children is an unusual finding but is not exceptional and is associated with nonspecific symptoms[25,26]. After 20 years of age, the rate of gallstone formation increases with each decade[27]. If GD occurs in 7%-11% of cases in a group of subjects under the age of 50 years, then calculi are detectable in 11%-30% of subjects aged 60-70 years and in 33%-50% of those over 90 years of age. The amount of cholesterol in the bile is supposed to increase with age[28]. This is caused by dyslipoproteinemia that results in a linear increase in cholesterol excretion into the bile and by the reduced synthesis of bile acids due to the dropped activity of the enzyme cholesterol 7α-hydroxylase (CYP7A1)[29]. The xenobiotic receptor, pregnant X receptor (PXR), has a role in the pathogenesis of cholesterol GD[30]. PXR prevents cholesterol GD via its coordinated regulation of the biosynthesis and transport of bile salts in the liver and intestine. Cholesterol precipitation is prevented by increases in concentrations of biliary bile salts and a reduced cholesterol saturation index (CSI)[30]. Loss of PXR sensitized mice to lithogenic diet-induced cholesterol GD, characterized by decreases in biliary concentrations of bile salts and phospholipids and increases in the CSI and formation of cholesterol crystals. The decreased bile acid pool size in PXR-/- mice that received lithogenic diets was associated with reduced expression of CYP7A1, the rate-limiting enzyme of cholesterol catabolism and bile acid formation. The reduced expression of CYP7A1 most likely resulted from activation of PXR and induction of fibroblast growth factor 15 in the intestine[30].
There is a negative correlation between age and the amount of synthesized bile acids and a positive correlation between cholesterol levels and age. Furthermore, hemoperfusion of the gallbladder wall is noted to be reduced with age due to the presence of sclerotic changes. This contributes to the dysfunction of the gallbladder, its infection and inflammation with exudation into the lumen of the organ.
Gender: The female gender is a generally recognized risk factor of GD[10,24,31-33]. Marschall HU and Einarsson C[34] assume that age and sex are profoundly associated with the incidence of gallstone disease; the metabolic risk factors for gallstone disease are different between men and women[1,29]. In reproductive-aged women, the risk of cholelithiasis is 2-3 times higher than that in men[10]. The reasons for this have not been fully elucidated. Pregnancies also contribute to formation of stones in the gallbladder[10,22,33]. GD is particularly common in multiparas (parity 4 or more). Gender differences and frequent GS detections in pregnant women are linked with hormonal background[10]. Elevated estrogen levels are known to increase cholesterol excretion into the bile by causing its supersaturation with cholesterol. During pregnancy, in addition to the elevated level of estrogens, gallbladder evacuation function suffers, giving rise to bile sludge and gallstones. Hormone replacement therapy (HRT) with estrogen-containing agents in postmenopausal women[35] and the use of hormonal oral contraceptives[19] may increase the risk of symptomatic GS. Use of HRT is positively associated with an increased risk of symptomatic GS in this population. This confirms trial data and additionally shows effects of duration of use and increased risk associated with past use[36]. Opinions regarding the association between gallbladder disease and oral contraceptives differ[19]. This may be associated with the fact that the effect of estrogens is dose-dependent. Therefore, the currently available low-dose estrogen-gestagen combination oral contraceptives have a lower risk for GD[10].
Regarding gender, despite of the higher absolute frequency of GS in females with cirrhosis, the risk of cholelithiasis in cirrhotic males is much higher than in the healthy population[2]. Fornari et al[37] claimed that cirrhosis is a risk factor for GD in males and suggested that a high level of estrogens could play a role by an impairment of gallbladder emptying, as observed also in pregnant women. Age, sex and body mass index (BMI), relevant factors for GS development in the general population, are much less important in patients affected by cirrhosis where the main factor to be considered is the degree of impairment of underlying liver disease[2].
Genetic factors: There is growing evidence that GS formation may be genetically determined[38]. The risk of GS formation is 2-4 times higher in individuals whose relatives suffer from GD[32,39]. In cases of family GD, genetic factors play a prevailing role and are characterized by autosomal dominant inheritance[31,40]. Genetic susceptibility contributes to the etiology of gallbladder diseases, as shown by multiple epidemiological studies. Murine experiments have shown that there is a lithogenicity gene[41]. A major gallstone susceptibility locus (Lith6) was identified in 2003 by quantitative trait locus mapping in mice. Two attractive positional and functional candidate genes in apolipoprotein B mRNA-editing protein (APOBEC1) and peroxisome proliferator-activated receptor gamma (PPARG) are located in this interval. In the investigated German samples, no evidence of association of APOBEC1 and PPARG with gallstone susceptibility was detected. Systematic fine mapping of the complete Lith6 region is required to identify the causative genetic variants for gallstone in mice and humans[42]. From quantitative trait locus mapping in inbred mice, Kovacs P et al[43] identified the Nr1h4 gene encoding the nuclear bile salt receptor FXR (farnesoid X receptor) as a candidate gene for the cholesterol gallstone susceptibility locus Lith7. Genome wide scans of inbred strains of mice have linked the genes encoding the hepatocanalicular cholesterol transporter. ATP binding cassette (ABC) G5 and G8 (ABCG5/G8) are sterol export pumps which regulate biliary cholesterol absorption and excretion. Supersaturation of bile with cholesterol is a primary step in the formation of cholesterol gallstones. The function of this transporter and the results of the genetic study taken together indicate that in gallstone-susceptible carriers of the ABCG8 19H allele, cholesterol cholelithiasis is secondary to increased hepatobiliary cholesterol secretion[44]. The formation of GS, supersaturated with cholesterol in bile, is determined by genetic and environmental factors. The linkage and association studies identified the cholesterol transporter ABCG5/G8 as a genetic determinant of GS formation, or LITH gene, in humans. The interaction of susceptible gene polymorphisms with age, sex and BMI in GD is unclear. Carriers of ABCG5 604Q or ABCG8 D19H polymorphisms have an increased risk of GD independent of age, sex and BMI[45]. The T400K polymorphism in ABCG8 may be associated with the incidence of GD in males[46]. The genes associated with the development of GD are assumed to be located mainly on chromosomes 3, 4, 9 and 11[47]. The increased expression of 3-hydroxy-3-methylglutaryl-coenzyme-A-reductase, the enzyme that regulates the synthesis of cholesterol in the body, has been earlier suggested to play the most major role[48]. Gene variants in the lipid metabolism pathway contribute to the risk of biliary tract stones and cancers, particularly of the bile duct[49]. With certain gene polymorphisms, there is an increased risk for systemic metabolic disturbances, leading to the higher secretion of cholesterol into the bile and to gallbladder dysfunction[17,44,46]. Genetic polymorphisms in apolipoprotein genes may be associated with alteration in lipid profile and susceptibility to GD[5,50]. The APOA1-75 G/A polymorphism is associated with gallstone disease and shows sex-specific differences. On the other hand, APOA1 M2(+/-) and APOC3 SstI polymorphisms may not be associated with gallstone disease. Haplotype analysis is a better predictor of risk for GD[51]. It was recently presented that a common polymorphism in the low-density lipoprotein receptor-related protein-associated protein (LRPAP1) gene might be associated with GD[52]. Mutations of the gene encoding the hepatocanalicular phosphatidylcholine transporters may lead to reduced lecithin secretion into the bile and its increased lithogenicity[53,54]. Association was stronger in subjects with cholesterol gallstones (odds ratio = 3.3), suggesting that His19 might be associated with a more efficient transport of cholesterol into the bile[17]. Cholesterol 7alpha-hydrolase (CYP7A1) is an enzyme that catalyzes the first, rate-limiting reaction of a cholesterol catabolic pathway. Recently, a common c.-278A > C polymorphism (rs3808607: G > T) has been described in the CYP7A1 gene, associated with altered plasma lipid levels. Authors concluded that CYP7A1 promoter polymorphism is not a valuable marker of GD susceptibility in a Polish population[52].
Mucin, a major component of mucus, plays an important role in GS formation. The molecular mechanisms of mucin overproduction, however, still remain unknown. Several mucin genes (MUC) have been implicated in various diseases and gel-forming mucin genes (MUC2, MUC5AC, MUC5B, and MUC6) were recognized to be the important components of digestive mucus. Furthermore, epidermal growth factor receptor (EGFR) might regulate the function of MUC5AC. MUC5AC is over-expressed in GD, despite of the decrease in the expression of EGFR mRNA. MUC5AC may be related to mucus hypersecretion[55]. The SNPs at MUC1 and MUC2 are significantly associated with GS in men but not in women. These genes can work jointly to further increase susceptibility to GS in a Chinese population[56].
Being overweight and obesity: Being overweight and obesity are important risk factors of cholelithiasis[24,31,33,57]. Obesity is accompanied by increased synthesis and excretion of cholesterol into bile. At the same time, the amount of produced cholesterol is directly proportional to being overweight[8]. Weight cycling, independent of BMI, may increase the risk of GD in men. Larger weight fluctuation and more weight cycles are associated with greater risk[58]. The beta3-adrenergic receptor (ADRB3) is a transmembrane receptor highly expressed in adipose tissue and thought to be involved in the regulation of lipolysis. ADRB3 is also highly expressed in gallbladder tissue where it may be involved in gallbladder contraction. Klass et al[59] indicate that the ADRB3 Trp64Arg polymorphism is associated with gallstone disease, thereby representing a genetic marker that identifies subjects at higher risk for gallstone formation. Low-calorie diets used in obese patients give rise to ointment-like bile and stones in 25% of cases. In the case of bypass surgery for obesity, the likelihood of cholelithiasis is even higher: 50% of patients are found to have GS within 6 mo postoperatively. Weight loss is accompanied by the elevated levels of mucin and calcium in the cystic bile, thereby giving rise to biliary sludge and stones in the gallbladder.
Diet: A high intake of cholesterol increases its bile level[31]. A low-fiber diet slows transit of the intestinal contents, which promotes the increased formation and absorption of secondary bile acids and the enhanced lithogenic properties of bile[22]. Refined carbohydrates increase cholesterol saturation of bile while small doses of alcohol have the opposite effect. Epidemiological studies in the United States have demonstrated that a daily intake of 2-3 cups of coffee reduces the risk for GS formation[60]. Long-term parenteral nutrition promotes gallbladder dilatation and hypokinesia and gives rise to gallstones[48].
Liver and pancreatic diseases: In liver cirrhosis, GS are detectable in 30% of patients[61,62]. It is stated that subjects with HBsAg[63] and viral hepatitis C have an increased risk for GS formation. Hepatic metabolic dysfunction and bile duct lesions are mentioned among its possible causes[57]. In primary biliary cirrhosis, bile duct stones (more commonly pigment ones) are encountered in 39% of patients. The incidence of GD increases in fatty hepatosis[64]. Patients with diabetes mellitus are at a higher risk for GD, which is linked with hypercholesterolemia observed in this disease[31,65]. Immune resistance associated with the polymorphism of genes encoding receptors in adipocytes: retinoid X receptor and peroxisome proliferators-activated receptor promotes the occurrence of cholelithiasis, as shown by the Chinese investigators’ data[66].
Drug: Estrogens, prednisolone, cyclosporine, azathioprine, sandostatin[67], clofibrate, nicotinic acid and a number of other long-term drugs increase the risk for GD[68,69]. Oral contraceptives increase the incidence of GD in younger women, especially in the early period of their use of oral contraceptives[70]. Sixty-eight point eight percent of SLE patients on corticosteroid therapy had cholelithiasis[71]. The data, presented in these articles, suggest that corticosteroids and oral contraceptives, which contain hormones related to steroid hormones, may be regarded as a model system of cholelithiasis development in man.
Long-term corticosteroid therapy is well known to cause dyslipoproteinemia, characterized by elevated plasma total cholesterol, triglycerides and low-density lipoprotein cholesterol. The major catabolic pathway for cholesterol is its transformation into bile acids, involving P450 cytochrome and subsequent bile excretion from the body. The elevated level of total cholesterol may change a bile acid/cholesterol ratio and lead to the formation of GS in patients with SLE or in patients who use oral contraceptives.
Cytostatic therapy during organ transplantation increases the risk of cholelithiasis. Stone formation is noted in 13%-60% of acromegaly patients taking octreotide (sandostatin) and becomes particularly high when it is discontinued[67,72]. Ceftriaxone frequently causes transient biliary precipitation and its probability increases if the child is over 12 mo of age, the dose is over 2 g/d, or the duration is over five days. Ceftriaxone, a third-generation cephalosporin, is widely used for treating infection during childhood. It is mainly eliminated in the urine, but approximately 40% of a given dose is unmetabolized and secreted into bile[73]. The risk for cholelithiasis increases in constitutive obesity and in the case of long-term high-dose insulin therapy and insulin resistance[74]. Gallstones appear to be a marker for insulin resistance, even in non-diabetic, nonobese men[75].
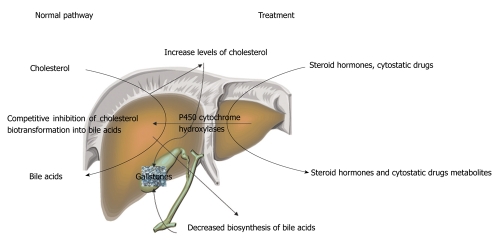
Long-term therapy with each of these agents enhances cholesterol excretion into bile and results in its supersaturation with cholesterol through competitive inhibition of bile acid synthesis from cholesterol on cytochrome Р450[71] (Figure 1).
Figure 1.
Diagram of steroid hormones-induced inhibition of cholesterol catabolism.
The defect in the key enzyme of the classical pathway of bile acid synthesis, cholesterol 7α-hydroxylase (CYP7A1), has been associated with a decrease in bile acid production via the classical pathway, which is compensated by activation of the alternative acidic pathway[76]. In these individuals, hepatic cholesterol contents are increased and, in adults, LDL hypercholesterolemia and cholesterol GS are commonly present[77]. Genetic variation in genes involved in steroid biosynthesis, metabolism and signal transduction have been suggested to play a role in GD. An association for cholelithiasis risk between short alleles for both c.1092+3607 (CA) 5-27 and c.172 (CAG) 5-32 repeat polymorphisms of the estrogen receptor-beta and androgen receptor was found in individuals of Greek descent[78]. Occurring cholesterol metabolic disturbances are attended by decreased gallbladder motor activity, which also promotes GS formation.
Low socioeconomic status and a poor hygiene level are currently stated among the risk factors of GD[79].
By using logistic regression multivariate analysis, authors[32] from Saudi Arabia note the following significant risk factors for GD: female sex, family history of gallstone disease and past history of pancreatitis. Moreover, age, education, blood pressure, smoking, coffee intake, being overweight, diabetes mellitus, number of pregnancies and use of oral contraceptives were not significant risk factors[32]. The data presented by the authors does not correspond well with the above mentioned and raises a question about the correlation of race and gallstone disease development. Apparently, a multicenter multinational investigation is required.
Factors that contribute to cholesterol precipitation and crystallization core formation
Mucin-glycoprotein gel is one of the most important and identified pronucleators. Mucins are high-molecular-weight glycoproteins containing oligosaccharide side-chains attached to serine or threonine residues of the apomucin backbone by O-glycosidic linkages[80]. Mucins can be divided into two classes: gel forming and membrane-associated. Bile mucin has two main domains: one rich in serine, threonine and proline, which contains the majority of the covalently-bound carbohydrates; and another, nonglycosylated domain, enriched in serine, glutamic acid, glutamine and glycine, which binds hydrophobic ligands such as bilirubin. In health, mucin is constantly secreted by the gallbladder mucosa; however, its secretion increases if lithogenic bile is present. Secretory mucins are gel forming and may increase bile viscosity. The biochemical composition of hepatic bile is modified during residence in the gallbladder, contributing to sludge formation. An increased expression of gel-forming mucin, such as MUC5AC and MUC2, was found in patients with hepatolithiasis[81]. Wang and coworkers[82] described a positive correlation between MUC1 and MUC5AC expression, indicating a gene-gene interaction that might affect the accumulation of mucin gel and cholesterol GS formation. Bile mucin is derived from pure hepatic bile, gallbladder-concentrated bile, and mucin secreted by the bile duct epithelium. In patients with biliary sludge, mucin concentration was higher in bile collected by endoscopic retrograde cholangiography than in gallbladder bile[80]. The biochemical composition of hepatic bile is modified during residence in the gallbladder, contributing to sludge formation.
Bilirubin is frequently found in the center of cholesterol stones, which allows us to think that cholesterol crystals may precipitate as protein-pigment complexes in the gallbladder.
Factors that lead to impaired gallbladder function (contraction, absorption, secretion)
Cholesterol precipitates are constantly formed in the normal gallbladder. Its contraction removes cholesterol crystals and mucus clumps, preventing the formation of stones[83]. This is also favored by the slightly acidic medium of bile. Gallbladder filling and emptying could be impaired in patients with GD[84]. GS formation is associated with poorer contractility and larger gallbladder volume[85]. It is likely that an increase in gallbladder volume could result in impaired gallbladder motility and bile stasis, which may encourage GS formation[86]. Cholestasis in the gallbladder with its preserved concentrating function substantially increases the risk of stone formation.
Gallbladder emptying is difficult in flatulence, pregnancy[87], on switching to complete parenteral nutrition, in prompt weight loss, long-term starvation[29], celiac disease, iron-deficiency anemia[88] and gallbladder cholesterosis[89]. With age, there is a reduction in the sensitivity and number of receptors to cholecystokinin, motilin and other stimuli of the motor activity of the gallbladder receptor apparatus. There is evidence for certain cholecystokinin receptor A gene polymorphisms that increase the rate of cholelithiasis due to impaired gallbladder motility[90]. Increased expression of the gene encoding the synthesis of type II receptor to pituitary polypeptide that activates adenylate cyclase in the tissue of the gallbladder, resulting in its impaired motility, is involved in the development of GD[91].
Somatostatin, atropine and methylscopolamine lower gallbladder contractility. Morphine exerts a cholecystokinetic effect but concurrently induces spasm in the sphincter of Oddi.
A few investigators attribute gallbladder smooth muscle hypokinesia to excess cholesterol in the cytoplasmic membranes of myocytes. The defective contraction of muscle cells with excessive cholesterol levels in the plasma membrane is due to an increased expression of caveolin-3 proteins Cav-3 that results in the sequestration of CCK-1 receptors in the caveolae, probably by inhibiting the functions of Galpha (i3) proteins[92].
Contractility of the gallbladder may be impaired by its denervation after surgery of the hepatopancreatoduodenal area or gastrectomy with bypass[93-96]. A notable reduction in the number of neurons in the gallbladder wall was observed in Chagas patients, in comparison with non-Chagas subjects[97].
Factors that lead to impaired enterohepatic circulation of bile acids
Small bowel diseases accompanied by severe malabsorption (gluten enteropathy, Crohn’s disease, etc.) result in impaired bile acid absorption[22]. The rate of stone formation amounts to as high as 26.4% in Crohn’s disease with predominant localization in the terminal small bowel.
At the same time, there is no difference in the rate of GS formation between men and women. There is no age-dependence characteristic of GD[48]. Cholesterol stones are generally formed in Crohn’s disease; however, there is evidence that pigment stones may be formed in this disease.
Ileectomy: Subtotal and total hemicolectomies increase the risk of GS formation.
Biliary fistulas: External drainage or biliary fistulas resulting from the pathological process, such as in xanthogranulomatous cholecystitis, promote massive loss of bile acids, which is not offset even by their intensive compensatory synthesis. Resection, diseases of the small bowel, with the pathological process being located in the terminal portion, and biliary fistulas lead to impaired enterohepatic circulation of bile acids and, as a result, to dyscholia and GD.
Composition of gallstones
Stones in the gallbladder and/or bile ducts are a morphological substrate of GD. The major components of virtually all types of GS are free unesterified cholesterol, unconjugated bilirubin, bilirubin calcium salts, fatty acids, calcium carbonates and phosphates, and mucin glycoproteins.
Three main categories of gallstones can be identified according to their predominant chemical composition, cholesterol and pigment stones[2]: (1) cholesterol stones, constituting as high as 75% of all gallstones in GD[10,98]; (2) pigment stones; and (3) mixed stones.
White or yellowish cholesterol gallstones are present in the gallbladder; they are round or oval in shape, light (they do not sink in water) and, when ignited, burn with a bright flame. When sectioned, they are radial in structure due to the radial alignment of cholesterol crystals. Cholesterol and mixed stones comprise mainly of cholesterol monohydrate (it is at least 70% in the cholesterol stones[22]) and a mixture of calcium salts, bile acids, pigments and glycoprotein, which may be present in the center of a gallstone and generate radial or concentric precipitates. Scanning and transmission electron microscopic studies of the microstructure of lithogenic bile have indicated that lamellar vesicles with incorporated lipophilic and hydrophilic compounds are not only a precursor, but also a major structural component of cholesterol stones[99]. Methods of study that determine the spatial relationships between the major components of lithogenic bile during crystallization are of great importance. The data on the structural relationship between glycoproteins and cholesterol in the GS are obtained from histochemical studies using light microscopy.
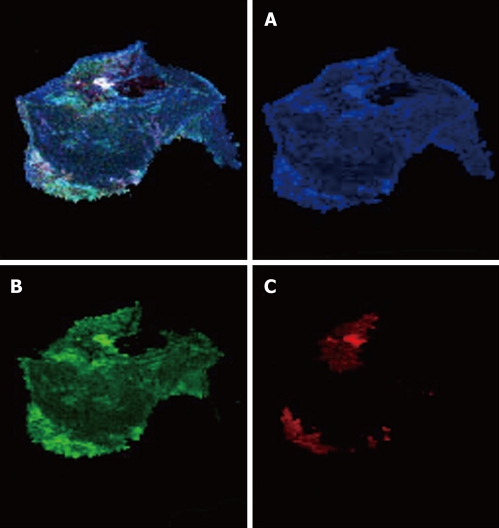
Color cathodoluminescence scanning electron microscopy (CCLSEM) studies of cholesterol GS (Figure 2) have shown that their major components are cholesterol and protein constituents (Figure 2A and B, respectively). Bilirubin is arranged as individual embedments onto the surface of the section of a stone (Figure 2С)[71,100,101].
Figure 2.
Color cathodoluminescence scanning electron microscopy micro images of cholesterol gallstones. The application of the computer program “Adobe Photoshop” (software) and color contrast by the color cathodoluminescence scanning electron microscopy (CCLSEM) technique permitted the determination of cholesterol, bilirubin and protein within the stone. CCLSEM micrographs of cholesterol (A), protein (B), bilirubin (C) were obtained after color separation[100]. The major components of the gallstones under examination were cholesterol (A) and protein (B). They were detected all over the entire surface of the scanned gallstone while rare bilirubin insertions (C) were seen only at the periphery of the gallstone.
Pigment GS are those that contain less than 30% cholesterol. These are black (compact and small) and brown (softer and large) pigment stones. The black pigment stones account for 20%-30% of the gallstones in GD and are more frequently encountered in the elderly. They are composed predominantly of calcium bilirubinate, phosphate and carbonate without a cholesterol impurity[102-105]. They have different shapes, are more commonly very small and numerous, greenish black in color, compact, but fragile. There are also brown pigment stones, very common in east Asia, which form due to bile stasis, parasites, incomplete polymerization of calcium hydrogen bilirubinate, saturated fatty acids and bacterial infection with enzymatic hydrolysis of biliary lipids[2]. The brown stones are chiefly located in the bile duct and amount to about 10%-20% of the stones that are formed in GD. The brown pigment stones contain calcium bilirubinate, less polymerized than that in the black pigment stones, as well as cholesterol and calcium palmitate and stearate. For pigment stones, supersaturation of bile with unconjugated bilirubin plays a major role, which results in its agglomeration[103]. Chronic hemolytic anemias are a major risk factor of bilirubin stone formation[104]. About 30% of patients with thalassemia major (TM) suffer from GD[105]. Recent studies have shown that a variant TATA-box in the promoter region of the UDP-glucuronosyltransferase 1A1 (UGT1A1) gene is associated with the development of cholelithiasis[105]. The coding region mutation (G71R) of the UGT1A1 gene was higher in Asians than those in Caucasians. The combined TATA-box variants and G71R mutations of the UGT1A1 is associated with cholelithiasis in beta-thal/Hb E[106]. It has been thought that intrahepatic stones are brown pigment stones (bilirubin carbonate stones). It became clear that the intrahepatic stones contained high levels of free bile acids and that bacterial infection, which deconjugates the glycine and taurine conjugations, is involved in the pathogenesis of GS. The fatty acid analysis demonstrated high levels of free saturated fatty acids in the GS as well as the involvement of phospholipases, which break down phospholipids in bile, particularly phospholipase A1[107].
Purely calcific stones that are composed of calcium carbonate are very rare in adults[48,108]. In contrast, calcium carbonate gallstones are relatively common in children. An increase in mucin producing epithelial cells in gallbladders from children containing calcium carbonate stones was demonstrated. This supports the hypothesis that cystic duct obstruction leading to increased gallbladder mucin production may play a role in the development of calcium carbonate gallstones in children[108].
Mixed cholesterol-calcific-pigment stones are most common: they sink in water and burn poorly; when cut, they have a lamellar pattern. The causes and factors which induce the alternation of layers and their chemical heterogeneity remain unknown. The mixed stones have various shapes and sizes. The data obtained by CCLSEM suggest that the composition and structure of single and multiple mixed GS are different[100,101]: (1) the single mixed GS display a protein-cholesterol composition in the core; (2) the multiple mixed GS exhibit a protein-bilirubin composition in the core; and (3) moreover, the single and multiple mixed GS necessarily contain a protein component that is arranged along the stone section plane. Whether bile glycoproteins are implicated in the formation of cholesterol stones is still debated. The data of qualitative and quantitative biochemical studies of the pronucleation activity of mucinic glycoproteins are in doubt and without agreement.
Knowledge of the chemical, structural and elemental composition of GS is essential for the etiopathogenesis of GD. To identify the predisposing factors for GS formation, X-ray diffraction powder analysis, atomic absorption spectroscopy and various biochemical estimations were carried out. In the present study, trace elemental analysis revealed calcium as the major constituent element, in addition to the iron, magnesium and zinc in the majority of GS. Patients with GS exhibited increased serum total bilirubin and conjugated bilirubin levels and liver function parameters (serum glutamic pyruvic transaminase, serum glutamic oxaloacetic transaminase and alkaline phosphatase). In patients with GS, higher concentrations of malondialdehyde, significantly higher glutathione disulfide/glutathione (GSH) ratio, reduced total GSH levels and significantly decreased antioxidant enzymes activities (superoxide dismutase, catalase and glutathione peroxidase) were found than in patients without GS. Further studies are needed to establish whether the observed differences are a cause or an effect of GS formation. Such studies could ultimately result in the development of new strategies for the treatment of GS and might provide clues for the prevention of GS formation[109].
PATHOGENESIS OF CHOLESTEROL STONES
The pathogenesis of GD is suggested to be multifactorial and probably develops from complex interactions between many genetic and environmental factors[1,34]. Unphysiological biliary supersaturation from hypersecretion of cholesterol, gallbladder hypomotility and the accumulation of mucin gel contribute to the formation of cholesterol GS, while black pigment stones derive from the precipitation of calcium hydrogen bilirubinate where pigment supersaturation and deposition of inorganic salts, phosphate and calcium bicarbonate accelerate the nucleation. Pigment supersaturation is common in hemolytic disorders, enterohepatic cycling of unconjugated bilirubin and ileal disorders and/or surgery[110]. Cholesterol GD results from a biochemical imbalance of lipids and bile salts in the gallbladder bile[30].
Cholesterol stones are formed in the gallbladder due to impaired relationships between the major bile components, cholesterol, phospholipids and bile acids[111]. The pathophysiology of GS formation involves three steps: saturation, crystallization and growth. Bile cholesterol supersaturation is an obligatory, but not the only, factor that contributes to GS formation. An important role in this is played by the state of pronucleating and antinucleating factors and the functional state of the gallbladder.
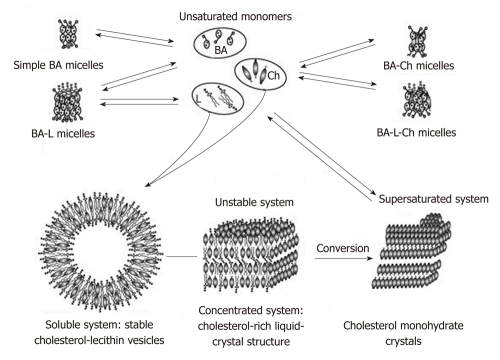
The biochemical composition and physicochemical properties of bile are modified when it is located in the gallbladder. Diminished evacuatory function of the gallbladder with its preserved concentrating capacity may give rise to biliary sludge and GS. In excess cholesterol or deficiency of phospholipids and/or bile acids (a high cholesterol saturation index), bile cholesterol is transported, not only in the form of mixed micelles, but also as phospholipid vesicles. Cholesterol-supersaturated unilamellar and then multilamellar vesicles that are less stable are formed. Nuclear receptors (NRs) play a key role in the transcriptional control of critical steps of hepatobiliary transport and phase I/II metabolism of endo- and xenobiotics such as bile acids and drugs. Apart from these metabolic roles, NRs may also play a key role in the control of hepatic inflammation. Hereditary and acquired alterations of NRs contribute to our understanding of the pathogenesis of cholestasis and GD. Moreover, NRs may represent attractive drug targets for these disorders[112]. Cholesterol nucleation is known to be an initial stage in the formation of cholesterol GS[113]. The present-day interpretation of the mechanisms responsible for cholesterol transport and formation of cholesterol monohydrate crystal in the bile suggests that cholesterol molecules nucleate from the liquid-crystalline phase (a mesophase) after the aggregation and possible fusion of cholesterol-rich unilamellar vesicles[99,114,115] (Figure 3). Under certain conditions, cholesterol can aggregate and precipitate in them as cholesterol monohydrate crystals to give rise to the core of a GS.
Figure 3.
Formation of molecular structures in the system containing bile acids, lecithin and cholesterol. Cholesterol-supersaturated vesicles can stick together and agglomerate to form multilayer (liposomal) liquid-crystal structures. When gallbladder contractility is decreased, liposomes may be converted to solid cholesterol monocrystals. BA: Bile acids; L: Lecithin; Ch: Cholesterol.
The important factor in such mesophasic nucleation is associated with further interaction between the monohydrate crystals and the molecules of protein and unconjugated bilirubin. All these organic substances are precursors in the lithogenic bile and structural components of most human GS[116].
Polarizing light microscopy is the main technique for visualization of cholesterol crystal formation processes in normal and lithogenic bile[117,118]. This technique has revealed that cholesterol crystallizes from bile via metastable intermediates[119]. Loginov et al[100] have shown that mixed (single and multiple) stones are composed of alternating concentric, cholesterol-rich and bilirubin-rich layers. The reason for this alternation and the periodic emergence of layers of various compositions remain unclear. By taking into account the data on the zonal stratification of bile on its drying and the relationship of the formation of cholesterol and bilirubin the deposits to the dehydration or watering of a solution, it can be presumed that the layering of stones depends on bile concentrations in a period of lithogenesis[120]. Cholesterol can crystallize even when the concentration of a bile solution is outside or slightly below the normal range. Bilirubin precipitation increases as lithogenic bile concentrates progressively. Thus, the concentrating or watering of a bile solution may be of great importance in the formation of cholesterol- and bilirubin-containing layers in the GS.
Bile proteins and bilirubin, in addition to cholesterol crystals, can be a matrix in stone formation. Mucin-glycoprotein gel is one of the most important and identified pronucleators. It should be noted the mucus of the gallbladder in normalcy constantly secretes the mucin; however, its secretion increases due to inflammation[121]. Chronic inflammation of the gallbladder wall and mucin hypersecretion are considered important factors in the pathogenesis of cholesterol GD. The results support a promoting effect of gallbladder mucin hypersecretion by lipid peroxidation leading to rapid formation of cholesterol crystals in gallbladder bile. These findings suggest that besides hypersecretion of cholesterol in bile, chronic inflammation of the gallbladder wall is implicated in the pathogenesis of cholesterol GD[121].
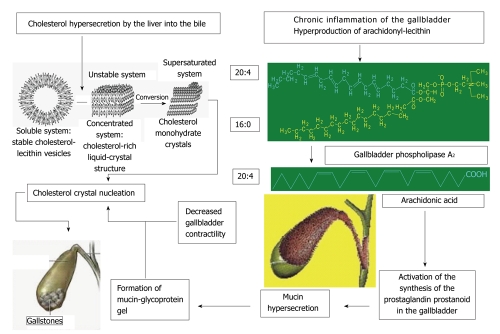
Bacterial infection is of great significance in the development of inflammation in GD. In health, bile is sterile as it has bactericidal activity[122]. When there are changes in bile composition or cholestasis in the gallbladder, bacteria can rise into the gallbladder through the bile duct and promote lithogenesis. Cystic bile destabilized by chronic inflammation of gallbladder wall contains high arachidonyl-lecithin levels (Figure 4). The observed increase in the activity of the phospholipase А2 secreted by bacteria leads to the hydrolysis of phospholipids and the accumulation of free fatty acids, including arachidonic acid[107]. The latter activates the generation of prostaglandins, thromboxanes and leukotrienes to cause mucin glycoproteins to be hypersecreted by the gallbladder mucosa. In infection, cholic acid is converted to lithocholic acid. The higher production of lithocholic acid in the cystic bile promotes aggregation of cholesterol monohydrate crystals.
Figure 4.
It shows a diagram of gallstone formation by taking into account the above impaired bile production and excretion processes.
In parallel with this, there are morphological changes in the gallbladder mucosa. The surface epithelium passes into goblet, mucus cells that secret much mucus, the columnar epithelium flattens and microvilli are lost. This results in impaired water and electrolyte absorption processes. Mucin and mucus hypersecretion gives rise to a parietal colloid solution that is turned to viscoelastic glycoprotein-mucin gel. The latter promotes the aggregation of phospholipid vesicles and the nucleation and precipitation of cholesterol monohydrate crystals and/or bilirubin. Cholesterol monohydrate crystals, mucus glycoprotein mucin bands and calcium bilirubinate granules form the basis for biliary sludge and a pigmented matrix of the core of most cholesterol gallstones.
Hypersecretion is induced by the increased expression of one of the genes encoding the synthesis of mucin (MUC5AC) and by the decreased expression of the epidermal growth factor receptor gene involved in the regulation of mucin synthesis, which are observed in all patients with GD[55]. The elevated levels of glycosaminoglycans mainly due to a sulfated fraction are characteristic.
In addition to mucin, the proteins that accelerate cholesterol precipitation include N-aminopeptidase, immunoglobulins and phospholipases C. The antinucleators include apolipoproteins А1 and А2, which slow cholesterol precipitation, aspirin and other nonsteroidal anti-inflammatory drugs.
The bulk of intrahepatic stones are formed due to biliary tract infection[123]. The neck of the gallbladder hosts the biggest bacterial load in comparison with the body and the fundus. This difference might be attributed to the presence of Rokitansky-Aschoff sinuses, the main histological characteristic of the region[124]. This is frequently the opportunistic flora (Escherichia coli, streptococcus, staphylococcus and typhoid bacillus) that, by setting in motion its capsular O-antigen, can persist in the GS for decades[125]. Intrahepatic stones contain abundant free fatty acids and free bile acids due to the deconjugation with bacterial enzymes.
Bacteria are readily cultured from cholesterol stones with pigment centers, allowing for analysis of their virulence factors. Bacteria sequestered in cholesterol stones cause infectious manifestations but less than bacteria in pigment stones. Possibly, because of their isolation, cholesterol stone bacteria are less often present in bile and blood, induce less immunoglobulin G, are less often killed by a patient’s serum and demonstrate fewer infectious manifestations than pigment stone bacteria[126]. The O-antigen capsule genes are bile induced and the capsule produced by the enzymes of this operon is specifically required for biofilm formation on cholesterol GS. Salmonella enterica serovar Typhi can establish a chronic, asymptomatic infection of the human gallbladder, suggesting that this bacterium utilizes novel mechanisms to mediate enhanced colonization and persistence in a bile-rich environment. GS are one of the most important risk factors for developing carriage and authors have previously demonstrated that salmonellae form biofilms on human GS in vitro[125]. Thus, the microorganisms induce increased mucin production and destroy both components that solubilize cholesterol in the micelles by inducing its crystallization. The performed investigations indicate that stones of various compositions are formed depending on the species of the microorganism that is responsible for biliary tract inflammation. Thus, the bacteria that produce beta-glucuronidase and mucus or beta-glucuronidase only give rise to pigment or mixed stones while the microorganisms that produce only mucus or do not produce any of these factors are more common in the cores of cholesterol stones[127].
The genetic material of Clonorchis sinensis and Ascaris lumbricoides worms may be found in the GS[128,129]. Clonorchis sinensis and Ascaris lumbricoides may be related to biliary stone formation and development[128].
Foreign bodies, such as suture materials, clips, swallowed metal or plastic fragments, or parasites, may become foci of nucleation. Surgical clips are the most common cause of iatrogenic cholelithiasis[23]. The stones’ growth rate is 3-5 mm per year and in some cases it may be more[22,130].
TREATMENT FOR GALLSTONE DISEASE
The treatment of cholelithiasis is symptomatic and chiefly aims at removing the stones from the gallbladder or bile ducts. When the cause of the disease is known, the conditions resulting in cholelithiasis, such as hemolytic anemia, obesity, diabetes mellitus, etc, are treated.
Surgery has long remained the exclusive form of therapy for GD. The achievements in bile molecular biology and biochemistry have extended the views of intricate bile production and excretion processes and the mechanisms responsible for formation of GS and their structure. This could expand indications for medical treatment in patients with GD. Therefore, surgical and medical treatments for cholelithiasis are equally used today. The basic treatments for GD are: (1) cavitary cholecystectomy endoscopic cholecystectomy; (2) litholytic therapy (LT); (3) extracorporeal shock wave lithotripsy (ESWL); (4) extracorporeal shock wave lithotripsy + Litholytic therapy; and (5) percutaneous transhepatic LT.
The final choice of treatment policy must be eventually determined by a joint decision between a therapist, surgeon and patient. This paper will outline the basic principles of medical therapy for cholelithiasis.
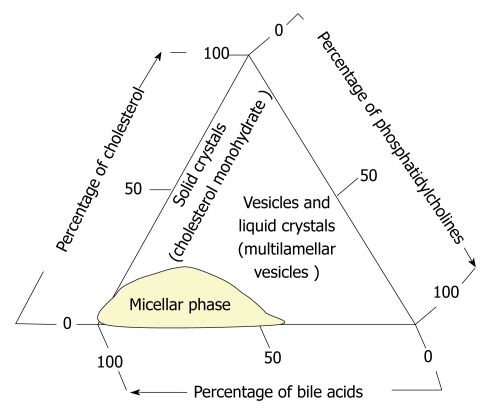
The second half of the last century was marked by the emergence of new medical treatments for GD: litholytic therapy (stone dissolution) and lithotripsy (stone shattering). About 30% of patients with gallbladder stones may undergo litholytic therapy[22]. GS dissolution is based on the pathophysiology of cholepoiesis and choleresis in cholelithiasis and is carried out with bile acids. Scientists established experimentally that the ratio between the concentration of bile acids leads to a redistribution of phases in a triangular coordinate system[114] (Figure 5).
Figure 5.
The phase state of the main bile components (cholesterol, phosphatidylcholines, bile acids) in the triangular coordinate system[114].
This principle underlies the dissolution of GS by using bile acids drugs. For this, litholytic drugs containing chenodeoxycholic or ursodeoxycholic acid (UDCA) are used. Preference is given to UDCA-containing agents. They are more effective and have virtually no side effects[48]. With administration of the agents, there is elimination of bile acid deficiency, inhibition of hepatic synthesis of cholesterol and its secretion into the bile, as well as intestinal absorption, ultimately resulting in a decreased bile cholesterol level and stone dissolution.
In health, the proportion of UDCA is not greater than 5% in the total bile acid pool, whereas it is more than 60% of all bile acids after three months or more of administration of oral UDCA-containing preparations[131]. The increased total pool of bile acids at the expense of polar UDCA causes a reduction in bile cholesterol saturation and promotes a gradual cholesterol solubilization from the gallstones. The administration of UDCA outside the intestine through the feedback system suppresses the biosynthesis of cholesterol, which also lowers the bile cholesterol saturation index. Reductions in cholesterol and potentially toxic primary acids in the total pool are followed by decreased cholesterol levels in the hepatocytic membranes[89]. This normalizes performance of the carriers of bile acids and phospholipids on the canalicular and basolateral membranes of the hepatocytes, which elevates the amount of bile acids and phospholipids in the canalicular bile and also decreases the bile cholesterol saturation index[132]. In vitro studies have demonstrated that UDCA reduces the levels of cholesterol and the intensity of lipid peroxidation in the myocyte cytoplasmic membrane of the gallbladder and diminishes its mucin secretion[133]. Even short-term treatment with UDCA preparations corrects impaired gallbladder motility, thus showing their choleretic activity[134,135].
For successful litholytic therapy, definite criteria should be met for selection of patients with cholelithiasis: (1) the stone should be cholesterol or mixed; (2) the size of the stones should not be greater than 1.5 cm; and (3) the gallbladder should fully preserve its function and be packed with stone not more than ¼ of the fasting volume; the cystic duct and common bile duct should preserve their patency; enterohepatic circulation of bile acids should be preserved.
The dose of a drug depends on body weight. The daily dose of bile acids should be increased in obese patients[22]. For the highest therapeutic effect, the drug should be taken in a single daily dose overnight, for its highest concentration in the gallbladder at a relative functional rest and during the maximum cholesterol synthesis[48]. Rarely, with the use of the drug there may be diarrhea. In these cases, 1/3 of the daily dose should be taken in the morning and the rest in the evening.
The efficiency of litholytic therapy is shown to depend largely on its use at the early stages of GD when compact stones have not been formed yet. Drug therapy is performed long-term (from 6 mo to 2 years or more), necessarily with ultrasound guidance and biochemical blood tests carried out every three months during therapy. When the stones are reduced in size, it is advisable to continue the therapy for 3-6 mo until they are completely dissolved. If there is no reduction in the sizes of gallstones within 12 mo of the initiation of litholytic therapy, the latter should be stopped[48]. Low-cholesterol diet and dietary intake of bran are indicated during and after the therapy[48]. Ursotherapy is not a contraindication in the treatment of pregnant women with GD[22].
When selecting the patients correctly, the efficiency of litholytic therapy with UDCA is as high as 60%-90%: (1) in the presence of “floating” cholesterol small stone, it is up to 90%; (2) with single mixed gallstones < 1 cm in diameter, it is up to 75%; and (3) with multiple mixed gallstones with the maximum diameter of < 1 cm, it is up to 60%.
The result of therapy depends on the size of a stone; cholesterol stones less than 5 mm are best dissolved irrespective of the risk factors predisposing to the disease[136]. Single stones are dissolved less well than multiple ones (the latter have a more optimal ratio of the surface of stones to the volume of the gallbladder containing bile acid preparations). The highest effect is noted in young patients. Successful therapy proves to be more frequent when GD is detected early and much rarer when there is a long history of the disease due to stone calcification. When gallbladder contractility is preserved, successful therapy is predicted to be much more optimistic[22].
Unfortunately, GS may again form after their successful dissolution. After successful oral LT, recurrent stones are annually about 10% during 5 years, more frequently during the first 2 years, and then their frequency decreases. The risk for recurrence is less in patients with a primary single stone than in those who have been earlier found to have multiple stones. For the prevention of stone recurrences, it is necessary to continue small-dose UDCA therapy, which results in a significant reduction in the bile lithogenicity index and prevents recurrent stone formation[48].
Contact litholysis
Contact litholysis is a variant of litholytic therapy. If contact litholysis is used, a substance that dissolves cholesterol stones is injected just into the gallbladder or bile ducts. Only cholesterol stones are prone to dissolution; their size and number are of no fundamental importance. Methyltretbutyl ether and propionic ether are used to dissolve stones in the gallbladder and bile ducts, respectively. Dissolution occurs within 4-16 h. The multicenter study covering 803 patients in 21 European medical centers has shown the high efficiency of contact litholysis. Puncture was successful in 761 (94.8%) patients and stones were dissolved in 95.1% of cases. After litholysis, biliary sludge remained in the gallbladder in 43.1% patients. The technique may be successfully used to dissolve fragments remaining after ESWL[22]. This procedure can be the method of choice in treating GD patients at high intraoperative risk. It may be employed both in patients with significant clinical manifestations and biliary colic episodes and those with asymptomatic GD.
From the physiological and molecular biochemical bases of the structural and functional state of the major components of bile, it is clear that, besides bile acids, phospholipids can solubilize cholesterol. The solubilizing properties of phosphatidylcholines (lecithins) are shown to be largely due to the fatty acid that is in the second position of a phospholipid molecule. This has given an impetus to design novel agents for dissolution of cholesterol gallstones containing conjugates of bile acids and fatty acids with a chain length of 14 to 22 carbon atoms linked by an amide bond[119,137]. The amide bond prevents the compound from splitting in the intestine. The first laboratory studies have demonstrated that the conjugates of bile acids and fatty acids do show a cholesterol-solubilizing effect[119]. The conjugates of bile acids with arachidonic acid, arachidyl-amino-cholanoid, have the best solubilizing effect. It has been indicated in vitro and in vivo (in mice) that these compounds are able to prevent the formation of cholesterol crystals and to dissolve them in animals on a lithogenic diet[119,137].
ESWL has substantially extended the capabilities of medical treatment in patients with GD and could achieve a positive effect in those with gallstones up to 3 cm in diameter. The technique is based on shock wave generation. Pressure that is 1000 times greater than the atmospheric one is achieved in the focus within 30 nsec. Because soft tissues absorb little energy, its bulk falls on a stone, causing its destruction. The technique is used as a preparatory stage for further oral litholytic therapy. There are strict indications for this type of therapy.
Criteria for selection of patients for lithotripsy are as follows: (1) single radiolucent cholesterol stones not more than 3 cm in diameter; (2) multiple radiolucent stones (not more than 3) 1-1.5 cm in diameter; (3) the volume of stones is < 1/2 of that of the gallbladder; (4) a functioning gallbladder; (5) normal bile duct patency; (6) contraindications to ESWL; (7) the presence of coagulopathy or anticoagulant therapy; and (8) the presence of cavitary mass along the course of a shock wave. Approximately 20% of patients with GD meet the criteria for ESWL.
Stone shattering into small fragments occurs after 1-3 sessions. When patients are correctly selected for ESWL, stones fragmentation can be achieved in 90%-95% of cases. Lithotripsy is considered successful if stones less than 5 mm in diameter can be fragmented. ESWL yields good results when minor (< 20 mm) single stones are shattered. There are a low percentage of positive results if large dense and multiple stones are available. After lithotripsy, stone fragments are mainly excreted independently. Shock wave lithotripsy is generally used in combination with litholytic therapy that should be continued within six months after the last session of lithotripsy. The adverse reactions of lithotripsy are rare if indications are correctly chosen and the procedure is strictly followed. The most common reactions are biliary colic and, occasionally, minor signs of cholecystitis, hyperaminotransferasemia[22]. Biliary colic is eliminated by the use of spasmolytics and analgesics. Shattering of large gallstones by a few sessions in combination with litholytic therapy prevents the development of obstructive jaundice after lithotripsy.
High recurrence rates in the late period following lithotripsy are the most essential limitation to apply this technique[138]. ESWL has also shown to be effective in 90% of the common bile duct stones refractory to endoscopic treatment[139]; however, a recurrence is observed in 14.5% of patients within 10 years[140]. There are data on the relative safety and efficiency of ESWL in patients with incorporated biliary tract stones and a high surgical risk[141,142].
Potential GD-preventing drugs
Among the GD-preventing drugs, ezetimibe is noteworthy[143]. This agent prevents the formation of cholesterol stones in mice by reducing cholesterol absorption (by 35% in the animals on a lithogenic diet and by 90% in the controls) and bile cholesterol saturation index (by 60% on a lithogenic diet), intensifying bile flow, and enhancing the secretion of bile salts (by 60%), phospholipids (by 44%) and glutathione (by 100%), which is associated with the slightly increased expression of bile acid carriers. According to the preliminary data, the major effect of ezetimibe in man is to lower cholesterol absorption[144]. The drug is also effective in resorbing cholesterol stones by producing excess unsaturated micelles. Moreover, it increases the time of cholesterol crystallization in patients[145].
The long-term use of magnesium preparations has been demonstrated to prevent the occurrence of clinical forms of GD. Magnesium deficiency may cause dyslipidemia and insulin hypersecretion[146,147].
There is evidence for the administration of melatonin for the prevention of GD. Melatonin is considered to lower bile cholesterol by reducing the rate of its absorption by the intestinal epithelium and by increasing the rate of its conversion to bile acids[148]. Of great importance in the prevention of recurrent gallstones are the following factors: (1) to avoid inactivity[24,48]. Patients with GD are recommended to exercise (graduated walking of at least 1 km daily; daily exercises associated with the tension of prelum abdominal and the elevation of intraabdominal pressure); (2) to keep a dietary pattern (frequent, fractional) and low-cholesterol diet; (3) to eliminate being overweight; (4) to avoid long-term starvation periods and intake of cholesterol synthesis-increasing drugs[22]; and (5) to have gallbladder ultrasonography at least once a year.
CONCLUSION
In conclusion, the achievement in the study of the physiology of bile formation and the pathogenesis of gallstone disease has allowed expanding indications for therapeutic treatment of GD and reducing the number of patients who undergo surgical treatment. It should be noted that notable advances have been made in studying the mechanisms responsible for the formation of GS, which could extend the capabilities of their dissolution and shattering conservatively. Because GD is a multifactorial disease, its treatment remains symptomatic. Because the etiology and pathogenesis of GD is still not well defined and strategies for prevention and efficient non-surgical therapies are missing, further studies are required[1]. This makes investigators continue so that researchers have new data to allow progress in the treatment of cholelithiasis. From a public health standpoint, it is not only important to study the background prevalence of gallstone disease regionally, but also to explore the demographic and biological markers related to the development of gallstone disease. If we can predict which factors contribute to the development of GD, we can prevent it by controlling these factors.
ACKNOWLEDGMENTS
The author to express thanks to Professor Gennadiy Vasilievich Saparin and Peter Valentinovich Ivannikov for receiving cholesterol gallstones image by CCLSEM. The author expresses his gratitude to Professor Lyudmila Yuryevna Ilchenko for advice in preparing the article and to doctors Myagkova Ekaterina Alexandrovna and Sazonova Anna Alexandrovna for assistance in preparation of the article.
Footnotes
Peer reviewers: Canhua Huang, Professor, The State Key Lab of Biotherapy, Sichuan University, No. 1, Keyuan Rd 4, Gaopeng ST High Tech Zone, Chengdu 610041, Sichuan Province, China; Professor, Dr. Takuji Tanaka, The Tohkai Cytopathology Institute: Cancer Research and Prevention, Minami-Uzura, Gifu City 500-8285, Japan
S- Editor Wu X L- Editor Roemmele A E- Editor Zhang DN
References
- 1.Sun H, Tang H, Jiang S, Zeng L, Chen EQ, Zhou TY, Wang YJ. Gender and metabolic differences of gallstone diseases. World J Gastroenterol. 2009;15:1886–1891. doi: 10.3748/wjg.15.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conte D, Fraquelli M, Giunta M, Conti CB. Gallstones and liver disease: an overview. J Gastrointestin Liver Dis. 2011;20:9–11. [PubMed] [Google Scholar]
- 3.Belousov Yu V. Pediatric Gastroenterology. Up-to-date guide. Moscow: Exma; 2006. p. 112. [Google Scholar]
- 4.Méndez-Sánchez N, Zamora-Valdés D, Flores-Rangel JA, Pérez-Sosa JA, Vásquez-Fernández F, Lezama-Mora JI, Vázquez-Elizondo G, Ponciano-Rodríguez G, Ramos MH, Uribe M. Gallstones are associated with carotid atherosclerosis. Liver Int. 2008;28:402–406. doi: 10.1111/j.1478-3231.2007.01632.x. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Cuén J, Aguilar-Medina M, Arámbula-Meraz E, Romero-Navarro J, Granados J, Sicairos-Medina L, Ramos-Payán R. ApoB-100, ApoE and CYP7A1 gene polymorphisms in Mexican patients with cholesterol gallstone disease. World J Gastroenterol. 2010;16:4685–4690. doi: 10.3748/wjg.v16.i37.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temel RE, Brown JM. A new framework for reverse cholesterol transport: non-biliary contributions to reverse cholesterol transport. World J Gastroenterol. 2010;16:5946–5952. doi: 10.3748/wjg.v16.i47.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagaudinov KG, Saidov SS, Garilevich BA, Zubkov AD, Abdulaev RA, Ovakimian GS. [Improvement of extracorporeal shockwave cholelithotripsy in the comprehensive treatment of cholelithiasis] Klin Med (Mosk) 2007;85:56–59. [PubMed] [Google Scholar]
- 8.Doggrell SA. New targets in and potential treatments for cholesterol gallstone disease. Curr Opin Investig Drugs. 2006;7:344–348. [PubMed] [Google Scholar]
- 9.Goldacre MJ, Duncan ME, Griffith M, Davidson M. Trends in mortality from appendicitis and from gallstone disease in English populations, 1979-2006: study of multiple-cause coding of deaths. Postgrad Med J. 2011;87:245–250. doi: 10.1136/pgmj.2010.104471. [DOI] [PubMed] [Google Scholar]
- 10.Novacek G. Gender and gallstone disease. Wien Med Wochenschr. 2006;156:527–533. doi: 10.1007/s10354-006-0346-x. [DOI] [PubMed] [Google Scholar]
- 11.Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/s0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- 12.Miquel JF, Covarrubias C, Villaroel L, Mingrone G, Greco AV, Puglielli L, Carvallo P, Marshall G, Del Pino G, Nervi F. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris. Gastroenterology. 1998;115:937–946. doi: 10.1016/s0016-5085(98)70266-5. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 14.Xu P, Yin XM, Zhang M, Liang YJ. [Epidemiology of gallstone in Nanjing City in China] Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:928. [PubMed] [Google Scholar]
- 15.Sampliner RE, Bennett PH, Comess LJ, Rose FA, Burch TA. Gallbladder disease in pima indians. Demonstration of high prevalence and early onset by cholecystography. N Engl J Med. 1970;283:1358–1364. doi: 10.1056/NEJM197012172832502. [DOI] [PubMed] [Google Scholar]
- 16.Everhart JE, Yeh F, Lee ET, Hill MC, Fabsitz R, Howard BV, Welty TK. Prevalence of gallbladder disease in American Indian populations: findings from the Strong Heart Study. Hepatology. 2002;35:1507–1512. doi: 10.1053/jhep.2002.33336. [DOI] [PubMed] [Google Scholar]
- 17.Buch S, Schafmayer C, Völzke H, Becker C, Franke A, von Eller-Eberstein H, Kluck C, Bässmann I, Brosch M, Lammert F, et al. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet. 2007;39:995–999. doi: 10.1038/ng2101. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed M, Diggory R. The correlation between ultrasonography and histology in the search for gallstones. Ann R Coll Surg Engl. 2011;93:81–83. doi: 10.1308/003588411X12851639107070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuart GS, Tang JH, Heartwell SF, Westhoff CL. A high cholecystectomy rate in a cohort of Mexican American women who are postpartum at the time of oral contraceptive pill initiation. Contraception. 2007;76:357–359. doi: 10.1016/j.contraception.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruhl CE, Everhart JE. Gallstone disease is associated with increased mortality in the United States. Gastroenterology. 2011;140:508–516. doi: 10.1053/j.gastro.2010.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsukanov VV, Nozdrachev KG, Tonkikh IuL, Bronnikova EP, Kupershteĭe EIu. [The mechanism of reverse cholesterol transport and cholelithiasis in Northern ethnic groups] Klin Med (Mosk) 2007;85:33–35. [PubMed] [Google Scholar]
- 22.Ilychenko АА. Gallstone disease. Lechashchiy Vrach. 2004;4:27–33. [Google Scholar]
- 23.Chong VH. Iatrogenic biliary stone. Surg Technol Int. 2005;14:147–155. [PubMed] [Google Scholar]
- 24.Völzke H, Baumeister SE, Alte D, Hoffmann W, Schwahn C, Simon P, John U, Lerch MM. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion. 2005;71:97–105. doi: 10.1159/000084525. [DOI] [PubMed] [Google Scholar]
- 25.Cozcolluela Cabrejas MR, Sanz Salanova LA, Martínez-Berganza Asensio MT, Gómez Herrero H, Mellado Santos JM, Miranda Orella L, Forradellas Morales A. [Childhood cholelithiasis in a district hospital] An Pediatr (Barc) 2007;66:611–614. doi: 10.1157/13107397. [DOI] [PubMed] [Google Scholar]
- 26.Poddar U. Gallstone disease in children. Indian Pediatr. 2010;47:945–953. doi: 10.1007/s13312-010-0159-2. [DOI] [PubMed] [Google Scholar]
- 27.Bergman S, Sourial N, Vedel I, Hanna WC, Fraser SA, Newman D, Bilek AJ, Galatas C, Marek JE, Monette J. Gallstone disease in the elderly: are older patients managed differently? Surg Endosc. 2011;25:55–61. doi: 10.1007/s00464-010-1128-5. [DOI] [PubMed] [Google Scholar]
- 28.Wang DQ. Aging per se is an independent risk factor for cholesterol gallstone formation in gallstone susceptible mice. J Lipid Res. 2002;43:1950–1959. doi: 10.1194/jlr.m200078-jlr200. [DOI] [PubMed] [Google Scholar]
- 29.Grigorieva IN. Major risk factors of cholelithiasis. Rossiyskiy Zhurnal Gastroenterologii, Gepatologii i Koloproktologii. 2007;6:17–19. [Google Scholar]
- 30.He J, Nishida S, Xu M, Makishima M, Xie W. PXR prevents cholesterol gallstone disease by regulating biosynthesis and transport of bile salts. Gastroenterology. 2011;140:2095–2106. doi: 10.1053/j.gastro.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin J, Han TQ, Fei J, Jiang ZY, Zhang Y, Yang SY, Jiang ZH, Cai XX, Huang W, Zhang SD. [Risk factors of familial gallstone disease: study of 135 pedigrees] Zhonghua Yi Xue Za Zhi. 2005;85:1966–1969. [PubMed] [Google Scholar]
- 32.Abu-Eshy SA, Mahfouz AA, Badr A, El Gamal MN, Al-Shehri MY, Salati MI, Rabie ME. Prevalence and risk factors of gallstone disease in a high altitude Saudi population. East Mediterr Health J. 2007;13:794–802. [PubMed] [Google Scholar]
- 33.Ko CW. Risk factors for gallstone-related hospitalization during pregnancy and the postpartum. Am J Gastroenterol. 2006;101:2263–2268. doi: 10.1111/j.1572-0241.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 34.Marschall HU, Einarsson C. Gallstone disease. J Intern Med. 2007;261:529–542. doi: 10.1111/j.1365-2796.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- 35.Cirillo DJ, Wallace RB, Rodabough RJ, Greenland P, LaCroix AZ, Limacher MC, Larson JC. Effect of estrogen therapy on gallbladder disease. JAMA. 2005;293:330–339. doi: 10.1001/jama.293.3.330. [DOI] [PubMed] [Google Scholar]
- 36.Hart AR, Luben R, Welch A, Bingham S, Khaw KT. Hormone replacement therapy and symptomatic gallstones - a prospective population study in the EPIC-Norfolk cohort. Digestion. 2008;77:4–9. doi: 10.1159/000113897. [DOI] [PubMed] [Google Scholar]
- 37.Fornari F, Civardi G, Buscarini E, Cavanna L, Imberti D, Rossi S, Sbolli G, Di Stasi M, Buscarini L. Cirrhosis of the liver. A risk factor for development of cholelithiasis in males. Dig Dis Sci. 1990;35:1403–1408. doi: 10.1007/BF01536748. [DOI] [PubMed] [Google Scholar]
- 38.Zimmer V, Lammert F. Genetics in liver disease: new concepts. Curr Opin Gastroenterol. 2011;27:231–239. doi: 10.1097/MOG.0b013e3283444862. [DOI] [PubMed] [Google Scholar]
- 39.Attili AF, De Santis A, Attili F, Roda E, Festi D, Carulli N. Prevalence of gallstone disease in first-degree relatives of patients with cholelithiasis. World J Gastroenterol. 2005;11:6508–6511. doi: 10.3748/wjg.v11.i41.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsika D, Grjibovski A, Einarsson C, Lammert F, Lichtenstein P, Marschall HU. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology. 2005;41:1138–1143. doi: 10.1002/hep.20654. [DOI] [PubMed] [Google Scholar]
- 41.Khanuja B, Cheah YC, Hunt M, Nishina PM, Wang DQ, Chen HW, Billheimer JT, Carey MC, Paigen B. Lith1, a major gene affecting cholesterol gallstone formation among inbred strains of mice. Proc Natl Acad Sci USA. 1995;92:7729–7733. doi: 10.1073/pnas.92.17.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schafmayer C, Völzke H, Buch S, Egberts J, Spille A, von Eberstein H, Franke A, Seeger M, Hinz S, Elsharawy A, et al. Investigation of the Lith6 candidate genes APOBEC1 and PPARG in human gallstone disease. Liver Int. 2007;27:910–919. doi: 10.1111/j.1478-3231.2007.01536.x. [DOI] [PubMed] [Google Scholar]
- 43.Kovacs P, Kress R, Rocha J, Kurtz U, Miquel JF, Nervi F, Méndez-Sánchez N, Uribe M, Bock HH, Schirin-Sokhan R, et al. Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J Hepatol. 2008;48:116–124. doi: 10.1016/j.jhep.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 44.Grünhage F, Acalovschi M, Tirziu S, Walier M, Wienker TF, Ciocan A, Mosteanu O, Sauerbruch T, Lammert F. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 2007;46:793–801. doi: 10.1002/hep.21847. [DOI] [PubMed] [Google Scholar]
- 45.Kuo KK, Shin SJ, Chen ZC, Yang YH, Yang JF, Hsiao PJ. Significant association of ABCG5 604Q and ABCG8 D19H polymorphisms with gallstone disease. Br J Surg. 2008;95:1005–1011. doi: 10.1002/bjs.6178. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Jiang ZY, Fei J, Xin L, Cai Q, Jiang ZH, Zhu ZG, Han TQ, Zhang SD. ATP binding cassette G8 T400K polymorphism may affect the risk of gallstone disease among Chinese males. Clin Chim Acta. 2007;384:80–85. doi: 10.1016/j.cca.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Qin J, Han TQ, Yuan WT, Fei J, Jiang ZH, Zhang J, Wang Y, Huang W, Zhang SD. [Study on the position of genes responsible for gallstone disease in Chinese population] Zhonghua Wai Ke Za Zhi. 2006;44:485–487. [PubMed] [Google Scholar]
- 48.Kharitonova LA. Cholelithiasis in children: issues of choice of therapeutic tactics. Russkiy Meditsinskiy Zhurnal. 2003;11:787–790. [Google Scholar]
- 49.Andreotti G, Chen J, Gao YT, Rashid A, Chen BE, Rosenberg P, Sakoda LC, Deng J, Shen MC, Wang BS, et al. Polymorphisms of genes in the lipid metabolism pathway and risk of biliary tract cancers and stones: a population-based case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2008;17:525–534. doi: 10.1158/1055-9965.EPI-07-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Báez S, Tsuchiya Y, Calvo A, Pruyas M, Nakamura K, Kiyohara C, Oyama M, Yamamoto M. Genetic variants involved in gallstone formation and capsaicin metabolism, and the risk of gallbladder cancer in Chilean women. World J Gastroenterol. 2010;16:372–378. doi: 10.3748/wjg.v16.i3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixit M, Choudhuri G, Saxena R, Mittal B. Association of apolipoprotein A1-C3 gene cluster polymorphisms with gallstone disease. Can J Gastroenterol. 2007;21:569–575. doi: 10.1155/2007/329342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juzyszyn Z, Kurzawski M, Modrzejewski A, Sulikowski T, Pawlik A, Czerny B, Droździk M. Low-density lipoprotein receptor-related protein-associated protein (LRPAP1) gene IVS5 insertion/deletion polymorphism is not a risk factor for gallstone disease in a Polish population. Dig Liver Dis. 2008;40:122–125. doi: 10.1016/j.dld.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Mbongo-Kama E, Harnois F, Mennecier D, Leclercq E, Burnat P, Ceppa F. MDR3 mutations associated with intrahepatic and gallbladder cholesterol cholelithiasis: an update. Ann Hepatol. 2007;6:143–149. [PubMed] [Google Scholar]
- 54.Rosmorduc O, Poupon R. Low phospholipid associated cholelithiasis: association with mutation in the MDR3/ABCB4 gene. Orphanet J Rare Dis. 2007;2:29. doi: 10.1186/1750-1172-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim HJ, Kim SH, Chae GB, Lee SJ, Kang CD. Increased expression of mucin 5AC mRNA and decreased expression of epidermal growth-factor receptor mRNA in gallstone patients. Tohoku J Exp Med. 2008;214:139–144. doi: 10.1620/tjem.214.139. [DOI] [PubMed] [Google Scholar]
- 56.Chuang SC, Juo SH, Hsi E, Wang SN, Tsai PC, Yu ML, Lee KT. Multiple mucin genes polymorphisms are associated with gallstone disease in Chinese men. Clin Chim Acta. 2011;412:599–603. doi: 10.1016/j.cca.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Chang TS, Lo SK, Shyr HY, Fang JT, Lee WC, Tai DI, Sheen IS, Lin DY, Chu CM, Liaw YF. Hepatitis C virus infection facilitates gallstone formation. J Gastroenterol Hepatol. 2005;20:1416–1421. doi: 10.1111/j.1440-1746.2005.03915.x. [DOI] [PubMed] [Google Scholar]
- 58.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Weight cycling and risk of gallstone disease in men. Arch Intern Med. 2006;166:2369–2374. doi: 10.1001/archinte.166.21.2369. [DOI] [PubMed] [Google Scholar]
- 59.Klass DM, Lauer N, Hay B, Kratzer W, Fuchs M. Arg64 variant of the beta3-adrenergic receptor is associated with gallstone formation. Am J Gastroenterol. 2007;102:2482–2487. doi: 10.1111/j.1572-0241.2007.01430.x. [DOI] [PubMed] [Google Scholar]
- 60.Leitzmann MF, Willett WC, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E. A prospective study of coffee consumption and the risk of symptomatic gallstone disease in men. JAMA. 1999;281:2106–2112. doi: 10.1001/jama.281.22.2106. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Liu D, Ma Q, Dang C, Wei W, Chen W. Factors influencing the prevalence of gallstones in liver cirrhosis. J Gastroenterol Hepatol. 2006;21:1455–1458. doi: 10.1111/j.1440-1746.2006.04465.x. [DOI] [PubMed] [Google Scholar]
- 62.Déry L, Galambos Z, Kupcsulik P, Lukovich P. [Cirrhosis and cholelithiasis. Laparoscopic or open cholecystectomy?] Orv Hetil. 2008;149:2129–2134. doi: 10.1556/OH.2008.28450. [DOI] [PubMed] [Google Scholar]
- 63.Hsing AW, Gao YT, McGlynn KA, Niwa S, Zhang M, Han TQ, Wang BS, Chen J, Sakoda LC, Shen MC, et al. Biliary tract cancer and stones in relation to chronic liver conditions: A population-based study in Shanghai, China. Int J Cancer. 2007;120:1981–1985. doi: 10.1002/ijc.22375. [DOI] [PubMed] [Google Scholar]
- 64.Loria P, Lonardo A, Lombardini S, Carulli L, Verrone A, Ganazzi D, Rudilosso A, D'Amico R, Bertolotti M, Carulli N. Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol. 2005;20:1176–1184. doi: 10.1111/j.1440-1746.2005.03924.x. [DOI] [PubMed] [Google Scholar]
- 65.Guraya SY. Reappraisal of the management of cholelithiasis in diabetics. Saudi Med J. 2005;26:1691–1694. [PubMed] [Google Scholar]
- 66.Chang SC, Rashid A, Gao YT, Andreotti G, Shen MC, Wang BS, Han TQ, Zhang BH, Sakoda LC, Leitzmann MF, et al. Polymorphism of genes related to insulin sensitivity and the risk of biliary tract cancer and biliary stone: a population-based case-control study in Shanghai, China. Carcinogenesis. 2008;29:944–948. doi: 10.1093/carcin/bgn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paisley AN, Roberts ME, Trainer PJ. Withdrawal of somatostatin analogue therapy in patients with acromegaly is associated with an increased risk of acute biliary problems. Clin Endocrinol (Oxf) 2007;66:723–726. doi: 10.1111/j.1365-2265.2007.02811.x. [DOI] [PubMed] [Google Scholar]
- 68.Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. 2007;99:3C–18C. doi: 10.1016/j.amjcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Schiemann U, Ferhat A, Götzberger M, Kaiser C, Stief J, Landgraf R, Dieterle C. Prevalence of cholecystolithiasis and its management among kidney/pancreas-transplanted type 1 (insulin-dependent) diabetic patients. Eur J Med Res. 2008;13:127–130. [PubMed] [Google Scholar]
- 70.Khan MK, Jalil MA, Khan MS. Oral contraceptives in gall stone diseases. Mymensingh Med J. 2007;16:S40–S45. [PubMed] [Google Scholar]
- 71.Reshetnyak TM, Saparin GV, Ivannikov PV, Reshetnyak VI. Corticosteroids and Cholelithiasis in Systemic Lupus Erythematosus. Scholarly Research Exchange. 2009;2009:9. [Google Scholar]
- 72.Sherlock S, Dooley J. Diseases of the liver and biliary system. 9th ed. Oxford: Blackwell Sci Pub; 1993. pp. 44–58. [Google Scholar]
- 73.Soysal A, Eraşov K, Akpinar I, Bakir M. Biliary precipitation during ceftriaxone therapy: frequency and risk factors. Turk J Pediatr. 2007;49:404–407. [PubMed] [Google Scholar]
- 74.Liu CM, Tung TH, Tsai ST, Liu JH, Tsai YK, Chen VT, Tam TN, Lu HF, Wang KK, Hsu CT, et al. Serum insulin, insulin resistance, beta-cell dysfunction, and gallstone disease among type 2 diabetics in Chinese population: a community-based study in Kinmen, Taiwan. World J Gastroenterol. 2005;11:7159–7164. doi: 10.3748/wjg.v11.i45.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang Y, Sung E, Ryu S, Park YW, Jang YM, Park M. Insulin resistance is associated with gallstones even in non-obese, non-diabetic Korean men. J Korean Med Sci. 2008;23:644–650. doi: 10.3346/jkms.2008.23.4.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, et al. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002;110:109–117. doi: 10.1172/JCI15387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kitsiou-Tzeli S, Giannatou E, Spanos I, Nicolaidou P, Fretzayas A, Tzetis M, Lazaris D, Kanavakis E, Tsezou A. Steroid hormones polymorphisms and cholelithiasis in Greek population. Liver Int. 2007;27:61–68. doi: 10.1111/j.1478-3231.2006.01385.x. [DOI] [PubMed] [Google Scholar]
- 79.Momiyama M, Wakai K, Oda K, Kamiya J, Ohno Y, Hamaguchi M, Nakanuma Y, Hsieh LL, Yeh TS, Chen TC, et al. Lifestyle risk factors for intrahepatic stone: findings from a case-control study in an endemic area, Taiwan. J Gastroenterol Hepatol. 2008;23:1075–1081. doi: 10.1111/j.1440-1746.2007.05258.x. [DOI] [PubMed] [Google Scholar]
- 80.Vilkin A, Geller A, Levi Z, Niv Y. Mucin gene expression in bile of patients with and without gallstone disease, collected by endoscopic retrograde cholangiography. World J Gastroenterol. 2009;15:2367–2371. doi: 10.3748/wjg.15.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sasaki M, Nakanuma Y, Kim YS. Expression of apomucins in the intrahepatic biliary tree in hepatolithiasis differs from that in normal liver and extrahepatic biliary obstruction. Hepatology. 1998;27:54–61. doi: 10.1002/hep.510270110. [DOI] [PubMed] [Google Scholar]
- 82.Wang HH, Afdhal NH, Gendler SJ, Wang DQ. Targeted disruption of the murine mucin gene 1 decreases susceptibility to cholesterol gallstone formation. J Lipid Res. 2004;45:438–447. doi: 10.1194/jlr.M300468-JLR200. [DOI] [PubMed] [Google Scholar]
- 83.Maximenko VB. Impaired concentration and motor evacuatory functions of the gallbladder in cholecystolithiasis. Rossiyskiy Zhurnal Gastroenterologii, Gepatologii i Koloproktologii. 2006;4:24–28. [Google Scholar]
- 84.Cerçi SS, Ozbek FM, Cerçi C, Baykal B, Eroğlu HE, Baykal Z, Yildiz M, Sağlam S, Yeşildağ A. Gallbladder function and dynamics of bile flow in asymptomatic gallstone disease. World J Gastroenterol. 2009;15:2763–2767. doi: 10.3748/wjg.15.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang SM, Yao CC, Pan H, Hsiao KM, Yu JK, Lai TJ, Huang SD. Pathophysiological significance of gallbladder volume changes in gallstone diseases. World J Gastroenterol. 2010;16:4341–4347. doi: 10.3748/wjg.v16.i34.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olokoba AB, Bojuwoye BJ, Olokoba LB, Wahab KW, Salami AK, Braimoh KT, Inikori AK. The relationship between gallstone disease and gall bladder volume. Niger J Clin Pract. 2008;11:89–93. [PubMed] [Google Scholar]
- 87.Bolukbas FF, Bolukbas C, Horoz M, Ince AT, Uzunkoy A, Ozturk A, Aka N, Demirci F, Inci E, Ovunc O. Risk factors associated with gallstone and biliary sludge formation during pregnancy. J Gastroenterol Hepatol. 2006;21:1150–1153. doi: 10.1111/j.1440-1746.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 88.Pamuk GE, Umit H, Harmandar F, Yeşil N. Patients with iron deficiency anemia have an increased prevalence of gallstones. Ann Hematol. 2009;88:17–20. doi: 10.1007/s00277-008-0557-x. [DOI] [PubMed] [Google Scholar]
- 89.Mansurov KhKh, Mirodzhov GK. Pathogenetic therapy for gallbladder cholesterosis. Rossiyskiy Zhurnal Gastroenterologii, Gepatologii i Koloproktologii. 2005;6:45–48. [Google Scholar]
- 90.Srivastava A, Pandey SN, Dixit M, Choudhuri G, Mittal B. Cholecystokinin receptor A gene polymorphism in gallstone disease and gallbladder cancer. J Gastroenterol Hepatol. 2008;23:970–975. doi: 10.1111/j.1440-1746.2007.05170.x. [DOI] [PubMed] [Google Scholar]
- 91.Zhang ZH, Wu SD, Gao H, Shi G, Jin JZ, Kong J, Tian Z, Su Y. Expression of pituitary adenylate cyclase-activating polypeptide 1 and 2 receptor mRNA in gallbladder tissue of patients with gallstone or gallbladder polyps. World J Gastroenterol. 2006;12:1468–1471. doi: 10.3748/wjg.v12.i9.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao Z, Schmitz F, Pricolo VE, Biancani P, Behar J. Role of caveolae in the pathogenesis of cholesterol-induced gallbladder muscle hypomotility. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1641–G1649. doi: 10.1152/ajpgi.00495.2006. [DOI] [PubMed] [Google Scholar]
- 93.Yi SQ, Ohta T, Tsuchida A, Terayama H, Naito M, Li J, Wang HX, Yi N, Tanaka S, Itoh M. Surgical anatomy of innervation of the gallbladder in humans and Suncus murinus with special reference to morphological understanding of gallstone formation after gastrectomy. World J Gastroenterol. 2007;13:2066–2071. doi: 10.3748/wjg.v13.i14.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kobayashi T, Hisanaga M, Kanehiro H, Yamada Y, Ko S, Nakajima Y. Analysis of risk factors for the development of gallstones after gastrectomy. Br J Surg. 2005;92:1399–1403. doi: 10.1002/bjs.5117. [DOI] [PubMed] [Google Scholar]
- 95.Nakamura K, Ogoshi K, Makuuchi H. Clinicopathological study of cholelithiasis following gastric cancer surgery. Eur Surg Res. 2005;37:29–35. doi: 10.1159/000083145. [DOI] [PubMed] [Google Scholar]
- 96.Quesada BM, Kohan G, Roff HE, Canullán CM, Chiappetta Porras LT. Management of gallstones and gallbladder disease in patients undergoing gastric bypass. World J Gastroenterol. 2010;16:2075–2079. doi: 10.3748/wjg.v16.i17.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crema E, Ribeiro LB, Adad SJ, Ectchebehere RM, Martins Júnior A, Silva AA. Gallbladder neuron count in cholelithiasis patients with and without Chagas disease. Rev Soc Bras Med Trop. 2007;40:15–17. doi: 10.1590/s0037-86822007000100003. [DOI] [PubMed] [Google Scholar]
- 98.vanBerge-Henegouwen GP, Venneman NG, van Erpecum KJ, Portincasa P. Drugs affecting biliary lipid secretion and gallbladder motility: their potential role in gallstone treatment and prevention. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:185–191. doi: 10.2174/1568008054064940. [DOI] [PubMed] [Google Scholar]
- 99.Loginov AS, Chebanov SM, Marakhovskiĭ IuKh, Goncharik II. [Microstructure of vesicular agglomerates of the lithogenic bile] Biull Eksp Biol Med. 1989;108:251–254. [PubMed] [Google Scholar]
- 100.Loginov AS, Chebanov SM, Petrakov AV, Saparin GV, Obyden SK, Ivannikov PV. Investigation of cholesterol, bilirubin, and protein distribution in human gallstones by color cathodoluminescence scanning electron microscopy and transmission electron microscopy. Scanning. 1998;20:17–22. doi: 10.1002/sca.1998.4950200103. [DOI] [PubMed] [Google Scholar]
- 101.Loginov AS, Chebanov SM, Saparin GV, Obyden SK, Reshetnyak VI. Microstructure of gallstones according to the color cathodoluminescence scanning electron microscopy. Russian Gastroenterol J. 1998;4:26–29. [Google Scholar]
- 102.Isayeva GSh. Possible involvement of Helicobacter bacteria in the pathogenesis of hepatobiliary diseases. Rossiyskiy Zhurnal Gastroenterologii, Gepatologii i Koloproktologii. 2008;4:14–22. [Google Scholar]
- 103.Vasavda N, Menzel S, Kondaveeti S, Maytham E, Awogbade M, Bannister S, Cunningham J, Eichholz A, Daniel Y, Okpala I, et al. The linear effects of alpha-thalassaemia, the UGT1A1 and HMOX1 polymorphisms on cholelithiasis in sickle cell disease. Br J Haematol. 2007;138:263–270. doi: 10.1111/j.1365-2141.2007.06643.x. [DOI] [PubMed] [Google Scholar]
- 104.Leff DR, Kaura T, Agarwal T, Davies SC, Howard J, Chang AC. A nontransfusional perioperative management regimen for patients with sickle cell disease undergoing laparoscopic cholecystectomy. Surg Endosc. 2007;21:1117–1121. doi: 10.1007/s00464-006-9054-2. [DOI] [PubMed] [Google Scholar]
- 105.Origa R, Galanello R, Perseu L, Tavazzi D, Domenica Cappellini M, Terenzani L, Forni GL, Quarta G, Boetti T, Piga A. Cholelithiasis in thalassemia major. Eur J Haematol. 2009;82:22–25. doi: 10.1111/j.1600-0609.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- 106.Tankanitlert J, Morales NP, Fucharoen P, Fucharoen S, Chantharaksri U. Association between promoter and coding region mutations of UDP-glucuronosyltransferase 1A1 and beta-thalassemia/Hb E with cholelithiasis. Eur J Haematol. 2008;80:351–355. doi: 10.1111/j.1600-0609.2007.01010.x. [DOI] [PubMed] [Google Scholar]
- 107.Uchiyama K, Kawai M, Tani M, Terasawa H, Tanimura H, Yamaue H. Pathogenesis of hepatolithiasis based on the analysis of components of intrahepatic stones. Hepatogastroenterology. 2007;54:1798–1804. [PubMed] [Google Scholar]
- 108.Sayers C, Wyatt J, Soloway RD, Taylor DR, Stringer MD. Gallbladder mucin production and calcium carbonate gallstones in children. Pediatr Surg Int. 2007;23:219–223. doi: 10.1007/s00383-006-1867-5. [DOI] [PubMed] [Google Scholar]
- 109.Kaur T, Kaur S. Pathophysiological conditions in cholelithiasis formation in North Indian population: spectroscopic, biophysical, and biochemical study. Biol Trace Elem Res. 2010;138:79–89. doi: 10.1007/s12011-010-8618-0. [DOI] [PubMed] [Google Scholar]
- 110.Carey MC. Pathogenesis of gallstones. Am J Surg. 1993;165:410–419. doi: 10.1016/s0002-9610(05)80932-8. [DOI] [PubMed] [Google Scholar]
- 111.Dikkers A, Tietge UJ. Biliary cholesterol secretion: more than a simple ABC. World J Gastroenterol. 2010;16:5936–5945. doi: 10.3748/wjg.v16.i47.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Claudel T, Zollner G, Wagner M, Trauner M. Role of nuclear receptors for bile acid metabolism, bile secretion, cholestasis, and gallstone disease. Biochim Biophys Acta. 2011;1812:867–878. doi: 10.1016/j.bbadis.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 113.Small DM. The staging of cholesterol gallstones with respect to nucleation and growth. In: Paumgartner J, Stiehl A, Gerok W, editors. Falk Symposium 29. Bile acids and lipids. England: MTP Press Limited; 1981. pp. 291–300. [Google Scholar]
- 114.Carey MC, Cohen D. Biliary transport of cholesterol in vesicles, micelles and liquid crystals. In: Paumgartner G, Stiehl A, Gerok W, editors. Falk Symposium 45. Bile Acids and the Liver. England: MTP Press Limited; 1987. pp. 287–300. [Google Scholar]
- 115.Cohen DE, Kaler EW, Carey MC. Cholesterol carriers in human bile: are "lamellae" involved? Hepatology. 1993;18:1522–1531. [PubMed] [Google Scholar]
- 116.Been JM, Bills PM, Lewis D. Microstructure of gallstones. Gastroenterology. 1979;76:548–555. [PubMed] [Google Scholar]
- 117.Chijiiwa K, Hirota I, Noshiro H. High vesicular cholesterol and protein in bile are associated with formation of cholesterol but not pigment gallstones. Dig Dis Sci. 1993;38:161–166. doi: 10.1007/BF01296790. [DOI] [PubMed] [Google Scholar]
- 118.Holan KR, Holzbach RT, Hermann RE, Cooperman AM, Claffey WJ. Nucleation time: a key factor in the pathogenesis of cholesterol gallstone disease. Gastroenterology. 1979;77:611–617. [PubMed] [Google Scholar]
- 119.Konikoff FM, Cohen DE, Carey MC. Phospholipid molecular species influence crystal habits and transition sequences of metastable intermediates during cholesterol crystallization from bile salt-rich model bile. J Lipid Res. 1994;35:60–70. [PubMed] [Google Scholar]
- 120.Loginov AS, Chebanov SM, Saparin GV, Obyden SK. The morphology and composition of cholesterol, protein, and bilirubin deposits in dried human bile: cathodoluminescence and backscattered electron imaging. Scanning. 1998;20:442–446. doi: 10.1002/sca.1998.4950200604. [DOI] [PubMed] [Google Scholar]
- 121.Jüngst C, Sreejayan N, Eder MI, von Stillfried N, Zündt B, Spelsberg FW, Kullak-Ublick GA, Jüngst D, von Ritter C. Lipid peroxidation and mucin secretagogue activity in bile of gallstone patients. Eur J Clin Invest. 2007;37:731–736. doi: 10.1111/j.1365-2362.2007.01853.x. [DOI] [PubMed] [Google Scholar]
- 122.Hofmann AF. Biliary secretion and excretion in health and disease: current concepts. Ann Hepatol. 2007;6:15–27. [PubMed] [Google Scholar]
- 123.Wu SD, Yu H, Sun JM. Bacteriological and electron microscopic examination of primary intrahepatic stones. Hepatobiliary Pancreat Dis Int. 2006;5:228–231. [PubMed] [Google Scholar]
- 124.Manolis EN, Filippou DK, Papadopoulos VP, Kaklamanos I, Katostaras T, Christianakis E, Bonatsos G, Tsakris A. The culture site of the gallbladder affects recovery of bacteria in symptomatic cholelithiasis. J Gastrointestin Liver Dis. 2008;17:179–182. [PubMed] [Google Scholar]
- 125.Crawford RW, Gibson DL, Kay WW, Gunn JS. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect Immun. 2008;76:5341–5349. doi: 10.1128/IAI.00786-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stewart L, Griffiss JM, Jarvis GA, Way LW. Bacteria entombed in the center of cholesterol gallstones induce fewer infectious manifestations than bacteria in the matrix of pigment stones. J Gastrointest Surg. 2007;11:1298–1308. doi: 10.1007/s11605-007-0173-4. [DOI] [PubMed] [Google Scholar]
- 127.Stewart L, Grifiss JM, Jarvis GA, Way LW. Biliary bacterial factors determine the path of gallstone formation. Am J Surg. 2006;192:598–603. doi: 10.1016/j.amjsurg.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 128.Jang JS, Kim KH, Yu JR, Lee SU. Identification of parasite DNA in common bile duct stones by PCR and DNA sequencing. Korean J Parasitol. 2007;45:301–306. doi: 10.3347/kjp.2007.45.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, Choi DW, Jang KT, Lee NY, Kim S, et al. Gallstones and Clonorchis sinensis infection: a hospital-based case-control study in Korea. J Gastroenterol Hepatol. 2008;23:e399–e404. doi: 10.1111/j.1440-1746.2007.05242.x. [DOI] [PubMed] [Google Scholar]
- 130.Mayev IV, Dicheva DT, Buragina TA. Diagnosis and treatment of biliary sludge in patients with ulcerative disease. Rossiyskiy Zhurnal Gastroenterologii, Gepatologii i Koloproktologii. 2007;4:68–72. [Google Scholar]
- 131.Mekhtiyev SN, Grinevich VB, Kravchuk YuA, Bogdanov RN. Biliary sludge: unsolved problems. Lechashchiy Vrach. 2007;6:24–28. [Google Scholar]
- 132.Marschall HU, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundström R, et al. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476–485. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 133.Jüngst C, Sreejayan N, Zündt B, Müller I, Spelsberg FW, Hüttl TP, Kullak-Ublick GA, del Pozo R, Jüngst D, von Ritter C. Ursodeoxycholic acid reduces lipid peroxidation and mucin secretagogue activity in gallbladder bile of patients with cholesterol gallstones. Eur J Clin Invest. 2008;38:634–639. doi: 10.1111/j.1365-2362.2008.01995.x. [DOI] [PubMed] [Google Scholar]
- 134.Guarino MP, Carotti S, Sarzano M, Alloni R, Vanni M, Grosso M, Sironi G, Maffettone PL, Cicala M. Short-term ursodeoxycholic acid treatment improves gallbladder bile turnover in gallstone patients: a randomized trial. Neurogastroenterol Motil. 2005;17:680–686. doi: 10.1111/j.1365-2982.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 135.Mas MR, Comert B, Mas N, Yamanel L, Ozotuk H, Tasci I, Jazrawi RP. Effects of long term hydrophilic bile acid therapy on in vitro contraction of gallbladder muscle strips in patients with cholesterol gallstones. World J Gastroenterol. 2007;13:4336–4339. doi: 10.3748/wjg.v13.i32.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stawarski A, Iwańczak B, Iwańczak F. [Predisposing factors and results of pharmalogical treatment using ursodeoxycholic acid of gallbladder stones in children] Pol Merkur Lekarski. 2006;20:199–202. [PubMed] [Google Scholar]
- 137.Keizman D, Goldiner I, Leikin-Frenkel A, Konikoff FM. [Innovations in the medical treatment of gallstones and fatty liver: FABACs (Fatty Acid Bile Acid Conjugates)] Harefuah. 2008;147:344–39, 373, 372. [PubMed] [Google Scholar]
- 138.Rabenstein T, Radespiel-Tröger M, Höpfner L, Benninger J, Farnbacher M, Greess H, Lenz M, Hahn EG, Schneider HT. Ten years experience with piezoelectric extracorporeal shockwave lithotripsy of gallbladder stones. Eur J Gastroenterol Hepatol. 2005;17:629–639. doi: 10.1097/00042737-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 139.Amplatz S, Piazzi L, Felder M, Comberlato M, Benvenuti S, Zancanella L, Di Fede F, de'Guelmi A, Bertozzo A, Farris P, et al. Extracorporeal shock wave lithotripsy for clearance of refractory bile duct stones. Dig Liver Dis. 2007;39:267–272. doi: 10.1016/j.dld.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 140.Carrilho-Ribeiro L, Pinto-Correia A, Velosa J, Carneiro De Moura M. A ten-year prospective study on gallbladder stone recurrence after successful extracorporeal shock-wave lithotripsy. Scand J Gastroenterol. 2006;41:338–342. doi: 10.1080/00365520500483256. [DOI] [PubMed] [Google Scholar]
- 141.Xu Z, Wang LX, Zhang NW, Hou CS, Ling XF, Xu Y, Zhou XS. Clinical application of plasma shock wave lithotripsy in treating impacted stones in the bile duct system. World J Gastroenterol. 2006;12:130–133. doi: 10.3748/wjg.v12.i1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shim CS, Moon JH, Cho YD, Kim YS, Hong SJ, Kim JO, Cho JY, Kim YS, Lee JS, Lee MS. The role of extracorporeal shock wave lithotripsy combined with endoscopic management of impacted cystic duct stones in patients with high surgical risk. Hepatogastroenterology. 2005;52:1026–1029. [PubMed] [Google Scholar]
- 143.Ahmed MH. Ezetimbe as potential treatment for cholesterol gallstones: the need for clinical trials. World J Gastroenterol. 2010;16:1555–1557. doi: 10.3748/wjg.v16.i13.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zúñiga S, Molina H, Azocar L, Amigo L, Nervi F, Pimentel F, Jarufe N, Arrese M, Lammert F, Miquel JF. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int. 2008;28:935–947. doi: 10.1111/j.1478-3231.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 145.Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134:2101–2110. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ko CW. Magnesium: does a mineral prevent gallstones? Am J Gastroenterol. 2008;103:383–385. doi: 10.1111/j.1572-0241.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- 147.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Long-term effect of magnesium consumption on the risk of symptomatic gallstone disease among men. Am J Gastroenterol. 2008;103:375–382. doi: 10.1111/j.1572-0241.2007.01696.x. [DOI] [PubMed] [Google Scholar]
- 148.Koppisetti S, Jenigiri B, Terron MP, Tengattini S, Tamura H, Flores LJ, Tan DX, Reiter RJ. Reactive oxygen species and the hypomotility of the gall bladder as targets for the treatment of gallstones with melatonin: a review. Dig Dis Sci. 2008;53:2592–2603. doi: 10.1007/s10620-007-0195-5. [DOI] [PubMed] [Google Scholar]