Abstract
Aortic valve disease (AVD) occurs in 2.5% of the general population and often requires surgical intervention. Aortic valve malformation (AVM) underlies the majority of cases, suggesting a developmental etiology. Elastin haploinsufficiency results in complex cardiovascular problems, and 20–45% of patients have AVM and/or AVD. Elastin insufficient (Eln+/−) mice demonstrate AVM and latent AVD due to abnormalities in the valve annulus region. The objective of this study was to examine extracellular matrix (ECM) remodeling and biomechanical properties in regional aortic valve tissue and determine the impact of early AVM on late AVD in the Eln+/− mouse model. Aortic valve ECM composition and remodeling from juvenile, adult, and aged stages were evaluated in Eln+/− mice using histology, ELISA, immunohistochemistry and gelatin zymography. Aortic valve tissue biomechanical properties were determined using micropipette aspiration. Cartilage-like nodules were demonstrated within the valve annulus region at all stages identifying a developmental abnormality preceding AVD. Interestingly, maladaptive ECM remodeling was observed in early AVM without AVD and worsened with late AVD, as evidenced by increased MMP-2 and MMP-9 expression and activity, as well as abnormalities in ADAMTS-mediated versican processing. Cleaved versican was increased in the valve annulus region of aged Eln+/− mice, and this abnormality correlated temporally with adverse alterations in valve tissue biomechanical properties and the manifestation of AVD. These findings identify maladaptive ECM remodeling in functional AVM as an early disease process with a progressive natural history, similar to that seen in human AVD, emphasizing the importance of the annulus region in pathogenesis. Combining molecular and engineering approaches provides complementary mechanistic insights that may be informative in the search for new therapeutic targets and durable valve bioprostheses.
Keywords: valve development, aortic valve annulus, elastin haploinsufficiency, micropipette aspiration, biomedical engineering
1. Introduction
Aortic valve disease (AVD) is a significant health care problem affecting 2.5% of the population. Surgical intervention and re-intervention is common, and the incidence of aortic valve procedures is rapidly increasing (Nkomo et al., 2006; Roger et al., 2011). Aortic valve malformation (AVM) underlies the majority of AVD cases, suggesting a major genetic contribution, and independent environmental risk factors such as aging have been established (Cripe et al., 2004; Roberts and Ko, 2005; Tzemos et al., 2008). AVD manifests as stenosis and/or regurgitation and results in hemodynamic perturbations that often result in a progressive disease process. AVD is characterized in part by extracellular matrix (ECM) accumulation and disorganization, identifying maladaptive ECM remodeling as a hallmark of end stage AVD (Edep et al., 2000; Fondard et al., 2005; Hinton et al., 2006; Togashi et al., 2007); however, the impact of early ECM remodeling in the context of AVM without AVD is largely unknown.
The aortic valve is situated between the left ventricle and aorta, and consists of cusp and annulus regions (Gross and Kugel, 1931; Zimmerman and Bailey, 1962). The cusp is made up of highly organized ECM (fibrosa, spongiosa, ventricularis layers) and predominantly valve interstitial cells (VICs) (Hinton and Yutzey, 2011). The cusps are hinged to the annulus, a crown-shaped, collagen-rich support structure within the aortic root (Fig. 1A–B) (Anderson, 2000; Yacoub et al., 1999). Organized elastic fibers are localized to the ventricularis layer of the cusp and extend into the valve hinge (Tseng and Grande-Allen, 2011; Zimmerman and Bailey, 1962). Importantly, some elastic fiber components, but not elastic fiber filaments, are present in the valve annulus (Gross and Kugel, 1931). Human AVD studies have suggested that disease processes begin at the base of the valve cusp or hinge region (Otto et al., 1994; Thubrikar et al., 1986); however, research efforts in both human and animal models have focused almost exclusively on the cusp. As a result, the contribution of the annulus region to AVD pathogenesis remains largely unknown.
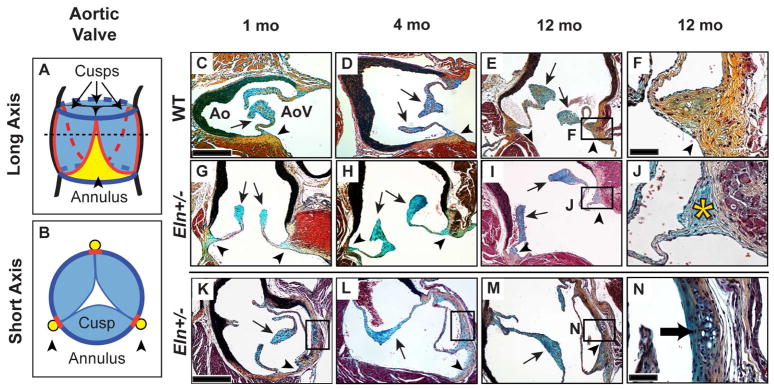
Fig. 1. Eln+/− aortic valves demonstrate cartilage-like nodules at all stages and annulus-specific proteoglycan accumulation at the aged stage.
Long (A, C–J) and short (B, K–N) axis sections of WT (C–F) and Eln+/− (G–N) aortic valves. The dotted line in A represents the plane of dissection shown in B. Pentachrome staining identified proteoglycans as blue in the cusp (arrows) and collagens as yellow in the annulus (arrowheads); this color coding is maintained in A and B with red lines representing the hinge. In aged (12 mo) Eln+/− valves, proteoglycan accumulation was observed in the annulus region (asterisk, J). Cartilage-like nodules were present in the Eln+/− annulus at all stages (black boxes, K–M; arrow N). AoV, aortic valve; Ao, aorta. Scale bars: 20 μm (F, J, N); 120 μm (all others).
Valve ECM composition determines its biomechanical properties (Schoen, 2008). Elastin, the primary component of elastic fibers, is responsible for valve extension and recoil, while collagen provides tissue strength and durability (Vesely, 1998). Proteoglycans provide cohesiveness between the fibrosa (collagen) and ventricularis (elastin) layers and contribute to valve tissue’s viscoelasticity. Biomechanical properties of valve tissue are usually determined by conventional physical testing (uniaxial or biaxial) strategies in large animals (Billiar and Sacks, 2000); however, these approaches are complicated in small animal models such as mice due to tissue size. We have recently demonstrated the feasibility of a modified micropipette aspiration approach to determine mechanical stiffness in cusp and annulus regions of mouse aortic valve tissue (Krishnamurthy et al., 2011). Given the robust insight that targeted mutagenesis mouse models of valve disease provide, combining molecular and engineering applications facilitates elucidation of structure-function relationships in valve tissue.
Elastin haploinsufficiency is a complex clinical condition caused by loss of function mutations or deletions in the elastin gene (OMIM*130160) and is associated with aortic valve abnormalities in 20–45% of patients (Eronen et al., 2002; Keane et al., 1976). Elastin mutant mice recapitulate the human phenotype. Specifically, mice homozygous for a null mutation in the elastin gene (Eln−/−) demonstrate arterial obstruction reminiscent of supravalvular aortic stenosis and perinatal death (Li et al., 1998). We recently identified the Eln+/− mouse as a model of AVM with latent AVD (Hinton et al., 2010). Eln+/− aortic valves are characterized by elastic fiber fragmentation (including dispersion of fragments into the annulus), VIC activation, and notably proteoglycan accumulation in the valve annulus region, reminiscent of myxomatous change. Importantly, the latent manifestation of AVD (present in 70% of aged mice) provides a unique model to assess the natural history of valve disease pathogenesis. The effects of elastin haploinsufficiency on the progression of ECM remodeling abnormalities are unknown.
Valve ECM remodeling during normal embryonic development and postnatal maturation is mediated by MMP (Matrix Metalloproteinase) and ADAMTS (A Disintegrin-like And Metalloprotease domain with ThromboSpondin-type 1 Motifs) enzyme families (Visse and Nagase, 2003). The proteases MMP-2 and MMP-9 remodel the ECM via cleavage of specific proteins, including type I and III collagens, elastin and versican. The proteoglycanases ADAMTS-5 and ADAMTS-9 process intact versican to generate cleaved versican that harbors the DPEAAE neoepitope. There is accumulating evidence that elastin-versican interactions play a role in ECM homeostasis. Versican interferes with elastic fiber assembly, and versican loss of function promotes elastogenesis (Huang et al., 2006; Wu et al., 2005). However, the effects of reduced elastin on versican are unknown. Increased expression and activation of MMP-2 and MMP-9 and abnormal versican processing have been implicated in AVD (Kern et al., 2010; Rabkin et al., 2001; Togashi et al., 2007), but the impact of remodeling enzyme misregulation in AVM is unknown.
The goal of this study was to combine molecular and biomechanical approaches to determine regional structure-function relationships over time in the Eln+/− mouse model of latent AVD. We tested the hypothesis that elastin haploinsufficiency results in deterioration of mechanical properties due to annulus-specific maladaptive ECM remodeling. We show that the annulus region is the origin of structural aortic valve abnormalities in the Eln+/− mouse, and identify maladaptive ECM remodeling as an early disease process occurring in AVM before AVD. These results advance our understanding of valve disease pathogenesis and may provide insight for the development of new therapeutics and durable bioprostheses.
2. Results
2.1. Eln+/− aortic valves demonstrate ECM structural abnormalities including early nodule formation in the annulus region
At all stages, WT mouse aortic valve tissue in the annulus region consisted primarily of compact collagens, while tissue in the cusp region consisted predominantly of proteoglycans (Fig. 1), consistent with previous reports (reviewed in (Hinton and Yutzey, 2011)). The cartilage-like quality of the annulus region in normal wild type mice was established at different postnatal stages (Supplemental Fig. 1). In contrast to WT valves, there was marked proteoglycan accumulation in the annulus region of aged Eln+/− aortic valves (Fig. 1I, J) (Hinton et al., 2010). Interestingly, Eln+/− aortic valves showed abnormal nodules in subareas of the annulus region, specifically near the commissures and aortic-mitral continuity, which were not present in WT valves (Fig. 1K–N). These nodules were characterized by chondrocyte-like halo-cell morphology and positive Alcian blue staining; they were present at all stages and did not appear to change in size or appearance over time.
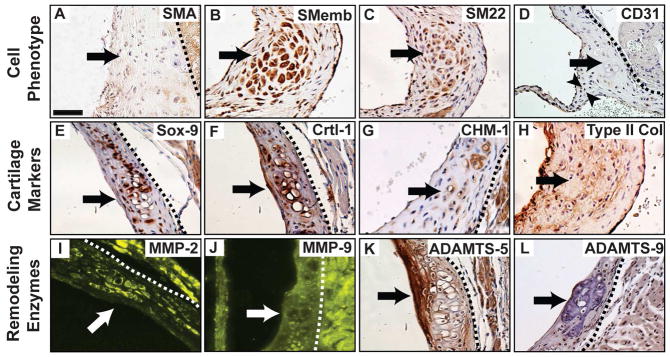
To further characterize the Eln+/− aortic valve annulus nodules, markers of cell phenotype (SMA, SMemb, SM22, CD31, Mac-3), cartilage (Sox-9, Crtl-1, CHM-1, type II Collagen) and remodeling enzymes (MMP-2, MMP-9, ADAMTS-5, ADAMTS-9) were investigated. SMemb and SM22 expression was detected, consistent with VIC activation at the aged stage, but SMA was absent (Fig. 2A–C). No macrophages were identified in the nodules, suggesting pathology was independent of inflammation (Supplemental Fig. 2). Interestingly, there was interstitial expression of the endothelial cell marker CD31 in the Eln+/− aortic valve annulus region, but not specifically the nodules (Fig. 2D). All cell phenotype markers were negative in age-matched WT control annulus tissue (Supplemental Fig. 3). Sox-9, Crtl-1, CHM-1 and type II collagen were expressed in the nodules in Eln+/− valves (Fig. 2E–H, Supplemental Fig. 1), suggesting pathology is due in part to aberrant cartilage development programs (Lincoln et al., 2006b; Wirrig et al., 2011). There was MMP-2 expression in the nodules and ADAMTS-5 expression around the nodules (Fig. 2I, K), demonstrating two different ECM remodeling processes within the annulus. These findings suggest that Eln+/− aortic valve pathogenesis begins in the annulus region and is characterized by ECM remodeling abnormalities and cartilage dysregulation.
Fig. 2. Characterization of cartilage-like nodules in the Eln+/− aortic valve annulus.
The nodules (arrows) were localized in the annulus region and characterized by cell phenotype (AD), cartilage markers (E–H), and remodeling enzymes (I–L). CD31 positive cells are present in the interstitium (arrowheads, D). All sections are in short axis; derived from aged mice, except panels D and G (adult). Scale bar: 20 μm. Dotted lines isolate valve tissue from aortic and/or myocardial tissue.
2.2. Eln+/− aortic valves demonstrate abnormal versican processing and quantitative differences in ECM composition with AVD
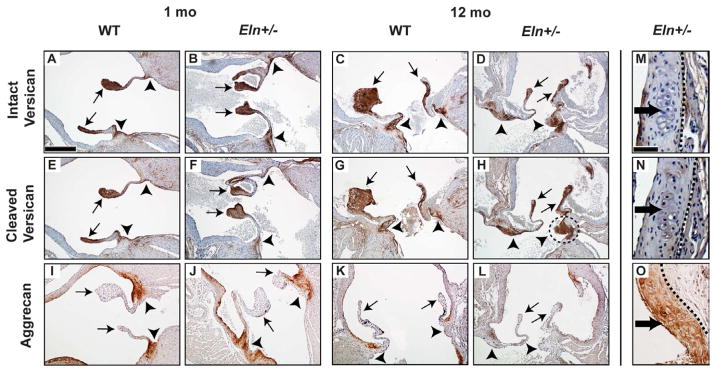
In WT valves, aggrecan was present in the annulus and absent in the cusps (Fig. 3I, J); and expression decreased with age (Supplemental Fig. 1 D–F). Intact and cleaved versican were localized predominantly to the cusps at all stages (Fig. 3A, B, E, F). However, in aged Eln+/− aortic valves, cleaved versican expression was increased in both cusp and annulus regions, compared to juvenile Eln+/− and aged WT valves (Fig. 3F–H), consistent with ADAMTS-5 expression in the annulus and cusps (Dupuis et al., 2011). The nodules were negative for both intact and cleaved versican (Fig. 3M, N), but positive for aggrecan (Fig. 3O), suggesting two distinct remodeling processes characterized by different proteoglycans and remodeling enzymes.
Fig. 3. Abnormal regional versican processing in Eln+/− aortic valves.
Increased cleaved versican was shown in the aged (12 mo) Eln+/− annulus region (dotted circle, H) compared to age matched WT (G), consistent with proximate ADAMTS-5 expression (Fig. 2K). Neither intact nor cleaved versican was expressed in the nodules (dark arrows, M–N), but aggrecan was (O). Cusps (arrows), annulus (arrowheads). Sections are oriented in long (A–L) or short (M–O) axis as illustrated in Fig. 1. Scale bars: 60 μm (A–L); 20 μm (M–O). For short axis sections, stage is 1 mo for M and N, and 12 mo for O. Dotted lines isolate valve tissue from aortic and/or myocardial tissue.
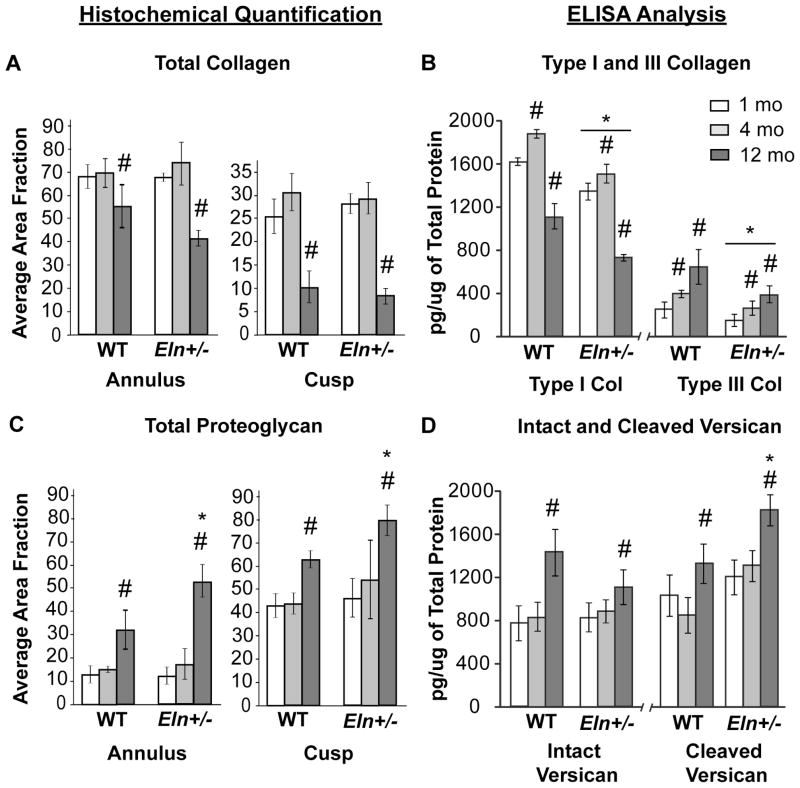
The total collagen content was decreased at the aged stage in both Eln+/− and WT aortic valves (Fig. 4A). There was a dramatic decrease in type I collagen at the aged stage compared with earlier stages for both Eln+/− and WT valves (p<0.0001). In contrast, there was a gradual increase in type III collagen with age in both Eln+/− and WT mice (p<0.01). Eln+/− valves demonstrated significantly lower type I and III collagens at all stages compared to WT (Fig. 4B, p<0.0001), suggesting upregulation of different collagens (Lincoln et al., 2006a). Total proteoglycan content was significantly increased in aged Eln+/− valves (Fig. 4C) with significant increases in both intact and cleaved versican expression (Fig. 4D, p<0.0001). Further, the cleaved to intact versican ratio was significantly higher in Eln+/− compared to WT aortic valves (p<0.01, data not shown).
Fig. 4. Regional and temporal quantification of ECM proteins in Eln+/− aortic valves.
Histochemical (A, C) and ELISA-based (B, D) quantification was performed for collagens and proteoglycans. With advanced age, total collagen (A) and type I collagen (B) decreased and total proteoglycan (C), and intact and cleaved versican (D) increased in both Eln+/− and WT valves (p<0.0001) (WT values reported (Krishnamurthy et al., 2011)). For all stages, Eln+/− valves demonstrated decreased type I and III collagens, as compared to WT group (B, p<0.0001). In addition, aged Eln+/− valves showed increased total proteoglycan content in both regions (C, p<0.005), possibly due to increased cleaved versican (D, p<0.0001), as compared to WT group. (* different from WT; # different from 1 mo).
2.3. Eln+/− aortic valves demonstrate increased MMP-2 and MMP-9 expression and activity with functional AVM that worsens with AVD
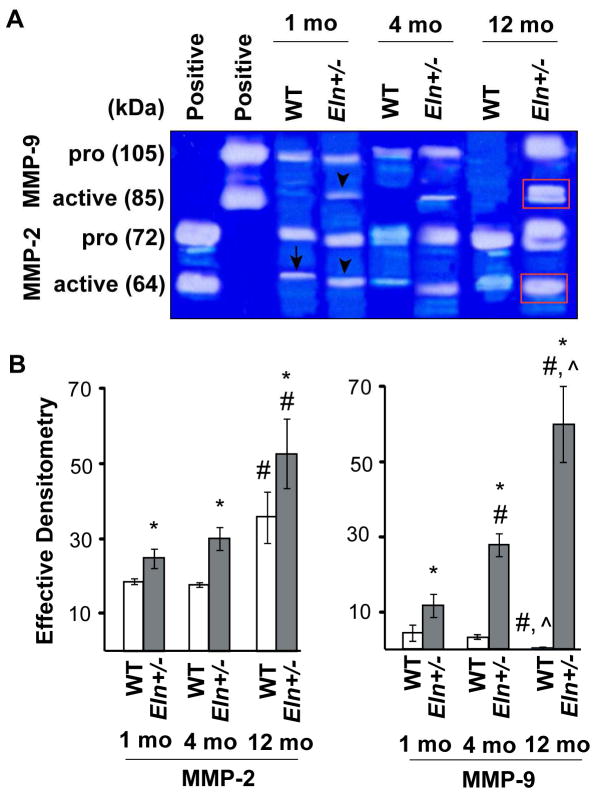
MMP-2 and MMP-9 were expressed in both WT and Eln+/− valves (Fig. 5). In aged Eln+/− valves, both MMP-2 and MMP-9 expression was unchanged in the cusp region and significantly increased in the annulus region (Figs. 5B″, D″, E, F), compared to WT (Figs. 5A″, C″). MMP-2 and MMP-9 activity in WT valves was modest, consistent with normal developmental remodeling and growth (Fig. 6A); however, with advanced age, MMP-2 increased and MMP-9 decreased (p<0.05). In contrast, both MMP-2 and MMP-9 activity was significantly increased in juvenile Eln+/− aortic valves before the onset of AVD (p<0.05), and increased dramatically at the aged stage after the onset of AVD (p<0.0001) (Fig. 6B), identifying early maladaptive ECM remodeling in AVM without AVD and worsening pathologic ECM remodeling with AVD.
Fig. 5. Aged Eln+/− aortic valves demonstrate increased MMP-2 and MMP-9 expression.
MMP-2 expression (A–B) was higher in both cusp and annulus tissue compared to MMP-9 expression (C–D). Both MMP-2 and MMP-9 expression was unchanged in the cusp region (p=NS, E) and increased in the annulus region (p<0.01, E) in aged Eln+/− valves (B″, D″), as compared to WT valves (A″, C″). MMP-2 and MMP-9 are stained green; cell nuclei, blue (ToPro3). Cusps (A′ – D′), annulus (A″ – D″). Sections are oriented in the long axis. Scale bars: 60 μm (A–D); 10 μm (all others). Dotted lines isolate valve tissue from aortic and/or myocardial tissue.
Fig. 6. Progressive increase in remodeling enzyme activity in Eln+/− aortic valve tissue.
Gelatin zymography showed modest MMP-2 (arrow, A) and MMP-9 activity in juvenile WT valves, consistent with normal developmental remodeling and growth. In Eln+/− aortic valves, both MMP-2 and MMP-9 activity was significantly increased at juvenile stage (arrowheads, A) before the onset of AVD (p<0.05, B), and increased dramatically at the aged stage after the onset of AVD (red boxes, A) (p<0.0001, B). (* different from WT; # different from 1 mo; ^ different from 4 mo, B).
2.4. Eln+/− aortic valves show a progressive decrease in regional tissue tensile stiffness
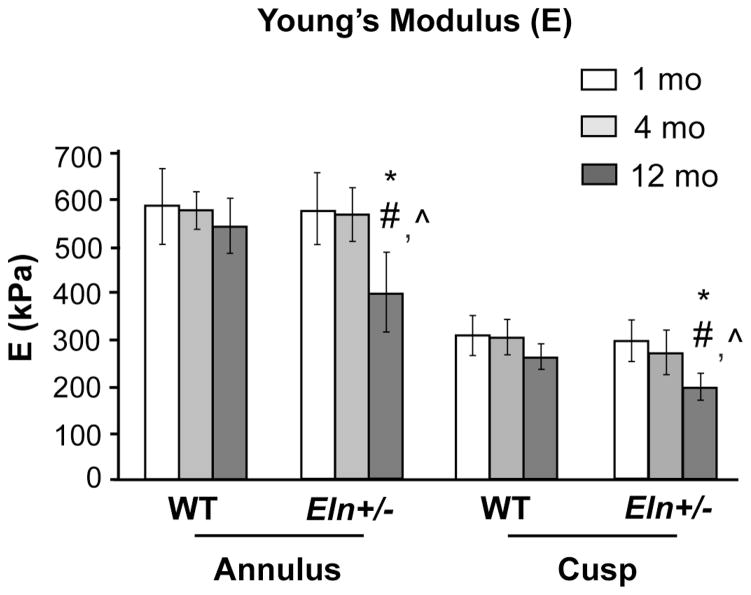
Young’s modulus in the aortic valve annulus region was significantly larger compared to the cusp region in both Eln+/− and WT valves at all stages (p<0.0001, Fig. 7). In Eln+/− valves, there was a significant decrease in tissue stiffness in both regions over time (p<0.0001, aged vs. juvenile and adult), and aged Eln+/− valves were significantly less stiff than age-matched WT valves (p<0.005). These biomechanical data correlate with histopathology findings and suggest that pathological remodeling alters ECM structure, resulting in changes in regional valve tissue biomechanical properties and ultimately AVD.
Fig. 7. Regional biomechanical dysfunction in aged Eln+/− mouse aortic valve tissue.
Young’s modulus, a measure of tissue stiffness, was significantly larger in the aortic valve annulus region, as compared to the cusp region in both Eln+/− and WT valves at all stages (p<0.0001). Young’s modulus decreases significantly in the aged (12 mo) Eln+/− cusp and annulus regions compared to age-matched WT (p<0.005) and younger Eln+/− mice (p<0.0001). (* different from WT; # different from 1 mo; ^ different from 4 mo).
3. Discussion
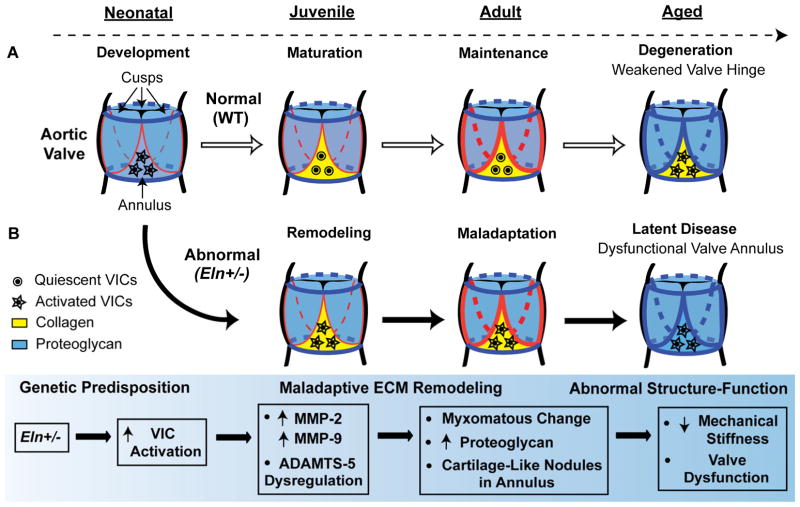
Valve motion is constant, and durable valve function typically continues through development, maturation, maintenance and senescence. Based on the findings of this study, we propose that a genetic predisposition to AVM leads to early cell-matrix abnormalities, characterized in part by maladaptive ECM remodeling, which progress and ultimately result in valve dysfunction (Fig. 8). ECM disorganization and VIC disarray are hallmarks of human AVD, and elastic fiber fragmentation is a universal finding (Hinton et al., 2006; Hinton and Yutzey, 2011; Schoen, 1997, 2008; Vesely, 1998). Studies in mice have identified subtle age-related degenerative ECM changes, including proteoglycan infiltration of the aortic valve annulus region, providing an important environmental contribution to the development of AVD (Krishnamurthy et al., 2011; Stephens et al., 2009). The Eln+/− mouse model of latent AVD demonstrates a marked exaggeration of the subtle age-related changes seen in WT mice and provides important insights into early disease mechanisms and the progressive nature of AVD pathogenesis. Our results demonstrate that early disease processes are characterized by structural changes in the valve annulus and maladaptive ECM remodeling preceding the manifestation of AVD.
Fig. 8. Working model of regional structure-function relationships in latent AVD.
Color coding is based on the pattern of pentachrome stain with proteoglycans as blue and collagens as yellow. Normal valves (A, white arrows) undergo development, maturation, maintenance and age-associated degeneration over time (dotted arrow). The valve annulus (yellow) and hinge (red) mature during early postnatal growth. Abnormal valves (B, black arrows) are characterized by a genetic predisposition to AVM (Eln+/−), early maladaptive ECM remodeling mediated in part by VIC activation, cell-matrix abnormalities disproportionately affecting the annulus region, and ultimately abnormal structure-function due to biomechanical failure at the hinge and valve dysfunction. We speculate that pathogenesis is initiated in the valve annulus region resulting in maladaptive ECM remodeling, and overt disease manifests later due to structural failure at the valve hinge.
Valve ECM remodeling is mediated by MMP and ADAMTS remodeling enzyme families. Our results demonstrate the presence of early maladaptive ECM remodeling in Eln+/− aortic valves that is characterized by MMP and ADAMTS abnormalities in the context of AVM without AVD, suggesting that this remodeling plays a central role in the manifestation of disease and is not only a secondary finding of end-stage pathology. The observed remodeling abnormalities markedly worsen after the onset of AVD, consistent with the idea that the disease state itself, e.g., the hemodynamic perturbations that result from a stenotic or regurgitant valve, can cause remodeling abnormalities. We show that versican and aggrecan demonstrate complementary expression patterns in regional mouse aortic valve tissue and change over time, and these proteoglycans are substrates of both MMPs (remodeling) and ADAMTSs (processing). Because MMP activity is modulated by versican, there may be a potential feedback mechanism whereby remodeling enzymes and proteoglycans are reciprocally regulated, consistent with abnormalities observed in the Adamts9 deficient mouse (Kern et al., 2010; Ra and Parks, 2007). These findings support the idea that ECM remodeling abnormalities are present in functional valve tissue and cause ECM compositional changes, contributing to the onset of AVD. The mechanisms causing early upregulation of remodeling enzymes warrants further investigation.
The findings of the present study advance the idea that AVD pathogenesis originates in the valve annulus region. We show postnatal expression patterns of cartilage markers in regional aortic valve tissue, consistent with the idea that cartilage programs contribute to the embryonic development of the whole valve (Lincoln et al., 2007; Wirrig et al., 2007), but regulate postnatal valve maintenance in the annulus region only. In this context, we speculate that areas of high shear stress on the elastic fiber rich (ventricularis) side of the valve, primarily at the hinge, may trigger VIC activation or other cell adaptations (involving non-resident hematopoietic cells or aberrant endothelial cells), which in turn contribute to disease processes originating in the annulus and affecting cartilage programs (Lincoln et al., 2006b; Wirrig et al., 2011). Interestingly, the Eln+/− nodules are positive for SMemb and SM22 expression and negative for SMA, potentially identifying functionally different subsets of activated VICs (Owens et al., 2004). These potential cell response patterns may direct the distinct histopathologic forms of valve disease, i.e, myxomatous change vs. fibrocalcific degeneration (Hinton and Yutzey, 2011; Schoen, 2008). In addition to the Eln+/− mouse, three targeted mutagenesis mouse models demonstrate similar aortic valve annulus phenotypes with localized cartilage-type matrix abnormalities, namely the Adamts9, Fibulin-4 and Ets-1 mutant mice (Gao et al., 2010; Hanada et al., 2007; Kern et al., 2010), suggesting disease manifestation in the annulus region of the valve may be generalizable. Overall, our data identify cartilage dysregulation as a central process in early AVD pathogenesis and implicate the valve annulus as the origin of disease.
While the role of collagens, proteoglycans and elastic fibers in normal valve development and mature valve structure is well-established, not much is known about the interaction of these ECM proteins in AVD. Versican is known to interact with other ECM components, including type I collagen, tenascin-R and hyaluronan, and studies have shown that versican interferes with elastic fiber assembly by preventing tropoelastin to bind the microfibrillar scaffold (Merrilees et al., 2011; Wu et al., 2005). Increased versican together with lack of mature elastic fiber deposition is associated with heightened cell proliferation (Merrilees et al., 2011), consistent with the observation of increased cell proliferation and increased versican in Eln+/− valves (Hinton et al., 2010). Conversely, reduction in intact versican promotes elastogenesis, suggesting an active versican-elastin interaction that is not restricted to structural considerations and is important for ongoing cell-matrix homeostasis (Huang et al., 2006). Elastic fiber fragmentation in Eln+/− valves may lead to upregulation of versican expression and/or abnormal versican processing, such that increased cleaved versican may reflect a compensatory mechanism to stabilize elastic fiber architecture. Our finding that versican processing is altered in Eln+/− valves is consistent with recent studies exploring versican-ADAMTS dysregulation, and suggests that the cleaved to intact versican ratio may provide a useful index to quantify versican processing defects (Fu et al., 2011; Kern et al., 2010).
ECM composition affects valve function, including tissue-level biomechanical properties (Schoen, 1997; Vesely, 1998). In this study, the biomechanical properties of Eln+/− mouse aortic valves are consistent with previously reported values in canine and porcine valve and aortic sinus stiffness studies (Liao et al., 2008; Merryman et al., 2006; Thubrikar et al., 1980). Compromised tissue stiffness in the cusp and annulus regions of Eln+/− aortic valves is consistent with the observed decrease in type I and III collagens and increase in proteoglycans. Type III collagen is associated with tissue tensile strength at high strains and is increased in an age-dependent manner, consistent with previous studies (Stephens et al., 2009; Stephens and Grande-Allen, 2007). Abnormal distribution of mechanical loading across the Eln+/− valve with increasing shear stress on the elastin-rich ventricular side of the hinge due to hemodynamic perturbation of latent AVD likely triggers annulus-specific ECM remodeling abnormalities. Tseng et al describes a significant role for elastin in flexure (Tseng and Grande-Allen, 2011), and the observation in Eln+/− valves that elastic fiber fragments extend into the annulus suggests an anatomical area of high vulnerability to disease. Overall, our results show that alterations in regional valve tissue biomechanical properties (function) correspond with both changes in ECM composition (structure) and remodeling enzyme activity (mechanism).
In summary, combining molecular approaches with engineering measures is important to fully understand developmental programs that result in AVM, factors that contribute to degeneration, and mechanisms of AVD. These insights may be informative in elucidating AVD pathogenesis and natural history in the context of translational research efforts such as novel therapeutics and durable valve bioprostheses.
4. Experimental Procedures
4.1. Animals
Elastin insufficient (Eln+/−) mice were maintained on an isogenic C57BL/6 background (Hinton et al., 2010; Li et al., 1998). Aortic valves were isolated from Eln+/+ (wild type, WT) and Eln+/− mice of mixed gender at three stages: juvenile (1 month old (mo)), adult (4 mo), and aged (12 mo). Genotyping was performed as previously described (Hinton et al., 2010). All protocols were approved by the Institutional Animal Care and Use Committee at Cincinnati Children’s Hospital Medical Center.
4.2. Histochemistry
To evaluate regional ECM composition and organization, histochemistry was performed on 5 μm sections using Movat’s modified pentachrome stain which colors elastic fibers black, collagen fibers yellow, proteoglycans blue, muscle red, and cell nuclei purple (Hinton et al., 2006). Aortic valve tissue was processed in both long (cross section, Fig. 1A) and short axes (en face, Fig. 1B). Three mice per genotype per stage were examined.
4.3. Immunohistochemistry
Antibodies directed against SMA (Sigma, 1:500), SMemb (Abcam, 1:500), SM22 (Abcam, 1:75), CD31 (Abcam, 1:50), Mac-3 (BD Biosciences, 1:20), Sox-9 (Abcam, 1:500), Crtl-1 (Iowa Developmental Hybridoma Bank, 1:10), CHM-1 (Lifespan Biosciences, 1:50), type II Collagen (Abcam, 1:200), MMP-2 (Abcam, 1:300), MMP-9 (Abcam, 1:300), ADAMTS-5 (Abcam, 1:100), ADAMTS-9 (1:50, Santa Cruz Biotechnology), Aggrecan (Chemicon, 1:100), Intact Versican (Millipore, 1:200), Cleaved Versican (Thermo Fisher, 1:100), were used to determine protein expression and localization using immunofluorescence (MMP-2 and MMP-9) or streptavidin/biotin colorimetry and diaminobenzidine detection (all others) (Hinton et al., 2006). Quantitative analysis was performed to determine average area fraction of MMP-2 and MMP-9 expression in regional aortic valve tissue using NIS software (Nikon Instruments, Melville NY), as previously described (Krishnamurthy et al., 2011). Three mice per genotype per stage were used. Antigen retrieval was performed using Proteinase K, Chondroitinase ABC or heat mediated citrate buffer. Fluorescent images were captured with a Zeiss LSM 510 confocal microscope and LSM version 3.2 SP2 software. Bright field images were obtained using a Nikon Ti-U microscope and NIS software.
4.4. Histochemical quantification and ELISA
Quantitative analysis of histochemical staining intensity was performed to determine average area fraction of collagens and proteoglycans in regional aortic valve tissue, using NIS software (Nikon Instruments, Melville NY)(Krishnamurthy et al., 2011). In addition, ELISA was performed on the whole valve (cusp and annulus regions) to quantify type I Collagen (Thermo Scientific, 1:1000), type III Collagen (Rockland, 1:5000), Intact Versican (Millipore, 1:2000) and Cleaved Versican (Thermo Fisher, 1:2000)(Hurley et al., 2010). Aortic valve tissue was isolated and homogenized in lysis buffer (1% Triton-X in PBS) containing protease inhibitors. Protein expression was normalized to total protein content as determined by Bradford assay using Coomassie Plus Assay Kit (Thermo Fisher Scientific). For the software based histochemical quantification, two representative sections each from three animals (n=3) per genotype per stage were used to evaluate area fractions in cusp and annulus regions. For ELISA, one whole aortic valve was used for each experiment and three experiments (n=3) were performed per genotype per stage.
4.5. Gelatin zymography
To quantify MMP-2 and MMP-9 activity, aortic valve tissue was subjected to gelatin zymography (Zhao et al., 2006). Aortic valve tissue was isolated and pooled (4 per experiment) and homogenized on ice in cold TNC buffer. Three experiments were performed for each stage and genotype. Total protein content was estimated for the collected supernatant. The gels were stained with Coomassie Blue R-250 and destained; active MMP-2 and MMP-9 were detected as clear bands against a blue background. Molecular weight markers (Bio-Rad) and positive controls (purified active MMP-2 and MMP-9, Calbiochem) were used for calibration. Both the pro and cleaved (active) forms of the MMPs are detected by zymography, but only the cleaved forms were quantified using a custom MATLAB-based (Mathworks Inc) band densitometry analysis normalized to the total protein content (Narmoneva et al., 2005).
4.6. Biomechanical testing
The regional biomechanical properties of aortic valve tissue ex vivo were examined using the micropipette approach that we developed recently (Krishnamurthy et al., 2011). Ten mice per genotype per stage were studied. Briefly, the mouse aortic valve tissue was dissected to reveal cusp and annulus regions in situ, and mounted on a microscope slide. The valve surface was accessed from the ventricular side using a micromanipulator and glass micropipette drawn to an inner diameter of 50–60 μm; upon contact, aspiration pressure was applied in a step-wise manner (Jones et al., 1999). Videos of tissue aspiration were recorded, and the aspiration length and radius were measured. Linear regression was used to curve fit the data. Young’s modulus (E) as a measure of tissue stiffness was determined using a half-space model equation (Guilak et al., 2005; Theret et al., 1988).
4.7. Statistical analysis
Multivariate ANOVA and post-hoc Bonferroni multiple comparison tests (SPSS Inc.) were used to determine effects on protein expression, MMP activity and Young’s modulus by region (cusp, annulus), genotype (Eln+/−, WT) and stage (juvenile, adult, aged). All variables were reported as means ± standard deviation; differences were considered significant at p<0.05.
Supplementary Material
Highlights.
Eln+/− aortic valves show early matrix remodeling abnormalities.
Maladaptive remodeling occurs prior to the onset of disease.
The aortic valve annulus region is the origin of disease.
Acknowledgments
We thank Dr. Santanu Chakraborty, Dr. Kathryn Rafferty, Abdul Sheikh, Jennifer Hurley and Bob Nielsen for their assistance. We also thank Dr. Dean Y. Li (University of Utah) for providing the elastin knockout mice. This study was supported by the AHA 09PRE2230162 (VKK), Cincinnati ESU Research Travel Grant (VKK), and the National Institutes of Health AR15768 (FG), DK078814 (DAN), and HL085122 (RBH).
Abbreviations
- ADAMTS
A Disintegrin-like And Metalloprotease domain with ThromboSpondin-type 1 Motifs
- AVD
Aortic Valve Disease
- AVM
Aortic Valve Malformation
- CHM
Chondromodulin
- Crtl
Cartilage-Link Protein
- ECM
Extracellular Matrix
- MMP
Matrix Metalloproteinase
- SMA
α-Smooth Muscle Actin
- SMemb
Non Muscle Myosin Heavy Chain
- VIC
Valve Interstitial Cell
Footnotes
There are no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RH. Clinical anatomy of the aortic root. Heart. 2000;84:670–673. doi: 10.1136/heart.84.6.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp--Part I: Experimental results. J Biomech Eng. 2000;122:23–30. doi: 10.1115/1.429624. [DOI] [PubMed] [Google Scholar]
- Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–143. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Dupuis LE, McCulloch DR, McGarity JD, Bahan A, Wessels A, Weber D, Diminich AM, Nelson CM, Apte SS, Kern CB. Altered versican cleavage in ADAMTS5 deficient mice; A novel etiology of myxomatous valve disease. Developmental Biology. 2011;357:152–164. doi: 10.1016/j.ydbio.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edep ME, Shirani J, Wolf P, Brown DL. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc Pathol. 2000;9:281–286. doi: 10.1016/s1054-8807(00)00043-0. [DOI] [PubMed] [Google Scholar]
- Eronen M, Peippo M, Hiippala A, Raatikka M, Arvio M, Johansson R, Kahkonen M. Cardiovascular manifestations in 75 patients with Williams syndrome. J Med Genet. 2002;39:554–558. doi: 10.1136/jmg.39.8.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondard O, Detaint D, Iung B, Choqueux C, Adle-Biassette H, Jarraya M, Hvass U, Couetil JP, Henin D, Michel JB, Vahanian A, Jacob MP. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26:1333–1341. doi: 10.1093/eurheartj/ehi248. [DOI] [PubMed] [Google Scholar]
- Fu Y, Nagy JA, Brown LF, Shih SC, Johnson PY, Chan CK, Dvorak HF, Wight TN. Proteolytic cleavage of versican and involvement of ADAMTS-1 in VEGF-A/VPF-induced pathological angiogenesis. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2011;59:463–473. doi: 10.1369/0022155411401748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Kim GH, Mackinnon AC, Flagg AE, Bassett B, Earley JU, Svensson EC. Ets1 is required for proper migration and differentiation of the cardiac neural crest. Development. 2010;137:1543–1551. doi: 10.1242/dev.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L, Kugel MA. Topographic Anatomy and Histology of the Valves in the Human Heart. Am J Pathol. 1931;7:445–447. [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Alexopoulos LG, Haider MA, Ting-Beall HP, Setton LA. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons isolated by cartilage homogenization. Ann Biomed Eng. 2005;33:1312–1318. doi: 10.1007/s10439-005-4479-7. [DOI] [PubMed] [Google Scholar]
- Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, Kanaar R, Essers J. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ Res. 2007;100:738–746. doi: 10.1161/01.RES.0000260181.19449.95. [DOI] [PubMed] [Google Scholar]
- Hinton RB, Adelman-Brown J, Witt S, Krishnamurthy VK, Osinska H, Sakthivel B, James JF, Li DY, Narmoneva DA, Mecham RP, Benson DW. Elastin haploinsufficiency results in progressive aortic valve malformation and latent valve disease in a mouse model. Circ Res. 2010;107:549–557. doi: 10.1161/CIRCRESAHA.110.221358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, Hinek A, Wight TN. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370–377. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- Hurley JR, Balaji S, Narmoneva DA. Complex temporal regulation of capillary morphogenesis by fibroblasts. Am J Physiol Cell Physiol. 2010;299:C444–453. doi: 10.1152/ajpcell.00572.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WR, Ting-Beall HP, Lee GM, Kelley SS, Hochmuth RM, Guilak F. Alterations in the Young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J Biomech. 1999;32:119–127. doi: 10.1016/s0021-9290(98)00166-3. [DOI] [PubMed] [Google Scholar]
- Keane JF, Fellows KE, LaFarge CG, Nadas AS, Bernhard WF. The surgical management of discrete and diffuse supravalvar aortic stenosis. Circulation. 1976;54:112–117. doi: 10.1161/01.cir.54.1.112. [DOI] [PubMed] [Google Scholar]
- Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010;29:304–316. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy VK, Guilak F, Narmoneva DA, Hinton RB. Regional structure-function relationships in mouse aortic valve tissue. J Biomech. 2011;44:77–83. doi: 10.1016/j.jbiomech.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102:1783–1787. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29:1065–1074. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln J, Florer JB, Deutsch GH, Wenstrup RJ, Yutzey KE. ColVa1 and ColXIa1 are required for myocardial morphogenesis and heart valve development. Developmental dynamics: an official publication of the American Association of Anatomists. 2006a;235:3295–3305. doi: 10.1002/dvdy.20980. [DOI] [PubMed] [Google Scholar]
- Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Developmental Biology. 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006b;294:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Merrilees MJ, Beaumont BW, Braun KR, Thomas AC, Kang I, Hinek A, Passi A, Wight TN. Neointima formed by arterial smooth muscle cells expressing versican variant v3 is resistant to lipid and macrophage accumulation. Arterioscler Thromb Vasc Biol. 2011;31:1309–1316. doi: 10.1161/ATVBAHA.111.225573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merryman WD, Huang HY, Schoen FJ, Sacks MS. The effects of cellular contraction on aortic valve leaflet flexural stiffness. J Biomech. 2006;39:88–96. doi: 10.1016/j.jbiomech.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Narmoneva DA, Oni O, Sieminski AL, Zhang S, Gertler JP, Kamm RD, Lee RT. Self-assembling short oligopeptides and the promotion of angiogenesis. Biomaterials. 2005;26:4837–4846. doi: 10.1016/j.biomaterials.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104:2525–2532. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. doi: 10.1161/01.CIR.0000155623.48408.C5. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen FJ. Aortic valve structure-function correlations: role of elastic fibers no longer a stretch of the imagination. J Heart Valve Dis. 1997;6:1–6. [PubMed] [Google Scholar]
- Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- Stephens EH, de Jonge N, McNeill MP, Durst CA, Grande-Allen KJ. Age-Related Changes in Material Behavior of Porcine Mitral and Aortic Valves and Correlation to Matrix Composition. Tissue Eng Part A. 2009;16:867–878. doi: 10.1089/ten.tea.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis. 2007;16:672–682. [PubMed] [Google Scholar]
- Theret DP, Levesque MJ, Sato M, Nerem RM, Wheeler LT. The application of a homogeneous half-space model in the analysis of endothelial cell micropipette measurements. J Biomech Eng. 1988;110:190–199. doi: 10.1115/1.3108430. [DOI] [PubMed] [Google Scholar]
- Thubrikar M, Piepgrass WC, Bosher LP, Nolan SP. The elastic modulus of canine aortic valve leaflets in vivo and in vitro. Circ Res. 1980;47:792–800. doi: 10.1161/01.res.47.5.792. [DOI] [PubMed] [Google Scholar]
- Thubrikar MJ, Aouad J, Nolan SP. Patterns of calcific deposits in operatively excised stenotic or purely regurgitant aortic valves and their relation to mechanical stress. Am J Cardiol. 1986;58:304–308. doi: 10.1016/0002-9149(86)90067-6. [DOI] [PubMed] [Google Scholar]
- Togashi M, Tamura K, Nitta T, Ishizaki M, Sugisaki Y, Fukuda Y. Role of matrix metalloproteinases and their tissue inhibitor of metalloproteinases in myxomatous change of cardiac floppy valves. Pathol Int. 2007;57:251–259. doi: 10.1111/j.1440-1827.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- Tseng H, Grande-Allen KJ. Elastic fibers in the aortic valve spongiosa: a fresh perspective on its structure and role in overall tissue function. Acta biomaterialia. 2011;7:2101–2108. doi: 10.1016/j.actbio.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, Webb GD, Siu SC. Outcomes in adults with bicuspid aortic valves. Jama. 2008;300:1317–1325. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31:115–123. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circulation research. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Wirrig EE, Hinton RB, Yutzey KE. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol. 2011;50:561–569. doi: 10.1016/j.yjmcc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirrig EE, Snarr BS, Chintalapudi MR, O’Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A. Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Developmental Biology. 2007;310:291–303. doi: 10.1016/j.ydbio.2007.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Yacoub MH, Kilner PJ, Birks EJ, Misfeld M. The aortic outflow and root: a tale of dynamism and crosstalk. Ann Thorac Surg. 1999;68:S37–43. doi: 10.1016/s0003-4975(99)00745-6. [DOI] [PubMed] [Google Scholar]
- Zhao H, Ito A, Sakai N, Matsuzawa Y, Yamashita S, Nojima H. RECS1 is a negative regulator of matrix metalloproteinase-9 production and aged RECS1 knockout mice are prone to aortic dilation. Circ J. 2006;70:615–624. doi: 10.1253/circj.70.615. [DOI] [PubMed] [Google Scholar]
- Zimmerman J, Bailey CP. The surgical significance of the fibrous skeleton of the heart. J Thorac Cardiovasc Surg. 1962;44:701–712. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.