Abstract
Studies of tick salivary glands (SGs) and their components have produced a number of interesting discoveries over the last four decades. However, the precise neural and physiological mechanisms controlling SG secretion remain enigmatic. Major studies of SG control have identified and characterized many pharmacological and biological compounds that activate salivary secretion, including dopamine (DA), octopamine, γ-aminobutyric acid (GABA), ergot alkaloids, pilocarpine (PC), and their pharmacological relatives. Specifically, DA has shown the most robust activities in various tick species, and its effect on downstream actions in the SGs has been extensively studied. Our recent work on a SG dopamine receptor has aided new interpretations of previous pharmacological studies and provided new concepts for SG control mechanisms. Furthermore, our recent immunohistochemical studies have suggested that multiple neuropeptides are involved in SG control. Myoinhibitory peptide (MIP) and SIFamide have been identified in the neural projections reaching the basal cells of acini type II and III. Pigment-dispersing factor (PDF)-immunoreactive neural projections reach type II acini, and RFamide- and tachykinin-immunoreactive projections reach the SG ducts, but the chemical nature of these immunoreactive materials are unidentified yet. Here, we briefly review previous pharmacological studies and provide a revised summary of SG control mechanisms in ticks.
Keywords: Dopamine (DA), dopamine receptors, neuropeptides, tick salivary gland acini, synganglion
Introduction
Ticks are a notorious group of hematophagous arthropods that feed on their hosts for long durations of several hours to more than a week and increase their body weight up to 100 times during feeding. Tick salivary secretions injected into the feeding site manipulate their host hemostasis and immune system, allowing for the long feeding period. Additionally, another essential salivary gland (SG) function is osmoregulation. During feeding, blood meal is digested, and excess water is expelled back into the host though the SGs. In Dermacentor andersoni and Amblyomma americanum, up to 75% of the ingested volume is expelled (Kaufman and Phillips, 1973; Sauer and Hair, 1971). A pair of female tick SGs is composed of three different types of acini (I, II, and III) that together form a grape-like structure. Each acinus contains several types of cells and different categories of secretory vesicles (Binnington, 1978; Coons and Roshdy, 1973). Acini II and III are considered the major structures that produce secretory proteins/compounds and participate in osmoregulation during feeding, while acinus I is thought to be involved in absorption of water in free-living ticks (Gaede et al., 1997; Rudolph and Knulle, 1974). After several days of feeding, acini II and III undergo a remarkable morphological transformation without a change in cell number. The nuclei and cytoplasm of most cells enlarge during feeding, resulting in an overall increase in acini mass (Sauer et al., 1995).
Pharmacological studies have implicated several components involved in the process of salivary secretion: dopamine (DA), octopamine, γ-aminobutyric acid (GABA), ergot alkaloids, and the muscarinic acetylcholine receptor agonist pilocarpine (PC) (Kaufman, 1976; Needham and Pannabecker, 1983; Pannabecker and Needham, 1982; Lindsay and Kaufman, 1986; Kaufman and Wong, 1983; Hsu and Sauer, 1975; Kaufman, 1978; also see review Bowman and Sauer, 2004). Recent immunohistochemical studies have suggested important roles of multiple neuropeptides (Šimo et al., 2009a; Šimo et al., 2009b). However, the precise roles of these components and their mechanisms in the complex physiological processes of the salivary secretion are still elusive.
In this review, we summarize previous findings about the biological components involved in the control of salivary fluid secretions. Recent discoveries of DA receptor expression in the SGs provide new insights into earlier pharmacological studies. We will also focus on recently described neuropeptidergic innervations of tick SGs.
Biological components in control of the tick salivary gland
A major tool in the studies of tick SGs has been the assessment of secretory activities of isolated SGs by measuring the volume of salivary secretion or the increased weight of ligated SGs. This in vitro assay has been widely used to identify the chemical components that modulate/activate the SGs.
Among many pharmacological agents examined, DA is the most active compound that directly induces salivary secretion in isolated SGs (Bowman and Sauer, 2004; Kaufman, 1976; Lindsay and Kaufman, 1986; McSwain et al., 1992a; McSwain et al., 1992b; Sauer et al., 2000; Sauer et al., 1995; Sauer et al., 1994; Schmidt et al., 1981). In addition, injection of DA into the hemocoel of a live tick induces mouthpart movement, the typical behavioral component of feeding. A more comprehensive model of DA action on the SG has been proposed (Sauer et al., 2000) in which DA activates two independent signaling pathways: cAMP-dependent signal transduction, which leads to fluid secretion, and a calcium-dependent signaling pathway, which stimulates prostaglandin E2 (PGE2) release through protein kinases (Mane, 1985; Mane et al., 1988; Needham and Sauer, 1979; Needman and Sauer, 1975). PGE2 in the salivary secretions is involved in immunosuppression and possibly vasodilation (Oliveira et al., 2011). Additionally, PGE2 has an autocrine or paracrine role within the SG itself, inducing the secretion of bioactive salivary proteins via intracellular Ca2+ mobilization (Sauer et al., 2000).
Octopamine also stimulated salivary secretion in A. americanum but at three orders of magnitude less than DA (Needham and Pannabecker, 1983; Pannabecker and Needham, 1982). Phentolamine, a nonselective alpha-adrenergic antagonist, did not have effect on octopamine-stimulated secretion in vitro, whereas the phenothiazine drug thioridazine effectively inhibited both DA- and octopamine-stimulated secretions (Pannabecker and Needham, 1982). The authors concluded that octopamine most likely interacts with other catecholamine receptors, perhaps DA receptors. However, Šimo et al., (2011) demonstrated that octopamine does not activate D1 receptors in heterologous expression systems, indicating that octopamine probably activates receptor(s) other than DA receptors in SGs.
GABA and its receptor(s) may also be involved in tick SG secretion control. GABA treatment of isolated A. hebraeum SGs potentiated the effect of DA on fluid secretion, and this modulation by GABA was blocked by the GABA-A receptor antagonists picrotoxin and bicuculline (Lindsay and Kaufman, 1986). These two antagonists showed the same effect on spiperone, a drug that acts in a similar way to GABA. Sulpiride, a spiperone antagonist, also prevented GABA-induced potentiation of the DA salivation effect. These results suggest that sulpiride and spiperone likely interact with GABA receptor(s) and that GABA has a modulatory role in DA-induced fluid secretion (Lindsay and Kaufman, 1986).
It is unlikely that ergot alkaloids are endogenous signaling molecules in tick SGs. Nevertheless, ergot injection stimulates fluid excretion in tick, which led to the speculation of a separate ergot alkaloid sensitive receptor (Kaufman and Wong, 1983). Ergot alkaloids interact with multiple biogenic amine receptors, including serotonin (5-HT) receptors and histamine receptors. Lower than millimolar concentrations of 5-HT and histamine have no effective on tick salivary secretion (Kaufman and Phillips, 1973). Thus, ergot-induced effects are unlikely via 5-HT or histamine receptors in tick SGs. Ergot alkaloid has been suggested to be independent from DA because sulpiride inhibited ergot alkaloid action without affecting DA-stimulated secretion (Kaufman and Wong, 1983). However, Šimo et al. (2011) showed that ergot alkaloid activates D1 receptors resulting in the elevation of cAMP in a heterologous expression system.
PC is a cholinomimetic that has been frequently used for injection or topical application to induce tick oral secretion (Hsu and Sauer, 1975; Kaufman, 1978; McSwain et al., 1992a; Ribeiro and Spielman, 1986; Tatchell, 1967). In A. hebraeum, injection of PC stimulated brisk fluid secretion, which fell to a half maximum after approximately 13 minutes, with a low rate of continued secretion up to 3 hours following injection. On the other hand, DA-induced salivary secretion lasted only for 40–50 min (Kaufman, 2000). Because PC failed to stimulate secretion in isolated SGs (Needman and Sauer, 1975), it is thought to induce salivary secretion indirectly through the central nervous system (CNS) (Kaufman, 1978). Injection of isosmotic solutions such as 1.2% NaCl, 11.2% sucrose or 2.3% urea into tick hemolymph also stimulated the salivary secretion in A. hebraeum. This study suggested that sensory or stretch receptor(s) sensitive to hemolymph osmolarity or volume, respectively, leads to saliva secretion (Kaufman et al. 2000).
Recent studies using RNA interference (RNAi) determined other gene products that may be involved in tick salivary secretion. For example, RNAi of secretory vesicle-associated synaptobrevin in A. americanum inhibited secretion of anticoagulant protein in PGE2-stimulated SGs (Karim et al., 2002; Karim et al., 2004). Recently, RNAi of an aquaporin (AQP) water channel in Ixodes ricinus completely inhibited DA-stimulated secretion in isolated SGs. Although AQP1 dsRNA-treated ticks exhibited a dramatic decrease in body weight and an increase in hemolymph osmolarity, they were still able to feed successfully (Campbell et al., 2010).
Dopamine as a paracrine signal
Until recently, the source of DA that is likely the initial command for salivary secretion has not been clear. An early study employing histofluorescent methods (Falck and Torp, 1962) for detecting catecholamines revealed the presence of DA/noradrenaline in the synganglia and axons innervating Boophilus microplus SGs (Binnington and Stone, 1977), leading to a speculation that DA stimulates fluid secretion in tick SGs via its action as a synaptic neurotransmitter. However, electrochemical and radioenzymatic assays found large quantities of DA in the SGs as well as in the synganglion of A. hebraeum, which is in conflict with the hypothesis of dopaminergic synapses in SGs. Also, injection of high doses of reserpine, a drug that depletes synaptic monoamines, did not significantly reduce the DA content in SGs (Kaufman et al., 1999).
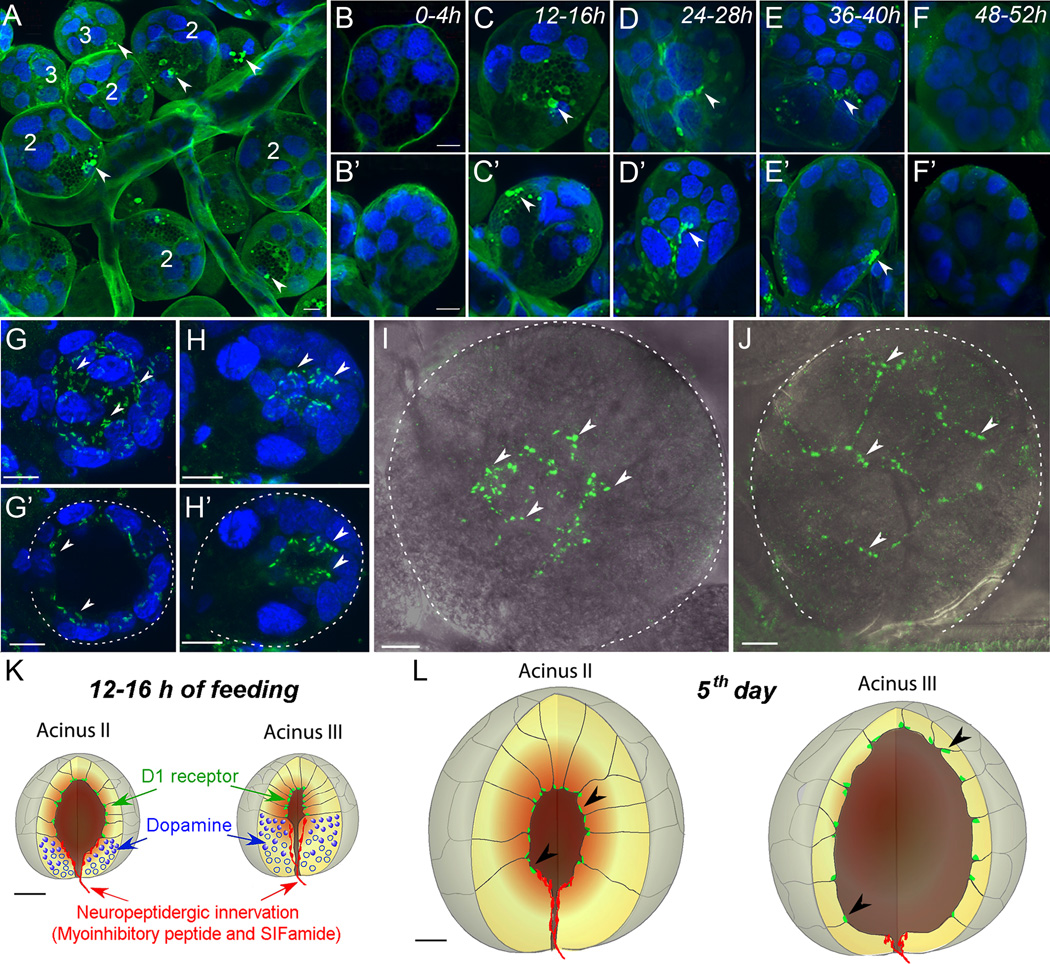
Recently, we found large DA-immunoreactive vesicles and their surrounding regions in the basal cells of type II and III acini (Fig. 1 A–F’) of Ixodes scapularis SGs. These vesicles were only observed between 12 and 40 h post-attachment in females (Fig. 1 A–F’), suggesting a role for DA in the early phases of feeding (Šimo et al., 2011). This finding was corroborated by an independent electron microscopy study showing the temporal dynamics of the large vesicles released from acini basal cells in the first two days of feeding (Grigorieva and Amosova, 2008). We have found that the dopaminergic basal cells are innervated by axon terminals containing the neuropeptides (see below) myoinhibitory peptide (MIP) and SIFamide, leading to the hypothesis that these neuropeptides are regulatory factors for DA synthesis or release by SG acini (Fig. 1 K).
Figure 1.
Dopamine and D1 receptor immunohistochemistry in the salivary glands of female I. scapularis during various feeding phases. (A) A region of salivary glands with clustered acini II (2) and III (3) 12–16 h after attachment. (B to F) Acini II 0–52 h after attachment to the host. (B’ to F’) Acini III at the same time-points. Note that positive staining (green marked with arrowheads) was detected in both acini II and III in the vesicles and their surrounding regions but only at 12–40 h post-attachment. The images are z-stacks of multiple confocal layers with thicknesses of 20 µm (A) or 2–5 µm (B to F’). (G) Whole acinus II of unfed female shown as a 27-µm-thick composite image. (G’) Optical section (10 µm) of acinus II demonstrating the D1 receptor immunoreactivity. (H) Whole acinus III of an unfed female shown as a composite image of 34 µm thickness. (H’) Optical section (5 µm) of acinus III demonstrating the apical location of D1 receptor immunoreactivity. (I) Acinus II and (J) III of a female 5 days post-attachment. Confocal composite images (10 µm) overlaid with the differential interference contrast images are shown. Schematic diagram showing the D1 (green), dopamine (blue), and neuropeptidergic innervation (red) in acini II and III of a 12–16 h post-attachment female (K) and 5 day post-attachment female (L). Arrowheads in G to L indicate the scattered patches of D1 receptor immunoreactivity on the luminal side of the acini. Dotted lines in G’, H’, I, and J indicate the acini boundary. Blue in A to H’ is 4', 6-diamidino-2-phenylindole (DAPI) staining for nuclei. Scale bars represent 10 µm. Modified from Šimo et al. (2011).
Dopamine receptors in salivary glands
At least three DA receptors have been identified in homology-based searches in the I. scapularis genome. They are named for their orthologous relationships with mammalian DA receptors: D1, D1-like, and D2 receptor (Šimo et al., 2011). Tissue-specific PCR founds D1 transcripts in SGs and synganglia. Immunohistochemistry for the D1 receptor revealed scattered patches of reactivity in the junctions between cells on the luminal surface of acini II and III (Fig. 1 G–L). This finding supports the paracrine hypothesis that DA released from basal cells in acini II and III activates the luminal D1 receptors within SGs. Reporter assays using the recombinant D1 receptor in mammalian cells showed that DA activated both calcium mobilization and cAMP elevation (Šimo et al., 2011). The D1 receptor expressed in a mammalian cell line was reactive to high dose of norepinephrine with 20x less lower sensitivity than to DA (Meyer et al., 2011; Šimo et al., 2011) but not to 10 µM octopamine (Šimo et al., 2011). Recently also the D1-like receptor was identified (Mayer et al., 2011) and we found that it is expressed in I. scapularis SGs (unpublished data, Šimo, Koči and Park). Further pharmacological characterization of these receptors in reporter assay systems will provide additional insights into previous pharmacological studies performed on isolated tick SGs.
Neuropeptidergic control of salivary glands
Nervous projections from the synganglion to the SGs have been previously described (Binnington and Tatchell, 1973; Chow and H, 1974; Megaw, 1977; Obenchain, 1974; Obenchain and Oliver, 1975, 1976; Saito, 1960). The innervation of SGs by a pair of nerves arising from the second pedal ganglia was described for the first time in Haemaphysalis flava (Saito 1960). Later, several authors reported that different SG regions are innervated by four pairs of nerves: salivary nerve 1 as a branch of the palpal nerve, salivary nerves 2–3 as branches of the lateral “sympathetic” nerve and salivary nerve 4 as a branch of the paraspiracular nerve (Binnington, 1978; Obenchain and Oliver, 1976). Specific innervation of nongranular and granular acini by axon terminals indicates direct neural control of SG activity, although their cellular sources in the CNS are unknown (Balashov et al., 1983; Coons and Alberti, 1999).
Recent studies based on neuropeptide mapping in ticks have revealed several neuropeptides involved in SG innervation. At least five antibodies raised against myoinhibitory peptide (MIP), SIFamide, pigment-dispersing factor (PDF), RFamide and tachykinin (TK) have revealed the peptidergic nature of SG innervation (Šimo et al., 2009a; Šimo et al., 2009b). So far, axonal projections from four distinct types of central neurons innervating SGs have been traced, and each neuronal type innervates specific regions or acini of the SGs (Šimo et al., 2009a; Šimo et al., 2009b).
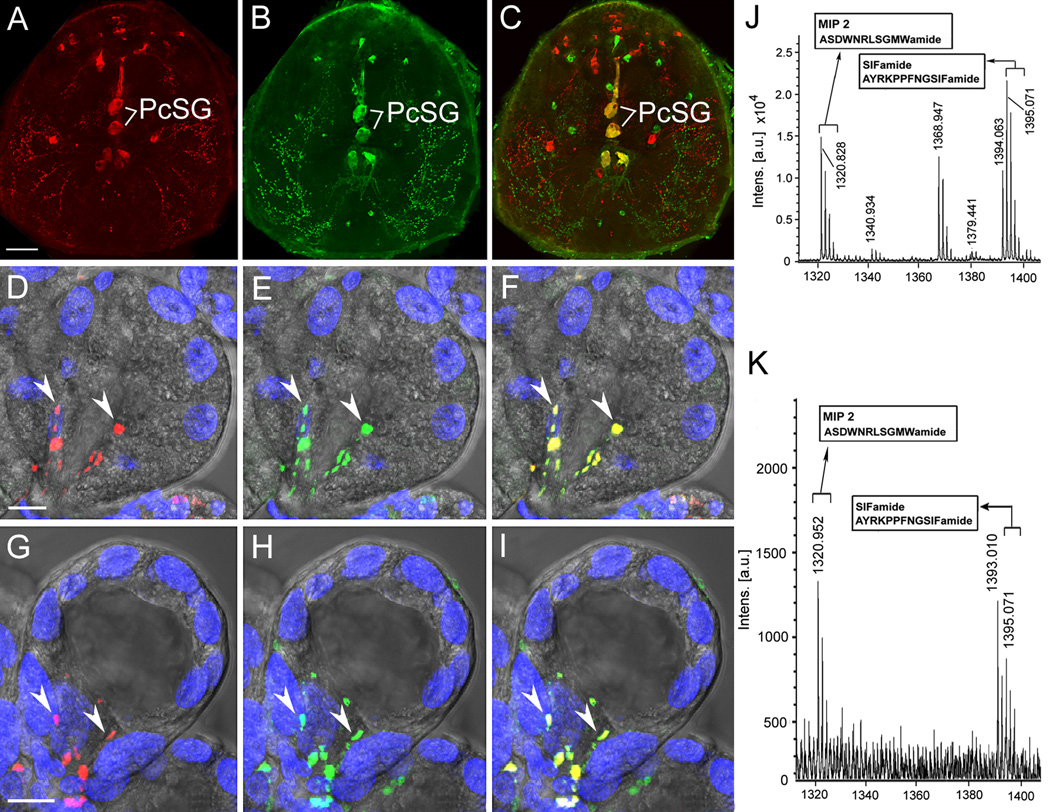
MIP and SIFamide immunostaining and in situ hybridization revealed a pair of giant protocerebral neurons (PcSG) innervating type II and III acini in I. scapularis SGs (Šimo et al., 2009b). The anatomy and innervation patterns of these neurons are very similar in diverse tick species (Figs. 2 A–C 3A, 4A). The axonal projections exit the synganglion through the palpal nerve and salivary nerve 1 (Figs. 3A, 4A), branch along the salivary duct surface, and terminate within SG acini types II and III but not type I (Figs. 2 D–I, 3B, D–F, 4 A, B). Comparative morphology of the terminal arborizations of these neurons in phylogenetically younger tick genera such as Dermacentor, Amblyomma or Rhipicephalus revealed bifurcated shapes in both type II and III acini (Figs. 3 B, E,F, 4 A,B), whereas evolutionary older genus, Ixodes, has 4–5 and 3–4 terminal containing varicosities in acini II and III, respectively (Figs. 2D–I, 3D, 4B). Terminal arborization morphology might be correlated with the number of neuropeptide-controlled cells.
Figure 2.
Myoinhibitory peptide (MIP) and SIFamide in I. scapularis synganglion and salivary glands acini. Double staining with antibodies to MIP (red; A) and SIFamide (green; B) revealed different types of neurons, including protocerebral salivary glands neurons PcSG, coexpressing MIP and SIFamide, shown as yellow in the merged image (C). Axon terminals in type II (D–F) and III (G–I) acini from PcSG neurons in a 1 day post-attachment female; MIP (red), SIFamide (green), and merge (yellow). Arrowheads indicate immunoreactive axon terminals. DAPI stained nuclei are blue. Scale bar is 50 µM for (A–C) and 10 µM for (D–I). MALDI-TOF mass spectra of synganglia extract (J) and salivary gland extract (K). Two large peaks, [M + H]+ 1,395.07 and 1,320.94, were commonly observed in both extracts. Modified from Šimo et al. (2009b) with permission from Journal of Comparative Neurology.
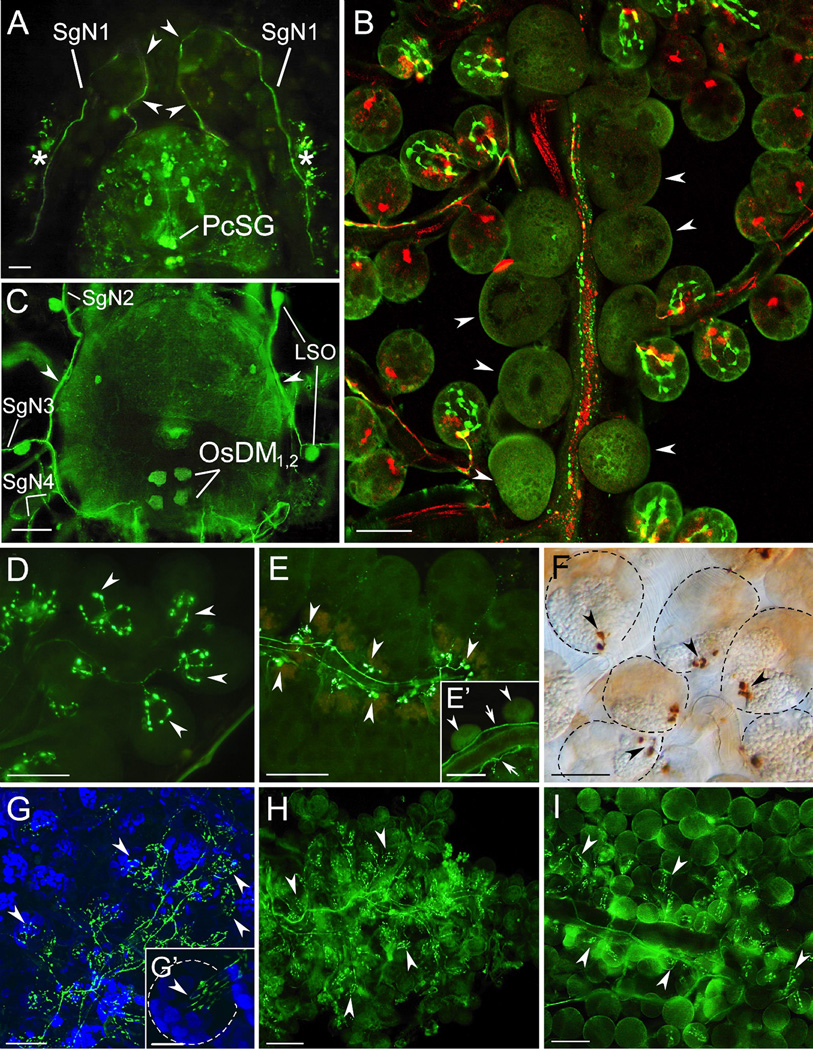
Figure 3.
Myoinhibitory peptide (MIP) and pigment dispersing factor (PDF) immunoreactivity (IR) in synganglia and salivary gland (SG) innervations in different tick species. (A) Pre-esophageal dorsal site of synganglion stained with MIP antibody in an unfed I. ricinus female. Two axons (arrowheads) from protocerebral salivary gland neurons (PcSG) excite the synganglion through the palpal nerve and salivary nerve 1 (SgN1) and innervate the SG acini II and III (asterisk). (B) Merged confocal z-stacks of an unfed R. appendiculatus female SG showing double staining with antibodies to PDF (green) and MIP (red). Note the PDF-like IR in axons containing varicosities along salivary ducts and multiple axon terminals exclusively on acini type II, whereas axons containing MIP-like IR terminate as bifurcated axon terminals on both acini types II and III. Acini I located along the main salivary duct are not innervated (arrowheads). (C) PDF-like-IR in the dorsal site of syngaglion in an unfed R. appendiculatus female. Four opistosomal neurons (OsDM1, 2) excite IR axons to the lateral nerve (lateral plexus, arrowheads) and SG nerves 2–4 (SgN2–4). Four lateral segmental organs (LSO) sitting on SgN2 and 3 were also PDF-like positive. (D–F) MIP-like IR (green in D and E, dark brown in F) axon terminals (arrowheads) in SG acini II and III in in females from 3 tick species. (D) Unfed I. ricinus. (E) Four-day fed Amblyomma variegatum larvae, (E’) MIP-like IR axon running along the main salivary duct (arrows) while acini type I (arrowhead) are not innervated. (F) Unfed D. reticulatus. Note that multiple branches of axon terminals were observed in I. ricinus (D), whereas typical bifurcated axon terminals were detected in A. variegatum (E) and D. reticulatus (F). (G–I) PDF-like IR (green) innervation of SG in females from 3 tick species (arrowheads PDF-like IR axon terminals). (G) Unfed I. scapularis. (G’) Higher magnification of type II acinus. (H) Unfed I. ricinus. (I) Unfed D. reticulatus. Note that in all three cases, only type II acini are innervated (arrowheads). Blue in G and G’ is 4',6-diamidino-2-phenylindole (DAPI) staining for nuclei. Scale bars are 50 µm (for A and C) and 30 µm (for B and D–I). (C) Modified from Šimo et al. (2009a) with permission from Cell and tissue research.
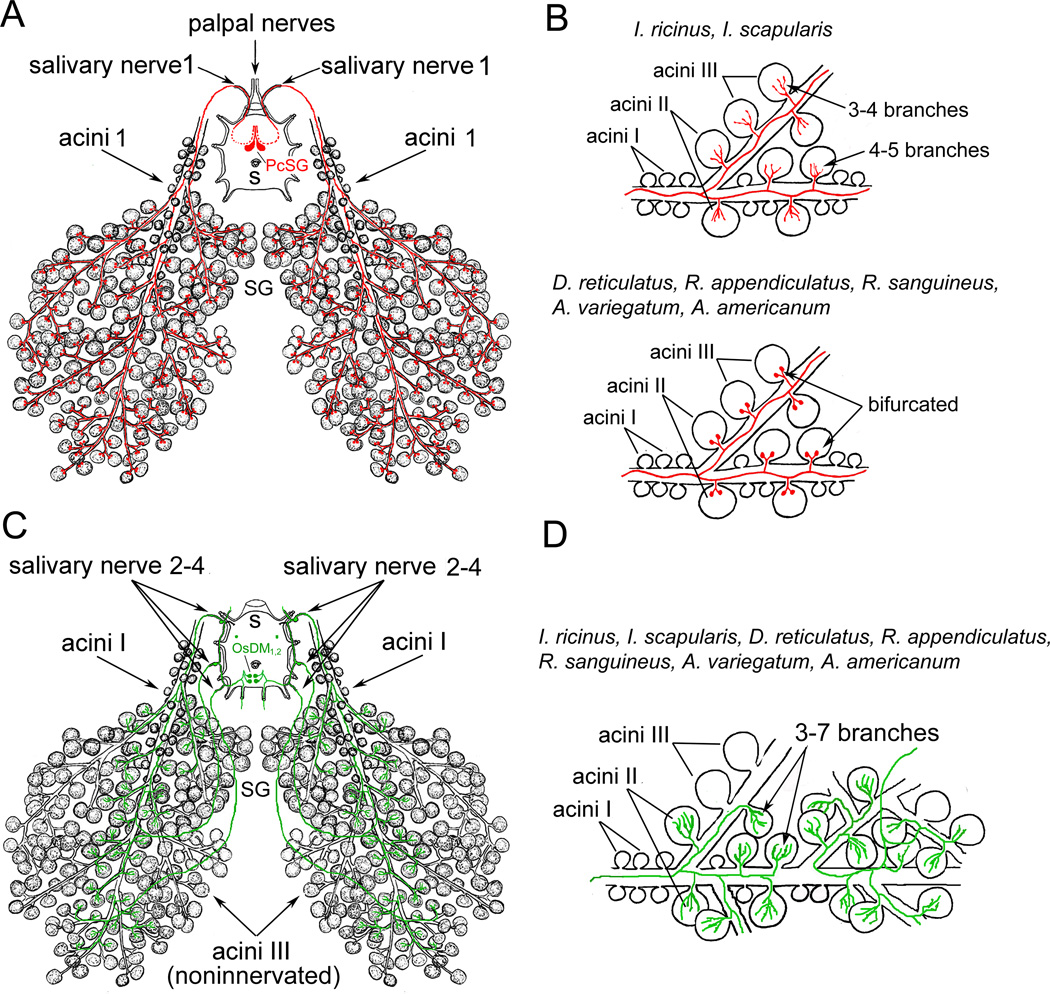
Figure 4.
Representations of identified central neurons innervating salivary glands (SGs). (A) Myoinhibitory peptide (MIP)-like immunoreactive (IR) axon terminals from giant protocerebral salivary glands neurons (PcSG, red) exit the synganglion through the palpal nerve and salivary nerve 1 and innervate acini types II and III. (B) I. ricinus and I. scapularis axon terminals of these neurons showed 4–5 and 3–4 branches containing varicosities in types II and III acini respectively. In D. reticulatus, R appendiculatus, R. sanguineus, A. variegatum, A. americanum these axon terminals lack varicosities and show typical bifurcating axon terminals. (C) Pigment dispersing factor (PDF)-like IR neurons (OsSG1,2, green) exit the synganglion through lateral and salivary nerves 2–4 to innervate specific type II acini . (D) Detailed view to PDF-like IR axon terminals in acini type II in seven different tick species. Branching axon terminals contain varicosities. Modified from Šimo et al. (2009a) with permission from Cell and tissue research.
Mature forms of MIP and SIFamide were detected in synganglia and in SG extract from an unfed I. scapularis female by matrix-assisted laser desorption/ionization-time of flight (MALDI TOF/TOF) (Fig 2. J, K). This finding confirms the immunohistochemical data, together suggesting that both of these highly conserved arthropod neuropeptides are delivered from synganglia to SG acini II and III via axonal projections (Šimo et al. 2009b). A recent publication by Šimo et al. (2011) suggests that these two neuropeptides may be involved in the regulation of DA synthesis/release (see above) in tick SGs implicated by the anatomy (Fig. 1K).
An antibody against PDF revealed two pairs of opistosomal SG neurons (OsSG) that exclusively innervate only type II acini (Šimo et al., 2009a). A single axon from each OsSG neuron enters the lateral nerve (lateral plexus) and projects three branches into salivary nerves 2–4 that terminate in the anterior, medial and posterior SG, respectively (Figs. 3C, 4C). The axon terminals of OsSG entering into the acini are characterized by 3–7 branches containing varicosities in all of the tick species that have been examined (Figs. 3B, G–I, 4 C, D). The morphology of PDF immunoreactive axon terminals within type II acini indicates that multiple cells in an acinus may be controlled by this innervation. Despite the fact that antiserum against Drosophila melanogaster PDF (NSELINSLLSLPKNMNDAamide) showed highly specific staining patterns, no PDF gene has been identified yet in the I. scapularis genome or in the transcriptomes of tick species. Therefore, it is possible that the PDF has not been identified because of its high sequence divergence or because of incomplete sequence of the genome. Alternatively, the immunoreactivity could be a consequence of cross-reactivity with an unknown protein. In either case, the immunohistochemistry revealed the anatomical structure of the PDF-immunoreactive neuronal projections onto the luminal surface of acini type II.
RFamide-like and tachykinin-like immunoreactive neuronal projections from synganglia through the palpal and salivary nerve 1 innervate the salivary ducts (data not shown). Numerous varicosities along these branching axons are present on the surface of salivary ducts (Šimo et al., 2009a). Similar types of SG innervations reactive for 5-HT, DA and octopamine have been described in several insect species (see review Ali, 1997), including a study that showed FMRFamide-like peptides stimulating fluid secretion from isolated SGs in the blowfly Calliphora vomitoria (Duve et al., 1992).
Summary and conclusion
Four decades of studies to understand the control mechanisms of tick salivary secretion have laid an important foundation for a model of the actions of DA and PGE2 (Bowman and Sauer, 2004; Sauer et al., 2000; Sauer et al., 1995). However, recent descriptions of DA localization and immunoreactivity necessitate revisions of this model. Furthermore, a better understanding of SG neuropeptidergic innervations has suggested at a more complex system for the control of SGs.
Recent discoveries of DA immunoreactive basal cells and luminal D1 receptors in SG acini II and III have led to a hypothesized paracrine function of DA (Šimo et al., 2011). In addition, neuropeptidergic projections reaching the DA immunoreactive SG basal cells suggested involvement of MIP and SIFamide in the control of DA cells. PcSG neurons produce both MIP and SIFamide and project their axonal branches to every acini type II and III. In addition to PcSG neurons, we have identified three additional different arborizations that are immunoreactive for PDF, tachykinin, and RFamide antibodies.
In conclusion, a number of biological components involved in the control of salivary secretion have been uncovered. DA receptor pharmacology currently delineates previous pharmacological studies of tick SGs. New resources, such as Ixodes genome sequences (Hill and Wikel, 2005), and technological advances, such as next generation sequencing and RNAi, will lead to a better understanding of the mechanisms controlling tick SGs. Understanding the mechanisms of tick SG control will reveal the target system, in the long term, for disrupting tick feeding and pathogen transmission.
Highlights.
Neuropeptidergic axon terminals innervate the basal cells in salivary gland acini.
The basal cells of acini are immunoreactive to dopamine antibody.
Dopamine D1 receptor is located on the luminal surface of apical cells of acini.
We propose a neuropeptidergic control of paracrine dopamine in the salivation.
Acknowledgement
We are grateful to Drs. Akira Mizoguchi and Jan Veenstra for providing the MIP and SIFamide antibodies. Funding support was provided by National Institutes of Health (NIH) Grant Numbers R01AI090062-01 and 1R21AI081136-01. This paper is contribution no. ### from the Kansas Agricultural Experiment Station, Manhattan, Kansas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali D. The aminergic and peptidergic innervation of insect salivary glands. Journal of Experimental Biology. 1997;200:1941–1949. doi: 10.1242/jeb.200.14.1941. [DOI] [PubMed] [Google Scholar]
- Balashov IUS, Raikhel AS, Hoogstraal H. In: An Atlas of ixodid tick ultrastructure. Raikhel AS, editor. Entomological Society of America; 1983. [Google Scholar]
- Binnington KC. Sequential changes in salivary gland structure during attachment and feeding of the cattle tick, Boophilus microplus. International Journal for Parasitology. 1978;8:97–115. doi: 10.1016/0020-7519(78)90004-8. [DOI] [PubMed] [Google Scholar]
- Binnington KC, Stone BF. Distribution of catecholamines in the cattle tick Boophilus microplus. Comparative Biochemistry and Physiology - Part C: Toxicology & Pharmacology. 1977;58:21–28. doi: 10.1016/0306-4492(77)90004-1. [DOI] [PubMed] [Google Scholar]
- Binnington KC, Tatchell RJ. The nervous system and neurosecretory cells of Boophilus microplus (Acarina, Ixodidae) Zeitschrift fur Wissenschaftliche Zoologie. 1973;185:193–206. [Google Scholar]
- Bowman AS, Sauer JR. Tick salivary glands: function, physiology and future. Parasitology. 2004;129 Suppl:S67–S81. doi: 10.1017/s0031182004006468. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Burdin M, Hoppler S, Bowman AS. Role of an aquaporin in the sheep tick Ixodes ricinus: assessment as a potential control target. International Journal for Parasitology. 2010;40:15–23. doi: 10.1016/j.ijpara.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Chow YS, Wang HC. Neurosecretory cells and their ultrastructures of Rhipicephalus sanguineus (Latreille) (Acarina, Ixodidae) Acta Arachnologica. 1974;25:53–67. [Google Scholar]
- Coons LB, Alberti G. The Acari-Ticks. In: Harrison FW, Foelix R, editors. Microscopic Anatomy of Invertebrates. Vol. 8B. New York: Wiley-Liss; 1999. pp. 267–514. Chelicerate Arthropoda. [Google Scholar]
- Coons LB, Roshdy MA. Fine structure of the salivary glands of unfed male Dermacentor variabilis (Say) (Ixodoidea: Ixodidae) Journal of Parasitology. 1973;59:900–912. [PubMed] [Google Scholar]
- Duve H, Johnsen AH, Sewell JC, Scott AG, Orchard I, Rehfeld JF, Thorpe A. Isolation, structure, and activity of -Phe-Met-Arg-Phe- NH2 neuropeptides (designated calliFMRFamides) from the blowfly Calliphora vomitoria. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2326–2330. doi: 10.1073/pnas.89.6.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck B, Torp A. New evidence for the localization of noradrenalin in the adrenergic nerve terminals. Medicina experimentalis. International journal of experimental medicine. 1962;6:169–172. doi: 10.1159/000135153. [DOI] [PubMed] [Google Scholar]
- Gaede K, Kn Uuml, Lle W. On the mechanism of water vapour sorption from unsaturated atmospheres by ticks. Journal of Experimental Biology. 1997;200:1491–1498. doi: 10.1242/jeb.200.10.1491. [DOI] [PubMed] [Google Scholar]
- Grigorieva LA, Amosova LI. Morphofunctional changes, and their significance, of salivary glands of ixodid ticks of subfamilies Ixodinae and Amblyomminae (Acari: Ixodidae) during nutrition. Zhurnal evoliutsionnoi biokhimii i fiziologii. 2008;44:622–635. [PubMed] [Google Scholar]
- Hill CA, Wikel SK. The Ixodes scapularis Genome Project: an opportunity for advancing tick research. Trends in parasitology. 2005;21:151–153. doi: 10.1016/j.pt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Hsu MH, Sauer JR. Ion and water balance in the feeding lone star tick. Comparative Biochemistry and Physiology Part A: Physiology. 1975;52:269–276. doi: 10.1016/s0300-9629(75)80085-5. [DOI] [PubMed] [Google Scholar]
- Karim S, Essenberg RC, Dillwith JW, Tucker JS, Bowman AS, Sauer JR. Identification of SNARE and cell trafficking regulatory proteins in the salivary glands of the lone star tick, Amblyomma americanum (L.) Insect Biochemistry and Molecular Biology. 2002;32:1711–1721. doi: 10.1016/s0965-1748(02)00111-x. [DOI] [PubMed] [Google Scholar]
- Karim S, Ramakrishnan VG, Tucker JS, Essenberg RC, Sauer JR. Amblyomma americanum salivary gland homolog of nSec1 is essential for saliva protein secretion. Biochemical and Biophysical Research Communications. 2004;324:1256–1263. doi: 10.1016/j.bbrc.2004.09.189. [DOI] [PubMed] [Google Scholar]
- Kaufman WR. The influence of various factors on fluid secretion by in vitro salivary glands of ixodid Ticks. Journal of Experimental Biology. 1976;64:727–742. doi: 10.1242/jeb.64.3.727. [DOI] [PubMed] [Google Scholar]
- Kaufman WR. Actions of some transmitters and their antagonists on salivary secretion in a tick. American Journal of Physiology. 1978;235:R76–R81. doi: 10.1152/ajpregu.1978.235.1.R76. [DOI] [PubMed] [Google Scholar]
- Kaufman WR. Actions of some transmitters and their antagonists on salivary secretion in a tick. American Physiological Society. 2000:R76–R77. doi: 10.1152/ajpregu.1978.235.1.R76. [DOI] [PubMed] [Google Scholar]
- Kaufman WR, Phillips JE. Ion and water balance in the Ixodid tick Dermacentor andersoni I Routes of ion and water excretion. Journal of Experimental Biology. 1973:523–536. [Google Scholar]
- Kaufman WR, Phillips JE. Ion and water balance in the Ixodid tick Dermacentor andersoni II Mechanism and control of salivary secretion. Journal of Experimental Biology. 1973;58:537–547. [Google Scholar]
- Kaufman WR, Sloley BD, Tatchell RJ, Zbitnew GL, Diefenbach TJ, Goldberg JI. Quantification and cellular localization of dopamine in the salivary gland of the ixodid tick Amblyomma hebraeum. Experimental and Applied Acarology. 1999;23:251–265. [Google Scholar]
- Kaufman WR, Wong DL. Evidence for multiple receptors mediating fluid secretion in salivary glands of ticks. European Journal of Pharmacology. 1983;87:43–52. doi: 10.1016/0014-2999(83)90048-1. [DOI] [PubMed] [Google Scholar]
- Lindsay PJ, Kaufman WR. Potentiation of salivary fluid secretion in ixodid ticks: a new receptor system for gamma-aminobutyric acid. Canadian Journal of Physiology and Pharmacology. 1986;64:1119–1126. doi: 10.1139/y86-191. [DOI] [PubMed] [Google Scholar]
- Mane SD. Cyclic AMP-depended protein kinase from the salivary glands of the tick, Amblyomma americanum - partila purification and properties. Insect Biochemistry. 1985;15:777–787. [Google Scholar]
- Mane SD, Sauer JR, Essenberg RC. Molecular forms and free cAMP receptor of the cAM dependent protein kinase catalityc subunit isoforms from the lone star tick, Amblyomma americanum (L.) Insect Biochemistry. 1988;29 doi: 10.1016/s0965-1748(98)00103-9. [DOI] [PubMed] [Google Scholar]
- McSwain JL, Essenberg RC, Sauer JR. Oral secretion elicited by effectors of signal transduction pathways in the salivary glands of Amblyomma americanum (Acari: Ixodidae) Journal of Medical Entomology. 1992a;29:41–48. doi: 10.1093/jmedent/29.1.41. [DOI] [PubMed] [Google Scholar]
- McSwain JL, Masaracchia RA, Essenberg RC, Tucker JS, Sauer JR. Amblyomma americanum (L.): protein kinase C-independent fluid secretion by isolated salivary glands. Experimental Parasitology. 1992b;74:324–331. doi: 10.1016/0014-4894(92)90156-5. [DOI] [PubMed] [Google Scholar]
- Megaw MW. The innervation of the salivary gland of the tick, Boophilus microplus. Cell and Tissue Research. 1977;184:551–558. doi: 10.1007/BF00220978. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Ejendal KFK, Watts VJ, Hill AC. Molecular and pharmacological characterization of two D1-like dopamine receptors in the Lyme disease vector, Ixodes scapularis. Insect Biochemistry and Molecular Biology. 2011;41:563–571. doi: 10.1016/j.ibmb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Needham GR, Pannabecker TL. Effects of octopamine, chlordimeform, and demethylchlordimeform on amine-controlled tick salivary glands isolated from feeding Amblyomma americanum. Pesticide Biochemistry and Physiology. 1983;19:133–140. [Google Scholar]
- Needham GR, Sauer JR. Involvement of calcium and cyclic AMP in controlling ixodid tick salivary fluid secretion. Journal of Parasitology. 1979;65:531–542. [PubMed] [Google Scholar]
- Needman G, Sauer J. Control of fluid secretion by isolated salivary glands of the Lone Star tick. Journal of Insect Physiology. 1975;21:1893–1898. doi: 10.1016/0022-1910(75)90220-6. [DOI] [PubMed] [Google Scholar]
- Obenchain FD. Neurosecretory system of the American dog tick, Dermacentor variabilis (Acari: Ixodidae) Journal of Morphology. 1974;142:433–446. doi: 10.1002/jmor.1051420406. [DOI] [PubMed] [Google Scholar]
- Obenchain FD, Oliver JH., Jr Neurosecretory system of the American Dog Tick, Dermacentor variabilis (Acari: Ixodidae). II. Distribution of secretory cell types, axonal pathways and putative neurohemal-neuroendocrine associations; comparative histological and anatomical implications. Journal of Morphology. 1975;145:269–293. doi: 10.1002/jmor.1051450303. [DOI] [PubMed] [Google Scholar]
- Obenchain FD, Oliver JH., Jr Peripheral nervous system of the ticks, Amblyomma tuberculatum Marx and Argas radiatus Railliet (Acari: Ixodoidea) Journal of Parasitology. 1976;62:811–817. [PubMed] [Google Scholar]
- Oliveira CJ, Sa-Nunes A, Francischetti IM, Carregaro V, Anatriello E, Silva JS, Santos IK, Ribeiro JM, Ferreira BR. Deconstructing tick saliva: non-protein molecules with potent immunomodulatory properties. Journal of Biological Chemistry. 2011;286:10960–10969. doi: 10.1074/jbc.M110.205047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannabecker T, Needham GR. Effects of octopamine on fluid Secretion by isolated salivary glands of a feeding ixodid tick. Archives of Insect Biochemistry and Physiology. 1982;2:217–226. [Google Scholar]
- Ribeiro JM, Spielman A. Ixodes dammini: salivary anaphylatoxin inactivating activity. Experimental Parasitology. 1986;62:292–297. doi: 10.1016/0014-4894(86)90034-2. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Knulle W. Site and mechanism of water vapour uptake from the atmosphere in ixodid ticks. Nature. 1974;249:84–85. doi: 10.1038/249084a0. [DOI] [PubMed] [Google Scholar]
- Saito Y. The internal anatomy in each stage of Haemaphysalis flava Neuman,1897. Acta Medica et Biologica. 1960;8:189–239. [Google Scholar]
- Sauer JR, Essenberg RC, Bowman AS. Salivary glands in ixodid ticks: control and mechanism of secretion. Journal of Insect Physiology. 2000;46:1069–1078. doi: 10.1016/s0022-1910(99)00210-3. [DOI] [PubMed] [Google Scholar]
- Sauer JR, Hair JA. Water balance in the lone star Tick (Acarina: Ixodidae): the effects of relative humidity and temperature on weight changes and total water content. Journal of Medical Entomology. 1971;8:479–485. doi: 10.1093/jmedent/8.5.479. [DOI] [PubMed] [Google Scholar]
- Sauer JR, McSwain JL, Bowman AS, Essenberg RC. Tick salivary gland physiology. Annual Review of Entomology. 1995;40:245–267. doi: 10.1146/annurev.en.40.010195.001333. [DOI] [PubMed] [Google Scholar]
- Sauer JR, McSwain JL, Essenberg RC. Cell membrane receptors and regulation of cell function in ticks and blood-sucking insects. International Journal for Parasitology. 1994;24:33–52. doi: 10.1016/0020-7519(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Schmidt SP, Essenberg RC, Sauer JR. Evidence for a D1 dopamine receptor in the salivary glands of Amblyomma americanum (L.) Journal of cyclic nucleotide research. 1981;7:375–384. [PubMed] [Google Scholar]
- Šimo L, Koči J, Žitňan D, Park Y. Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS One. 2011;6:e16158. doi: 10.1371/journal.pone.0016158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L, Slovák M, Park Y, Žitňan D. Identification of a complex peptidergic neuroendocrine network in the hard tick, Rhipicephalus appendiculatus. Cell and Tissue Research. 2009a;335:639–655. doi: 10.1007/s00441-008-0731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L, Žitňan D, Park Y. Two Novel Neuropeptides in Innervation of the Salivary Glands of the Black-Legged Tick, Ixodes scapularis: Myoinhibitory Peptide and SIFamide. Journal of Comparative Neurology. 2009b;517:551–563. doi: 10.1002/cne.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatchell RJ. A modified method for obtaining tick oral secretion. Journal of Parasitology. 1967;53:1106–1107. [PubMed] [Google Scholar]