Abstract
Thrombospondin 1(TSP1) plays major roles in both physiologic and pathologic tissue repair. TSP1 through its type 1 repeats is a known regulator of latent TGF-β activation and plays a role in wound healing and fibrosis. Binding of the TSP N-terminal domain to cell surface calreticulin in complex with LDL-receptor related protein 1 stimulates intermediate cell adhesion, cell migration, anoikis resistance and collagen expression and matrix deposition in an in vivo model of the foreign body response. There is also emerging evidence that TSP EGF-like repeats alters endothelial cell-cell interactions and stimulate epithelial migration through transactivation of EGF receptors. The mechanisms underlying these functions of TSP1 and the implications for physiologic and pathologic wound repair and fibrosis will be discussed.
Keywords: thrombospondins, calreticulin, EGF receptors, wound repair, fibrosis, TGF-β
1. Introduction
Tissue repair is a highly orchestrated process involving cell specific, temporally and spatially regulated responses, which include hemostasis with platelet aggregation, deposition of a provisional extracellular matrix (ECM), inflammatory cell recruitment, fibroblast proliferation and angiogenesis, replacement of the provisional ECM with cell type specific ECM, and finally capillary and fibroblast regression with re-epithelialization (Diegelmann and Evans, 2004). In contrast, fibrosis represents dysregulation of tissue repair processes and is characterized by excessive deposition of a fibrillar collagen-rich ECM, induction and proliferation of contractile, ECM-producing myofibroblasts, and variable inflammation, the sum of which results in replacement of the normal parenchymal architecture with non-functional scar tissue.
The matricellular protein thrombospondin 1 (TSP1) is a complex multi- functional protein released from platelet α-granules, incorporated into the fibrin clot, and expressed by cell types that participate in wound healing responses in a temporally regulated manner (Agah et al., 2002; DiPietro et al., 1996; Murphy-Ullrich and Mosher, 1985; Raugi et al., 1987; Reed et al., 1993). TSP1 regulates multiple cellular events involved in tissue repair including hemostasis, cell adhesion, migration, proliferation, ECM expression and organization, and regulation of growth factor activity (Adams and Lawler, 2004, 2011). In addition to physiologic repair, TSP1 is also expressed at elevated levels in many tissues undergoing fibroproliferative remodeling and blockade of specific actions of TSP1 or loss of TSP1 expression can attenuate pathologic tissue remodeling (Daniel et al., 2007; Hugo, 2003; Poczatek et al., 2000).
There are excellent recent reviews which discuss the functions of the broader thrombospondin family (Adams and Lawler, 2011) or the role of Group A thrombospondins in wound healing, ischemia, and the foreign body response (Kyriakides and Maclauchlan, 2009). In this review, we will focus primarily on aspects of TSP1 function relevant to tissue repair and fibrosis which have not been addressed extensively elsewhere. These functions include latent TGF-β activation, signaling of intermediate cell adhesion and collagen stimulation through calreticulin (CRT)-LRP1, and its emerging role as a regulator of EGF receptor signaling.
2. TSP1 activation of latent TGF-β
2.1 Background and mechanisms
In the late 1980s we observed effects of platelet derived TSP1 on the growth of bovine aortic endothelial cells which could not be blocked by cocktails of available anti-TSP1 antibodies. This led us to investigate possible contaminating proteins in our TSP1 preparations. At the suggestion of Dr. Vishva Dixit, we treated TSP1-stimulated cultures with anti-TGF-β neutralizing antibodies, since TGF-β is abundant in platelet α-granules. Interestingly, our effects on endothelial growth and morphology in TSP1-treated cultures were reversed by blockade of TGF-β activity (Murphy-Ullrich et al., 1992). Further investigation of platelet TSP1 preparations provided by several different labs all showed traces of bioactive TGF-β. TSP1 specifically binds to purified radiolabeled active TGF-β and TGF-β bioactivity co-eluted with TSP1 in platelet releasates (Murphy-Ullrich et al., 1992). Since heparin, α2-macroglobulin or fibronectin could not disrupt TSP-TGF-β binding and decorin had only a modest displacement effect, it was initially thought that TSP1-TGF-β complex formation functioned to protect TGF-β from endogenous inhibitors or perhaps prevent sequestration in the extracellular matrix. Platelet TSP1 depleted of “contaminating” TGF-β still retained TGF-β-dependent effects on endothelial growth inhibition. This activity was explained by the observation that incubation of TSP1 depleted of associated TGF-β activity with either purified large or small latent TGF-β complex converts the latent growth factor to the biologically active form (Schultz-Cherry and Murphy-Ullrich, 1993; Schultz-Cherry et al., 1994b). TSP1 binds to the latent TGF-β complex through specific sequences in the type 1 repeats or thrombospondin repeats (TSRs) and induces activation through stimulation of a conformation change in the latent complex. Importantly, activation occurs independent of proteolytic activity and does not require interactions with cells (Schultz-Cherry et al., 1994a; Schultz-Cherry and Murphy-Ullrich, 1993). Furthermore, as TGF-β can retain its biological activity when bound to TSP1, this suggests that TSP1 might facilitate presentation of active TGF-β to cell surface receptors. (Murphy-Ullrich et al., 1992; Schultz-Cherry et al., 1994b). This would be consistent with other mechanisms of latent TGF-β activation such as integrin-dependent or mechano-dependent activation identified subsequent to our findings with TSP1 (Munger et al., 1999; Wipff et al., 2007). Activation via these mechanism requires a) tethering of the latent TGF-β complex into the extracellular matrix, typically through binding of the latent TGF-β binding protein to fibrillin or fibronectin, b) interactions of the latent complex via the RGD sequence in the latency associate peptide with cell surface molecules such as integrins, and c) alterations of latent complex folding partly through cytoskeletal-generated contractile forces (Hinz, 2009; Munger and Sheppard, 2011). Although direct protein-protein interactions between latent TGF-β and TSP1 are sufficient to induce conformational changes in the latent complex and TSP1 fragments and peptides can induce activation in vitro and in vivo, it is indeed possible that TSP1 interactions with its cellular receptors modulate activation of latent TGF-β in certain cellular systems in vivo. Antibodies to cell surface receptors for TSP1, avβ3 integrin and CD47, inhibit activation of TGF-β in tamoxifen-treated mammary carcinoma cells (Harpel et al., 2001) and macrophage CD36 is required for latent TGF-β activation by TSP1 in bleomycin-stimulated pulmonary fibrosis (Chen et al., 2009; Yehualaeshet et al., 2000; Yehualaeshet et al., 1999). CD36 is a scavenger receptor for both oxidized LDL and TSP1, and oxidized LDL reduces TSP1 binding to mouse peritoneal macrophages and latent TGF-β activation by these cells (Sakamoto et al., 2005). However, oxidized LDL also reduced latent TGF-β activation by TSP1 in a cell free system (Sakamoto et al., 2005), suggesting oxidized LDL acts potentially both by steric inhibition of soluble TSP1 binding to the latent complex through an unknown mechanism and by blocking localization of the latent complex to the cell surface for activation. There is also emerging evidence that TSP1 binding to the latent complex might be critical for mechanical activation of TGF-β. Although earlier studies had shown apparently normal TGF-β activity in platelets from TSP1 null mice, further studies showed that a small but reproducible level of TGF-β activated by platelets under shear flow is indeed TSP1-dependent (Abdelouahed et al., 2000; Ahamed et al., 2009). Furthermore, in neonatal sheep lungs, mechanical stretch increased TSP1 expression and TGF-β activity (Warburton and Kaartinen, 2007). The ability of TSP1 to release and/or activate matrix-bound latent TGF-β has not been directly addressed. In addition, the capacity of extracellular matrix bound TSP1 to activate TGF-β has not been directly addressed and it is not known whether TSP1 must be released from the matrix to bind and activate latent TGF-β. Further studies are needed to more clearly define the role of extracellular matrix and cell surface interactions in regulating TSP1-dependent TGF-β activation.
Two sequence motifs in the TSRs of TSP1 are required for binding and activation of latent TGF-β: KRFK and WxxW (Figure 1). Binding of the KRFK sequence, located between the 1st and 2nd type 1 repeats of TSP1, to the LAP of the latent complex is necessary for activation (Schultz-Cherry et al., 1995). KRFK peptide or TSP1 binding to the small latent complex induces a conformational change in the latent complex and in the LAP as determined by alterations in the circular dichroism spectra (Jablonsky, Jackson, Su, Muccio, and Murphy-Ullrich, unpublished data). Furthermore, binding of TSP1 to free LAP prevents LAP from conferring latency on the mature domain (Ribeiro et al., 1999). The RFK sequence of TSP1 binds to the (L54SKL) sequence which is conserved in the LAP regions of TGF-βs1-3 (Ribeiro et al., 1999). We showed that this LSKL sequence is critical for maintenance of latency through mediating binding of the LAP to the R94KPK sequence in the type II receptor binding region of the mature domain (Young and Murphy-Ullrich, 2004a) and the importance of the LSKL sequence for maintenance of latency has recently been confirmed by further biochemical and structural studies (Shi et al., 2011; Walton et al., 2010). Shi et al recently showed that lysine26 in the LSKL (lysine55 from signal peptide) sequence of the latency associated peptide (LAP) is a “fastener residue” in the latency lasso which comprises a structural constraint necessary for maintaining latency (Shi et al., 2011) and integrin binding to LAP disrupts this lasso. Similarly, we showed that KRFK/TSP1 activates the latent complex by competitively disrupting the latency interaction between the LSKL sequence in the LAP and the RKPK sequence in the mature domain of TGF-β (Young and Murphy-Ullrich, 2004a). This new structural information coupled with our biochemical approaches suggests a common molecular basis for activation of the latent complex. The critical role of lysine26 is consistent with our use of LSAL peptide as an inactive control for LSKL. Interestingly, other proteins with RFK motifs (neuropilin-1 has an RKFK sequence, F-spondin has KRFK in TSR repeat 6, and ADAMTS1 has KTFR) have also been shown to activate latent TGF-β (Attur et al., 2009; Bourd-Boittin et al., 2011; Glinka and Prud’homme, 2008). This suggests that the “RFK” mechanism might be a common mechanism of latent TGF-β activation utilized by additional proteins. The second TGF-β binding motif in each of the three TSRs (WxxW) binds to VLAL sequences in the both the LAP and the active domain of TGF-β and facilitates the ability of KRFK to activate latent TGF-β, possibly acting as a “docking site” to correctly orient the KRFK sequence with its complementary site on the latent TGF-β molecule (Young and Murphy-Ullrich, 2004b). WxxW peptides alone do not activate latent TGF-β, but can be used to competitively block both TSP1 binding to the mature domain and activation of the latent TGF-β complex by TSP1. The role of c-mannosylation of these tryptophan resides in mediating TSP1 binding to the latent complex is unknown and represents a potential level of control of these interactions (Hofsteenge et al., 2001). C-mannosylation of WSPW peptide increased binding to heat shock cognate protein 70 and indirectly increased TGF-β signaling through TAK1, suggesting that this post-translational modification can affect protein-protein interactions (Ihara et al., 2010).
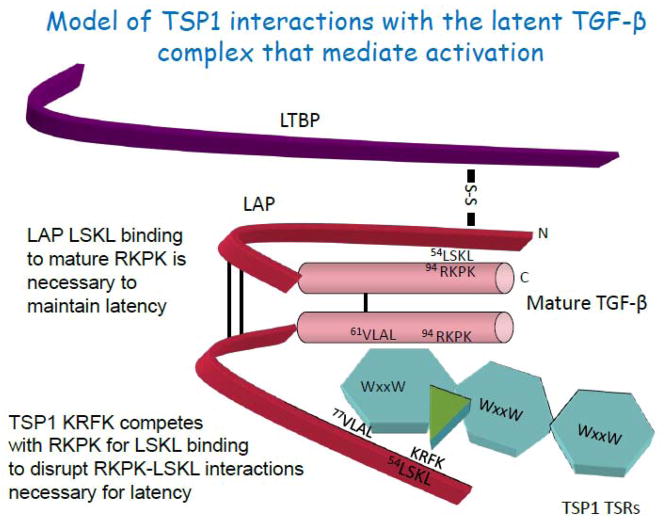
Figure 1. TSP1 interactions with latent TGF-β that induce activation.
The type 1 repeats of TSP1 (TSRs) mediate the binding of TSP1 to the small latent complex consisting of the N-terminal pro-domain, known as the latency associated peptide (LAP), and the C-terminal portion of the latent complex, known as mature TGF-β. The C-terminal portion of the mature TGF-β homodimer binds to TGF-β signaling receptors. Binding of the LSKL sequence at the amino terminus of the LAP to the RKPK sequence in the receptor binding region of the mature domain is necessary to confer latency. The small latent complex is a disulfide-bonded homodimer (S-S bonds in black) which is covalently bound to the fibrillin-like latent TGF-β binding protein (LTBP) at cysteine 33 of the LAP. The tryptophan-rich motifs (WSxW) present in each of the 3 TSRs of TSP1 bind to the VLAL sequence in the mature domain. This interaction is necessary for activation of latent TGF-β by TSP1 or recombinant TSRs, but in itself is not sufficient for activation. The WSxW sequences in the TSRs also bind to the VLAL sequence in the LAP domain, although the significance of this interaction for activation is not clear. The KRFK sequence of TSP1 which is located at the N-terminal portion of the 2nd TSR recognizes the LSKL sequence in the LAP and competitively disrupts LSKL-RKPK binding, thus displacing LAP from the mature domain, resulting in access of the mature domain for TGF-β receptor binding. The LSKL sequence has been shown to be critical for latency (Shi et al., 2011; Walton et al., 2010; Young and Murphy-Ullrich, 2004a). This stick model is not intended to accurately represent the relative sizes of the proteins or to convey specific information regarding the folding of either the TSRs or mature TGF-β.
2.2 Biological roles
In vitro studies have shown that TSP1 activates latent TGF-β secreted by multiple cell types including endothelial cells, mesangial cells, hepatic stellate cells and skin, lung, and cardiac fibroblasts, T cells, and macrophages (Breitkopf et al., 2005; Mimura et al., 2005; Murphy-Ullrich and Poczatek, 2000; Poczatek et al., 2000; Schultz-Cherry and Murphy-Ullrich, 1993; Yang et al., 2009; Yehualaeshet et al., 1999; Yevdokimova et al., 2001; Zhou, 2004; Zhou et al., 2006). Peptides such as LSKL or WxxW which block TSP1 binding to the latent complex or antibodies which block TSP1-dependent TGF-β activation such as Mab 133 have been used to establish the involvement of endogenous TSP1 in TGF-β activation in a number of disease conditions and physiologic processes (Table 1) (Belmadani et al., 2007; Crawford et al., 1998; Daniel et al., 2004; Kondou et al., 2003; Lu et al., 2011). Initial evidence for an in vivo role of TSP1 in latent TGF-β activation was shown by the ability of the KRFK peptide administered in the perinatal period to partially rescue the abnormal TSP-1 null phenotype, in particular airway epithelial hyperplasia and pancreatic islet hyperplasia/acinar hypoplasia (Crawford et al., 1998). Furthermore, treatment of wild type mice with the LSKL blocking peptide in the perinatal period replicated features of the TSP1 knockout phenotype in the airways and pancreas. Double knockout of both β6 integrin and TSP1 results in a phenotype distinct from either single knockout that is characterized by severe inflammation, cardiac degeneration, and epithelial hyperplasia, suggesting both separate and synergistic roles in regulating latent TGF-β activation (Ludlow et al., 2005). However, it is likely that the primary role for TSP1 in controlling TGF-β activation is during injury, under stress, and in pathologic conditions, rather than during development. The expression of TSP1 is induced by factors associated with systemic diseases with fibrotic end organ involvement including high glucose, reactive oxygen species, and angiotensin II (Wang et al., 2002; Wang et al., 2004b; Yevdokimova et al., 2001; Zhou et al., 2006). Indeed there is evidence from studies utilizing TSP1 antagonist peptides and diabetic TSP1 knockout mice that TSP1 is a major factor in the development of fibrotic end organ complications in diabetes (Belmadani et al., 2007; Daniel et al., 2007; Lu et al., 2011). Diabetic rats with abdominal aortic coarctation development left ventricular dysfunction and interstitial myocardial fibrosis. Treatment with intraperitoneal injections of LSKL, but not LSAL control peptide, reduced cardiac fibrosis, Smad phosphorylation, and improved left ventricular function (Belmadani et al., 2007). Similarly, treatment of Akita mice, a model of type 1 diabetes, with intraperitoneal LSKL reduced urinary TGF-β activity and renal phospho-Smad2/3 levels and improved markers of tubulointerstitial injury and podocyte function (Lu et al., 2011). Interestingly, several studies have shown that TSP1 is involved in alveolar macrophage-dependent TGF-β activation in mouse and rat models of bleomycin-induced pulmonary fibrosis and treatment with either TSP1 or CD36 antagonist peptides can ameliorate lung fibrosis and reduce active TGF-β (Chen et al., 2009; Yehualaeshet et al., 2000). Yet a study using bleomycin-treated TSP1 null mice showed no protection from pulmonary fibrosis or reduction in Smad phosphorylation (Ezzie et al., 2011). The reason for this discrepancy is not clear. However, it is possible that the potentially increased nitric oxide-dependent peroxynitrite mediated damage in the absence of the nitric oxide inhibitory function of TSP1 in the TSP1 null mouse might exacerbate lung fibrosis in response to the oxidant bleomycin.
Table 1.
Diseases associated with TSP1 regulation of TGF-β activation
| Disease | Organ/Tissue | In vitro | In vivo model | TSP1 antagonist | References |
|---|---|---|---|---|---|
| Diabetes | Kidney | Mesangial cells in high glucose; glycated albumin | Mice, STZ and Akita, TSP1 null; USF2 transgenic | GGWSHW LSKL Mab 133 |
Poczatek 2000; Yedokimova 2001; Wahab 2005; Yang 2004; Daniel 2007; Liu 2007, Lu & Miao 2011 |
| Diabetes/Hypertension | Heart | Rat cardiac fibroblasts; mesangial cells | Rats, STZ with aortic coarctation | LSKL Mab 133 |
Naito 2004; Zhou 2006; Belmadani 2007 |
| Mesangial Proliferative glomerulonephritis | Kidney | Rats, Anti-Thy-1 antibody injury | LSKL, AAWSHW Anti-sense oligonucleotides |
Daniel 2004; Daniel 2003 | |
| Chronic Kidney Disease | Kidney | Rat, UUO | LSKL | Xie XS, 2010 | |
| Bleomycin-induced lung fibrosis | lung | Lung fibroblasts (bleomycin, IL-4) | Rats, mice Bleomycin-treated |
LSKL, WSxW in vitro; CD36 binding peptides in vivo | Zhou 2004; Yehualaeshet 2000, Chen2009 |
| Asthma airway remodeling | lung | airway epithelium-fibroblast co-cultures | LSKL | Morishima 2001 | |
| Liver fibrosis | liver | Rat, DMN-induced | LSKL | Kondou 2003 | |
| Hepatic stellate cells: PDGF, TNF-α | LSKL | Breitkopf 2004 | |||
| Hepatocytes, bile acid | LSKL | Myung 2006 | |||
| Scleroderma | skin | Scleroderma fibroblasts | Anti-sense oligos, Mab 133, GGWSHW | Mimura 2005 | |
| Systemic Sclerosis | Fibroblast contractility | LSKL, siRNA | Chen Y 2011 | ||
| Autoimmune disease | Dermis | Dendritic cells; Human peripheral blood T cells | Mab133, LSKL | Derks 2007 | |
| Eye | RPE | Anti-TSP antibody, TSP1 (−/−) | Futagami 2007; Zamiri 2005 |
||
| NK cells | Anti-TGF-β antibody | Pierson 1996 | |||
| MS/Brain inflammation | EAE model of MS with Ang II | LSKL, candesartan | Lanz 2010 | ||
| Th17 in EAE | EAE MOG peptide/TSP1 knockout | TSP1 (−/−) | Yang 2009 | ||
| Rheumatoid Arthritis | Synovium | RA synovial fibroblasts | Rats, Collagen- induced arthritis | Adenoviral TSP1 | Jou 2005; Pohlers 2007 |
| Wound healing | Skin | Dermal fibroblast cultures | Mouse, excisional wound healing; | TSP1 (−/−); KRFK, KFK- fatty acid | Nor 2005, Cauchard 2004 |
| Cancer | glioma | Antibodies to TSP1 | Sasaki 2001 | ||
| Tamoxifen, MCF7, T47D mammary carcinoma | WSHW, anti-CD47, anti-αvβ3 antibody | Harpel 2001 | |||
| Mice, subcutaneous B16F10 melanoma | RFK-2nd TSR | Miao 2001 | |||
| Mice, A431 squamous cell carcinoma | RFK-TSR | Yee 2004 | |||
| Tumor stoma collagen and nanoparticle delivery | HSTS26T fibrosarcoma, Mu89 melanoma | losartan | Diop-Frimpong 2011 |
2.3 TSP1-dependent TGF-β activation in wound healing
One of the roles of TSP1 in dermal wound healing appears to be regulation of latent TGF-β activation. The phenotype of excisional wound healing in the TSP1 null mouse is consistent with a decrease in local TGF-β activation (Agah et al., 2002) and is characterized by a delay in macrophage recruitment and capillary angiogenesis and a persistence of granulation tissue, neovascularization, and inflammation (Nor et al., 2005). Topical treatment of TSP1 null wounds with the KRFK activating peptide largely rescued the TSP1 null wound phenotype (Nor et al., 2005). TGF-β levels in these wounds were increased following KRFK treatment and the effects of the KRFK peptide were blocked by a pan-specific anti-TGF-β antibody. While these data suggest that TSP1 plays a role in local activation of TGF-β during wounding, the studies of Agah et al., concluded that the decreased active and total TGF-β in the wounds of TSP1 or TSP1/TSP2 null mice is indirect and primarily due to defects in macrophage recruitment to wounds (a major source of TGF-β in wounds) leading to an overall reduction in TGF-β rather than a defect in activation (Agah et al., 2002). Despite this controversy, it is clear that TSP1 has the potential to modify the wound healing process. Subcutaneous implantation of TSP1 soaked sponges increased levels of active TGF-β, gel contraction and fibroblast migration (Sakai et al., 2003). Overexpression of TSP1 in keloids and in scleroderma correlates with increased TGF-β activity (Chen et al., 2011; Chipev et al., 2000; Mimura et al., 2005). Others have used a derivative of the KRFK sequence, KFK coupled to a fatty acyl moiety to locally activate TGF-β and increase TIMP-1, which reduces MMP-induced elastin and collagen degradation when applied to dermal fibroblast cultures (Cauchard et al., 2004). Systemic administration of the LSKL blocking peptide did not reduce Smad signaling or impair dermal wound healing in diabetic mice, although, these studies did not address the effects of direct LSKL administration to the wounds and it is not known if local dermal levels of LSKL following systemic intraperitoneal peptide administration are sufficient to alter local TGF-β activation (Lu et al., 2011). It remains to be determined whether either positive or negative modulation of TSP1 action represents a viable therapeutic strategy to modify either defective or excessive wound repair processes in vivo.
3. TSP transactivation of the EGFR through E123
TSP1 is well known to regulate the actions of multiple different growth factors, including TGF-β activation, VEGF, FGF, and PDGF. Emerging evidence also suggests a role for the EGF-like repeats of TSP1 in transactivation of EGF-family receptors in epithelial and endothelial cells. TSP1 can disrupt endothelial barrier function through stimulation of tyrosine phosphorylation of endothelial cell-cell junctional proteins (Goldblum et al., 1999). It was initially thought that TSP1 induced disruption of cell-cell junctions through its ability to stimulate focal adhesion disassembly and actin cytoskeletal reorganization similar to the actions of SPARC (Goldblum et al., 1994; Liu et al., 2009b). However, the CRT binding sequence of TSP1 (hep I peptide) had no effect on barrier function. Subsequent studies showed that the EGF-like modules of (E123) of TSP1,2, and 4 stimulated EGFR tyrosine phosphorylation and downstream activation of phospholipase Cγ and EGFR-dependent A431 epidermoid carcinoma epithelial cell migration in scratch assays (Liu et al., 2009a). Although antibodies to the EGFR ectodomain block E123 stimulation of EGFR phosphorylation, direct binding of E123 was not detected, suggesting activation by indirect mechanisms (Liu et al., 2009a). E123 stimulation increased MMP9 activity and MMP inhibition significantly reduced E123 stimulated EGFR phosphorylation, suggesting a possible scenario in which E123 activates MMPs to liberate cell surface bound EGFR ligands for EGFR binding (Liu et al., 2009a). Furthermore, TSP1 stimulated EGFR and ErbB2 phosphorylation in lung microvascular endothelial cells and TSP1, E123, and EGF induced endothelial barrier dysfunction in cells overexpressing EGFR (Garg et al., 2011). The in vivo actions of E123 transactivation of EGFR family members remains to be elucidated. However, the E123 region might have broad activities in endothelial and epithelial function in wound healing and tumorigenesis through modulation of cell-cell interactions, similar to the role of the E123 domains in synaptogenesis (Risher, 2012).
4. TSP1 N-terminal domain signaling through the CRT-LRP1 co-complex
Induction of the state of intermediate adhesion is a characteristic function of matricellular proteins (Greenwood and Murphy-Ullrich, 1998; Murphy-Ullrich, 2001; Sage and Bornstein, 1991). This is characterized by cellular de-adhesion involving re-organization of the focal adhesion scaffold with dispersal of vinculin and α-actinin dissociation with the integrin β-subunit (Greenwood et al., 2000) in the presence of clustered integrin in a spread cell. The intermediate adhesive state is proposed to facilitate cell migration and survival, thus enhancing tissue repair and remodeling, consistent with the known roles of matricellular proteins on cell adhesion in vitro and on wound healing, repair, and fibrosis in vivo (Murphy-Ullrich, 2001).
It has long been recognized that in vitro substrates of immobilized TSP1 weakly and variably support cell attachment but fail to promote cell adhesion with cytoskeletal organization and focal adhesion formation (Adams and Lawler, 1993; Lahav et al., 1987; Murphy-Ullrich and Hook, 1989; Tuszynski et al., 1987). In addition, exposure of cells to soluble TSP1 either prior to or at the time of plating inhibits cell attachment and spreading on fibronectin substrates and presentation of soluble TSP to adherent cells with organized focal adhesions induces loss of focal adhesions as visualized by interference reflection microscopy (Murphy-Ullrich and Hook, 1989; Murphy-Ullrich and Mosher, 1987). The de-adhesive activity of TSP1 and TSP2 was localized to amino acids 19–35 and can be mimicked by the soluble, isolated N-terminal domain of TSP1 or by peptides comprising this sequence (Murphy-Ullrich et al., 1993). Although we initially speculated that transmembrane heparan sulfate proteoglycans such as syndecans would be the receptor for this heparin-binding sequence (Sun et al., 1989), further studies showed that TSP1 stimulation of focal adhesion disassembly was not blocked by heparanse treatment of cells and a cell surface form of the ER calcium-binding and chaperone protein, CRT, was identified through affinity binding approaches as the hep I binding receptor (Goicoechea et al., 2000; Murphy-Ullrich et al., 1993). Lysines at amino acid 24 and 32 were shown to be important for hep I function and these amino acids have also been shown to be critical for TSP1-CRT binding as shown by both protein binding and molecular dynamics simulations (Goicoechea et al., 2000; Yan et al., 2010, 2011). TSP1 engagement of cell surface CRT initiates signaling in the FAK, ERK, and PI3K/Akt pathways which culminates in transient down regulation of Rho activity, critical for focal adhesion disassembly and actin stress fiber reorganization (Greenwood et al., 1998; Orr et al., 2002; Orr et al., 2004). In addition to focal adhesion disassembly, TSP1 signaling through the CRT- LDL-receptor related protein 1 (LRP1) co-complex stimulates random and directed cell migration of endothelial cells and fibroblasts (Orr et al., 2003a; Orr et al., 2003b). Signaling is initiated when TSP1 binds to CRT which induces increased CRT association with the scavenger receptor LRP1 and transient association of LRP1 with Gαi protein (Orr et al., 2003b). Hep I itself does not bind to LRP1, although other portions of the TSP1 N-terminal domain bind to LRP1 (Wang et al., 2004a). Molecular modeling of the TSP1-CRT complex suggest that TSP1 binding to CRT induces a more open CRT conformation with an increase in the rotational angle between the CRT N and P domains (Yan et al., 2010, 2011) to potentially expose an otherwise cryptic LRP1 binding site and could account for the enhanced association between CRT and LRP1 upon TSP1 ligation of CRT. LRP1 is a ligand for cell surface CRT in mediating clearance of apoptotic cells, both when presented in cis (same cell) or in trans (on different cells) (Gardai et al., 2005; Gold et al., 2010). Macrophage mediated clearance of apoptotic cells is generally considered to aid in resolution of inflammation and fibrosis (Wynn and Barron, 2010). However, this effect is independent of TSP1-CRT binding, since CRT null MEFs expressing CRT lacking the TSP binding site could be phagocytosed by murine macrophages, although CD47 inactivation of SIRPα is required to prevent phagocytosis of viable cells (Gardai et al., 2005). Li et al showed that TSP1 binding to cell surface CRT is required for TSP1 stimulation of T cell motility through CD47(Li et al., 2005). The role of TSP1-CD47 binding in regulating inhibition of phagocytosis by CRT-LRP1 was not addressed and the possibility of cross-talk between the N and C terminal domains remains an open question (Chao et al., 2010; Gardai et al., 2005). Preliminary studies to determine whether TSP1 binding to CRT (hep I peptide) would affect LRP1 clearance of matrix metalloproteinases failed to show any differences in MMP activity (Bing Sun, Mariya Sweetwyne, Joanne Murphy-Ullrich, unpublished data). Factors that regulate CRT localization at the cell surface represent another mechanism to regulate TSP1 functions mediated by direct interactions of TSP1 with CRT and also potentially cross-regulate TSP1-LRP1 and TSP1-CD47 mediated signaling (Chao et al., 2010; Jeffery et al., 2011; Tarr et al., 2010). This possibility is intriguing especially given the recent finding that CD47 is modified by heparan sulfate glycosaminoglycan chains (Kaur et al., 2011; Roberts, 2012). Although the recombinant, trimeric TSP1 N-terminal domain (NoC1) and the hep I peptide bind CRT and signal identically to the intact TSP molecule, there remains the possibility that folding of the C-terminus might affect TSP1-CRT interactions similar to how the calcium binding at the C-terminus can regulate binding of the N-terminus to integrins and other ligands (Calzada et al., 2008). Although the CRT binding sequence is conserved in both TSP1 and TSP2, we only have direct evidence for TSP2 stimulation of focal adhesion disassembly. TSP2 signaling of cell migration, anoikis resistance or collagen production through cell surface CRT remains unknown.
Adherent cells require signals from the extracellular matrix for survival. This occurs through both integrin-dependent and independent signals. Loss of cell adhesion signals result in apoptosis which has been termed “anoikis” (Frisch and Ruoslahti, 1997; Frisch and Screaton, 2001). Anoikis has physiologic roles especially during development and during resolution of wound healing, but dysregulated anoikis is associated with certain diseases (Chiarugi and Giannoni, 2008). Pathways activated by TSP1 signaling through CRT-LRP1 such as ERK and PI3K are associated with cell survival and we hypothesized that activation of survival pathways during down regulation of cell adhesion might provide a survival advantage during tissue remodeling. Indeed, hep I, TSP1, and NoC1 all prevented apoptotic cell death of fibroblasts in suspension (Pallero et al., 2008). Prevention of anoikis depended on TSP1 interactions with CRT as CRT null cells stably expressing CRT lacking the TSP1 binding site were not rescued from anoikis by TSP1. TSP1 rescue from anoikis is dependent on Akt signaling, but not ERK. Interestingly, TSP1/hep I had no effect on cell death in adherent cells. The in vivo significance of TSP1 prevention of anoikis remains to be elucidated, although it is reasonable to suggest that this TSP1 function could impact early cellular responses to injury.
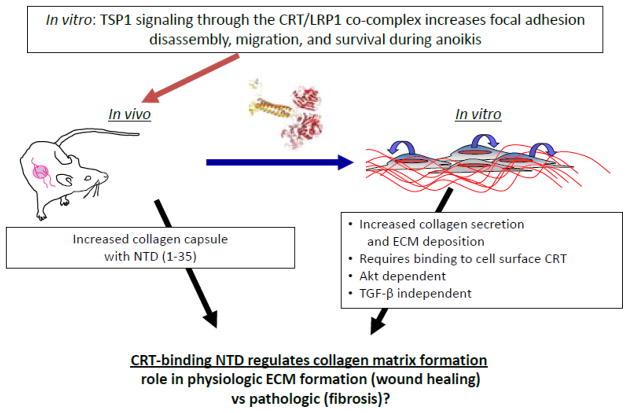
To begin to address the in vivo role of TSP1 signaling through the CRT-LRP1 co-complex, we initiated local expression of the CRT binding sequence during tissue remodeling in the foreign body response (Bonadio et al., 1999; Puolakkainen et al., 2005; Sweetwyne et al., 2010). Surgical sponges impregnated with type I collagen loaded with plasmid for expression of GFP-tagged, secreted CRT binding sequence of TSP1were implanted subcutaneously in wild type mice. Invading cells ingest the collagen and are locally transfected to express secreted TSP1 CRT binding fragment. Given the reduced granulation tissue in excisional dermal wounds in TSP1 null mice and the known in vitro functions of TSP1 signaling through the CRT-LRP1 co-complex in stimulating cell migration and in preventing anoikis (Orr et al., 2003a; Pallero et al., 2008; Polverini et al., 1995), we predicted that wild-type mice overexpressing the TSP1 CRT binding sequence at the implant site would exhibit increased granulation tissue due to an increase in endothelial cell and fibroblast migration and survival. Instead, local overexpression of the TSP1 CRT binding sequence by invading cells at the sponge margins stimulated formation of a dense, highly organized collagenous capsule (Sweetwyne et al., 2010). The collagen capsule acted as a physical barrier for later cell migration. Stimulation of collagen capsule formation was not the result of increased myofibroblast infiltration, but rather, as shown in in vitro studies, due to direct stimulation of collagen protein expression by TSP1 binding to cell surface CRT in an Akt-dependent, TGF-β independent manner (Sweetwyne et al., 2010). The contribution of TSP1-CRT signaling to collagen matrix production in wound healing and pathologic tissue remodeling is unknown. Since the N-terminus can be readily proteolyzed from the C-terminus and there is evidence that the N-terminus can be localized to sites of wound healing (Hogg et al., 1994; Hogg et al., 1993; Lee et al., 2006), it is reasonable to speculate that the N-terminal domain of TSP1 might signal events critical to early phases of tissue repair through engagement of cell surface CRT (Figure 2).
Figure 2. The N-terminal CRT binding sequence of TSP1 in tissue repair.
Previous in vitro studies showed that binding of the N-terminus of TSP1 to cell surface CRT activated intracellular signaling through engagement of LRP1 by membrane associated CRT. TSP1 signaling though CRT induced focal adhesion disassembly, stimulation of random and directed cell migration, and resistance to anoikis. In vivo studies in which local overexpression of the TSP1 CRT binding sequence occurs in a mouse model of the foreign body response and subsequent in vitro studies showed that TSP1 signaling through CRT also induces increased expression of type I collagen in an Akt-dependent, TGF-β independent manner. These data suggest a novel role of the N-terminal domain of TSP1 in wound repair and tissue remodeling.
5. Discussion
The factors that determine whether TSP1 participation in tissue repair stimulates physiologic or pathologic remodeling are not clear. One obvious determinant is the nature and persistence of the stimulus for increased TSP1 expression, such as glucose, angiotensin II, other growth factors (PDGF), and reactive oxygen species. Factors which regulate expression of TSP receptors and interactions of specific TSP domains with these receptors also modulate cellular responses to TSPs. Furthermore, TSP1 can be presented to cells either as a matrix-bound protein or in soluble form and as an intact protein or as fragments. Depending on the protease, isolated N and C-terminal domains can be either soluble or insoluble molecules. Cleavage by cathepsin G results in a soluble N-terminal domain in vitro (Hogg et al., 1993), whereas, TSP1 cleaved by ADAMTS-1 results in wound ECM-bound N-terminal domain (NTD) and soluble C-terminal domain (Lee et al., 2006). The trimeric, but not the monomeric, form of the C-terminal domain mediates matrix deposition of TSP1(Adams et al., 2008).
Interestingly, our studies have shown that TSP1 up regulates collagen I expression through both its N- and C-terminal domains. This is somewhat unusual in that the N- and C-terminal domains typically regulate distinct and sometimes opposing cellular behaviors (Elzie and Murphy-Ullrich, 2004). It remains to be determined if interactions of TSP1 with a particular cell in a specific tissue in a specific disease milieu can trigger multiple responses through distinct receptors simultaneously. Obviously cellular expression of specific complements of TSP1 (and EGF-family) receptors and the expression of other molecules which bind to TSP to either mask or expose particular receptor sites play a role in determining the ultimate “outcome” of TSP binding to cells. Proteolysis of the N and C terminus and their solubility or ECM localization can also modulate cellular responses/interactions with TSP in the context of receptor binding in the absence of other domains. Interestingly, most functional studies have examined TSP1 actions in the context of soluble TSP1 rather than TSP1 bound to the matrix. There are also likely to be temporally or spatially-regulated factors that dictate cellular responses to TSP1. For example, in wound healing, TSP1 signaling of intermediate adhesion with focal adhesion disassembly, increased cell migration, and anoikis resistance might facilitate early events in cellular responses to injury, whereas TSP1 signaling of collagen expression through CRT- LRP1 might preferentially occur in cells which are fully adherent and non-motile. Similarly, persistence of TSP1 expression following the initial phases of wound healing or re-expression due to fibrotic stimuli could lead to TGF-β activation and stimulation of fibrotic matrix production, whereas TSP1 control of TGF-β activation in early wound healing events appears necessary for normal wound healing. Given these multiple and sometimes contradictory functions of TSP1, one should interpret phenotypes of knockout animals with caution. Both local and systemic functions of TSP1, such as inhibition of nitric oxide signaling, can impact phenotypes and perhaps mask more subtle biological activities (Roberts, 2012). The potential regulation of growth factors such as TGF-β and growth factor receptors such EGFR and ErbB2 by TSP further implicates thrombospondins as key regulators of tissue remodeling and compounds the complexity of interpreting phenotypes. Rescue of knockout phenotypes by local overexpression of specific TSP1 domains or TSP1 mutated in specific receptor binding sequences could prove useful in elucidating the complexity of in vivo roles of TSP1.
Acknowledgments
Grant support: HL79644, DK078038, HL084223(JMU), T32 HL07918 (MTS); R13 DK08753
Abbreviations
- CRT
calreticulin
- EGF
epidermal growth factor
- LAP
latency associated peptide
- LRP1
LDL-receptor related protein 1
- TSP
thrombospondin
- TGF-β
transforming growth factor-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelouahed M, Ludlow A, Brunner G, Lawler J. Activation of platelet-transforming growth factor beta-1 in the absence of thrombospondin-1. J Biol Chem. 2000;275:17933–17936. doi: 10.1074/jbc.275.24.17933. [DOI] [PubMed] [Google Scholar]
- Adams JC, Bentley AA, Kvansakul M, Hatherley D, Hohenester E. Extracellular matrix retention of thrombospondin 1 is controlled by its conserved C-terminal region. J Cell Sci. 2008;121:784–795. doi: 10.1242/jcs.021006. [DOI] [PubMed] [Google Scholar]
- Adams JC, Lawler J. Diverse mechanisms for cell attachment to platelet thrombospondin. J Cell Sci. 1993;104:1061–1071. doi: 10.1242/jcs.104.4.1061. [DOI] [PubMed] [Google Scholar]
- Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamed J, Janczak CA, Wittkowski KM, Coller BS. In vitro and in vivo evidence that thrombospondin-1 (TSP-1) contributes to stirring- and shear-dependent activation of platelet-derived TGF-beta1. PLoS One. 2009;4:e6608. doi: 10.1371/journal.pone.0006608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attur MG, Palmer GD, Al-Mussawir HE, Dave M, Teixeira CC, Rifkin DB, Appleton CT, Beier F, Abramson SB. F-spondin, a neuroregulatory protein, is up-regulated in osteoarthritis and regulates cartilage metabolism via TGF-beta activation. Faseb J. 2009;23:79–89. doi: 10.1096/fj.08-114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani S, Bernal J, Wei CC, Pallero MA, Dell’italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171:777–789. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- Bourd-Boittin K, Bonnier D, Leyme A, Mari B, Tuffery P, Samson M, Ezan F, Baffet G, Theret N. Protease profiling of liver fibrosis reveals the adam metallopeptidase with thrombospondin type 1 motif, 1 as a central activator of TGF-beta. Hepatology. 2011 doi: 10.1002/hep.24598. [DOI] [PubMed] [Google Scholar]
- Breitkopf K, Sawitza I, Westhoff JH, Wickert L, Dooley S, Gressner AM. Thrombospondin 1 acts as a strong promoter of transforming growth factor beta effects via two distinct mechanisms in hepatic stellate cells. Gut. 2005;54:673–681. doi: 10.1136/gut.2004.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada MJ, Kuznetsova SA, Sipes JM, Rodrigues RG, Cashel JA, Annis DS, Mosher DF, Roberts DD. Calcium indirectly regulates immunochemical reactivity and functional activities of the N-domain of thrombospondin-1. Matrix Biol. 2008;27:339–351. doi: 10.1016/j.matbio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchard JH, Berton A, Godeau G, Hornebeck W, Bellon G. Activation of latent transforming growth factor beta 1 and inhibition of matrix metalloprotease activity by a thrombospondin-like tripeptide linked to elaidic acid. Biochem Pharmacol. 2004;67:2013–2022. doi: 10.1016/j.bcp.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, Majeti R, Weissman IL. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Leask A, Abraham DJ, Kennedy L, Shi-Wen X, Denton CP, Black CM, Verjee LS, Eastwood M. Thrombospondin 1 is a key mediator of transforming growth factor beta-mediated cell contractility in systemic sclerosis via a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Tissue Repair. 2011;4:9. doi: 10.1186/1755-1536-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang X, Weng D, Tian L, Lv L, Tao S, Chen J. A TSP-1 synthetic peptide inhibits bleomycin-induced lung fibrosis in mice. Experimental and Toxicologic Pathology. 2009;61:59–65. doi: 10.1016/j.etp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Chipev CC, Simman R, Hatch G, Katz AE, Siegel DM, Simon M. Myofibroblast phenotype and apoptosis in keloid and palmar fibroblasts in vitro. Cell Death Differ. 2000;7:166–176. doi: 10.1038/sj.cdd.4400605. [DOI] [PubMed] [Google Scholar]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56:2982–2989. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65:459–468. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- Elzie CA, Murphy-Ullrich JE. The N-terminus of thrombospondin: the domain stands apart. Int J Biochem Cell Biol. 2004;36:1090–1101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Ezzie ME, Piper MG, Montague C, Newland CA, Opalek JM, Baran C, Ali N, Brigstock D, Lawler J, Marsh CB. Thrombospondin-1-deficient mice are not protected from bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;44:556–561. doi: 10.1165/rcmb.2009-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Garg P, Yang S, Liu A, Pallero MA, Buchsbaum DJ, Mosher DF, Murphy-Ullrich JE, Goldblum SE. Thrombospondin-1 opens the paracellular pathway in pulmonary microvascular endothelia through EGFR/ErbB2 activation. Am J Physiol Lung Cell Mol Physiol. 2011;301:L79–90. doi: 10.1152/ajplung.00287.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Y, Prud’homme GJ. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J Leukoc Biol. 2008;84:302–310. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea S, Orr AW, Pallero MA, Eggleton P, Murphy-Ullrich JE. Thrombospondin mediates focal adhesion disassembly through interactions with cell surface calreticulin. J Biol Chem. 2000;275:36358–36368. doi: 10.1074/jbc.M005951200. [DOI] [PubMed] [Google Scholar]
- Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. Faseb J. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum SE, Ding X, Funk SE, Sage EH. SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc Natl Acad Sci U S A. 1994;91:3448–3452. doi: 10.1073/pnas.91.8.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum SE, Young BA, Wang P, Murphy-Ullrich JE. Thrombospondin-1 induces tyrosine phosphorylation of adherens junction proteins and regulates an endothelial paracellular pathway. Mol Biol Cell. 1999;10:1537–1551. doi: 10.1091/mbc.10.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood JA, Murphy-Ullrich JE. Signaling of de-adhesion in cellular regulation and motility. Microsc Res Tech. 1998;43:420–432. doi: 10.1002/(SICI)1097-0029(19981201)43:5<420::AID-JEMT8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Greenwood JA, Pallero MA, Theibert AB, Murphy-Ullrich JE. Thrombospondin signaling of focal adhesion disassembly requires activation of phosphoinositide 3-kinase. The Journal of biological chemistry. 1998;273:1755–1763. doi: 10.1074/jbc.273.3.1755. [DOI] [PubMed] [Google Scholar]
- Greenwood JA, Theibert AB, Prestwich GD, Murphy-Ullrich JE. Restructuring of focal adhesion plaques by PI 3-kinase. Regulation by PtdIns (3,4,5)-p(3) binding to alpha-actinin. J Cell Biol. 2000;150:627–642. doi: 10.1083/jcb.150.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpel JG, Schultz-Cherry S, Murphy-Ullrich JE, Rifkin DB. Tamoxifen and estrogen effects on TGF-beta formation: role of thrombospondin-1, alphavbeta3, and integrin-associated protein. Biochem Biophys Res Commun. 2001;284:11–14. doi: 10.1006/bbrc.2001.4922. [DOI] [PubMed] [Google Scholar]
- Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11:120–126. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- Hofsteenge J, Huwiler KG, Macek B, Hess D, Lawler J, Mosher DF, Peter-Katalinic J. C-mannosylation and O-fucosylation of the thrombospondin type 1 module. J Biol Chem. 2001;276:6485–6498. doi: 10.1074/jbc.M008073200. [DOI] [PubMed] [Google Scholar]
- Hogg PJ, Jimenez BM, Chesterman CN. Identification of possible inhibitory reactive centers in thrombospondin 1 that may bind cathepsin G and neutrophil elastase. Biochemistry. 1994;33:6531–6537. doi: 10.1021/bi00187a021. [DOI] [PubMed] [Google Scholar]
- Hogg PJ, Owensby DA, Chesterman CN. Thrombospondin 1 is a tight-binding competitive inhibitor of neutrophil cathepsin G. Determination of the kinetic mechanism of inhibition and localization of cathepsin G binding to the thrombospondin 1 type 3 repeats. J Biol Chem. 1993;268:21811–21818. [PubMed] [Google Scholar]
- Hugo C. The thrombospondin 1-TGF-beta axis in fibrotic renal disease. Nephrol Dial Transplant. 2003;18:1241–1245. doi: 10.1093/ndt/gfg159. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Manabe S, Ikezaki M, Inai Y, Matsui IS, Ohta Y, Muroi E, Ito Y. C-Mannosylated peptides derived from the thrombospondin type 1 repeat interact with Hsc70 to modulate its signaling in RAW264.7 cells. Glycobiology. 2010;20:1298–1310. doi: 10.1093/glycob/cwq096. [DOI] [PubMed] [Google Scholar]
- Jeffery E, Peters LR, Raghavan M. The polypeptide binding conformation of calreticulin facilitates its cell-surface expression under conditions of endoplasmic reticulum stress. The Journal of biological chemistry. 2011;286:2402–2415. doi: 10.1074/jbc.M110.180877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Kuznetsova SA, Pendrak ML, Sipes JM, Romeo MJ, Li Z, Zhang L, Roberts DD. Heparan sulfate modification of the transmembrane receptor CD47 is necessary for inhibition of T cell receptor signaling by thrombospondin-1. The Journal of biological chemistry. 2011;286:14991–15002. doi: 10.1074/jbc.M110.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondou H, Mushiake S, Etani Y, Miyoshi Y, Michigami T, Ozono K. A blocking peptide for transforming growth factor-beta1 activation prevents hepatic fibrosis in vivo. J Hepatol. 2003;39:742–748. doi: 10.1016/s0168-8278(03)00377-5. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal. 2009;3:215–225. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav J, Dardik R, Stein O. Endothelial cell thrombospondin and its possible role in cell adhesion. Semin Thromb Hemost. 1987;13:352–360. doi: 10.1055/s-2007-1003511. [DOI] [PubMed] [Google Scholar]
- Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, Iruela-Arispe ML. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. Embo J. 2006;25:5270–5283. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SS, Forslow A, Sundqvist KG. Autocrine regulation of T cell motility by calreticulin-thrombospondin-1 interaction. J Immunol. 2005;174:654–661. doi: 10.4049/jimmunol.174.2.654. [DOI] [PubMed] [Google Scholar]
- Liu A, Garg P, Yang S, Gong P, Pallero MA, Annis DS, Liu Y, Passaniti A, Mann D, Mosher DF, Murphy-Ullrich JE, Goldblum SE. Epidermal growth factor-like repeats of thrombospondins activate phospholipase Cgamma and increase epithelial cell migration through indirect epidermal growth factor receptor activation. J Biol Chem. 2009a;284:6389–6402. doi: 10.1074/jbc.M809198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Mosher DF, Murphy-Ullrich JE, Goldblum SE. The counteradhesive proteins, thrombospondin 1 and SPARC/osteonectin, open the tyrosine phosphorylation-responsive paracellular pathway in pulmonary vascular endothelia. Microvasc Res. 2009b;77:13–20. doi: 10.1016/j.mvr.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-beta activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol. 2011;178:2573–2586. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow A, Yee KO, Lipman R, Bronson R, Weinreb P, Huang X, Sheppard D, Lawler J. Characterization of integrin beta6 and thrombospondin-1 double-null mice. J Cell Mol Med. 2005;9:421–437. doi: 10.1111/j.1582-4934.2005.tb00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K. Constitutive thrombospondin-1 overexpression contributes to autocrine transforming growth factor-beta signaling in cultured scleroderma fibroblasts. Am J Pathol. 2005;166:1451–1463. doi: 10.1016/s0002-9440(10)62362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Munger JS, Sheppard D. Cross Talk among TGF-beta Signaling Pathways, Integrins, and the Extracellular Matrix. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, Höök M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993;268:26784–26789. [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Hook M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989;109:1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Mosher DF. Localization of thrombospondin in clots formed in situ. Blood. 1985;66:1098–1104. [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Mosher DF. Interactions of thrombospondin with cells in culture: rapid degradation of both soluble and matrix thrombospondin. Semin Thromb Hemost. 1987;13:343–351. doi: 10.1055/s-2007-1003510. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Schultz-Cherry S, Höök M. Transforming growth factor-β complexes with thrombospondin. Mol Biol Cell. 1992;3:181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nor JE, Dipietro L, Murphy-Ullrich JE, Hynes RO, Lawler J, Polverini PJ. Activation of Latent TGF-beta1 by Thrombospondin-1 is a Major Component of Wound Repair. Oral Biosci Med. 2005;2:153–161. [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Elzie CA, Kucik DF, Murphy-Ullrich JE. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J Cell Sci. 2003a;116:2917–2927. doi: 10.1242/jcs.00600. [DOI] [PubMed] [Google Scholar]
- Orr AW, Pallero MA, Murphy-Ullrich JE. Thrombospondin stimulates focal adhesion disassembly through Gi- and phosphoinositide 3-kinase-dependent ERK activation. J Biol Chem. 2002;277:20453–20460. doi: 10.1074/jbc.M112091200. [DOI] [PubMed] [Google Scholar]
- Orr AW, Pallero MA, Xiong WC, Murphy-Ullrich JE. Thrombospondin induces RhoA inactivation through FAK-dependent signaling to stimulate focal adhesion disassembly. J Biol Chem. 2004;279:48983–48992. doi: 10.1074/jbc.M404881200. [DOI] [PubMed] [Google Scholar]
- Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003b;161:1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallero MA, Elzie CA, Chen J, Mosher DF, Murphy-Ullrich JE. Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis. Faseb J. 2008;22:3968–3979. doi: 10.1096/fj.07-104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poczatek MH, Hugo C, Darley-Usmar V, Murphy-Ullrich JE. Glucose stimulation of transforming growth factor-beta bioactivity in mesangial cells is mediated by thrombospondin-1. Am J Pathol. 2000;157:1353–1363. doi: 10.1016/s0002-9440(10)64649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverini PJ, DiPietro LA, Dixit VM, Hynes RO, Lawler J. Thrombospondin 1 knock out mice show delayed organization and prolonged neovascularization of skin wounds. Faseb J. 1995;9:A272. [Google Scholar]
- Puolakkainen PA, Bradshaw AD, Brekken RA, Reed MJ, Kyriakides T, Funk SE, Gooden MD, Vernon RB, Wight TN, Bornstein P, Sage EH. SPARC-thrombospondin-2-double-null mice exhibit enhanced cutaneous wound healing and increased fibrovascular invasion of subcutaneous polyvinyl alcohol sponges. J Histochem Cytochem. 2005;53:571–581. doi: 10.1369/jhc.4A6425.2005. [DOI] [PubMed] [Google Scholar]
- Raugi GJ, Olerud JE, Gown AM. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987;89:551–554. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Puolakkainen P, Lane TF, Dickerson D, Bornstein P, Sage EH. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993;41:1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biology. 2012 doi: 10.1016/j.matbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DD, Miller TW, Rogers NM, Yaob M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biology. 2012 doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- Sakai K, Sumi Y, Muramatsu H, Hata K, Muramatsu T, Ueda M. Thrombospondin-1 promotes fibroblast-mediated collagen gel contraction caused by activation of latent transforming growth factor beta-1. J Dermatol Sci. 2003;31:99–109. doi: 10.1016/s0923-1811(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Miyazaki A, Tamagawa H, Wang GP, Horiuchi S. Specific interaction of oxidized low-density lipoprotein with thrombospondin-1 inhibits transforming growth factor-beta from its activation. Atherosclerosis. 2005;183:85–93. doi: 10.1016/j.atherosclerosis.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, Lawler J, Murphy-Ullrich JE. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-β. J Biol Chem. 1994a;269:26783–26788. [PubMed] [Google Scholar]
- Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-β secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-β in a chemically defined system. J Biol Chem. 1994b;269:26775–26782. [PubMed] [Google Scholar]
- Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Mosher DF, Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J Biol Chem. 1989;264:2885–2889. [PubMed] [Google Scholar]
- Sweetwyne MT, Pallero MA, Lu A, Van Duyn Graham L, Murphy-Ullrich JE. The calreticulin-binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling. Am J Pathol. 2010;177:1710–1724. doi: 10.2353/ajpath.2010.090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr JM, Young PJ, Morse R, Shaw DJ, Haigh R, Petrov PG, Johnson SJ, Winyard PG, Eggleton P. A mechanism of release of calreticulin from cells during apoptosis. J Mol Biol. 2010;401:799–812. doi: 10.1016/j.jmb.2010.06.064. [DOI] [PubMed] [Google Scholar]
- Tuszynski GP, Rothman V, Murphy A, Siegler K, Smith L, Smith S, Karczewski J, Knudsen KA. Thrombospondin promotes cell-substratum adhesion. Science. 1987;236:1570–1573. doi: 10.1126/science.2438772. [DOI] [PubMed] [Google Scholar]
- Walton KL, Makanji Y, Chen J, Wilce MC, Chan KL, Robertson DM, Harrison CA. Two distinct regions of latency-associated peptide coordinate stability of the latent transforming growth factor-beta1 complex. The Journal of biological chemistry. 2010;285:17029–17037. doi: 10.1074/jbc.M110.110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Herndon ME, Ranganathan S, Godyna S, Lawler J, Argraves WS, Liau G. Internalization but not binding of thrombospondin-1 to low density lipoprotein receptor-related protein-1 requires heparan sulfate proteoglycans. Journal of cellular biochemistry. 2004a;91:766–776. doi: 10.1002/jcb.10781. [DOI] [PubMed] [Google Scholar]
- Wang S, Shiva S, Poczatek MH, Darley-Usmar V, Murphy-Ullrich JE. Nitric oxide and cGMP-dependent protein kinase regulation of glucose-mediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells. J Biol Chem. 2002;277:9880–9888. doi: 10.1074/jbc.M108360200. [DOI] [PubMed] [Google Scholar]
- Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem. 2004b;279:34311–34322. doi: 10.1074/jbc.M401629200. [DOI] [PubMed] [Google Scholar]
- Warburton D, Kaartinen V. When the lung is stretched, could it be thrombospondin via TGFbeta1 peptide activation? J Physiol. 2007;584:365. doi: 10.1113/jphysiol.2007.144394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Murphy-Ullrich JE, Song Y. Structural insight into the role of thrombospondin-1 binding to calreticulin in calreticulin-induced focal adhesion disassembly. Biochemistry. 2010;49:3685–3694. doi: 10.1021/bi902067f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Murphy-Ullrich JE, Song Y. Molecular and structural insight into the role of key residues of thrombospondin-1 and calreticulin in thrombospondin-1-calreticulin binding. Biochemistry. 2011;50:566–573. doi: 10.1021/bi101639y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Vega JL, Hadzipasic M, Schatzmann Peron JP, Zhu B, Carrier Y, Masli S, Rizzo LV, Weiner HL. Deficiency of thrombospondin-1 reduces Th17 differentiation and attenuates experimental autoimmune encephalomyelitis. Journal of autoimmunity. 2009;32:94–103. doi: 10.1016/j.jaut.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehualaeshet T, O’Connor R, Begleiter A, Murphy-Ullrich JE, Silverstein R, Khalil N. A CD36 synthetic peptide inhibits bleomycin-induced pulmonary inflammation and connective tissue synthesis in the rat. Am J Respir Cell Mol Biol. 2000;23:204–212. doi: 10.1165/ajrcmb.23.2.4089. [DOI] [PubMed] [Google Scholar]
- Yehualaeshet T, O’Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol. 1999;155:841–851. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevdokimova N, Wahab NA, Mason RM. Thrombospondin-1 is the key activator of TGF-beta1 in human mesangial cells exposed to high glucose. J Am Soc Nephrol. 2001;12:703–712. doi: 10.1681/ASN.V124703. [DOI] [PubMed] [Google Scholar]
- Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-beta. The Journal of biological chemistry. 2004a;279:38032–38039. doi: 10.1074/jbc.M405658200. [DOI] [PubMed] [Google Scholar]
- Young GD, Murphy-Ullrich JE. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-beta complex. J Biol Chem. 2004b;279:47633–47642. doi: 10.1074/jbc.M404918200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Hagood JS, Murphy-Ullrich JE. Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic signaling. Am J Pathol. 2004;165:659–669. doi: 10.1016/s0002-9440(10)63330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Poczatek MH, Berecek KH, Murphy-Ullrich JE. Thrombospondin 1 mediates angiotensin II induction of TGF-beta activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys Res Commun. 2006;339:633–641. doi: 10.1016/j.bbrc.2005.11.060. [DOI] [PubMed] [Google Scholar]