Abstract
The present study describes the generation of a knock-in mouse model to address the role of type II procollagen (Col2a1) alternative splicing in skeletal development and maintenance. Alternative splicing of Col2a1 precursor mRNA is a developmentally-regulated event that only occurs in chondrogenic tissue. Normally, chondroprogenitor cells synthesize predominantly exon 2-containing mRNA isoforms (type IIA and IID) while Col2a1 mRNA devoid of exon 2 (type IIB) is the major isoform produced by differentiated chondrocytes. Another isoform, IIC, has also been identified that contains a truncated exon 2 and is not translated into protein. The biological significance of this IIA/IID to IIB splicing switch is not known. Utilizing a splice site targeting knock-in approach, a 4 nucleotide mutation was created to convert the 5 splice site of Col2a1 exon 2 from a weak, non-consensus sequence to a strong, consensus splice site. This resulted in apparent expression of only the IIA mRNA isoform, as confirmed in vitro by splicing of a type II procollagen mini-gene containing the 5′ splice site mutation. To test the splice site targeting approach in vivo, homozygote mice engineered to retain IIA exon 2 (Col2a1+ex2) were generated. Chondrocytes from hindlimb epiphyseal cartilage of homozygote mice were shown to express only IIA mRNA and protein at all pre- and post-natal developmental stages analyzed (E12.5, E16.5, P0, P3, P7, P14, P28 and P70). As expected, type IIB procollagen was the major isoform produced in wild type cartilage at all post-natal time points. Col2a1+ex2 homozygote mice are viable, appear healthy and display no overt phenotype to date. However, research is currently underway to investigate the biological consequence of persistent expression of the exon 2-encoded conserved cysteine-rich domain in post-natal skeletal tissues.
Keywords: type II procollagen, alternative splicing, precursor (pre-) mRNA, cartilage, chondrocyte, splice site, knock-in mutation, transgenic mouse
1. Introduction
Type II collagen is the major extracellular matrix (ECM) component in cartilage. It is synthesized as a homotrimeric procollagen molecule consisting of a triple helical domain flanked by a 5′ amino propeptide and a 3′ carboxy propeptide; the propeptides are subsequently cleaved resulting in mature triple helical collagen molecules that form fibrils in the ECM (Canty and Kadler, 2005; Eyre, 2002; Eyre et al., 2006; Minns and Steven, 1977; Prockop and Kivirikko, 1995). The type II procollagen gene (Col2a1) is alternatively-spliced by mechanisms that regulate the inclusion, exclusion or truncation of exon 2 (McAlinden et al., 2004; McAlinden et al., 2008). Exon 2 encodes a 69 amino acid cysteine-rich (CR), von Willebrand Factor C-like domain within the amino (NH2) propeptide that is highly conserved between species (Bornstein, 2002). Two isoforms of Col2a1 (type IIA and type IIB) were initially identified by the inclusion (IIA) or exclusion (IIB) of exon 2 (Ryan and Sandell, 1990). Expression of these isoforms is known to occur in a developmentally-regulated manner in chondrogenic tissue whereby chondroprogenitor cells express mainly the IIA isoform while differentiated chondrocytes express predominantly IIB mRNA (Lui et al., 1995; Ng et al., 1993; Oganesian et al., 1997; Sandell et al., 1991; Sandell et al., 1994). Recently, we identified two additional alternatively-spliced isoforms named IIC and IID (McAlinden et al., 2008) (Fig 1A). Type IIC mRNA is formed by the use of an alternative 5′ splice site in exon 2 resulting in a truncated transcript containing premature stop codons. We hypothesize that this splicing event does not result in production of a protein but is important for regulating expression of the other Col2a1 isoforms that are translated to protein (McAlinden et al., 2008). Type IID procollagen differs from the IIA isoform by the presence of an additional amino acid at the 3′ end of the exon 2-encoded CR domain; this extra amino acid (arginine in mouse; tryptophan in human) does not alter the remaining protein coding sequence. Expression patterns of IID mRNA were found to be similar to that of IIA mRNA during chondrogenic differentiation of human mesenchymal stem cells and expression levels of IID mRNA were found to be approximately one third less abundant than IIA mRNA isoforms in this system (McAlinden et al., 2008). In addition, utilization of a TaqMan®-based real time PCR assay to quantify absolute levels of Col2a1 IIA, IIB, IIC and IID mRNA isoforms showed that, in wild type mouse epiphyseal cartilage tissue, the IIA isoform was more abundant than the IID isoform (McAlinden et al., unpublished results). It is not known at this stage whether functional redundancy exists between IIA and IID procollagen proteins.
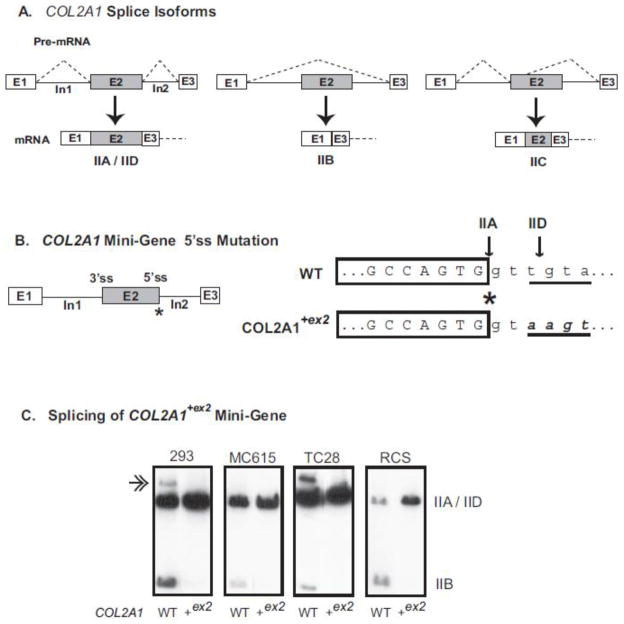
Fig. 1.
COL2A1 spliced isoforms and the effect of exon 2 splice site mutation on pre-mRNA alternative splicing. Panel A shows all potential alternative splicing events involving COL2A1 exon 2 described to date (McAlinden et al., 2008). Panel B shows the composition of the human COL2A1 mini-gene containing full-length exons 1, 2 and 3 and intervening introns 1 and 2. The position of the 3′ splice site (3′ss) and 5′ splice site (5′ss) of exon 2 are indicated. Asterisk denotes the exon-intron junction at the 5′ss where uppercase nucleotides represent exonic sequence and lowercase nucleotides represent intronic sequence with respect to the IIA splicing event. The position of both the IIA and IID splice sites are shown by the arrows. The underlined four nucleotides denote the intronic splice site sequence that was mutated (bold, italic) to create the consensus 5′ss sequence present in the COL2A1+ex2 mini-gene. Panel C shows autoradiograph images of cDNA products derived from splicing of wild type or mutant mini-genes in cell lines HEK-293 (293), MC615, TC28 and RCS. The position of IIA/IID and IIB products are indicated. Double arrow represents a slower-migrating band formed by heteroduplex formation of mismatched IIA and IID cDNA strands (Claus et al., 2010; McAlinden et al., 2008). Note disappearance of this band as a result of the IIA splice site mutation which disrupts the IID exon-intron splice site.
Previous studies from our laboratory have shown that exon 2 is alternatively spliced due to the presence of a weak, non-consensus 5′ splice site sequence and an adjacent stem loop cis element (McAlinden et al., 2005). With this knowledge, we devised a knock-in strategy to alter the 5′ splice site sequence of Col2a1 exon 2 such that it would conform to a strong consensus sequence and, in theory, would be more efficiently recognized by the splicing machinery. Subsequently, type IIA procollagen would be the only isoform synthesized regardless of the status of chondrocyte differentiation, while type IIB procollagen synthesis would be inhibited. The present study describes this splice site knock-in strategy in detail and that it was successful in vitro to specifically favor production of the exon 2-containing IIA procollagen isoform. We also describe generation of viable homozygote knock-in mice (Col2a1+ex2) and show that differentiated chondrocytes from these mice express only type IIA procollagen mRNA and protein instead of the IIB procollagen isoform that is normally expressed in post-natal cartilage. Interestingly, expression of IIA procollagen was still detectable in articular cartilage, growth plate cartilage and in the trabecular bone of hindlimbs from 10 week old homozygote mice. It should be noted that the α1(II) chain of type II procollagen and the α3 (XI) chain of type XI procollagen are encoded by the same gene (Eyre, 1987; Furuto and Miller, 1983). Therefore, the “IIA” exon 2-containing procollagen α3 chain, rather than the IIB form devoid of exon 2, will also be present within α1α2α3 type XI procollagen heterotrimers. This mouse model will provide insights into the importance of the developmentally-regulated alternative splicing switch of Col2a1 exon 2 in skeletal development and maintenance. In addition, the strategy described in the present study to generate knock-in transgenic mice can be applied to any alternatively-spliced gene to investigate biological function of specific protein isoforms in vivo.
2. Results
2.1. Conversion of the weak 5′splice site sequence of COL2A1 exon 2 to a consensus splice site sequence results in production of only IIA mRNA in vitro
Fig. 1B shows the four nucleotide mutation at the 5′ splice site of exon 2 that was created in the human COL2A1 mini-gene construct to generate a strong consensus splice site sequence. In theory, this mutation should alter the splicing mechanism such that exon 2 will always be included in the mature mRNA transcript. To test this, a human type II procollagen mini-gene (previously shown to be a reliable model system to study COL2A1 alternative splicing (McAlinden et al., 2005)) was utilized. Fig. 1C shows that splicing of the mutant mini-gene containing the four nucleotide 5′ splice site mutation in intron 2 (COL2A1+ex2) resulted in the production of type IIA mRNA regardless of cell type. This was confirmed by DNA sequencing (not shown) as well as visualization of cDNA band size (Fig. 1C). This splice site mutation also inhibits production of the type IID mRNA isoform, as shown by the disappearance of the slower-migrating cDNA band seen most clearly in HEK-293 and TC28 cells following splicing of the wild type mini-gene (Fig. 1C). The presence of this additional band is due to heteroduplex formation containing mismatched IIA and IID cDNA strands resulting in single-strand extensions, a hairpin fold or formation of another secondary structure (Claus et al., 2010; McAlinden et al., 2008). In addition, mutant COL2A1 mini-genes were generated containing both the 5′ splice site mutation and an upstream one base pair mutation to create an HaeIII restriction site, the location of which is shown in the targeting vector in Fig. 2A. The HaeIII site was created for genotyping purposes and was found to not alter splicing of the COL2A1 mini-gene in vitro (not shown).
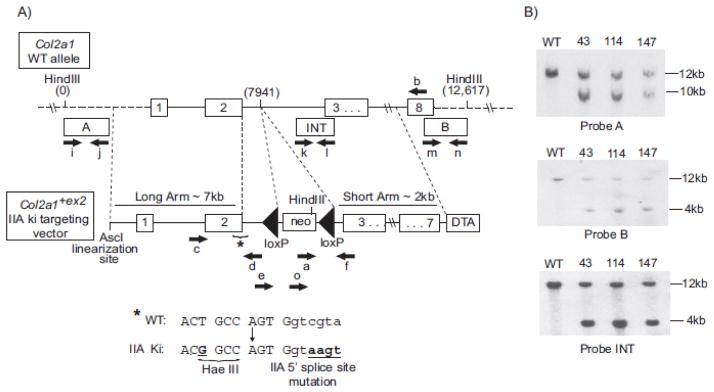
Fig. 2.
Knock-in targeting strategy and confirmation of homologous recombination in ES cells. Panel (A) shows a region of the wild type (WT) Col2a1 allele and the targeting vector to generate the Col2a1+ex2 knock-in (ki) allele. Location of all primers used to detect homologous recombination and presence of specific point mutations are shown by black arrows (primers a, b, c, d, e, f, and o). Primers i+j, k+l and m+n were used to amplify probes A, INT or B, respectively for use in Southern blotting. Sequences of all primers are shown in Table S1 (including primers g and h which were used to test for the presence of Cre). Asterisk (*) shows the region and the sequence of the Col2a1 allele containing the point mutation to create a HaeIII restriction site and the point mutations to create the consensus 5′ splice site sequence in intron 2. Bold, underlined nucleotides denote these point mutations. Uppercase letters represent exon sequence and lowercase letters represents intron sequence. Panel (B) shows radiolabeled Southern blots of HindIII digested genomic DNA from homologous recombinant ES clones 43, 114, and 147 and wild-type (WT) control. Southern blot probes A and B confirm homologous recombination within the long and short arms, respectively and probe INT confirms single integration. Expected DNA band sizes are: WT = 12.6 kb with probes A, B, and INT; IIA knock-in mutant = 9.8 kb with probe A and 4.2 kb with probes B and INT.
2.2. Generation of viable homozygote Col2a1+ex2 knock-in mice
To test the 5′ splice site targeting approach in vivo, we generated a Col2a1+ex2 targeting vector (Fig. 2) containing the same 4 nucleotide point mutations that were introduced into the COL2A1 mini-gene (Fig. 1B). The presence of these point mutations in addition to an upstream mutation to create a HaeIII restriction for genotyping purposes was confirmed in eight ES clones by PCR with primers Col2a1-F7 and Col2a1-R7 (Primers c + d, Fig. 2; Table S1), followed by sequencing and HaeIII restriction digest of the PCR products (not shown). Homologous recombination in three of these ES clones (numbers 43, 114, and 147) was then investigated by Southern blot analysis using probes A, B and internal (INT) (Fig. 2A). Fig. 2B confirms integration of the mutant Col2a1+ex2 targeting vector into the correct location within the murine Col2a1 gene by the presence of expected fragment sizes following HindIII digestion of genomic DNA (IIA knock-in mutant DNA fragment size = 9.8kb as detected with Probe A or 4.2 kb as detected with Probes B or INT). Probe INT also confirmed single integration of the knock-in vector. Each of the three positive ES cell clones displayed a normal karyotype (not shown). Injection of two independent positive ES clones (#43 and #147) into C57BL/6J blastocysts followed by implantation into pseudopregnant females resulted in chimeric pups. F1 offspring were generated by crossing male chimeric mice (>95% chimerism) with C57BL/6J female mice (Jackson Laboratory). Germline transmission of the mutant allele was confirmed by PCR analysis with primers Col2a1-loxP-Rev and Z1021 (primers f and o, Fig. 2; Table S1). Heterozygote mice were bred to EIIa–Cre mice to delete the loxP flanked neomycin selection cassette (Jackson Laboratory Strain Name: B6.FVB-Tg(EIIa-cre)C5379Lmgd/J; Stock Number: 003724). Removal of the neomycin cassette was confirmed by PCR with primers Col2a1-loxP-For and Col2a1-loxP-Rev (Primers e and f, Fig 2; Table S1) and the genotype of mice from each litter was always confirmed by PCR with primers Col2a1-loxP-For, Col2a1-loxP-Rev and Z1021 (Primers e, f and o; Fig 2, Table S1). Resulting heterozygous and homozygous mutant mice were viable, obtained with predicted Mendelian frequency and displayed no overt phenotype with respect to overall size and preliminary analysis of cartilage development from E16.5-P70.
2.3. Col2a1+ex2 homozygote knock-in mice express only the IIA Col2a1 mRNA isoform
A semi-quantitative PCR method was first carried out to analyze if the 5′ splice site mutation was functional in vivo. It should be noted that this method cannot distinguish between IIA and IID mRNA isoforms that are both likely to be expressed in chondrocytes from WT and heterozygous cartilage. Following extraction and reverse transcription of RNA from either whole hindlimbs (E12.5, E16.5) or from pooled hindlimb-derived epiphyseal and femoral head cartilage (P0-P70), PCR was carried out utilizing a previously published murine primer pair spanning alternative exon 2 (Hatano et al., 2002).
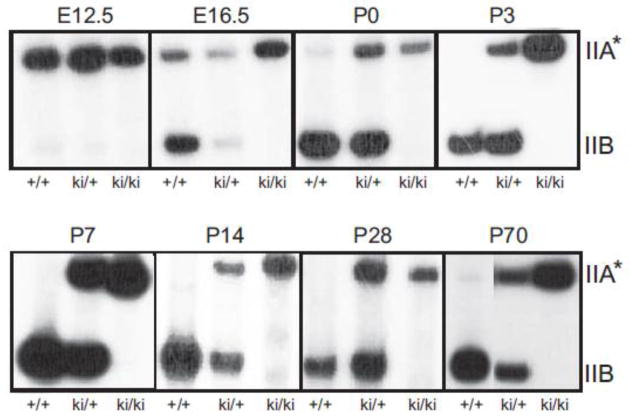
Fig. 3 shows [α-32P]dCTP-radiolabeled PCR products amplified from reverse transcribed RNA extracted from wild type (WT; +/+), heterozygous knock-in (ki/+) and homozygous knock-in (ki/ki) cartilage. In WT cartilage, E12.5 is the only time point showing predominant expression of the exon 2-containing isoforms of Col2a1. In WT tissue from E16.5 onwards, the developmentally-regulated splicing switch from IIA/IID to IIB mRNA is apparent and IIB mRNA appears to be the major mRNA product produced at all post-natal time points analyzed. Sequencing of the cDNA band from P7 WT cartilage confirmed the presence of the IIB isoform (not shown). A minor band representing exon 2-containing Col2a1 mRNA was detected at post-natal time points P0 and P70 in WT cartilage, but not at the other post-natal time points analyzed.
Fig. 3.
Expression of Col2a1 mRNA alternatively-spliced isoforms in limb cartilage from knock-in mouse litters at different developmental time points. The autoradiograph shows [α-32P]dCTP- labeled cDNA bands representing endogenous Col2a1 isoforms in wild type (+/+), heterozygote (ki/+) and homozygote knock-in (ki/ki) hindlimb cartilage at different embryonic (E) and post-natal (P) time points of development (E12.5-P70). IIA and IIB cDNA band sizes are ~ 485bp and ~277bp, respectively. *Note that in WT and ki/+ cells, in addition to exon 2-containing IIA mRNA isoforms, levels of IID mRNA isoforms may also be present. Only IIA mRNA isoforms will be present in homozygous tissue.
In E12.5 heterozygous tissue, exon 2-containing transcripts were predominant while IIB transcripts were barely detectable, similar to that found in WT E12.5 tissue. In heterozygous tissue from all other time points, two clear bands corresponding to Col2a1 cDNA containing or devoid of exon 2 were found at each time point, as expected (Fig. 3). It should be noted that this semi-quantitative PCR method over-estimates the ratio of IIB:IIA mRNA expressed in E16.5, P0 and P70 WT cartilage and in E16.5-P70 heterozygous cartilage due to more efficient amplification of the shorter IIB transcript during PCR (McAlinden et al., unpublished results). Future studies will involve in-depth analyses utilizing a new TaqMan®-based alternative transcript real time PCR assay, specifically designed to quantify absolute levels of all four Col2a1 isoforms identified to date (IIA, IIB, IIC, IID) (McAlinden et al., unpublished results). However, for the purpose of this present study, confirming the presence/absence of the IIA mRNA isoform in homozygous cartilage tissue at various time points of development was sufficient to address the success of the 5′ splice site targeting approach in vivo. Indeed, Fig. 3 shows that IIA mRNA is the only Col2a1 transcript synthesized in homozygous knock-in cartilage cells at all pre- and post-natal time points analyzed. Sequencing of the exon 2-containing cDNA band from P7 homozygous cartilage confirmed that it was the IIA isoform (not shown). Taken together, the data confirms that the splice site knock-in mutation was functional in vivo to specifically select for the type IIA mRNA procollagen isoform in homozygote knock-in mice.
2.4. Persistent expression of type IIA procollagen does not affect chondrocyte differentiation or endochondral ossification
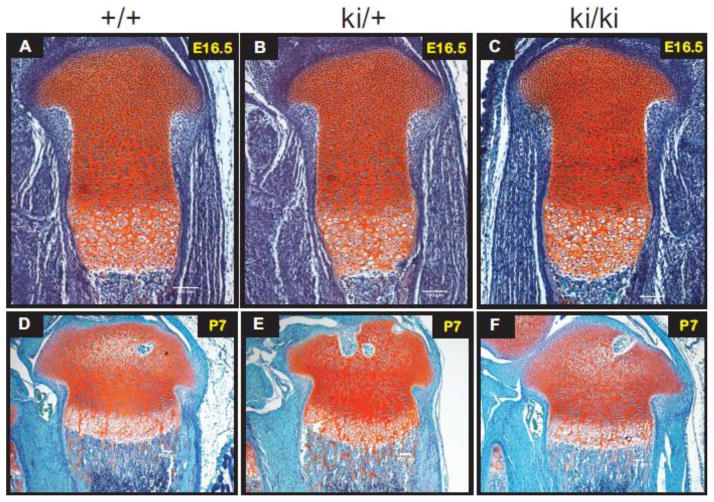
To investigate the general morphology of developing hindlimbs, Safranin O staining of paraffin-embedded tissue sections was carried out. Fig. 4 shows that, at E16.5 and P7, no apparent differences were found between WT, heterozygous and homozygous tissue with respect to the formation of columnar proliferating chondrocytes and hypertrophic chondrocytes of developing growth plate cartilage. In addition, no obvious differences were found when comparing the size of the proliferative and hypertrophic zones between all three genotypes. Endochondral ossification and secondary ossification center (SOC) formation also appeared normal between all three genotypes as shown by the presence of metaphyseal trabecular bone and epiphyseal vascular invasions, respectively (Fig 4D–F). No apparent morphological differences were found between all three genotypes when comparing Safranin-O sections from other post-natal time points (P14, P28, P70) (not shown).
Fig. 4.
Safranin-O staining of wild type (+/+), heterozygous (ki/+) and homozygous knock-in (ki/ki) hindlimb tissue sections. Positive Safranin-O staining (red) is shown throughout the cartilaginous region of the developing growth plate and trabecular bone in E16.5 developing tibiae (Panels A–C) and in P7 developing proximal tibiae (D–F). Scale bars = 100 μm.
2.5. Expression of only IIA procollagen protein persists up to P70 in Col2a1+ex2 homozygote hindlimbs
To investigate the effect of the 5′splice site mutation on synthesis and deposition of type IIA procollagen protein into the extracellular matrix (ECM) of homozygous cartilage tissue, dual fluorescence immunohistochemistry (IHC) was carried out on paraffin sections of mouse hindlimbs using a specific “anti-IIA” polyclonal antibody that recognizes the exon 2-encoded cysteine-rich (CR) domain within the amino (NH2) propeptide of type II procollagen (Oganesian et al., 1997) (shown in green), and an antibody that recognizes the triple helical domain of type II procollagen (Cremer and Kang, 1988) (shown in red) (Figs. 5–7). There is no antibody available that specifically recognizes the IIB isoform of type II procollagen. It should be noted that the “anti-IIA” antibody likely detects IIA procollagen as well as the IID procollagen isoform since they both differ by only one amino acid (McAlinden et al., 2008). Therefore, in WT and heterozygous cartilage tissue, green fluorescent staining will represent localization of IIA and IID procollagens in the ECM, but only IIA procollagen in homozygous tissue. In addition, although type XI procollagen is a minor collagen component of articular cartilage compared to type II procollagen (Eyre, 2002), the “anti-IIA” antibody will also detect the exon 2-encoded CR domain within the α3 chain of type XI procollagen since it is derived from the same gene that encodes the α1(II) chain of type II procollagen (Furuto and Miller, 1983). It should also be noted that this IHC data does not distinguish between cleaved IIA NH2-propeptide and unprocessed IIA procollagen proteins in the ECM. Therefore, given these facts and for the sake of simplicity, we will refer to “IIA procollagen” or IIA-containing protein” when describing the presence of green fluorescent staining patterns obtained with the anti-IIA antibody.
Fig. 5.
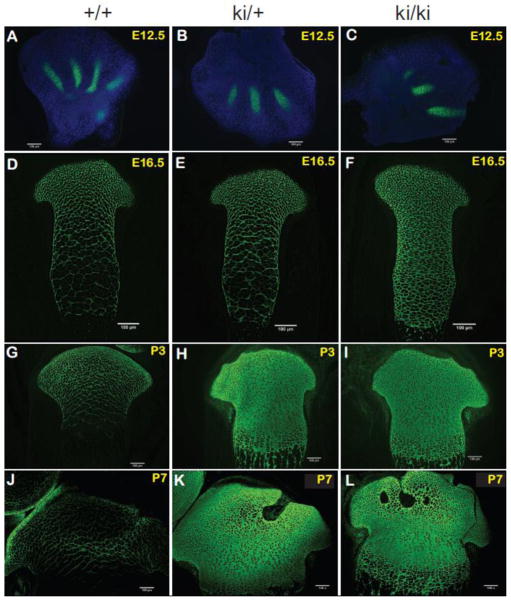
Localization of type IIA procollagen proteins by fluorescence immunohistochemistry. Dual immunohistochemistry was carried out using a polyclonal antibody that specifically recognizes the exon 2-encoded cysteine-rich (CR) domain of type II procollagen or the α3 chain of type XI procollagen (anti-IIA antibody) in combination with a polyclonal antibody against the type II collagen triple helical domain. However, each panel shows only anti-IIA antibody staining patterns (green) to better emphasize differences in localization patterns between WT, heterozygous (ki/+) and homozygous knock-in (ki/ki) tissue. Positive staining was found in the developing digits of E12.5 hindlimbs (Panels A–C), E16.5 developing tibiae (Panels D–F), P3 proximal tibiae (Panels G–I) and P7 proximal tibiae (Panels J–L). Scale bars = 100 μm.
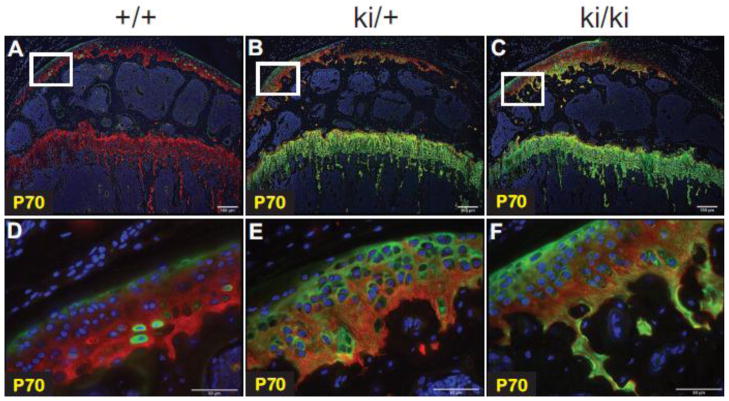
Fig. 7.
Type II procollagen protein expression in P70 proximal tibiae. Dual immunohistochemistry was done to detect type II procollagen proteins in wild type (+/+), heterozygous (ki/+) and homozygous (ki/ki) mice. Green staining represents anti-IIA positive proteins (i.e. the exon 2-encoded CR domain within the NH2-propeptide of type II procollagen or the α3 chain of type XI procollagen). Red fluorescent staining denotes the type II collagen triple helical domain. Areas of yellow fluorescence represent co-localization. Panels D, E and F are higher magnification images of the white boxed regions shown in Panels A, B and C, respectively. Scale bars = 100 μm (Panels A, B, C) or 50 μm (Panels D, E, F).
Figs. 5A–C show that only IIA procollagen was detectable in WT, heterozygous and homozygous cartilaginous regions of E12.5 developing digits. This is in agreement with the mRNA expression patterns shown in Fig. 3. No red signal, indicating the type II collagen triple-helical domain, was detected due to the fact that very little ECM has been laid down at this early stage, including a mature type II collagen fibrillar network.
In Figs. 5D–L, although dual IHC was carried out, data shows expression patterns obtained from the anti-IIA antibody only, to better emphasize differences in localization patterns of the IIA-containing proteins between WT, heterozygous and homozygous tissue. At E16.5, abundant IIA procollagen staining was seen in the most proximal region of developing WT and heterozygous tibiae since this region contained more chondroprogenitor cells synthesizing exon 2-containing Col2a1 mRNA (Fig. 5D–E). However, a “lacy” localization pattern was observed in the proliferative and hypertrophic zones since IIB mRNA was the major isoform generated in these differentiated chondrocytes, not IIA. This lacy distribution pattern is believed to indicate interterritorial matrix localization of remnant protein that was synthesized at earlier time points of development when chondrocytes were in a less differentiated state. However, in the E16.5 homozygous developing tibia, no “lacy” interterritorial localization patterns were observed since IIA procollagen was continually being synthesized and secreted into the ECM zones occupied by differentiated proliferating chondrocytes and hypertrophic chondrocytes (Fig. 5F). Similar differences in the localization patterns of IIA procollagen were found between WT and homozygous proximal tibiae at P3 (Fig. 5G–I) and P7 (Fig. 5J–L). In heterozygous tissue at P3 and P7, localization patterns of IIA procollagen resembled that found in homozygous tissue (Figs. 5H, I, K, L) unlike at E16.5 where expression patterns were more similar to WT (Figs. 5D, E). It should be noted that the P7 WT immunofluorescence signals (Fig. 5J) were over-exposed for the sole purpose of identifying the expression patterns of IIA procollagen; when analyzed at the same exposure as used for P7 heterozygous and homozygous tissue, minimal antibody staining was found. Interestingly, the most abundant staining in P7 WT tissue was identified in the peripheral regions of developing articular cartilage (Fig. 5J). Taken together, data presented in Fig. 5 confirms that the 5′splice site mutation results in continued synthesis and deposition of IIA procollagen protein into the ECM of Col2a1+ex2 homozygous knock-in cartilage protein up to post-natal day 7.
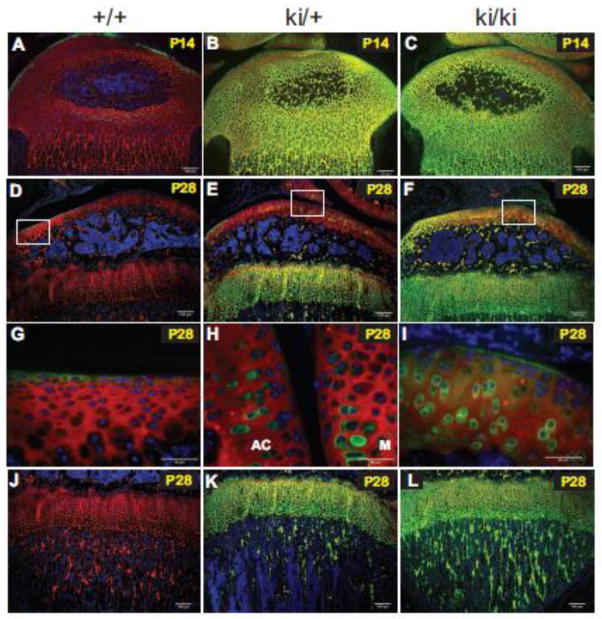
For P14 and P28 hindlimb tissue, it was necessary to show dual immunostaining data since WT epiphyseal cartilage at these post-natal time points showed almost no green staining with the anti-IIA antibody, except for the outermost region of the developing articular cartilage (Fig. 6A, D, G). Visualization of WT tissue sections was achieved by observation abundant expression of the type II procollagen triple helical domain (red staining) throughout the WT proximal tibia (Fig. 6A, D). Interestingly, there was no indication of co-localization (yellow fluorescence) of the IIA-containing protein and type II procollagen triple helical domain in the outermost region of developing articular cartilage in WT tissue at P7 (results not shown), P14 and P28 (Fig. 6A, D, G).
Fig. 6.
Type II procollagen protein expression patterns in P14 and P28 hindlimbs. Dual fluorescence immunohistochemistry was carried out to detect type II procollagen proteins in wild type (+/+), heterozygous (ki/+) and homozygous (ki/ki) proximal tibiae. Green staining represents anti-IIA positive proteins (i.e. the exon 2-encoded CR domain within the NH2-propeptide of type II procollagen or the α3 chain of type XI procollagen). Red fluorescent staining denotes the type II collagen triple helical domain. Areas of yellow fluorescence represent co-localization. Panels A-C, P14 tissue; Panels D-L, P28 tissue. Panels G, H and I are higher magnification images of the white boxed regions shown in Panels D, E and F, respectively. Note that the magnified image in Panel H has been rotated 90 degrees clockwise. AC=articular cartilage; M=meniscus (Panel H). Panels J–L show the metaphyseal trabecular bone region of developing tibiae. Scale bars = 100 μm (Panels A–F; J–L) or 50 μm (Panels G–I).
At P14, heterozygous and homozygous cartilage of the developing proximal tibia showed abundant expression of IIA-containing proteins and areas of yellow fluorescent staining represents co-localization of the IIA NH2-propeptide with the type II procollagen triple helical domain (Fig. 6B, C). In P28 homozygous and heterozygous knock-in cartilage tissue, persistent expression of IIA procollagen proteins were found in the growth plate cartilage ECM below the subchondral bone (Fig. 6E, F). No anti-IIA staining was found in WT growth plate cartilage tissue since IIB was the dominant isoform present (Fig. 6D). In P28 homozygous articular cartilage, a predominance of red staining in specific regions of the tissue (indicative of type II collagen triple helical domain) was found suggesting that the IIA NH2-propeptide had been cleaved from the procollagen molecule and removed from the ECM (Fig. 6F). This observation was even more apparent in articular cartilage from heterozygous tissue (Fig. 6E). Figs. 6G–I are higher magnification images of the white-boxed areas shown in Figs. 6D–F, respectively and highlights the presence of intracellular and pericellular staining of IIA-containing protein in and around articular chondrocytes. However, in WT tissue, any anti-IIA-reactive protein products were only found lining the outermost surface of articular cartilage (Fig. 6G) as also found in P14 WT cartilage (Fig. 6A).
The type II procollagen triple helical protein was also present in trabecular bone within the secondary center of ossification and within the metaphysis beneath the growth plate in WT, heterozygous and homozygous tissue (Figs 6A–C; D–F; J–L). However, only heterozygous and homozygous knock-in tissue showed persistent expression of IIA-containing proteins in the trabeculae of the epiphyseal subchondral bone (Figs. 6B,C, E, F) and of the metaphyseal bone (Fig. 6K, L).
Finally, at P70, expression of IIA-containing proteins was still visible in growth plate cartilage and in the trabeculae of the subchondral bone and metaphyseal bone of homozygous knock-in hindlimbs (Fig. 7C). Similar localization patterns were detected in heterozygous hindlimbs (Fig. 7B) whereas no IIA signal was detected in WT growth plate cartilage or trabecular bone (Fig. 7A). However, IIA-containing proteins were present in the outermost region of P70 WT articular cartilage and, interestingly, intracellular staining within chondrocytes of the deeper zones of articular cartilage was also detected (Fig. 7A) and shown more clearly in the magnified image (Fig. 7D). This finding explains why a minor band representing exon 2-containing mRNA was detected in chondrocytes from WT epiphyseal cartilage cells at P70 (Fig. 3). In heterozygous and homozygous aricular cartilage, IIA-containing protein staining was detected along the periphery of the tissue in addition to pericellular localization within the deeper zones (Figs. 7B, C, E, F). Very few areas containing intracellular staining were found, unlike in WT tissue (Figs. 7D, E, F).
2.6. Type IIA procollagen from P7 epiphyseal cartilage tissue is processed
To investigate whether type IIA procollagen synthesized in post-natal cartilage is processed similar to type IIB procollagen, P7 epiphyseal cartilage tissue was subjected to 4M guanidinium-HCl (Gn-HCl) extraction and resulting protein lysates were analyzed by gel electrophoresis and Western blotting. Utilizing the same anti-IIA polyclonal antisera that was used for immunohistochemistry in this study, Fig. 8A shows that IIA procollagen forms are present in the matrix of homozygous knock-in (ki/ki) cartilage, with lower amounts in heterozygous (ki/+) tissue and very low levels remaining in WT (+/+) tissue, as expected. This observation compares well with the immunohistochemistry results of P7 tissue sections (Figs. 5J–L). Since the anti-IIA antibody is unable to detect the processed IIA NH2-propeptide in these Western blots, the monoclonal antibody 1C10 was used to specifically detect native α1(II) collagen chains. Fig. 8B firmly concludes that the majority of IIA procollagen in ki/+ and ki/ki cartilage is processed whereby the NH2- and COOH- propeptides are removed to form native type II collagen. In addition, minor bands representing pN-IIA procollagen (i.e. procollagen containing the IIA NH2-propeptide but devoid of the COOH propeptide) were also detectable in ki/+ and ki/ki cartilage, but not in +/+ tissue (Fig. 8B). These pro-forms likely represent newly synthesized IIA procollagen chains.
Fig. 8.
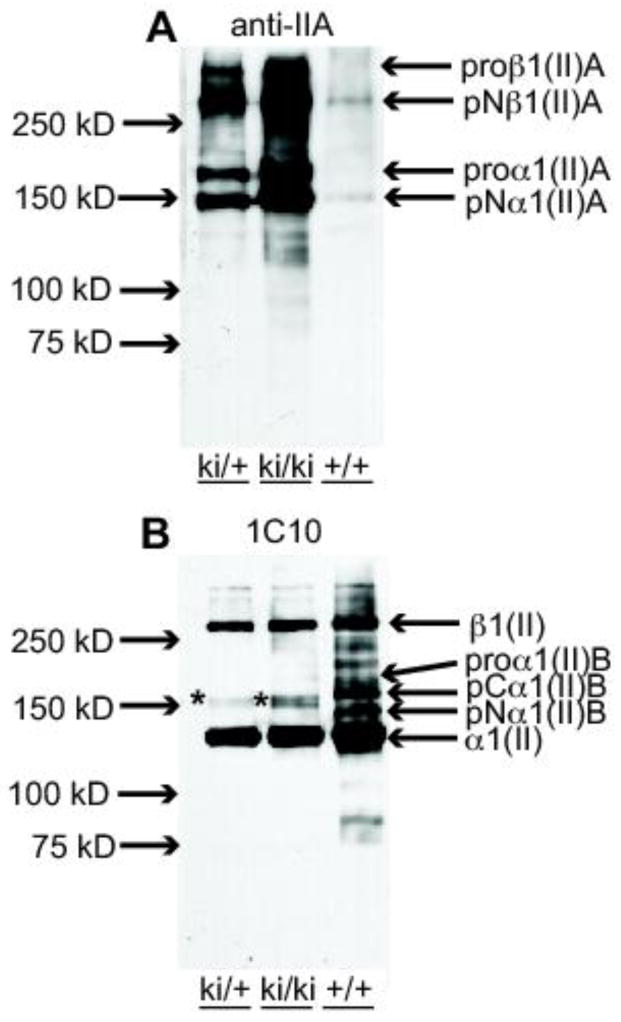
Analysis of type II procollagen processing in P7 epiphyseal cartilage. A. Western blot of 4M Gn-HCl extracts of epiphyseal cartilage from hindlimbs of P7 wild type (+/+), heterozygous (ki/+) and homozygous (ki/ki) mice probed with the anti-IIA polyclonal Ab (Oganesian et al., 1997). Weakly immunoreactive IIA-positive collagen bands in the 150 kDa to 250 kDa range are found in the +/+ lane; increasing intensities of these bands are found in the ki/+ and ki/ki bands, as expected. pNα1(II)A at 150 kDa represents procollagen molecules containing only the NH2-propeptide. The slower migrating band, proα1(II)A, represents procollagen chains containing both NH2- and COOH-propeptides. The two high molecular weight bands are dimers of the α1(II)A chains and indicated as proβ1(II)A and pNβ1(II)A as per nomenclature for collagen chains. B. Western blot of the same protein extracts described in A, but probed with monoclonal antibody 1C10 that recognizes native type II collagen (Fernandes et al., 2003). The major immunoreactive band in all three lanes is the fully processed α1(II) chain (devoid of NH2- and COOH-propeptides). The fully processed collagen chains in the ki/ki lane are derived only from type IIA procollagen. This antibody also reacts weakly with the 150 kDa band corresponding to pNα1(II)A (*). In the +/+ lane, type IIB procollagen processing intermediates are also detected, pCα1(II)B and pNα1(II)B. The highest molecular weight band, β1(II) represent dimers of the α1(II) chains. In ki/ki tissue, these dimers can only be derived from proα1(II)A collagen chains.
3. Discussion
Various approaches can be taken to analyze the impact of alternative splicing in mice. These include targeted deletion of the alternative exon of interest, creation of specific mutations to strengthen or obliterate a splice site, administration of oligonucleotides to redirect the splicing process or alteration of a particular splicing factor protein by its deletion, over-expression or expression of a dominant-negative mutant (Moroy and Heyd, 2007). To address the biological significance of Col2a1 alternative splicing during skeletal development in vivo, we chose a splice site targeting strategy to convert the weak 5′ splice site of alternative exon 2 to a strong, consensus splice site. Although previous studies in our laboratory have shown splicing factor TIA-1 to be involved in regulating Col2a1 exon 2 alternative splicing (McAlinden et al., 2007) there are likely to be many other factors involved. Therefore, a splicing factor-directed strategy would not be a reliable approach to specifically alter Col2a1 alternative splicing. For the splice site targeting strategy, our hypothesis was that optimization of the 5′ splice site would lead to constitutive inclusion of Col2a1 exon 2 in the final mature mRNA, similar to what was achieved for the EDA exon of fibronectin mRNA (Muro et al., 2003). Our approach was validated in vitro using a COL2A1 mini-gene system and in vivo by generation and analysis of homozygous Col2a1+ex2 mice.
We chose to generate a mouse model expressing only the IIA procollagen isoform rather than the IID isoform or the IIB isoform for a number of reasons. Firstly, the IID isoform had not yet been identified at the time when we had designed the splice site targeting strategy and had begun generating the knock-in mice. However, given the fact that the IIA isoform has been more commonly reported in the literature and the observations that the IIA isoform is more abundant than the IID isoform in WT mouse cartilage tissue (McAlinden et al., unpublished results) and in human mesenchymal stem cells induced to undergo chondrocyte differentiation (McAlinden et al., 2008), we still would have chosen to over-express the IIA isoform given the choice. Future studies will be targeted toward generating antibodies that will distinguish between the IIA and IID protein isoforms and address whether they have redundant or distinct biological functions.
A major reason against choosing to express only the IIB procollagen isoform stemmed from the fact that exon 2-containing Col2a1 mRNA isoforms are known to be expressed during early development of other non-chondrogenic tissues such as heart, brain and kidney (Bishop et al., 1994; Lui et al., 1995; Ng et al., 1993; Sandell et al., 1994). Thus, the potential of embryonic death or death shortly after birth by eliminating exon 2, was a concern. It is also possible that a proportion of these exon 2-containing mRNAs encode the α3 chain of type XI procollagen given the fact that the α1(II) chain and α3(XI) chain are encoded by the same gene (Furuto and Miller, 1983). In any case, expression of the exon 2-containing mRNA does suggest a role for the exon 2-encoded conserved cysteine-rich (CR) domain during early development. In fact, a recent study has shown that mouse embryos lacking Col2a1 exon 2 displayed a partially penetrant phenotype whereby forebrain development was disrupted (Leung et al., 2010). In addition, a decreased survival rate of mice lacking the homologous exon 2-encoded CR domain of the type I procollagen α1(I) chain has been reported (Bornstein et al., 2002).
Other rationales for generating a IIA procollagen knock-in mouse instead of a IIB-expressing mouse came from previous studies in our laboratory where we reported synthesis of the human trimeric IIA procollagen NH2-propeptide in vitro following transfection of mammalian CHO cells with an expression construct containing cDNA encoding exons 1–8 of the NH2-propeptide (McAlinden et al., 2002). However, when we attempted to generate a IIB NH2-propeptide using the same cDNA expression construct devoid of exon 2, no protein was ever detected in the conditioned medium (unpublished observations). In addition, mutations to obliterate the 5′ splice site of exon 2 in a COL2A1 mini-gene resulted in inhibition of the IIA isoform as expected but, surprisingly, no IIB mRNA was detected either (unpublished observations). These findings suggest that the ability to splice exon 2 and/or form exon 2-containing mRNA isoforms is important for the cell and that potentially undesirable effects may result by eliminating this conserved domain.
Unlike type II procollagen knock-out mice that were shown to die before or shortly after birth (Li et al., 1995), homozygote mice engineered to retain Col2a1 exon 2 (Col2a1+ex2) are viable, appear healthy and do not display any obvious skeletal phenotype. Analysis of a range of different developmental time points confirmed expression of IIA procollagen (but not the IIB procollagen) up to 10 weeks of age in homozygous hindlimb cartilage at the mRNA (Fig. 3) and protein level (Figs. 5–7). Since exon 2-containing Col2a1 isoforms are normally expressed during embryonic development, it was perhaps not surprising to find that cartilage development and chondrocyte differentiation appeared normal in homozygous mice at E16.5 (Fig. 4A–C). In addition, endochondral ossification and secondary ossification formation were also apparently normal (Fig. 4D–F), unlike in collagen II knock-out mice where an epiphyseal growth plate was not formed (Li et al., 1995). Taken together, it is apparent that although a type II collagen matrix is required for proper long bone development, the role of the Col2a1 alternative splicing switch does not involve regulation of chondrocyte differentiation. Therefore, mature proliferating chondrocytes and hypertrophic chondrocytes (that normally synthesize type IIB procollagen), can develop normally even though they are generating only type IIA procollagen (Figs. 5D–L).
However, alterations in cell signaling and/or ECM composition in homozygous cartilage are likely given the fact that the normal Col2a1 IIA:IIB ratios have changed. Previous reports point to the possibility that the IIA NH2-propeptide may function as a regulator of growth factor activity. One study showed that recombinant IIA propeptides could bind to BMP-2 and TGF-β in vitro (Zhu et al., 1999). Another study reported dorsalization of Xenopus embryos by over-expression of IIA procollagen, but not IIB procollagen; it was proposed that this phenotype was due to binding to BMP thereby inactivating its activity in the ECM, similar to the effects of chordin which also contains CR protein domains similar to that encoded by Col2a1 exon 2 (Larrain et al., 2000; O’Leary et al., 2004). In addition, structural analysis of the Col2a1 exon 2-encoded CR repeat domain has shown similarities to the fibronectin type I repeat domain, further suggesting the potential of ligand binding function (O’Leary et al., 2004). Therefore, it is possible that persistent expression of IIA procollagen in post-natal homozygous and heterozygous growth plate cartilage and trabecular bone extracellular matrix results in altered growth factor signaling mechanisms. In fact, preliminary micro-CT analyses has revealed lower metaphyseal trabecular bone mass in post-natal homozygote hindlimbs compared to age-matched WT control mice (unpublished observations). We are currently pursuing these findings further and will address whether this effect could be due to potential changes in BMP activity.
There is no conclusive evidence from previous studies reporting potential growth factor-regulating function (Larrain et al., 2000; Zhu et al., 1999) as to whether the IIA NH2- propeptide must be attached to the major collagen triple helical domain (i.e. trimeric pN-procollagen) or whether it can exert its function as a “free” NH2-propeptide following N-proteinase cleavage. From the dual immunohistochemistry data presented in this study, yellow fluorescent staining patterns were detected, suggesting co-localization of the IIA-NH2 propeptide with the major triple helical domain of type II collagen (Figs. 6 and 7). This data does not confirm what proportion of IIA NH2-propeptides are bound to the type II collagen triple helix as pN-procollagen and what proportion exist independently as free propeptide in the matrix. However, we have confirmed, at least in P7 epiphyseal cartilage tissue, that the majority of IIA procollagen in homozygous Col2a1+ex2 tissue is indeed processed whereby the NH2- and COOH- propeptides are removed (Fig. 8B). This finding is an important observation in collagen biochemistry. Minor bands representing the pro-forms of IIA procollagen were also detected and these likely represent the newly synthesized IIA procollagen pool prior to processing. ADAMTS-2 and ADAMTS-3 have been identified as the major N-proteinases that cleave the NH2-propeptide of type IIB procollagen (Colige et al., 2005; Fernandes et al., 2001; Le Goff et al., 2006), but whether these enzymes are also responsible for cleaving the IIA procollagen NH2-propeptide remains to be validated. Previous studies in our laboratory have shown that certain matrix metalloproteinase (MMP) enzymes are capable of cleaving recombinant trimeric IIA propeptide in vitro (Fukui et al., 2002) and it will be interesting to investigate whether MMPs contribute toward IIA procollagen processing in vivo.
Closer examination of type II procollagen immunolocalization patterns in P28 and P70 hind limbs apparently indicates removal of the IIA NH2-propeptide from the matrix of articular cartilage (Figs. 6, 7). Although it is known that the NH2-propeptide of type IIB procollagen is eventually removed from the ECM of articular cartilage following N-proteinase cleavage (Rebuck et al., 1999), the process by which this clearance occurs is not fully understood. In this study, intracellular staining of IIA-containing proteins was noted in chondrocytes from homozygous articular cartilage (Fig. 6I). It may be that re-uptake of the cleaved IIA NH2-propeptide is a potential mechanism to achieve propeptide clearance but further studies are needed to prove this.
While a decrease in IIA-containing protein expression was found in the ECM of articular cartilage of homozygous and heterozygous tissue from P14-P70, abundant expression of anti-IIA reactive proteins was still found in the matrix of growth plate cartilage and trabecular bone tissue (Figs. 6, 7). This could suggest that the cells of the growth plate and within the bone trabecular matrix may not process type IIA procollagen similar to articular cartilage cells. Alternatively, if procollagen processing does occur, then retention of cleaved IIA NH2-propeptide within growth plate/trabecular bone matrices is occurring, perhaps by specific interactions of the propeptide with other matrix components. It is also possible that a proportion of anti-IIA reactive proteins expressed in post-natal growth plate and trabecular bone matrices are derived from type XI procollagen. Type XI procollagen is normally localized in pericellular regions of cartilage where thinner collagen II fibrils are located (Keene et al., 1995; Poole et al., 1985; Warner et al., 2006). In addition, processing of the type XI procollagen NH2-propeptide is complex: the α1(XI) NH2-propeptide is initially retained, but later processed; the α2(XI) NH2-propeptide is rapidly removed after synthesis while the α3(XI) NH2-propeptide (normally the “IIB” form in differentiated chondrocytes) is not removed (Morris and Bachinger, 1987; Thom and Morris, 1991). Since the Col2a1+ex2 homozygotes are engineered to retain exon 2, type XI procollagen heterotrimers in these mice will contain the “IIA” form of α3(XI). Therefore, positive anti-IIA staining in growth plate and trabecular matrices is likely due, in part, to the presence of unprocessed α3(XI) procollagen chains within type XI heterotrimers. The presence of α3(XI) unprocessed chains may also explain the pericellular localization patterns in P28 and P70 homozygous and heterozygous articular cartilage (Figs. 6H, I; 7E, F).
In the superficial zone of developing articular cartilage, anti-IIA-reactive signals were found in WT as well as heterozygous and homozygous tissue (Figs. 6D–F; 7A–C). Since the superficial zone of articular cartilage consists primarily of a pericellular matrix (Poole et al., 1984), and hence thin collagen fibrils, then these anti-IIA staining patterns are likely due to the presence of α3(XI) unprocessed chains within type XI procollagen heterotrimers. It is interesting, however, as to why IIA-containing proteins were still found to be present in the superficial zone of WT tissue. It may be that these proteins were synthesized early in development when synthesis of IIA exon 2-containing isoforms was predominant, and expression has persisted over time. Alternatively, cells from the WT superficial zone may continue to express IIA-containing proteins at post-natal time points and secrete them into the pericellular matrix. Either way, the data suggests that the exon 2-encoded CR domain is necessary to maintain post-natal superficial zone articular cartilage. In addition, there is no conclusive evidence from these studies to explain the presence of intracellular anti-IIA signals within deep zone chondocytes from WT P70 articular cartilage (Fig. 7D). Whether this staining pattern is due to presence of the cleaved NH2-propeptide of type IIA procollagen or α3 (XI) unprocessed chains, has yet to be determined. Future studies involving in situ hybridizations and detailed protein analysis to distinguish type II procollagen and type XI procollagen chains will aid in deciphering what levels of anti-IIA-reactive products are derived from type II procollagen or type XI procollagen within articular cartilage, growth plate cartilage and trabecular bone matrices.
Type XI procollagen heterotrimers are also known to co-polymerize with type II collagen (Eyre, 2002; Mendler et al., 1989; Wu and Eyre, 1995) and function to nucleate the self assembly of collagen II fibrils and regulate their lateral growth (Blaschke et al., 2000). Therefore, it is possible that in the Col2a1+ex2 mice, alteration of type XI procollagen α3 chains to retain the globular exon 2-encoded CR domain may affect its functions in regulating collagen II fibril formation, diameter and/or organization. We intend to examine this further by electron microscopy approaches.
In summary, by inhibiting alternative splicing of Col2a1 and selecting to solely express the IIA procollagen isoform, we have generated viable homozygous mice that will permit us to study skeletal development at the post-natal level in order to understand why the conserved CR domain is not normally present in the ECM of post-natal cartilage or trabecular bone. There are many avenues to investigate such as addressing a potential unexplored role for type II procollagen in post-natal bone development based on our preliminary micro-CT data. It will also be important to develop a proteomics-type approach to investigate changes in the composition of cartilage and trabecular bone ECM as a consequence of the knock-in mutation.
4. Materials and methods
4.1. Construction of wild type and mutant COL2A1 mini-genes
A previously described wild type human COL2A1 mini-gene (McAlinden et al., 2005) was utilized to generate exon 2 splice site mutant mini-genes. The wild type mini-gene (~5.9kb) consisting of exons 1–3 and full-length introns 1 and 2 (Fig. 1B), was cloned into pcDNA3 vector (Invitrogen) under transcriptional control of the cytomegalovirus promoter. Mutant mini-genes containing the 5′splice site mutation within intron 2 (COL2A1+ex2) (Fig. 1B) with or without inclusion of an upstream HaeIII restriction site (Fig. 2A) were generated using the QuikChange™ site-directed mutagenesis kit (Stratagene). PCR mutagenesis was carried out over 18 cycles (95°C, 30s; 55°C, 1min; 68°C, 6min) and resulting PCR products were treated with DpnI (1h, 37°C) to digest parental, methylated DNA. Digested DNA (1 μl) was transformed into XL-1 Blue Supercompetent cells (Stratgene) and resulting colonies were screened for presence of the correct mutations.
4.2. Transient transfection of COL2A1 mini-genes and analysis of mini-gene spliced mRNA isoforms
The following cell lines were used for transfections: i) HEK-293 human embryonic kidney cells (ATCC), ii) MC615 murine vertebral chondrocytes(Mallein-Gerin and Olsen, 1993), iii) TC28/I2 chondrocytes from human costal cartilage (gift from Dr. Mary Goldring, Hospital for Special Surgery, NY) and iv) RCS (LTC) rat chondrosarcoma cells (Fernandes et al., 1997). Each mini-gene construct was transfected into every cell line using FuGENE™ 6 reagent (Roche Applied Science) following the manufacturer’s protocol. Briefly, 1–3 μg of the mini-gene construct was tranfected into cells at a ratio of 1:4 DNA:FuGene (μg:μl) for 5h in serum-free medium. Serum-containing medium was then added (final concentration of 10% fetal bovine serum) and the cells were cultured for a further 48h. Total RNA was harvested and purified using the RNeasy kit (Qiagen). Reverse-transcribed cDNA was amplified by PCR in the presence of [α-32P]dCTP (800Ci/mmol; Perkin Elmer). The primers pcDNA3-COL2A1-Ex1 (5′-CAAGCTTACATGATTCGGC-3′) and sp6 were used to amplify cDNA derived only from the exogenously transfected COL2A1 mini-genes. PCR was carried out for 20x cycles (95°C, 30s; 55°C, 30s; 72°C, 30s). Following addition of 6x loading dye (30% glycerol, 0.025% (w/v) bromophenol blue, 0.025% cyanol blue), an aliquot of resulting PCR products (10 μl) was electrophoresed through 6% polyacrylamide gels at 200V. Gels were then dried, exposed to X-ray film and developed the following day.
4.3. Generation of a IIA knock-in (Col2a1+ex2) targeting vector
A mouse 129SvEv BAC (bacterial artificial chromosome, BAC ID# bMQ-131018; Source Bioscience) containing the Col2a1 genomic locus was used to generate the IIA Col2a1+ex2 knock-in targeting vector (Fig. 2). Mutations were introduced into the BAC using galK-based recombineering (Warming et al., 2005), a method that manipulates BACs independently of restriction sites via homologous recombination in bacteria. Galk recombineering is a two-step procedure using galK-based positive and negative selection. In the initial recombination step, point mutations at the 5′ splice site of Col2a1 exon 2 and a silent mutation to create an engineered HaeIII restriction site (for genotyping purposes) were introduced into the BAC using 50bp Col2a1 homology arms flanking the galK gene. This cassette was generated via PCR using primers Col2a1-IIA-galKrd1-FOR and Col2a1-IIA-galKrd1-REV (Table S2). Clones were obtained via positive selection on minimal media plates containing galactose as the only carbon source. Correctly recombined clones were identified via directional PCR, restriction digest to confirm a lack of BAC rearrangements, and sequencing of PCR products spanning the desired mutations. Positive clones from the first round of recombineering were subjected to a second round of recombineering to remove the galK gene, leaving behind only the desired mutations. The recombination cassette was generated by annealing two 100 bp complementary oligos (Col2a1-galKrd2-FOR2 and Col2a1-galKrd2-REV2; Table S2) that span the Col2a1 homology arms used in step 1. Clones were obtained via negative selection on minimal media plates containing glycerol as the only carbon source and 2-deoxy-galactose (DOG) as the selection agent. Clones in which the galK cassette has been deleted will grow in the presence of DOG while those retaining the galK gene will not. Positive recombinants were identified via directional PCR, restriction digest, and sequencing. A loxP-neor-loxP expression cassette was inserted within a non-conserved region of Col2a1 intron 2 (approximately 350 bp downstream of exon 2) via another recombineering method (Lee et al., 2001) and confirmed by positive selection in ES cells. The targeting vector was generated from the engineered BAC using gap repair (Lee et al., 2001) into a plasmid containing a diphtheria toxin cassette for negative selection in ES cells. Resulting IIA Col2a1+ex2 targeting vector DNA was prepared using a Qiagen maxi-prep protocol, linearized by restriction digest with AscI and purified by phenol/chloroform extraction. Linearized targeting vector (40 μg) was then used for electroporation into EDJ22 embryonic stem (ES) cells, derived from 129S6/SvEvTac mice. For all other standard procedures including electroporation, detection of positive ES clones and blastocyst injections for generation of knock-in mice, see Supplemental Material.
4.4. Col2a1 mRNA expression in limb cartilage harvested from knock-in mouse litters
Hind limbs were harvested from litters collected at developmental time points E12.5, E16.5, P0, P3, P7, P14, P28 and P70. Whole limbs from E12.5 and E16.5 embryos were used for RNA extraction while limbs from P0–P70 pups were dissected to isolate epiphyseal and femoral head cartilage tissue which was subsequently pooled and used for RNA extraction. RNA from either whole limbs or isolated cartilage tissue was extracted using Trizol reagent (Invitrogen) following the manufacturer’s instructions and finally dissolved in 30–40 μl of RNAse free water and analyzed with a Nanodrop spectrophotometer. Reverse transcribed cDNA (20 μl) was diluted with a volume of sterile water (80 μl) and an aliquot of this cDNA mix (10 μl) was PCR amplified using specific primer pairs (Exon 1 forward primer: 5′ CAGGCCTCGCGGTGAG 3′; Exon 4 reverse primer: 5′ GTTCTCCATCTCTGCCACG 3′) to amplify a region of the endogenous mouse Col2a1 gene spanning exon 2 (Hatano et al., 2002). PCR was carried out in the presence of [α-32P]dCTP (800Ci/mmol; Perkin Elmer) for 25 cycles (95°C, 30 s; 60°C, 30 s; 72°C, 30 s). 10 μl of 6x loading dye (30% glycerol, 0.025% (w/v) bromophenol blue, 0.025% (w/v) cyanol blue) was added to each PCR amplified reaction mix and 10 μl of the PCR/dye mix was electrophoresed at 200 V through a 6% polyacrylamide gel. Gels were dried, exposed to X-ray film overnight and developed the following day.
4.5. Safranin-O staining of hindlimb tissue sections
Paraffin sections were treated with xylene, rehydrated through decreasing concentration of ethanol, stained in Weigert’s Hematoxylin for 5 min, washed in running water for 5 min and stained with 0.001% Fast Green for 3 min. Samples were then rinsed in 1% glacial acetic acid and stained in 0.1% Safranin O for 5 min. Samples were dehydrated and cleared by incubation in 95% alcohol, 100% alcohol and xylene. Mounting medium was applied and stained sections were cover-slipped.
4.6. Fluroescence immunohistochemistry to detect type II procollagen protein expression
Limbs were harvested and fixed in 10% neutral buffered formalin. Fixation times varied between 1–3 days depending on the age of the tissue. After fixation, samples were rinsed in sterile water and decalcified whenever necessary (E16.5-P70) for a period of time between 12 hrs – 3 weeks depending on the bone content of the limbs. Fixed, decalcified limbs were then dehydrated, embedded in paraffin and sectioned. Glass slides containing paraffin section (10 μm) were baked overnight at 56°C, de-paraffinized in xylene, rehydrated through different grades of ethanol and incubated with 1% hyaluronidase (Sigma) for 30 min at 37°C. Sections were rinsed with 1xPBS and blocked with 10% goat serum for 1 h at room temperature and then incubated overnight at 4°C with a rabbit polyclonal “anti-IIA” antibody that recognizes the exon 2-encoded cytseine-rich domain within the NH2- propeptide of type II procollagen (1/400 dilution) (Oganesian et al., 1997) and rat polyclonal antibodies against the triple helical domain of type II collagen (Cremer and Kang, 1988). Each antibody was diluted 1/400 and 1/100, respectively in 2% goat serum. Following 1x PBS washes, paraffin sections were incubated with species-specific secondary antibodies (1/250 dilution) that were conjugated to Alexa fluorescent dyes [Invitrogen: goat anti-rabbit Alexa 488; goat anti-rat Alexa 594] for 1 h at room temperature. DAPI mounting medium was applied following three rinses in 1x PBS and stained sections were cover-slipped. Human embryonic limb sections (day 54 or day 67 of development) obtained from the Laboratory of Developmental Biology at University of Washington in Seattle were used as positive controls for the primary antibodies. A Nikon Eclipse E800 fluorescence microscope was used to view the fluorescent images. The FITC and TRITC band pass filter sets were used to view sections labeled with Alexa 488 and 594 dyes, respectively and the DAPI filter set was used for viewing cell nuclei.
4.7. Collagen extraction, electrophoresis and Western blotting
Epiphyseal cartilage tissue was extracted from hind limbs of P7 wild type, heterozygous and homozygous Col2a1+ex2 knock-in mice under a dissecting microscope. Tissue was snap frozen and stored at −80°C until further analysis. Tissue was thawed on ice, weighed and extracted in a fixed volume of 4M guanidinium HCl (Gn-HCl), 50mM Tris-HCl, pH 7.4 containing protease inhibitors for 24 h at 4°C (Fernandes et al., 2001) to solubilize non-crosslinked type II collagen. Proteins in the 4M Gn-HCl extracts were resolved by SDS-PAGE, Western blotted and probed with the same anti-IIA polyclonal antisera as was used for immunohistochemistry (Oganesian et al., 1997) or with a monoclonal antibody (1C10) recognizing native type II collagen as we have described before (Fernandes et al., 2003).
Supplementary Material
Highlights.
Splice site targeting strategy inhibited type II procollagen alternative splicing
Knock-in mice synthesizing only type IIA procollagen were generated
IIA procollagen knock-in mice are viable with no apparent overt phenotype
Post-natal cartilage analysis in IIA knock-in mice currently in progress
Acknowledgments
We would like to thank the excellent staff members and services of the Transgenic Vectors Core (http://hopecenter.wustl.edu/cores/transgenicVectors), the Embryonic Stem Cell Core (http://escore.im.wustl.edu/) and the Mouse Genetics Core (http://mgc.wustl.edu/) at Washington University School of Medicine. We also thank Crystal Idleburg for histology services, Bennett Sprugel for technical assistance and Elizabeth DeLassus and John Freeman for microscopy assistance. Human tissue was obtained from the Laboratory of Developmental Biology, University of Washington, Seattle (which is funded by NIH grant 5R24HD000836). Thanks also to Dr. Thomas Hering and Dr. Debabrata Patra for critical reading of this manuscript. This work has received funding from NIH R21 grant AR053513 (AM) and a Pilot & Feasibility grant (AM) provided from NIH Musculoskeletal P30 Core Grant (P30 AR057235).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bishop PN, Reardon AJ, McLeod D, Ayad S. Identification of alternatively spliced variants of type II procollagen in vitreous. Biochem Biophys Res Commun. 1994;203:289–295. doi: 10.1006/bbrc.1994.2180. [DOI] [PubMed] [Google Scholar]
- Blaschke UK, Eikenberry EF, Hulmes DJ, Galla HJ, Bruckner P. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000;275:10370–10378. doi: 10.1074/jbc.275.14.10370. [DOI] [PubMed] [Google Scholar]
- Bornstein P. The NH(2)-terminal propeptides of fibrillar collagens: highly conserved domains with poorly understood functions. Matrix Biol. 2002;21:217–226. doi: 10.1016/s0945-053x(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Walsh V, Tullis J, Stainbrook E, Bateman JF, Hormuzdi SG. The globular domain of the proalpha 1(I) N-propeptide is not required for secretion, processing by procollagen N-proteinase, or fibrillogenesis of type I collagen in mice. J Biol Chem. 2002;277:2605–2613. doi: 10.1074/jbc.M106181200. [DOI] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Claus S, Aubert-Foucher E, Demoor M, Camuzeaux B, Paumier A, Piperno M, Damour O, Duterque-Coquillaud M, Galera P, Mallein-Gerin F. Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer cultures. J Cell Biochem. 2010;111:1642–1651. doi: 10.1002/jcb.22897. [DOI] [PubMed] [Google Scholar]
- Colige A, Ruggiero F, Vandenberghe I, Dubail J, Kesteloot F, Van Beeumen J, Beschin A, Brys L, Lapiere CM, Nusgens B. Domains and maturation processes that regulate the activity of ADAMTS-2, a metalloproteinase cleaving the aminopropeptide of fibrillar procollagens types I-III and V. J Biol Chem. 2005;280:34397–34408. doi: 10.1074/jbc.M506458200. [DOI] [PubMed] [Google Scholar]
- Cremer MA, Kang AH. Collagen-induced arthritis in rodents: a review of immunity to type II collagen with emphasis on the importance of molecular conformation and structure. Int Rev Immunol. 1988;4:65–81. doi: 10.3109/08830188809044771. [DOI] [PubMed] [Google Scholar]
- Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4:30–35. doi: 10.1186/ar380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR. Type XI or 1α2α3α collagen. In: Mayne R, Burgeson RE, editors. Structure and function of collagen types. Academic Press; New York: 1987. pp. 261–281. [Google Scholar]
- Eyre DR, Weis MA, Wu JJ. Articular cartilage collagen: an irreplaceable framework? European Cells Mat. 2006;12:57–63. doi: 10.22203/ecm.v012a07. [DOI] [PubMed] [Google Scholar]
- Fernandes RJ, Hirohata S, Engle JM, Colige A, Cohn DH, Eyre DR, Apte SS. Procollagen II amino propeptide processing by ADAMTS-3. Insights on dermatosparaxis. J Biol Chem. 2001;276:31502–31509. doi: 10.1074/jbc.M103466200. [DOI] [PubMed] [Google Scholar]
- Fernandes RJ, Schmid TM, Harkey MA, Eyre DR. Incomplete processing of type II procollagen by a rat chondrosarcoma cell line. Eur J Biochem. 1997;247:620–624. doi: 10.1111/j.1432-1033.1997.00620.x. [DOI] [PubMed] [Google Scholar]
- Fernandes RJ, Seegmiller RE, Nelson WR, Eyre DR. Protein consequences of the Col2a1 C-propeptide mutation in the chondrodysplastic Dmm mouse. Matrix Biol. 2003;22:449–453. doi: 10.1016/s0945-053x(03)00077-5. [DOI] [PubMed] [Google Scholar]
- Fukui N, McAlinden A, Zhu Y, Crouch E, Broekelmann TJ, Mecham RP, Sandell LJ. Processing of type II procollagen amino propeptide by matrix metalloproteinases. J Biol Chem. 2002;277:2193–2201. doi: 10.1074/jbc.M105485200. [DOI] [PubMed] [Google Scholar]
- Furuto DK, Miller EJ. Different levels of glycosylation contribute to the heterogeneity of alpha 1(II) collagen chains derived from a transplantable rat chondrosarcoma. Arch Biochem Biophys. 1983;226:604–611. doi: 10.1016/0003-9861(83)90329-6. [DOI] [PubMed] [Google Scholar]
- Hatano H, Sarkar G, Bolander ME. Development of a cellular model to study alternative splicing of type II collagen gene. J Orthop Res. 2002;20:516–519. doi: 10.1016/S0736-0266(01)00123-1. [DOI] [PubMed] [Google Scholar]
- Keene DR, Oxford JT, Morris NP. Ultrastructural localization of collagen types II, IX, and XI in the growth plate of human rib and fetal bovine epiphyseal cartilage: type XI collagen is restricted to thin fibrils. J Histochem Cytochem. 1995;43:967–979. doi: 10.1177/43.10.7560887. [DOI] [PubMed] [Google Scholar]
- Larrain J, Bachiller D, Lu B, Agius E, Piccolo S, De Robertis EM. BMP-binding modules in chordin: a model for signalling regulation in the extracellular space. Development. 2000;127:821–830. doi: 10.1242/dev.127.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff C, Somerville RP, Kesteloot F, Powell K, Birk DE, Colige AC, Apte SS. Regulation of procollagen amino-propeptide processing during mouse embryogenesis by specialization of homologous ADAMTS proteases: insights on collagen biosynthesis and dermatosparaxis. Development. 2006;133:1587–1596. doi: 10.1242/dev.02308. [DOI] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Leung AW, Wong SY, Chan D, Tam PP, Cheah KS. Loss of procollagen IIA from the anterior mesendoderm disrupts the development of mouse embryonic forebrain. Dev Dyn. 2010;239:2319–2329. doi: 10.1002/dvdy.22366. [DOI] [PubMed] [Google Scholar]
- Li SW, Prockop DJ, Helminen H, Fassler R, Lapvetelainen T, Kiraly K, Peltarri A, Arokoski J, Lui H, Arita M, et al. Transgenic mice with targeted inactivation of the Col2α1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9:2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Lui VC, Ng LJ, Nicholls J, Tam PP, Cheah KS. Tissue-specific and differential expression of alternatively spliced alpha 1(II) collagen mRNAs in early human embryos. Dev Dyn. 1995;203:198–211. doi: 10.1002/aja.1002030208. [DOI] [PubMed] [Google Scholar]
- Mallein-Gerin F, Olsen BR. Expression of simian virus 40 large T (tumor) oncogene in mouse chondrocytes induces cell proliferation without loss of the differentiated phenotype. PNAS. 1993;90:3289–3293. doi: 10.1073/pnas.90.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlinden A, Crouch EC, Bann JG, Zhang P, Sandell LJ. Trimerization of the amino propeptide of type IIA procollagen using a 14-amino acid sequence derived from the coiled-coil neck domain of surfactant protein D. J Biol Chem. 2002;277:41274–41281. doi: 10.1074/jbc.M202257200. [DOI] [PubMed] [Google Scholar]
- McAlinden A, Havlioglu N, Liang L, Davies SR, Sandell LJ. Alternative splicing of type II procollagen exon 2 is regulated by the combination of a weak 5 ′ splice site and an adjacent intronic stem-loop cis element. J Biol Chem. 2005;280:32700–32711. doi: 10.1074/jbc.M505940200. [DOI] [PubMed] [Google Scholar]
- McAlinden A, Havlioglu N, Sandell LJ. Regulation of protein diversity by alternative pre-mRNA splicing with specific focus on chondrogenesis. Birth Defects Res C Embryo Today. 2004;72:51–68. doi: 10.1002/bdrc.20004. [DOI] [PubMed] [Google Scholar]
- McAlinden A, Johnstone B, Kollar J, Kazmi N, Hering TM. Expression of two novel alternatively spliced COL2A1 isoforms during chondrocyte differentiation. Matrix Biol. 2008;27:254–266. doi: 10.1016/j.matbio.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlinden A, Liang L, Mukudai Y, Imamura T, Sandell LJ. Nuclear protein TIA-1 regulates COL2A1 alternative splicing and interacts with precursor mRNA and genomic DNA. J Biol Chem. 2007;282:24444–24454. doi: 10.1074/jbc.M702717200. [DOI] [PubMed] [Google Scholar]
- McAlinden A, Shim K-H, Ravindran S, Hering TM. Quantification of type II procollagen splice forms during chondrogenesis of ATDC5 cells using alternative transcript-q(PCR) (AT-qPCR) Unpublished results. unpublished results. (submitted for publication) [Google Scholar]
- Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989;108:191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minns RJ, Steven FS. The collagen fibril organization in human articular cartilage. J Anat. 1977;123:437–457. [PMC free article] [PubMed] [Google Scholar]
- Moroy T, Heyd F. The impact of alternative splicing in vivo: mouse models show the way. RNA. 2007;13:1155–1171. doi: 10.1261/rna.554607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NP, Bachinger HP. Type XI collagen is a heterotrimer with the composition (1 alpha, 2 alpha, 3 alpha) retaining non-triple-helical domains. J Biol Chem. 1987;262:11345–11350. [PubMed] [Google Scholar]
- Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LJ, Tam PP, Cheah KS. Preferential expression of alternatively spliced mRNAs encoding type II procollagen with a cysteine-rich amino-propeptide in differentiating cartilage and nonchondrogenic tissues during early mouse development. Dev Biol. 1993;159:403–417. doi: 10.1006/dbio.1993.1251. [DOI] [PubMed] [Google Scholar]
- O’Leary JM, Hamilton JM, Deane CM, Valeyev NV, Sandell LJ, Downing AK. Solution structure and dynamics of a prototypical chordin-like cysteine-rich repeat (von Willebrand Factor type C module) from collagen IIA. J Biol Chem. 2004;279:53857–53866. doi: 10.1074/jbc.M409225200. [DOI] [PubMed] [Google Scholar]
- Oganesian A, Zhu Y, Sandell LJ. Type IIA procollagen amino propeptide is localized in human embryonic tissues. J Histochem Cytochem. 1997;45:1469–1480. doi: 10.1177/002215549704501104. [DOI] [PubMed] [Google Scholar]
- Poole AR, Pidoux I, Reiner A, Choi H, Rosenberg LC. Association of an extracellular protein (chondrocalcin) with the calcification of cartilage in endochondral bone formation. J Cell Biol. 1984;98:54–65. doi: 10.1083/jcb.98.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Morphology of the pericellular capsule in articular cartilage revealed by hyaluronidase digestion. J Ultrastruct Res. 1985;91:13–23. doi: 10.1016/0889-1605(85)90071-0. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Rebuck N, Croucher LJ, Hollander AP. Distribution of two alternatively spliced variants of the type II collagen N-propeptide compared with the C-propeptide in bovine chondrocyte pellet cultures. J Cell Biochem. 1999;75:13–21. doi: 10.1002/(sici)1097-4644(19991001)75:1<13::aid-jcb2>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Sandell LJ. Differential expression of a cysteine-rich domain in the amino-terminal propeptide of type II (cartilage) procollagen by alternative splicing of mRNA. J Biol Chem. 1990;265:10334–10339. [PubMed] [Google Scholar]
- Sandell LJ, Morris N, Robbins JR, Goldring MB. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J Cell Biol. 1991;114:1307–1319. doi: 10.1083/jcb.114.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LJ, Nalin AM, Reife RA. Alternative splice form of type II procollagen mRNA (IIA) is predominant in skeletal precursors and non-cartilaginous tissues during early mouse development. Dev Dyn. 1994;199:129–140. doi: 10.1002/aja.1001990206. [DOI] [PubMed] [Google Scholar]
- Thom JR, Morris NP. Biosynthesis and proteolytic processing of type XI collagen in embryonic chick sterna. J Biol Chem. 1991;266:7262–7269. [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner LR, Brown RJ, Yingst SM, Oxford JT. Isoform-specific heparan sulfate binding within the amino-terminal noncollagenous domain of collagen alpha1(XI) J Biol Chem. 2006;281:39507–39516. doi: 10.1074/jbc.M608551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JJ, Eyre DR. Structural analysis of cross-linking domains in cartilage type XI collagen. Insights on polymeric assembly. J Biol Chem. 1995;270:18865–18870. doi: 10.1074/jbc.270.32.18865. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Oganesian A, Keene DR, Sandell LJ. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-beta1 and BMP-2. J Cell Biol. 1999;144:1069–1080. doi: 10.1083/jcb.144.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.