Abstract
The cytoarchitecture and cortical connections of the anterior cingulate, medial and dorsal premotor, and precentral region are investigated using the Nissl and NeuN staining method and the fluorescent retrodgrade tract tracing technique. There is a gradual stepwise laminar change in the cytoarchitectonic organization from the proisocortical anterior cingulate region, through the lower and upper banks of the cingulate sulcus, to the dorsolateral isocortical premotor and precentral motor regions of the frontal lobe. These changes are characterized by a gradational emphasis on the lower stratum layers (V and VI) in the proisocortical cingulate region to the upper stratum layers (II and III) in the premotor and precentral motor region. This is accompanied by a progressive widening of layers III and VI, a poorly delineated border between layers III and V and a sequential increase in the size of layer V neurons culminating in the presense of giant Betz cells in the precentral motor region. The overall patterns of corticocortical connections paralleled the sequential changes in cytoarchitectonic organization. The proisocortical areas have connections with cingulate motor, supplementary motor, premotor and precentral motor areas on the one hand and have widespread connections with the frontal, parietal, temporal and multimodal association cortex and limbic regions on the other. The dorsal premotor areas have connections with the proisocortical areas including cingulate motor areas and supplementary motor area on the one hand, and premotor and precentral motor cortex on the other. Additionally, this region has significant connections with posterior parietal cortex and limited connections with prefrontal, limbic and multimodal regions. The precentral motor cortex also has connections with the proisocortical areas and premotor areas. Its other connections are limited to the somatosensory regions of the parietal lobe. Since the isocortical motor areas on the dorsal convexity mediate voluntary motor function, their close connectional relationship with the cingulate areas form a pivitol limbic-motor interface that could provide critical sources of cognitive, emotional and motivational influence on complex motor function.
Keywords: Cerebral Cortex, Frontal Lobe, Limbic System, Motivation, Motor Behavior
INTRODUCTION
Early physiological studies have described organizations of the primary motor (MI) and supplementary motor (MII) representations in the frontal lobe of the rhesus monkey [31,202,203]. Subsequent physiological and anatomical investigations using more refined methodological approaches suggested multiple motor representations located in the precentral and premotor regions [41,44,45,53,60,61,99,125,128,130,157,158]. In recent years additional investigations have also outlined distinct motor areas in the cingulate cortex [34,63,97,111,112,117,125,153,175,176]. To date, a number of reports have described the cortical efferent and afferent connections of the precentral, premotor, supplementary [35,48,51,69,87,92,94,96,112–114,119,138,183–186] and cingulate motor areas [9,52,53,90,96,112–114,119,181]. Although it appears that there are significant differences in the corticocortical connections that characterize these motor areas, a systematic investigation of these patterns of connections has yet to be conducted. An analysis of the literature suggests a common cortical input to these motor regions is derived from the postcentral gyrus including the primary somatosensory cortex (SI) and other somatosensory representations of the parietal lobe such as the second somatosensory area (SII) and supplementary sensory area (SSA) [33,77,129,154].
In recent studies examining the architecture and connections of the somatosensory areas of the parietal lobe, it was proposed that these areas are organized into dual trends in the macaque monkey brain (27,120). According to this view, on a structural basis, the ventral part of the somatosensory cortex (head, face and neck representations) is mainly related to proisocortical areas of the insular cortex whereas the dorsal part of the somatosensory cortex (trunk and limb representations) is primarily related to the caudal cingulate region (area 23a) and proisocortical retrosplenial region (areas 29 and 30). Moreover these studies have shown a tripartite organization in both of these trends. Thus, from the proisocortical regions of the cingulate and insular cortex, laminar differentiation gradually progresses in three lines. The root line (proisocortical regions) of the dorsal somatosensory trend consists of areas with progressive laminar changes leading to the supplementary sensory area (SSA) of the caudal cingulated region, whereas the rootline of the ventral somatosensory trend includes the second somatosensory type of cortex (SII/PV – according to Krubitzer and Kaas, 1995, 2005) of the frontoparietal operculum [27,120]. The belt line is viewed as parasensory areas of the superior (dorsal trend) and inferior (ventral trend) parietal regions including areas 1, 2, PE (5) and PF on the basis of progressive laminar changes. The core line is formed by the dorsal and ventral parts of area 3 (SI) with marked granularity of the cortex. Furthermore, this tripartite organization showed specific intrinsic connections among the root, core and belt regions [27,33,68,120,154,191].
Since the somatosensory and somatomotor areas are integral parts of the cortical somatosensorimotor system, it would be of great interest to determine if the dorsal motor areas of the precentral and premotor regions are also organized in a manner similar to that of the dorsal somatosensory cortex. That is, can these motor areas be traced from the dorsomedial proisocortex (rostral cingulate region) in terms of a stepwise pattern of architectonic differentiation and a stepwise pattern of cortical interconnections? In the present study therefore, we first examined the cytoarchitecture of the precentral and premotor regions, MII, the rostral cingulate motor area (M3) and rostral portion of the cingulate gyrus using Nissl and NeuN stained material. Secondly, we delineated the cortical connections of these regions by placing injections of fluorescent retrograde tracers (FRT) in selected parts of these cortical regions. The results of our study indicate that like the dorsal somatosensory region, the dorsal somatomotor region is primarily affiliated on both cytoarchitectural and connectional grounds with the rostral (cingulate) proisocortex. Moreover, the connectional data indicate that these motor areas are also organized in a tripartite manner as observed for the somatosensory cortex. These findings advance our understanding of the organization of the precentral motor, premotor and anterior cingulate areas, and underscore the relevance of this brain region in conveying cognitive and higher-order emotional and motivational influences on voluntary motor function.
MATERIAL AND METHODS
Experimental material used to examine dorsomedial frontal lobe and anterior cingulated corticocortical connections was obtained from the brains of 9 adult rhesus monkeys (Macaca mulatta) using retrograde flourescent tract tracers. All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of South Dakota and carried out in an AAALAC approved facility. Guidelines for the ethical treatment of experimental animals outlined by the United States Department of Agriculture, National Institutes of Health and Society for Neuroscience were followed. Fluorescent retrograde tracing (FRT) techniques and intracortical microstimulation were used in experimental combinations for connectional analyses. For architectonic analyses the brains of 3 non-operated monkeys (Macaca mulatta) were embedded in paraffin and stained for Nissl substance with cresyl violet or thionin. The same brains were used in our previous studies examining the transitional nature of cytoarchitectonic organization in the frontoparietal cortex [27], and posterior cingulate and adjacent parietal cortex [120]. Immunohistochemical labeling with neuron specific nuclear protein (NeuN) marker was also conducted on 2 more rhesus monkey brains (Macaca mulatta) for architectonic investigation.
Neurourgical and Tract Tracer Injection Procedures
All neurosurgical procedures were performed using sterile methods. Preoperatively, each monkey was immobilized with atropine (0.5mg/kg) then ketamine hydrochloride (1mg/kg). The monkey was then intubated, placed on a mechanical ventilator and anesthetized with a mixture of 1.0–1.5% isofluorane and surgical grade air/oxygen. The monkey was subsequently placed into a cranial holding device and administered mannitol intravenously (1.0–1.5g/kg). For surgical exposure of the dorsolateral frontal region, a skin incision and bone flap was fashioned over the midline followed by a dural flap with its base attached to the superior sagittal sinus. For continued exposure the medial frontal and cingulate cortex, the bridging veins draining into the superior sagittal sinus were cauterized and separated.
Using a surgical microscope, injection of a retrograde neural tracer was then made into selected targets along the dorsolateral and medial wall of the cerebral hemisphere and cortex lining the banks of the cingulate sulcus. The retrograde tracers used were 3–5% fast blue (FB) in 0.1M phosphate buffer at pH 7.4, 3–4% diamidino-yellow (DY) in 0.1M phosphate buffer at pH 7.4, and 10% fluororuby (FR) (equal mixtures of 3,000 mw and 10,000 mw) in 0.9% saline at pH 7.4 [121]. Fast blue was purchased from Sigma-Aldrich (St. Louis, Missouri, catalog number F-5756) and Dr. Illing Plastics GMBH (Kunstoffe-Rohstoffe-Anlagen, Bergfeld 29, 64747 Breuberg, Germany) and DY from Sigma Aldrich (catalog number 0281) and Dr. Illing Plastics GMBH. Fluororuby was obtained from Molecular Probes (Eugene, Oregon; catalog numbers D-3308 (3,000 mw) and D-1817 (10,000 mw)). Each fluorescent tract tracer (FRT) was injected using a Hamilton microsyringe with the cannula tip inserted 2–3 mm below the cortical surface. The syringe was secured in a microinjection holder attached to a Kopf electrode micromanipulator unit (model 1460–61; David Kopf Instruments, Tujunga, CA). Only one penetration (total injected volume of 0.2–0.4 µl) of the selected tracer was made in the intended cytoarchitectonic region for each tract tracer experiment. Injection site Cases 5 and 6 reported in this study correspond to Cases 1 and 2 respectively, in our previous paper on amygdala interconnections with the cingulate motor cortex [122]. To minimize the number of animals used to accomplish the study goals, injection Cases 2 and 3 were performed in the same hemisphere (i.e., same animal experiment) as were injection Cases 5 and 6. Likewise, injection Cases 7 and 10 were made in the same hemisphere of one animal as were cases 12, 13 and 15. Finally, injection Cases 9 and 14 were also made in the same hemisphere.
Following the injection procedure, the surgical field was rinsed with 0.9% saline and swabbed then the dura was closed with sutures. The bone flap was replaced and anchored and the muscle and skin were closed. The animal was monitored and Bicillin L-A or amoxicillin was used as pre- and post-operative prophylaxis antibiotic and buprenorphin (0.01 mg/kg) was used as a post-operative analgesic.
Intracortical Microstimulation Procedure
Using electrophysiological microstimulation and a combination of ketamine (10 mg/kg) and diazepam (1.0 mg/kg), the leg representation of the primary motor cortex (MI or area 4), the face, arm and leg representations of the supplementary motor cortex (MII or area 6m), and movement representations within area 6DC were localized prior to making the FRT injection [62,107,118,123,178]. We would like to point out that MII [6, 203] correlates with the SMA, SMA-proper and M2 nomenclature of others [94,97,159,181,107,198,206]. Architectonically this field corresponds to areas 6m and F3 [91,100,155,177]. In case 10, the pre-supplementary motor area (preSMA) was defined as being directly anterior to the face/arm representation of MII and being unexcitable with microstimulation [103]. Area 6DR was also defined as being unexcitable and directly anterior to the area 6DC in which movements were elicited. Stimulation was performed using a Grass Square Pulse Stimulator system (model S28; Grass Technologies, West Warwick, RI) with an attached tungsten electrode (impedance 0.5–1.5 MΩ). The electrode was placed 100 µm below the pial surface and then advanced at 500 µm intervals. Movements were evoked using a train duration of 50 ms and pulse duration of 0.2 msec delivered at 330 Hz. Current intensity ranged between 1 and 90 µA. Threshold currents were determined and the evoked movements were recorded if noted by 2 observers. Each stimulation site location was recorded for reconstruction purposes. For accuracy, the recorded position of each electrode was made relative to general features of the surgical exposure in addition to gyral and sulcal landmarks and surface vessels. Electrode penetration sites were minimized to avoid tissue damage. After defining the borders of the movement representations in MI, MII, or area 6DC, injections of FRT were placed within each localized somatotopic representation or in the unresponsive cortex located anterior to 6DC and MII.
Histological and Immunohistochemical Procedures
Tract Tracing Cases
Each monkey receiving injection(s) of retrograde tract tracing compound survived for a period of 27–33 days, then was re-anesthetized using an overdose of pentobarbital and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in 0.1M phosphate buffer (PB) adjusted to a pH of 7.4. After fixation, the brain was cryoprotected by infusing 10% sucrose in 0.1M PB at a pH of 7.4 followed by a solution of 30% sucrose in 0.1M PB at a pH of 7.4. The central nervous system was removed and stored for 3 or 4 days in 30% sucrose in 0.1M PB at 4°C. The cerebral cortex was then frozen sectioned on a sliding microtome (American Optical 870, Buffalo, NY) at a thickness of 50 µm in cycles of 10. Thus, 10 sets of tissue sections through the entire cerebral cortex were saved and within each set, every tissue section was spaced at 500 µm intervals. The first set of tissue sections was used for architectonic analysis using standard histochemical methodology to evaluate the cortical architectonic region containing the injection site(s) and retrogradedly labeled neurons. To accomplish this, each cortical section was mounted on subbed slides, dried overnight at room temperature, defatted, stained for Nissl substance using thionin as described previously [120] then coverslipped with Permount (SP15-500, Fisher Chemicals, Fair Lawn, NJ). The second series of tissue sections was used for fluorescent tracer analysis (FB, DY and FR) and specifically, microscopic identification of the fluorescent injection site(s) and retrogradedly labeled cortical neurons. To accomplish this, after sectioning, each coronal section was immediately rinsed in PB, mounted on gelatin-coated slides, dried overnight at 4°C, coverslipped using D.P.X. mounting medium (Aldrich Chemical Company, Milwaukee, WI) and stored in a freezer. In the experimental tract tracing case with an injection of FR into the leg region of MI, a third series of tissue sections was processed for immunohistochemical visualization of FR using biotinylated anti-FR (Vector, Burlingame, CA). This was accomplished as described in our previous report [121] and carried out in addition to preparing the fluorescent tracer series for verification of retrograde transport.
Cytoarchitectural Cases
To study the cytoarchitecture of the anterior cingulate, dorsomedial premotor and precentral motor cortex, we used the paraffin embedded Nissl staining method in three non-operated cases. Briefly, the tissue was fixed in 0.1 M phosphate buffered formalin, embedded in paraffin, cut in the coronal plane at 35 µm and stained with thionin or cresyl violet as detailed in our previous reports [40,137,173]. To supplement these observations, we studied NeuN stained tissue sections in 2 additional monkey brains that were cut in the coronal plane at 50 µm in cycles of 10 as described in the previous paragraph. Although the Nissl and NeuN methods are compareable, an advantage of the NeuN method is that it selectively stains only nerve cells [126,201] in contrast to the Nissl method which stains nucleic acids, and thus the Nissl substance of neurons, glia and endothelial cells [11,85,147]. To accomplish the NeuN procedure, after perfusion fixation with 4% paraformaldehyde in 0.1M phosphate buffer, sucrose cryoprotection and tissue sectioning as described above, one complete set of tissue sections was rinsed in 0.05M tris-buffered saline adjusted to a pH of 7.4 (TBS). The tissue was then incubated in TBS with 5% normal goat serum (NGS) and 1.25% triton x-100 overnight at 4° C. Next, the sections were incubated in 5% NGS in TBS with mouse anti-neuronal nuclei (NeuN) monoclonal antibody (MAB 377, Chemicon, Temecula, CA) at a dilution of 1:1000 overnight at 4° C. Following thorough rinsing in TBS, the tissue sections were incubated in biotinylated anti-mouse IgG (BA-9200, Vector Laboratories, Burlingame, CA) at a dilution of 1:500 at room temperature for three hours then rinsed in TBS. All sections were then incubated in a solution of avidin-biotin peroxidase complex (ABC) (PK-6100, Vector Laboratories, Burlingame, CA) for 3 hours at room temperature, rinsed again in TBS, and incubated with the vector SG peroxidase substrate kit (SK-4700, Vector Laboratories, Burlingame, CA) for approximately 2–10 minutes, yielding a blue reaction product. The tissue sections were rinsed in TBS, mounted on subbed slides then dried at room temperature overnight. Finally, the sections were dehydrated in graded levels of increased concentrations of alcohol, cleared in xylene, then coverslipped using Permount.
Data Analysis
Using the set of serial sections prepared for fluorescent analysis, the entire cortical gray matter in every tissue section was evaluated for labeling (i.e., all even and odd numbered sections) using epiflourescent illumination on a BX-51 or BX-60 Olympus microscope (Leeds Precision, Minneapolis, MN). Using the same microscope, the locations of retrogradedly labeled neurons were plotted in every other tissue section (i.e., every even numbered section), and the peripheral boundary of each injection site was traced in all tissue sections containing the injection site. To accomplish this, a computer-controlled high resolution MAC 5000 motorized microscope stage (Ludl Electronic Products, Hawthorne, NY) attached to the microscope was electronically interfaced to a Neurolucida neuroanatomical data collection and analysis system (Micro Bright Field Bioscience, Williston, VT). The analysis system generated a digital image of each individual coronal tissue section containing the precise location of the plotted neurons and injection sites. Matching tissue sections from the Nissl stained series were then used to map the cytoarchitectonic organization of the coronal sections.
The collected data was reconstructed by transferring the data points (locations of retrograde labeled neurons and injection sites) from the charted serial tissue sections onto a surface image of the lateral, medial and ventral walls of the hemisphere. The surface images of each cortical case were generated from metrically calibrated digital photographs of the brain surface taken during surgery and at autopsy. Cytoarchitectonic boundaries were also transferred onto the surface images using the Nissl stained sections generated for each individual monkey case. Finally, using the same Nissl stained sections through the medial temporal lobe region, the architectonic boundaries were charted using Neurolucida, or drawn using a DL 2 Dokumator (Carl Zeiss/Jena, Germany) to determine the specific boundaries of the various hippocampal subfields. Once identified, the locations of each individual DY or FB labeled cell was transferred onto the hippocampal chartings for analysis. In Cases 10–14, intracortical microstimulation sites were also transferred onto the surface images of the cerebral cortex [107,118,123]. Correlation of the stimulation points and physiological boundaries of the movement representations with respect to the location of the injection site allowed us to determine if the injectate was confined to the intended somatotopic representation which was confirmed in all the cases presented in this report.
Representative examples of injections sites and retrogradedly labeled neurons were photographed using a SpotFlex 64 Mp digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) mounted on the BX- 51 Olympus microscope. The camera was interfaced with a Pentium 4 Dell Computer (Dell omputer Corp., Round Rock, TX, USA) to store the images. Adobe Photoshop (Adobe Systems, San Jose, CA, USA) was then used to prepare illustrations using the images. Only contrast and brightness features were adjusted to achieve publication quality representation of original microscopic data. Using Adobe Illustrator software (Adobe Systems, San Jose, CA, USA), line drawings of the lateral, medial and ventral cortical surfaces were constructed from original photographic images taken of the brain to demonstrate the location of the injection site (s) and distribution of retrograde labeling. Using Adobe Illustrator line drawings were made of representative coronal levels from selected Cases using the charted tissue sections.
RESULTS
Cytoarchitecture of the Rostral Cingulate, Dorsal Premotor and Dorsal Precentral Motor Cortex
The anterior cingulate region is described as area 24 whereas the premotor and precentral regions are comprised of areas 6 and 4 respectively [16]. Areas 6 and 4 extend from the cingulate sulcus medially onto the gyral cortex rostral to the central sulcus up to the level of the arcuate sulcus (Fig. 1). Since several studies have described architectonic characteristics of these cortical regions [6,14,15,97,100,110,155,166,168], we will concentrate in this report only on the salient features that depict progressive and distinguishing laminar characteristics of these regions.
Figure 1.
Schematic representation of lateral (upper diagram) and medial (lower diagram) surfaces of the cerebral cortex of the rhesus monkey (Macaca mulatta) modified according to Morecraft and colleagues [120]. The architectonic areas depicted are based upon previous findings for the occipital lobe [146], parietal lobe [137], inferior temporal and superior temporal sulci [172], superior temporal gyrus and supratemporal plane [75, 134], the insular, parietotemporal opercular and frontotemporal opercular areas [66, 75, 77, 109, 158], as well as the premotor [6], prefrontal [150, 151], cingulate [114, 122] and parahippocampal [12] regions. Abbreviations: as, arcuate sulcus; cc, corpus callosum; cf, calcarine fissure; cgs, cingulate sulcus; cs, central sulcus; G, gustatory cortex; hf, hippocampal fissure; Iag, agranular sector of insula; Idg, dysgranular sector of insula; IG, granular sector of insula; ios, inferior occipital sulcus; ips, intraparietal sulcus; KA, auditory koniocortex; lf, lateral fissure; ls, lunate sulcus; MI, primary motor cortex; MII, supplementary motor cortex; M3, rostral cingulate motor cortex; M4, caudal cingulate motor cortex; OFC, orbitofrontal cortex; ots, occipital temporal sulcus; paAc, para-auditory cortex caudal; paAlt, para-auditory cortex lateral; paAr,para-auditory cortex rostral; paI, parainsular cortex; ParaSub, parasubiculum; poms, medial parieto-occipital sulcus; pre-SMA, presupplementary motor cortex; PreSub, presubiculum; Pro, proisocortex; proA, proauditory cortex; ProM, proisocortical motor cortex; ProStr, prostriata; ProSub, prosubiculum;ps, principle sulcus; reipt, retroinsular parietal temporal cortex; reit, retroinsular temporal cortex; rf, rhinal fissure; RI, retroinsular cortex; ros, rostral sulcus; SII, secondary somatosensory cortex; sts, superior temporal sulcus; Sub, subiculum; TMA, transitional motor area; TSA, transitional sensory area.
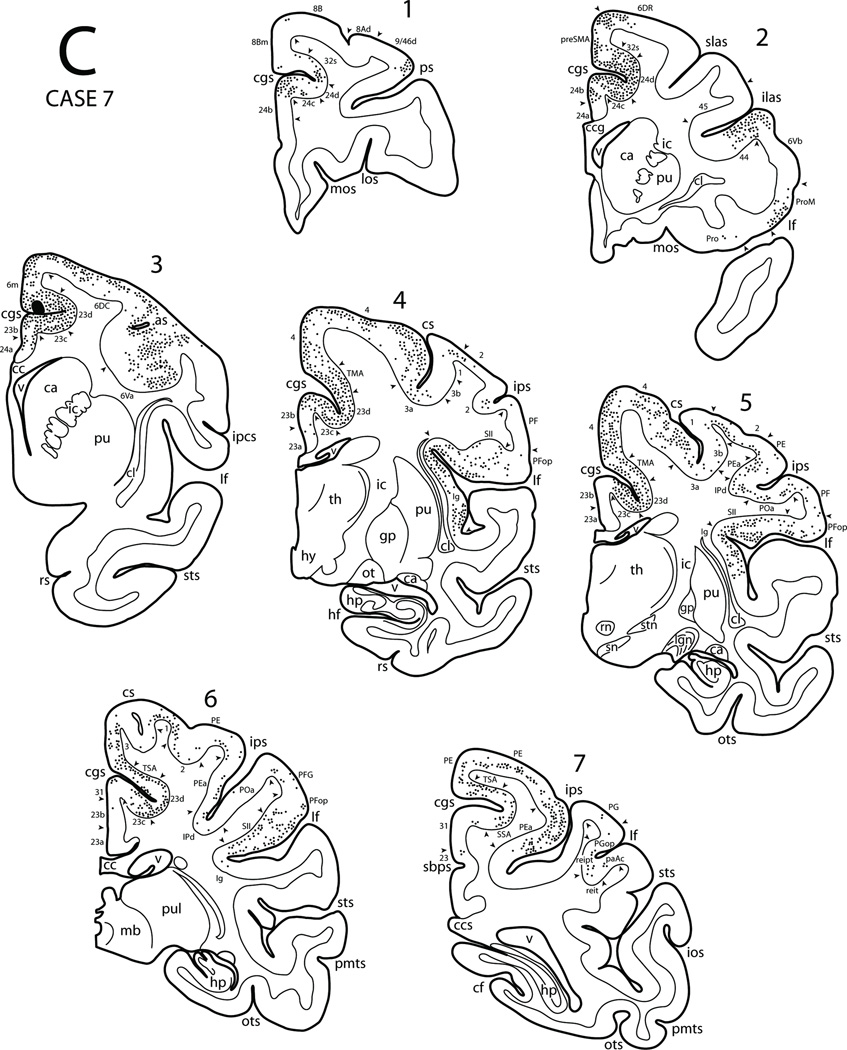
The rostral part of the cingulate cortex is comprised of four subdivisions which have been designated as areas 24a, 24b, 24c and 24d in the ventral-dorsal dimension [36,39,55,122,146,169,193]. The major portion of cingulate area 24 is considered to be proisocortical in nature. The ventral-most subdivision of the anterior cingulate, area 24a, extends from the dorsal surface of the corpus callosum to approximately the lower one-half of the cingulate gyrus (Fig. 1, bottom). It is characterized by densely packed, medium-sized neurons in the lower stratum layers (V and VI). Layers V and VI are inseparable and area 24a lacks layer IV neurons and its layer III contains mostly small-sized pyramidal neurons which are loosely arranged. Layer II has an irregular outer border and blends with layer III (Fig. 2A). Area 24b extends dorsally from area 24a into the medial edge of the lower bank of the cingulate sulcus (Fig. 1, bottom). Area 24b differs from area 24a by having deeply-stained pyramidal neurons forming a band in layer V while layer VI neurons are not as prominent as those in area 24a (Fig. 2B). Area 24c occupies the lower bank of the cingulate sulcus and has more organized deeply-stained pyramidal neurons in layer V as well as relatively more neurons in layer III. The outer border of layer II is better defined than that in areas 24a and 24b (Fig. 2C). Area 24d lies in the depth of the cingulate sulcus. This area stands out by having loosely arranged neurons in all layers and the pyramidal neurons are not as deeply stained as are those in areas 24a, b and c. It is possible to assume that this appearance may be due to the curvature effect in the sulcal depth (Fig. 2D). Although this may be the case, we feel that it is a distinct transitional region which is supported by the unique cytoarchitectural features and the fact that this region is specifically connected with the primary motor (MI) and primary somatosensory (SI) areas (Morecraft and Van Hoesen, 1992, see their Figs. 5A, 7C; Morecraft et al., 2004, see their Fig. 10, coronal sections 1 and 3). Areas 24c and d contain the rostral cingulate motor cortex (M3) [114,122].
Figure 2.
Bright-field photomicrographs of NeuN stained tissue sections showing the cytoarchitecture of the cingulate, medial frontal, dorsal premotor and motor regions. A, area 24a. B, area 24b. C, area 24c. D, area 24d. E, sulcal (s) area 6m. F, area 6m. G, area 6DR. H, area 6DC. I, area TMA. J, area 4. Scale bar in panel F = 1 mm and applies to panels A–F; Scale bar in panel J = 1 mm and applies to panels G–J.
Figure 5.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site in area 24/32 (shown in black in panel B) and cortical distribution of labeled neurons (black dots) in Case 1. Major sulci are opened to show the spatial distribution of labeling within the depths. For abbreviations, see Figure 1.
Figure 7.
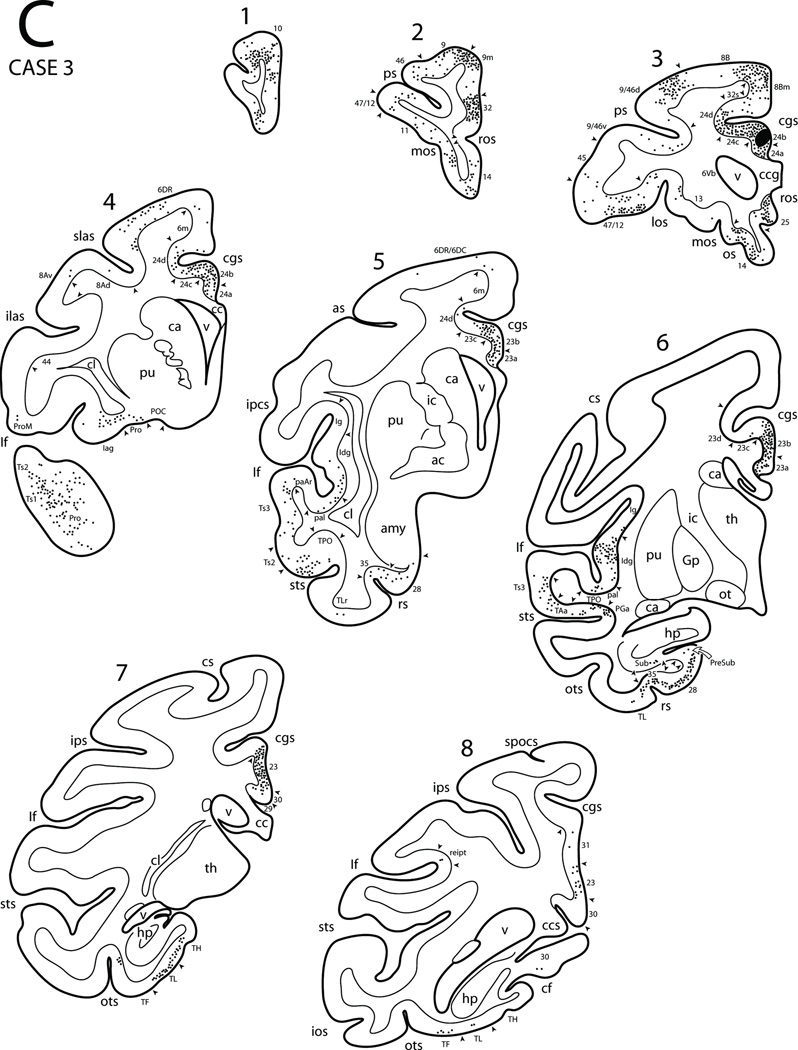
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site in area 24b and cortical distribution of labeled neurons in Case 3. Also shown are eight representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the cortical distribution of labeled neurons. Architectonic areas are indicated by the smaller font size and the small arrow heads depict the location of architectonic borders. Abbreviations: ac, anterior commissure; amy, amygdala; ca, caudate nucleus; cc, corpus callosum; ccg, genu of corpus callosum; ccs, splenium of corpus callosum; cl, claustrum; gp, globus pallidus; hp, hippocampus; ic, internal capsule;los, lateral orbital sulcus; mos, medial orbital sulcus; ot, optic tract; pu, putamen; spocs, superior postcentral sulcus; th, thalamus;v, ventricle. For other abbreviations, see Figure 1.
Figure 10.
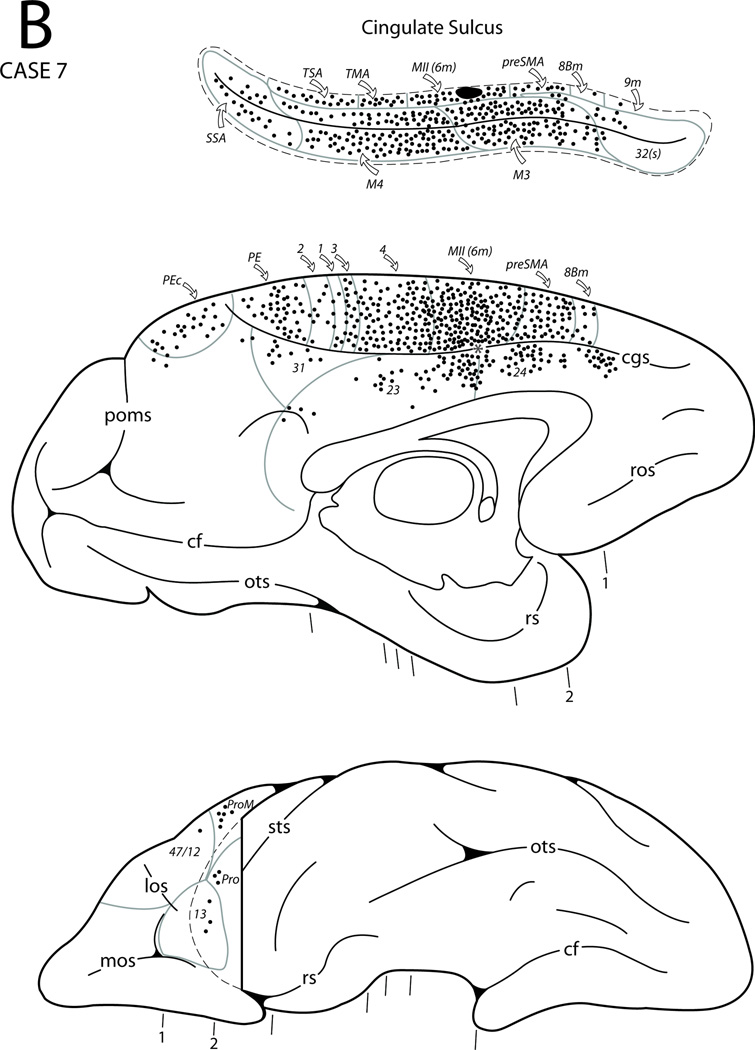
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site in sulcal area 6m and cortical distribution of labeled neurons in Case 7. The asterisk in the anterior cingulate sulcus (see panel B) marks the coronal level containing the injection site in the depth of the sulcus (see expanded cingulate sulcus and coronal section C3). Also shown area seven representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the distribution of labeled neurons. Abbreviations:hy, hypothalamus; pmts, posterior middle temporal sulcus; pul, pulvinar; rn, red nucleus; sbps, subparietal sulcus; sn, substantia nigra;stn, subthalamic nucleus. For other abbreviations see Figures 1, 3, 6, 7 and 9.
In the upper bank of the cingulate sulcus, there is a significant change in the architecture. The upper stratum layers (II and III) are more marked, that is, more cellular as compared with the lower stratum layers (V and VI). The cortex in this region of the cingulate sulcus contains various areas in the rostral to caudal direction in line with the dorsally-adjacent premotor and precentral regions. The rostral part of the upper bank of the cingulate sulcus contains the ventral extension of area 6m. Specifically, the sulcal part of area 6m is characterized by having prominent neurons in layer III which are mostly medium-sized, and virtually absent layer IV neurons. Layers V and VI contain medium to small sized pyramidal neurons with some large pyramidal cells. There is an emergence of mild columnarity as well (Fig. 2E). Further caudally, the upper bank of the cingulate cortex contains an area which has been designated the transitional motor area (TMA) [120]. TMA has all the characteristics of the dorsally adjacent area 4 although it lacks the large Betz cells (Fig. 2I).
Dorsal to the cingulate sulcus the cortex on the medial hemispheric surface contains three distinct areas in the premotor and precentral regions. The rostral part of area 6m has the characteristics of the premotor area on the dorsolateral hemispheric surface (see below). It contains prominent medium-sized pyramidal neurons in layers III and V abutting each other. They are more compact and darkly stained in comparison to those located dorsolaterally [6] (Fig. 2F). The caudal portion of area 6m contains scattered large Betz cell-like neurons in layer V. Collectively, area 6m corresponds to the physiologically characterized supplementary motor area (MII) (Fig. 1, bottom). Further caudally is located the medial portion of area 4. This area contains large Betz cells in layer V which are arranged into clusters (Fig. 2J). Physiologically, this region corresponds to the leg representation of the primary motor cortex (MI).
On the lateral surface, the dorsal portion of premotor area 6 can be divided into two major regions [6,101]. The rostral premotor area (area 6DR) is characterized by columnar appearance, having a typical agranular cortex, that is, an absence of layer IV. Pyramidal neurons of layers III and V almost abut forming a central band of medium-sized pyramidal neurons (Fig. 2G). Caudal to the rostral portion of area 6 (6DR) is a region that has been termed area 6DC. In this area the basic architectural characteristics are similar to the features of area 6DR. In addition however, area 6DC has large pyramidal cells scattered in layer V of the Betz cell type (Fig. 2H). Both divisions of the dorsal portion of area 6 extend ventrally to the level of the spur of the arcuate sulcus and into the dorsal bank of the upper limb of the arcuate sulcus Fig. 1, top). Caudal to area 6DC is the dorsal portion of area 4. Like area 6 this area is agranular, thus does not have a distinctive band of cells forming a layer IV. In this cortex, there are clusters of large pyramidal neurons of the Betz cell type in layer V. This area extends caudally into the rostral bank of the central sulcus where it borders area 3a in the depth of the central sulcus. These large pyramidal neurons are distributed in the leg, trunk and hand representations of MI.
Thus, it seems that one can follow successive laminar changes from the proisocortical cingulated cortex (areas 24a, b, c and d), leading to the TMA in the upper bank of the cingulate sulcus and areas 6m and 4 on the medial wall. Whereas in the cingulate cortex, there is greater emphasis on the lower stratum neurons, the cortex of the upper bank of the cingulate sulcus has prominent upper stratum layers. Area 6 on the medial and dorsolateral surface is characterized by the pyramidal neurons of layers III and V forming a band in the absence of layer IV neurons. The caudal portion of area 6 on the dorsal lateral surface of the cerebral hemisphere shows, in addition, evidence of scattered pyramidal neurons (of the Betz cell type) in layer V. The medial and dorsolateral portions of area 4, in contrast, have a significant number of large pyramidal neurons of the Betz-cell type in layer V. The dorsal portion of area 4 is preceded by TMA in the upper bank of the cingulate sulcus which also has few large neurons in layer V.
Fluorescent Retrograde Tracer Studies
The corticocortical connections from 15 experimental injection cases with fluorescent retrograde tracers (FRT) placed within the anterior cingulate, medial frontal and dorsofrontal cortex are described (Figs. 3,4). First we describe the cingulate injection cases followed by premotor (medial then lateral) and finally precentral motor injection cases (Figs. 5–16). We want to point out that the results obtained from each injection site cannot be considered representative of the connectivity of the entire area as the overall connections of a single region are likely to vary to some degree. Since, a the major goal of the present study was to investigate the overall trend of the corticocortical connections that characterize these different regions, proceeding from the anterior cingulate area through the medial and dorsolateral frontal regions, we elected to make a small injection in predetermined parts of each major architectonic area. The results of these findings are presented below.
Figure 3.
Composite diagram to show the location of the Fast blue (FB), diamidino-yellow (DY) and fluororuby (FR) injection site in Cases 1 through 15 (C1–C15) located in the anterior cingulate, cingulated motor, dorsal premotor and precentral motor areas. Abbreviations: ilas, inferior limb of arcuate sulcus; slas, superior limb of arcuate sulcus. For other abbreviations, see Figure 1.
Figure 4.
Low power photomicrographs of cingulate, dorsal premotor and motor areas to show the location of FRT injections in Nissl stained tissue preparations. The inset in each panel is a higher power fluorescent image of the injection site. A, Case 2. B, Case 3. C, Case 6. D, Case 10. E, Case 7. F, Case 11. G, Case 13. H, Case 9. I, Case 14. The asterisk in panel C denotes a small, localized gray matter infarction. Scale bar = 1mm and applies to all Nissl stained images. Abbreviations: DY, diamidino yellow; FB, fast blue. For other abbreviations, see Figure 1.
Figure 16.
Diagrammatic representation of the lateral (A) and medial (B) surfaces of the cerebral hemisphere to show the injection site in the leg region of M1 and cortical distribution of labeled neurons in Case 14. The box diagram above the medial wall in panel B shows the stimulation map in relation to the injection site location. Also shown are four representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the distribution of labeled neurons. Abbreviations: T, toe; Ta, tail. For other abbreviations see Figures 1, 3, 6, 7, 9, 10, 12 and 14.
Rostral Cingulate Gyrus Cases
In Case 1 an injection of fast blue (FB) was located in the anterior perigenual part of area 24 that extended into the caudal-most part of area 32 (Figs. 3, 5). Numerous labeled neurons were found rostral to the injection site in areas 32 and 10 as well as ventral to the injection site in areas 14 and 25. Dorsally, scattered neurons were noted in area 9m. Caudal to the injection site labeled cells were found in area 24. Few neurons were found in posterior cingulate area 23, retrosplenial areas 30 and 29 including the upper bank of the calcarine sulcus. Labeled neurons were also observed in the rostral cingulate sulcus that involved area 32s and 9m, as well as the fundus (area 24d) and lower bank (area 24c) more caudally corresponding to the region of the rostral cingulate motor cortex (M3). Caudally in the cingulate sulcus a small patch of labeled neurons were found in the lower bank of the sulcus corresponding to the region of the caudal cingulate motor cortex (M4) and supplementary sensory cortex (SSA, or area PEci). On the lateral surface of the hemisphere, labeled neurons occurred in prefrontal areas 8B, 9, 46 with some labeled neurons in areas 9/46d, 9/46v and 47/12. On the orbitofrontal surface of the prefrontal cortex discrete clusters of labeled neurons were found in areas 47/12, 13, 14, 11, 10 and Pro. In the cortex lining the Sylvian fissure, labeled cells were found in the granular (Ig), dysgranular (Idg) and agranular (Iag) parts of the insula, the gustatory area in the upper bank and in areas paI, paAr and proA of the lower bank. Labeling was also located in temporopolar area Pro as well as areas Ts1, Ts2, Ts3 and paAlt of the superior temporal gyrus (STG). In the superior temporal sulcus (STS), labeled neurons were observed in areas TAa, TPO and PGa of the upper bank of the sulcus. Very few labeled cells were noted in area IPa of the STS. In the ventromedial temporal region, labeling was observed in the temporopolar proisocortex (Pro), perirhinal (area 35), prorhinal and entorhinal (area 28) cortex (Fig. 17A). A few labeled cells were noted in the rostral portion of area TL (TLr) lateral to the rhinal sulcus. Posteriorly, labeled cells were found in the rostral parahippocampal gyrus including areas TF, TL and TH. In the hippocampal formation, FB labeled cells occurred in the rostral part of area CA1 (subfield according to Lorente de Nó, 1934) and throughout the prosubiculum, and subiculum. A few cells were also noted in the cortical transition area (CTA; Rosene and Van Hoesen, 1987), and parasubiculum.
Figure 17.
Photomicrographs showing representative examples of FRT labeled cells in six selected Cases. In each panel the cortical gray matter layers are indicated by Roman numerals. A) Fast blue labeled cells in area 28 following an injection of FB in perigenual areas 24/32 in Case 1. B) Fast blue and diamidino-yellow labeled cells in area TH following an injection of FB and DY in the rostral cingulate motor cortex in injection Case 5 (FB) and Case 6 (DY) respectively. Both injections were made in the same hemisphere of the same animal. C) Diamidino-yellow labeled cells in area SII following an injection of DY into the sulcal region of area 6m in the upper bank of the cingulate sulcus in Case 7. D) Fast blue labeled cells in area 4 (MI) following an injection of FB in the arm region of area 6m (MII) in Case 11. E) Diamidino-yellow labeled cells in area PEci (SSA) following an injection of DY into area 6DC in Case 13. F) Diamidino-yellow labeled cells (white arrows) in the caudal part of M3 (area 24d) following an injection of DY into the medial surface of MI (area 4) in Case 14. Scale bar in A = 200 µm and also applies to panels C, D and E. Scale bar in B = 100 µm. Scale bar in F = 50 µm. Abbreviations: LD, lamina dissecans.
In summary, the most rostral portion of the cingulate proisocortex seems to be related to the cingulate motor areas (M3 and M4) on the one hand and to selected areas in the prefrontal, rostral temporal, paralimbic as well as limbic areas including the insula.
In Case 2 a diamidino-yellow (DY) injection was placed in the rostral portion of area 24a above the corpus callosum (Figs. 3, 4A, 6). The location of this injection was caudal to that in Case 1. The basic pattern of the resulting label was similar to that in case one but with some differences. Thus, the labeled neurons were noted in areas 24, 32 and 25 rostrally as well as in areas 24 and 23 caudally with some labeled neurons in area 31, retrosplenial cortex (areas 29 and 30), and the upper bank of the calcarine sulcus corresponding to area prostriata [116]. In the cingulate sulcus, labeling was found in areas 32 and 9m, the cingulate motor areas (M3 and M4), and supplementary sensory cortex (SSAcorresponding to cytoarchitectonic area PEci). Other areas showing labeled neurons were in medial portions of areas 9 and 8B as well as the preSMA. On the orbitofrontal surface labeled neurons were noted in areas 10, 11, 47/12 and the rostral part of area 14. A few labeled neurons were also found in area 13 and in the orbital proisocortex (Pro). On the lateral surface of the frontal lobe, labeled neurons occurred in areas 6DR, 8B, 9, 10, 8Ad, 9/46d, 9/46v, 46, 47/12 and 45. A few DY labeled neurons were noted in parietal areas PG, Opt and PEa including PGm medially. Within the temporal lobe the Sylvian fissure contained a few labeled neurons in the gustatory area, in the ventral portion of the insular cortex (areas Iag, Idg and Ig) and in the adjacent portion of the para-insular region in the supratemporal plane (areas paI, paAr and proA). The temporal polar cortex displayed labeled neurons on its lateral surface in area Pro with distinct patches of labeled cells extending posteriorly in areas Ts1, Ts2 and Ts3 of the superior temporal gyrus. Areas TAa, TPO and PGa in the superior temporal sulcus also showed labeled neurons. Finally, labeled neurons were also observed in the ventromedial portion of the temporal lobe. These included the medial temporal polar proisocortex, perirhinal cortex, prorhinal cortex and entorhinal cortex as well as the rostral portion of areas TF, TL and TH of the parahippocampal gyrus. In the hippocampal region DY labeled cells were noted only in the subiculum.
Figure 6.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site in area 24a and cortical distribution of labeled neurons in Case 2. Abbreviations: los, lateral orbital sulcus; mos, medial orbital sulcus. For other abbreviations, see Figure 1.
In summary, the rostral portion of area 24a is connected with the cingulate motor areas and the rostral premotor area (6DR). Its other connections are directed from the prefrontal cortex, caudal parietal area PG, temporal lobe and the rostral portion of the superior temporal region as well as to the paralimbic and limbic areas.
In Case 3 an FB injection was placed slightly dorsal to the previous case involving primarily area 24b (Figs. 3, 4B, 7). The overall patterns of the projections were similar to those observed in the Case 2 with some notable differences. Thus, Case 3 showed additional labeling in MII, 6DC, and ProM in the frontal cortex and area PEc and PGop of the partetal cortex. This case also showed projections from area reipt and SII of the Sylvian fissure, area IPa of the STS as well as the presubiculum and rostral part of area CA1 of the hippocampal region. Unlike Case 2, labeling was not found in area Opt.
In summary, area 24b has distinct connections with the cingulate motor areas M3 and M4, supplementary motor area (MII) as well as premotor area 6DR. Its other connections are with the prefrontal cortex, the caudal parietal lobe, the rostral superior temporal region and paralimbic and limbic areas similar to those found with cases 1 and 2.
In Case 4 a single of injection of FB was placed in the supracallosal part of area 24 (Fig. 3) involving subsectors a and b (Case not illustrated). There was some spread of tracer in the region of the induseum griseum over the corpus callosum. Resulting label was noted rostrally in areas 24, 32, 25 and 14. Caudally, labeling occurred in areas 24, 23 and 31 and extended into the dorsal part of the retrosplenial region involving areas 29 and 30. Below the splenium of the corpus callosum, labeling extended from area 23 into the rostral part of the upper bank of the calcarine sulcus to involve area prostriata. Above the injection site, labeling occurred in area 32, and the lower bank and fundus of the cingulate motor areas M3 and M4. Significant labeling also occurred in the upper bank of the cingulated sulcus extending onto the medial wall of the hemisphere above the sulcus. This involved the medial part of areas 9 and 8Bm including the preSMA and MII. Caudally labeling was noted in the rostral part of the supplementary sensory area (SSA) of the upper bank of the cingulate sulcus. On the lateral surface, predominate label occurred in premotor areas 6DR and 6DC. Additional labeled neurons were noted in the rostroventral portion of area 6 (6V) and in adjacent area 44. In the parietal lobe, few labeled neurons were found in area PFG, PG and area POa of the ventral bank of the intraparietal sulcus. In the prefrontal cortex, labeling involved areas 8Ad, 8B, 9, 9/46d, 9/46v and 46. Some label occurred in area 45 within and around the inferior limb of the arcuate sulcus, area 47/12 as well as in area ProM. On the orbital surface of the frontal lobe, discrete patches of labeled neurons were noted in areas 47/12, 13 and 11 as well as in area 14 of the gyrus rectus. In the temporal lobe, loci of labeled neurons were noted in the Sylvian fissure that included the insula (Iag and Idg and Ig) as well as in SII, PFop and PGop in the upper bank. A few neurons were also noted in areas reipt and reit in the caudal-most part of the Sylvian fissure. In the lower bank of the Sylvian fissure labeling occurred in areas paI, paAr and proA. In the superior temporal gyrus, significant labeling occurred in the temporal polar cortex (Pro) and in areas Ts1, Ts2 and Ts3. Adjoining cortex in the superior temporal sulcus also displayed labeling in areas TAa, TPO, PGa and IPa. In the ventromedial temporal cortex, heavy labeling was noted in the perirhinal (area 35), prorhinal and entorhinal (area 28) regions and a few labeled cells were found in area TLr lateral to the rhinal sulcus. Labeling was also noted in the parahippocampal gyrus involving the rostral parts of areas TH, TL and TF. In the hippocampal region, fast blue labeled cells were found in the subiculum and prosubiculum. Finally, a few labeled neurons were noted in the CA1 sector of the hippocampus, parasubiculum, cortical transition area (CTA, see Rosene and Van Hoesen, 1987) and hippocampal-amygdaloid transition area (HATA, see Rosene and Van Hoesen, 1987).
In summary, rostral cingulate areas 24a and 24b are connected with the cingulate motor areas (M3 and M4), MII as well as with the lateral premotor areas 6DR and 6DC. Its other connections are with the prefrontal cortex, parietal cortex and superior temporal regions as well as the limbic and paralimbic areas.
Rostral Cingulate Sulcus Cases (Lower Bank)
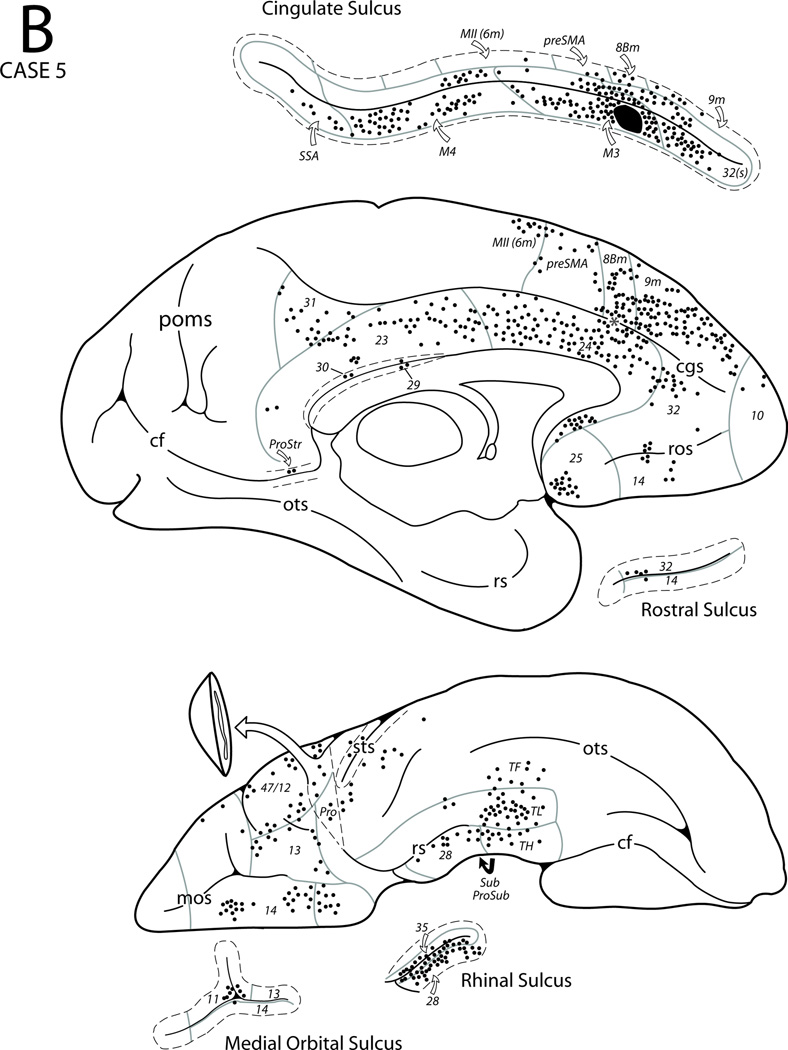
In Case 5 a single injection of FB was placed in the rostral portion of the lower bank of the cingulate sulcus in area 24c (the rostral portion of M3) (Figs. 3, 8). The resulting label was noted in the medial surface above the injection site in MII, preSMA, and in areas 8Bm, 9m and 10. Rostral and below the injection site labeled neurons were found in areas 24a and b with some clusters of labeled cells in areas 32, 25 and 14. Caudally, the labeled cells extended into area 24 and into the rostral part of areas 23a and 23b with some noted in areas 30, 29, 31 and prostriata. Within the cingulate sulcus, labeling was found in area 32s rostral to the injection site as well as in M3 (non-injected parts), M4, and SSA posterior to the injection site. In the premotor region distinct patches of labeled neurons were noted in areas 6DC and 6DR. In the frontal lobe cortex labeled neurons were found in areas 47/12, 13, 14 and Pro on the orbital surface. On the lateral surface of the prefrontal cortex, labeled neurons were observed in 8B, 8Ad, 9, 9/46d, 9/46v as well as the midportion of area 46 above and below the principal sulcus. Also, labeled cells were found in areas 47/12 and ProM as well as in ventral pre-motor areas 6Vb and 44 in the lower limb of the arcuate sulcus. In the Sylvian fissure, some labeling occurred in SII and significant numbers of labeled neurons were found in most of the insular cortex (Iag, Idg and Ig) except for its most caudal part. In addition, parainsular areas paI and proA also contained a few labeled cells and some were also found in area reipt more caudally. In the superior temporal region, a few labeled neurons were noted in areas Ts1 and Ts2 including the adjacent temporal proisocortex. Small clusters of neurons occurred in the upper bank of the superior temporal sulcus in areas TAa and TPO particularly at the mid-portion of the sulcus and a few labeled neurons were also noted in areas PGa and IPa in the fundus. In the parietal lobe, a few FB labeled neurons were observed in the ventral part of areas PFG and PG including opercular areas PFop and PGop. In the ventromedial temporal lobe, labeled neurons were observed in the perirhinal cortex with some labeled cells located in prorhinal and entorhinal cortex, and in the rostral part of the medial temporopolar proisocortex. Labeled neurons were also observed in areas TF, TL and TH of the parahippocampal gyrus (Fig. 17B). In the hippocampal region, FB labeled neurons were found in the prosubiculum and subiculum with very few in the cortical transition area (CTA).
Figure 8.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site in area 24c and cortical distribution of labeled neurons in Case 5. The asterisk in the anterior cingulate sulcus (see panel B) marks the coronal level containing the injection site in the depth of the sulcus (see expanded cingulate sulcus). For abbreviations see Figures 1, 3, 6 and 7.
In summary, the rostral portion of M3 is connected with the caudal cingulate motor area (M4) as well as with MII and premotor areas 6DR and 6DC. Its other connections are with the prefrontal, rostral inferioparietal, superior temporal, limbic and paralimbic areas including the insula.
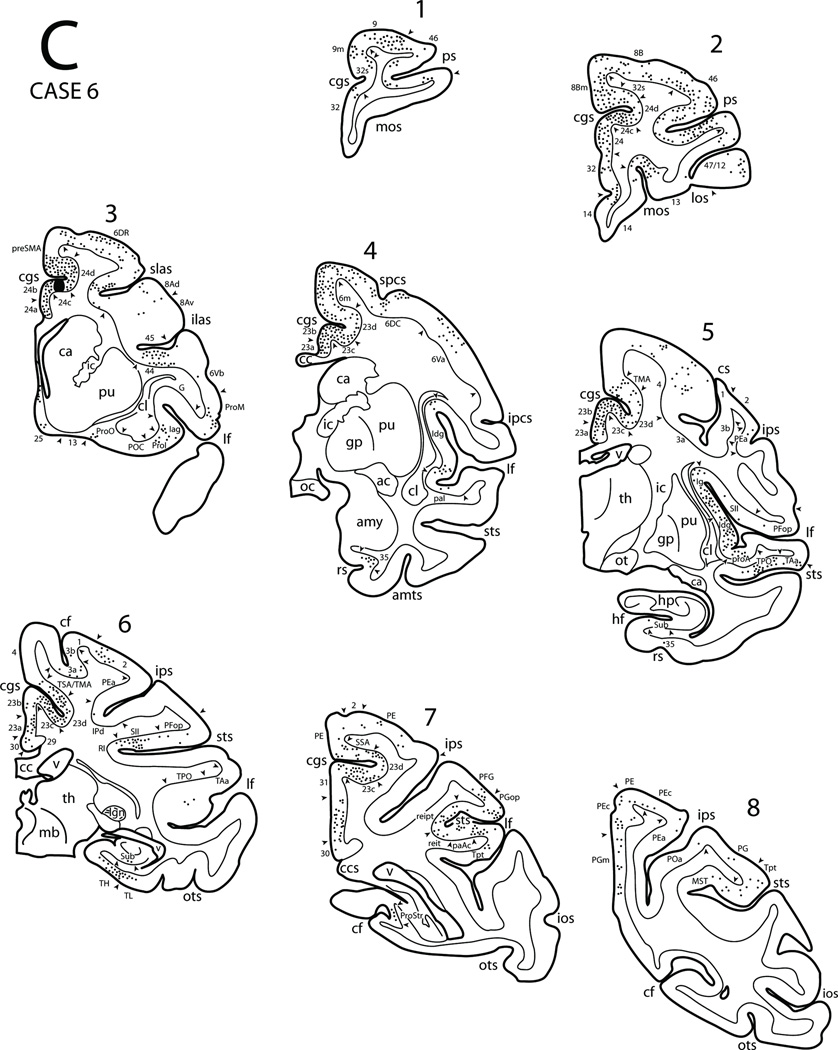
In Case 6 an injection of DY was placed in the lower bank of the rostral cingulate sulcus involving the mid-region of area 24c (M3) (Figs. 3, 4C, 9). This injection was located slightly caudal to the injection site in Case 5. The overall patterns of the resulting labeled neurons were similar to those observed in the Case 5 with some notable differences. For example, additional labeling in Case 6 was found in frontal areas 4, 6Va, and 45. Additional parietal areas also showed labeling in Case 6 including the dorsal parts of areas 3,1 and 2, areas IPd and PEa of the intraparietal sulcus, as well as areas PE and PEc of the superior parietal lobule. Furthermore, labeling was found in areas paAc and reit of the Sylvian fissure, MST of the STS, and area Tpt of the posterior temoporal region. Finally, although labeling was found in the subiculum in Case 6 (as noted in Case 5), we did not find labeling in the prosubiculum or the CTA as observed in Case 5. (Fig. 17B).
Figure 9.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site in area 24c and cortical distribution of labeled neurons in Case 6. The asterisk in the anterior cingulate sulcus (see panel B) marks the coronal level containing the injection site in the depth of the sulcus (see expanded cingulate sulcus and coronal section C3). Also shown are eight representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the distribution of labeled neurons. Abbreviations:amts, anterior medial temporal sulcus; amy, amygdala; ipcs, inferior precentral sulcus; lgn, lateral geniculate nucleus; mb, midbrain; oc, optic chiasm; spcs, superior precentral sulcus. For other abbreviations see Figures 1, 3, 6 and 7.
In summary, the mid-region of M3 has significant connections with the caudal cingulate motor area (M4), MII, premotor areas 6DR and 6DC as well as the precental motor area. Its other connections are with prefrontal, parietal, superior temporal, limbic and paralimbic areas including the insula.
Rostral Cingulate Sulcus Cases (Upper Bank)
In Case 7 a DY injection was placed in the upper bank of the cingulate sulcus and involved the sulcal portion of area 6m (MII) (Figs. 3, 4E, 10). On the medial surface, the resulting labeled neurons were observed throughout both banks of the cingulate sulcus except for its rostral-most portion. From rostral to caudal, in the upper bank of the cingulate sulcus labeled neurons were found in the sulcal part of areas 32 and 6m, and TMA, TSA and SSA. In the lower bank, the labeled neurons were noted in M3 and M4. The caudal-most portion of the cingulate region also displayed labeled neurons in areas 23, 31, and PEc. Labeled neurons were noted in the rostral portion of the cingulate gyrus mostly in area 24b with some noted in area 24a. In the cortex dorsal to the cingulate sulcus, labeled neurons were found in areas 8Bm, preSMA, MII (area 6m), the medial portion of area 4 as well as in the medial portions of areas 3, 1, 2 and PE. In sharp contrast to the previous case, in the prefrontal cortex only a few labeled cells were found in areas 46, 9/46d, 9/46v, 8B and orbital areas 13, Pro and 47/12. A significant number of labeled neurons occurred in the dorsal portion of area 4 including the rostral bank of the central sulcus. Labeled neurons were also found in premotor areas 6DC and 6DR as well as in area 8B. The ventral portion of premotor area 6 (areas 6Va and 6Vb), area ProM, and the adjacent caudal bank of the lower limb of the arcuate sulcus (area 44) also contained labeled neurons. In the upper bank of the Sylvian fissure, patches of labeled neurons occurred in areas SII, PFop, PGop and reipt (Fig. 17C). Labeled neurons were observed in the insula (Iag, Idg and Ig) as well as in areas RI, reit, paAlt and paAc in the lower bank of the Sylvian fissure. A few labeled neurons were found in areas Tpt, TPO and TAa of the upper bank of the STS. Finally, in the parietal lobe DY labeling occurred in the dorsal portions of areas 3, 1 and 2 as well as in areas PE, PEc, PEa, IPd, POa, PF, PFG and PG.
It seems that the patterns of labeled cells in this Case are different from those in the previous Cases. Sulcal area 6m is connected with cingulate motor areas (M3 and M4), the gyral part of MII, lateral premotor areas (6DR and 6DC) and MI. Its other connections are with postcentral and posterior parietal cortex. Sulcal area 6m is also connected with SII, other areas in the Sylvian fissure and insula. It has only limited connections with the prefrontal regions.
In Case 8 an injection of DY was placed into the upper bank of the cingulate sulcus in a location that was slightly caudal to Case 7 (Fig.3). This case is not illustrated. The overall patterns of the resulting labeled neurons were similar to those observed in Case 7 with some significant differences. For example, labeling in Case 8 was not found in prefrontal areas 8Bm, 8B,and 44, orbital areas 13 and Pro, as well as ventral premotor areas 6Va and 6Vb. Similarily, labeling was not found in parietal areas PF, PFG, PG, PFop, PGop and POa nor in areas reit or Tpt.
In summary, the basic pattern of the connections seen in this Case was similar to that seen in the previous Case with the exception of significant decline in the number of prefrontal, orbitofrontal and inferior parietal connections. Thus, the main connections of Case 8 were with the cingulated proisocortical areas, cingulate motor areas (M3 and M4) as well as with MII. This area is also connectionally related to premotor and precentral areas. Its other connections are with the dorsal and medial parietal regions and very minimal connections with the temporal and prefrontal regions.
In Case 9 an injection of FB was placed in the lip of the upper bank of the cingulate sulcus (Figs. 3, 4H, 11). The injection site involved the cortex of the TMA, near the junction of medial area 4 (see Figs. 11A and Fig 11B, panel 2). Heavy labeling spread rostrally in the upper bank of the cingulated sulcus involving sulcal area 6m. Caudal to the injection, site labeling occurred in TSA and SSA. Above the cingulate sulcus, labeling was noted in the preSMA, area 6m and medial area 4 as well as medial parietal areas 3, 1, 2, PE and PEc. In the lower bank of the cingulate sulcus there were patchy clusters of labeled neurons in cingulate motor areas (M3 and M4) as well as in SSA. Very few labeled neurons were noted in areas 24b, 23b, 31 and PGm. On the lateral frontal surface, labeling occurred in the dorsal part of area 4 as well as the premotor areas 6DC and 6DR. A few FB labeled cells were also noted in the ventral premotor area 6Va below the arcuate spur. In the lateral parietal cortex, labeled neurons were located in areas 3, 1, 2, PE, PEa and IPd. Within the Sylvian fissure, patches of labeling were noted in the posterior insula (Idg and Ig), SII, and in areas RI, reipt, reit and paAc. In the superior temporal region, very few labeled cells were noted in areas TAa and TPO of the STS.
Figure 11.
Diagrammatic representation of the lateral (A) and medial (B) surfaces of the cerebral hemisphere to show the injection site in the transitional motor area (TMA) and cortical distribution of labeled neurons in Case 9. Also shown are four representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the distribution of labeled neurons. For abbreviations see Figures 1, 3, 6, 7.
Thus, the pattern of labeling in this Case is basically similar to that of the previous Case. That is, TMA is connected minimally with the cingulate proisocortex (areas 24a and b) but significantly with cingulate motor areas (M3 and M4), MII, 6DR, 6DC and MI. Other connections are with the postcentral and posterior parietal cortex including SSA, SII and insula. Only minimal connections are established with the temporal lobe.
Medial and Dorsal Premotor and Motor Cases
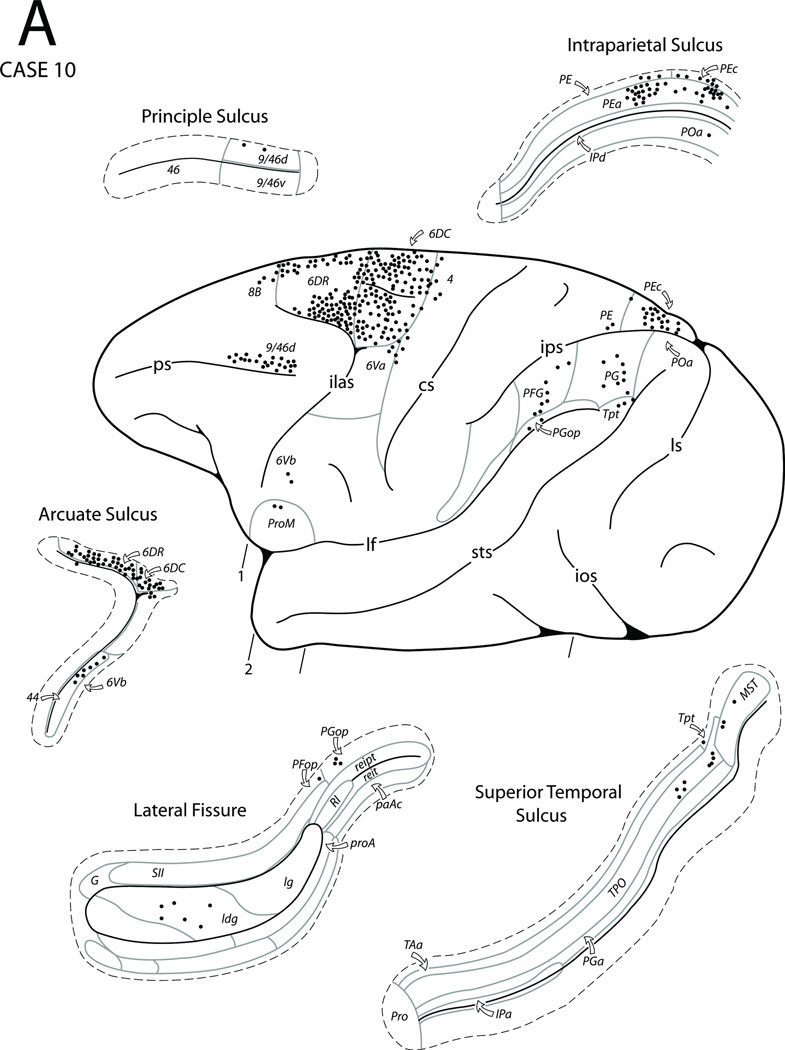
In Case 10 a FB injection was placed in the medial surface of the superior frontal gyrus and involved the area corresponding to the preSMA as determined by being unresponsive to microstimulation (Figs. 3, 4D, 12). The resulting labeled neurons on the medial surface were noted in area 6m (MII) and the preSMA as well as prefrontal areas 8Bm and 9m. The labeled neurons also extended into the rostral portion of the adjacent upper bank of the cingulate sulcus to involve the sulcal portions of areas 32 and 6m. Some neurons were also noted in TMA. In the lower bank and fundus of the cingulate sulcus clusters, of labeled neurons occurred in both cingulate motor areas as well as caudally within the SSA. Some labeled neurons were also noted in the areas 24a, 24b (cingulated proisocortex) and in area 31 of the cingulate gyrus as well as in areas PEc and PGm of the medial parietal cortex. On the lateral surface of the cerebral hemisphere FB labeling was largely confined to the dorsal region of the frontal lobe. A substantial number of labeled neurons occurred in premotor areas 6DC and 6DR while a few were found in the rostral part of area 4. Other areas displaying labeled neurons in the frontal lobe included the dorsocaudal portion of area 9/46d, area 44 in the caudal bank of the inferior limb of the arcuate sulcus, and a small patch of labeled cells in ProM as well as a few in ventral premotor areas 6Va and 6Vb. A few scattered patches of labeled neurons were also noted in the dysgranular portion of the insula. In the parietal lobe distinct patches of labeled neurons were observed in area PEc and the adjacent portion of area PEa as well as in areas PFG, PFop, PGop, and PG of the inferior parietal lobule. Finally, a few labeled neurons were noted in areas TPO, Tpt and MST of the caudal superior temporal sulcus.
Figure 12.
Diagrammatic representation of the lateral (A) and medial (B) surfaces of the cerebral hemisphere to show the injection site in the preSMA and cortical distribution of labeled neurons in Case 10. The box diagram below the medial wall in panel B shows the stimulation map in relation to the injection site location. Also shown are four representative coronal sections (C) taken at levels shown in panels A and B to depict the location of the injection site and the distribution of labeled neurons. Abbreviations: D, digit; El, elbow;NR, no response; Sh, shoulder. For other abbreviations see Figures 1, 3, 6, 79, and 10.
Thus, the main connections of the preSMA are with the cingulate proisocortex, cingulate motor areas, MII, lateral premotor areas and MI. Its other connections are with the posterior parietal cortex, posterior STS as well as SSA and insula.
In Case 11 the FB injection was placed into the arm representation of MII (architectonic area 6m) as defined electrophysiologically (Figs. 3, 4F,13). The basic pattern of labeled neurons was similar to Case 7 which had an injection in the sulcal portion of area 6m with some differences. The overall pattern of connections also resembled the previous preSMA injection case (Case 10) with some notable differences. On the medial surface, labeled neurons were noted in MII rostral and caudal to the injection site as well as in the caudal portion of the preSMA. The labeled neurons also extended into the adjacent portion of the upper bank of the cingulate sulcus to involve the sulcal portion of area 6m and the caudal part of the sulcal portion of area 32. Additionally, labeled neurons were observed in the depth and the lower bank of the cingulate sulcus involving both cingulate motor areas. Below the cingulate sulcus labeled neurons were noted in areas 24b and 23b of the cingulate gyrus adjacent to the cingulate motor areas. Caudally, labeled neurons were observed in areas 31, SSA, and PEc. On the lateral surface, numerous labeled neurons were noted in the dorsolateral portion of MII on the convexity and in premotor areas 6DR and 6DC. Unlike the preSMA injection case (Case 10), significant numbers of labeled cells were observed in the mid-portion of area 4 that extended into the rostral bank of the central sulcus (Fig. 17D). The caudal bank of the lower portion of the arcuate sulcus also displayed many labeled neurons involving area 44. Few labeled neurons were observed in areas 6Va, 6Vb and ProM. Few labeled neurons were also noted in areas 8B and 8Ad as well as the caudal portion of area 9/46d. Discrete loci of labeled neurons were observed in the pericentral portion of the Sylvian fissure (SII). Scattered clusters of labeled cells were found in the insula (Iag, Idg and Ig). In the parietal lobe discrete patches of labeled neurons were observed in areas 2, PE, PEc, PG and Opt. In the intraparietal sulcus a few labeled neurons were observed in areas IPd and POa in its depth and lower bank, respectively, of the intraparietal sulcus, and as well as in area PEa in its upper bank. Some labeled neurons were found in retroinsular area RI, and in areas PFop and PGop in the Sylvian fissure.
Figure 13.
Diagrammatic representation of the lateral (A) and medial (B) surfaces of the cerebral hemisphere to show the injection site in the arm region of MII and cortical distribution of labeled neurons in Case 11. The box diagram below the medial wall in panel B shows the stimulation map in relation to the injection site location. Abbreviations: Ea, ear, L, leg; Wr, wrist. For other abbreviations see Figures 1, 3, 6, 7, 10 and 12.
In summary, the mid-region of MII is connected with cingulate proisocortex, cingulate motor areas (M3 and M4), other parts of MII, premotor areas 6DC and 6DR as well as the upper portion of MI. Its other connections are with the parietal lobe including SII, SSA and insula as well as only minor connections with the prefrontal cortex.
In Case 12 FB was placed near the central portion of premotor area 6DR where stimulation failed to result in motor responses (Figs. 3, 14). Resulting labeled neurons were seen on the lateral surface in the cortex surrounding the area 6DR injection as well as in adjacent area 6DC and a few in the rostral part of area 4. Some labeling was observed in ventral area 6 and ProM as well as in areas 44 and 45 of the inferior limb of the arcuate sulcus. Beyond the arcuate sulcus, in the prefrontal cortex, labeling was found in areas 8Ad, 8B and the adjacent portions of areas 9/46d, 9 and 46. Caudal to the central sulcus, labeling was found in areas IPd and POa of the intraparietal sulcus, and PFG of the inferior parietal lobule. In the Sylvian fissure, labeled neurons were found in the insula (Iag, Idg and Ig sectors) as well as the rostral part of SII and areas RI, reit and reipt more caudally. In the lower bank of the Sylvian fissure, discrete clusters of labeled neurons were found in areas paI and proA. Patches of labeled neurons occurred in areas Ts2, Ts3 and Tpt of the superior temporal gyrus. The upper bank of the superior temporal sulcus also displayed significant labeling in areas TPO with occasional FB labeled cells in areas TAa, PGa and IPa. On the medial surface of the hemisphere, labeling was found in areas 9m, 8Bm, preSMA and 6m (MII) extending into the upper bank of the cingulate sulcus including the sulcal portions areas 32 and 6m as well as TMA. Some labeled neurons also occurred in the fundus and lower bank of the of the cingulate sulcus corresponding to the cingulate motor areas and in SSA. In the cingulate gyrus, labeled neurons were found in areas 24 and 23 as well as the retrosplenial cortex (area 29 and 30). More caudally, FB labeled cells occupied areas 31, PGm and PO. Few neurons were also observed in areas 47/12 and 13 of the orbitofrontal surface and TF of the parahippocampal gyrus.
Figure 14.
Diagrammatic representation of the lateral (A), medial and ventral (B) surfaces of the cerebral hemisphere to show the injection site in area 6DR and cortical distribution of labeled neurons in Case 12. The box diagram above the lateral wall in panel A shows the stimulation map in relation to the injection site location. Abbreviations: A, arm; Hp, hip. For other abbreviations see Figures 1, 3, 6, 7, 9 and 12.
Thus, premotor area 6DR is connected with the remainder of 6DR and 6DC, the cingulated proisocortex as well as the cingulate motor areas (M3, M4), TMA and MII. It has only limited connections with MI. Its other connections are with the posterior parietal cortex. Additionally, it has significant connections with prefrontal and temporal regions as well as the insula.
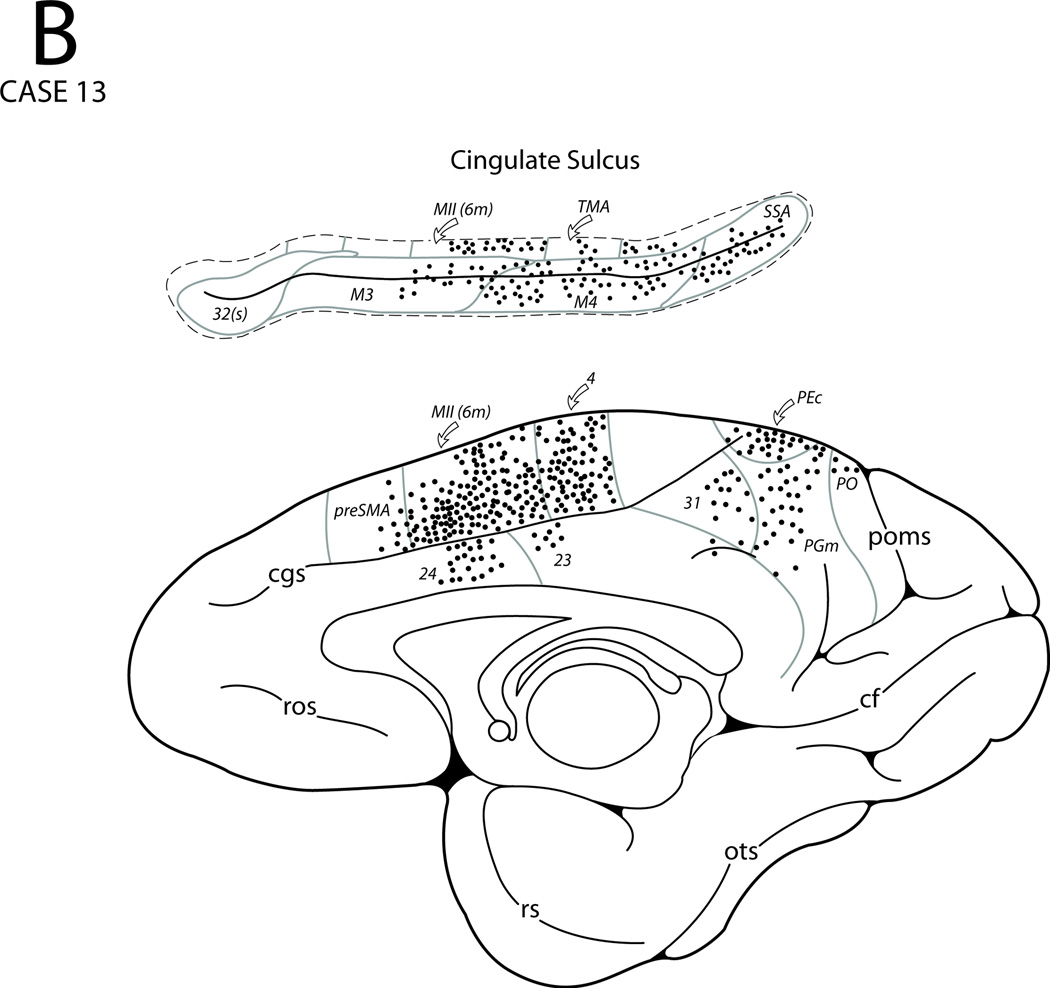
In Case 13 the DY injection involved the central portion of area 6DC as defined using intracortical microstimulation and sulcal landmarks as guides (Figs. 3, 4G, 15). On the lateral surface of the hemisphere, labeled neurons were observed in the surrounding area 6DC and also in area 6DR extending into the adjacent upper bank of the arcuate sulcus. Labeled neurons were also noticed in the dorsal part of area 4 on the gyral surface. Some labeled neurons were also found in ventral premotor area 6Va, the caudal and rostral parts of area 6DR, and in prefrontal areas 8B, 8Ad 9/46d. On the medial surface of the hemisphere, labeled neurons occurred in preSMA, area 6m (MII) as well as in medial area 4. From these regions labeling extended into the upper bank of the cingulate sulcus which involved area 6m, TMA and TSA. Labeled neurons also occurred in M3, M4 as well as SSA (Fig. 17E). Labeling was also found in areas 24, 31, PEc, PGm and PO. Beyond the central sulcus, labeled neurons occurred in areas 2, PE and PEc of the superior parietal lobule and in areas PEa and IPd of the intrapariteal sulcus. In the Sylvian fissure, a few scattered clusters of labeled neurons were found in SII and in areas reit and reipt. Labeled neurons were also noted in the Idg and Ig portions of the insula while very few labeled cells were observed in areas paI and proA. Ventral to the Sylvian fissure, a few labeled neurons were found in area Tpt of the superior temporal gyrus.
Figure 15.
Diagrammatic representation of the lateral (A) and medial (B) surfaces of the cerebral hemisphere to show the injection site in area 6DC and cortical distribution of labeled neurons in Case 13. The box diagram above the lateral wall in panel A shows the stimulation map in relation to the injection site location. For abbreviations see Figures 1, 3, 6, 7, 9, 10, 12 and 14.
In summary, premotor area 6DC is connected with cingulate proisocortical area 24 as well as with both cingulate motor areas and MII. It is also connected with premotor area 6DR, medial and dorsal portions of motor area 4, as well as with TMA, TSA and SSA. Its other connections are with the posterior parietal cortex and areas within the Sylvian fissure.
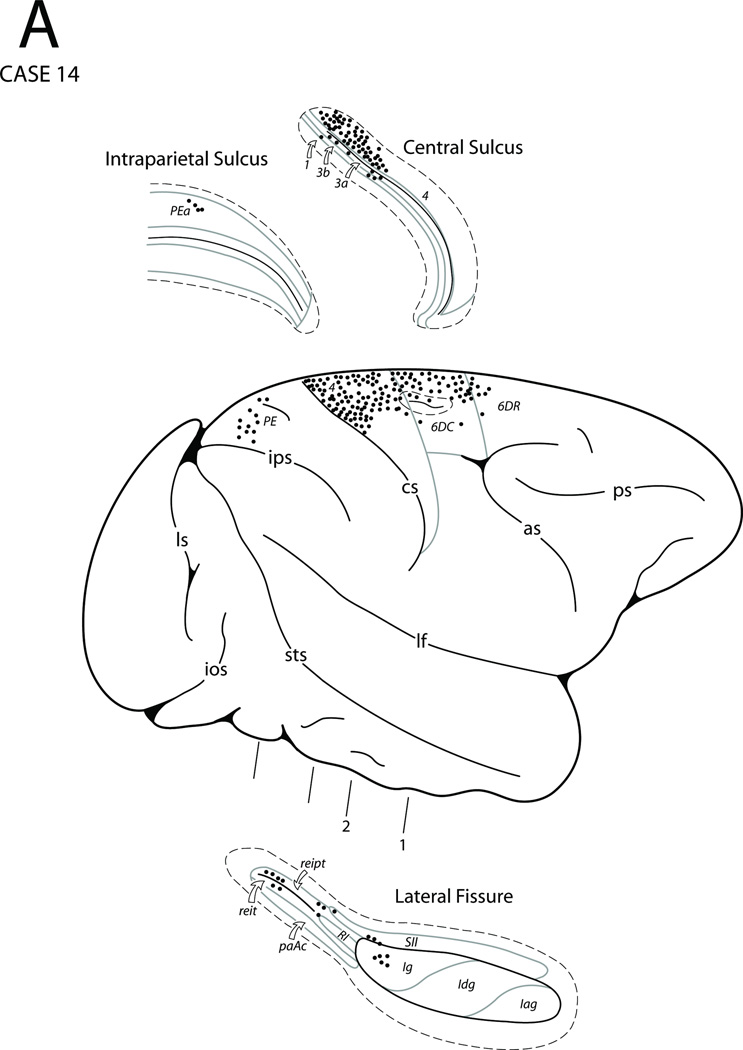
In Case 14 an injection of DY was placed in the medial part of area 4 (i.e., leg representation of MI) as defined physiologically (Figs. 3, 4I and 16). Compared to all of the previous Cases, labeling was highly localized. Thus, labeling occurred in medial area 4, the caudal part of area 6m (MII) as well as in medial parietal areas 3, 1, 2 and PE. Labeling was also seen in the upper bank of the cingulate sulcus including the caudal part of area 6m and in TMA, TSA and SSA. Distinct patches of labeled cells were noted in the depths of the cingulate sulcus corresponding to the cingulate motor areas (M3 and M4) (Fig. 17F). On the lateral surface, labeling was confined to the dorsal part of area 4 including the rostral bank of the central sulcus and to premotor area 6DC. A few labeled neurons were noted in the insula (Ig), the dorsal part of area 3 and in area PE and area PEa in the posterior part of the intraparietal sulcus. Finally, small patches of DY labeled cells were found in the caudal part of the Sylvian fissure within SII, as well as areas reipt and reit.
In Case 15 a fluororuby (FR) injection was placed on the lateral convexity in the dorsal-most part of the precentral gyrus corresponding to the leg region of MI which was localized physiologically (Fig. 3). This Case is not illustrated. The basic pattern of labeling was similar to that in Case 14 with minor differences. On the lateral cortical surface, labeled neurons were noted in area 4 and premotor area 6DC. Caudal to the injection site, labeled neurons were observed to extend into area 4 of the central sulcus. Labeling was noted in the dorsal part of the postcentral gyrus including areas 3, 1, 2, 5 (PE) and PEc. A few labeled neurons were found in area PEa of the intraparietal sulcus. In the Sylvian fissure a patch of FR labeled cells was found in the posterior region of the insula. Small groups of labeled neurons were also found in the caudal part of SII and areas reit and reipt of the posterior part of the Sylvian fissure. On the medial surface of the hemisphere, neurons were seen in areas 6m (MII), 4, 3, 1, 2 and PE including area PEc. Discrete patches of labeled neurons were seen in the cingulate sulcus in the caudal parts of both cingulate motor areas as well as in TMA, TSA and SSA. Finally, a few labeled cells were found in the caudal and dorsal part of area 24b.
In summary, the dorsal and medial portions of area 4 are connected with the cingulated proisocortex (area 24), cingulate motor areas (M3 and M4), MII, TMA, premotor area 6DC and the dorsal and medial portions of area 4. Their other connections are with post-central and posterior parietal areas including SSA and SII as well as the insula.
DISCUSSION
Traditionally the organization of the precentral and premotor areas has been considered in a rostrocaudal direction [30,31,54,58,71,81–83,86,187,188,202]. This notion has a great deal of implications since it has been shown in several studies that the caudal part of the precentral region, including the rostral bank of the central sulcus, contains neurons that are involved predominantly in the movement of the distal extremity and facial muscles. By contrast, the rostral precentral and premotor areas have been shown to be involved mainly in synergistic trunk and limb girdle musculature as well as in head and neck movement [30,31,81–83,202,203]. Over the past several years, however, it has become clear that areas other than the precentral and premotor regions may have important roles in motor function. For example, the supplementary motor area (MII) has been shown to be involved in preparation, anticipation, initiation and sequencing of unilateral and bilateral movements [25,29,59,131,182,189,199]. It also has been demonstrated that there are distinct motor representations in the premotor and precentral regions as well as in the cingulate cortex [10,53,63,112,119,125,128,156]. The level of understanding about the functional roles of other nearby regions, such as the arcuate cortex as well as the dorsal and ventral premotor areas, is becoming even more clear as a result of several recent neurophysiological and neuroanatomical studies [37,44,46,61,157–159,200]. Moreover, behavioral processes such as motivation, will, drive, and emotion that play a contributing role in motor activities is beginning to unfold with the development of functional imaging techniques [18,24,32,64,104,171]. Therefore it is pertinent to know how the precentral and premotor areas relate structurally to the dorsal and medial regions implicated in the cortical motor system. It is without question that this interrelationship is likely to play a significant role in complex, higher-order motor behaviors. With this in mind we will consider the sequential cytoarchitectural and connectional associations of the frontal motor areas with the adjacent medial proisocortical areas of the anterior cingulate cortex.
Architectonic Characteristics of the Anterior Cingulate and Dorsal Motor Areas
Anterior cingulate area 24 is proisocortical in nature and is characterized by a simple cortex with an ill-defined second layer, a sparse number of third layer neurons, an absence of a fourth layer, and darkly stained neurons in layers V and VI. There are four subdivisions of area 24 in the ventral to dorsal dimension. Area 24a has all the characteristics of a proisocortex (Fig. 2A). In adjacent area 24b there is a slight increase in the number of third layer neurons, and layers V and VI are separable (Fig. 2B). The next division is area 24c in the lower bank of the cingulate sulcus, which shows a better developed layer II, more pyramidal neurons in layer III, and prominent neurons in layers V and VI (Fig. 2C). Next to area 24c is area 24d located in the depth of the cingulate sulcus. In this area the neurons are more dispersed and the layers are narrowed. Both areas 24c and 24d form the rostral cingulate motor area (M3) [112,114,122].
Areas 24c and 24d are considered as transitional areas where the proisocortical type of cortex changes to the isocortical type in the upper bank of the cingulate sulcus. That is, at this location layers II and III are more prominent than layers V and VI. The upper bank of the rostral portion of the cingulated sulcus is known to contain an extension of medial area 6 (area 6m, MII or supplementary motor area), medial area 4, and the transitional motor area (TMA). All these areas have characteristically prominent neurons in layer III. Some of these areas contain few large-sized neurons in layer V such as in TMA, adjacent to the sulcal portion of area 4 (Fig. 2I). Above the upper bank of the rostral cingulate sulcus is medial area 8Bm, which is dysgranular, that is, having few layer IV neurons, and the preSMA, which is agranular. Medial areas 6 and 4 above the cingulate sulcus are also agranular. Area 6m has prominent neurons in layers III and V, whereas the medial portion of area 4 has a significant number of Betz cells in layer V (Fig. 2F, J). The dorsolateral portions of areas 6 and 4 continue to the level of the upper limb and spur of the arcuate sulcus. Lateral area 6, which has prominent layers III and V, has been divided into two major sectors. The caudal division, area 6DC, is characterized by scattered large Betz cells in layer V, whereas the rostral area 6DR is not (Fig. 2G, H). Finally dorsal area 4 (Fig. 2J), which contains the MI leg, arm and trunk representations, has large clusters of Betz cells in layer V [6]. Thus, it seems that the dorsal precentral and premotor areas can be viewed as having progressed in a stepwise structural manner from the proisocortex of the cingulate gyrus toward the isocortical areas of the upper bank of the cingulate sulcus (areas 4 and 6m as well as TMA). Further stepwise pyramidalization occurs in the premotor areas, from area 6DR without Betz cells as in, to area 6DC with few Betz cells, and to area 4 with a heavy concentration of Betz cells. Thus, the dorsal motor trend can be characterized by a stepwise laminar change from the proisocortex of the cingulate cortex up to the level of spur of the arcuate sulcus [139,141,168]. Such stepwise architectonic changes from the cingulate cortex medially to the dorsal motor regions have been proposed by several investigators [134,139,140,167,168].
A number of recent studies have analyzed the cytoarchitecture of the macaque anterior cingulated region [19,193,195], premotor cortex [7,100,155], and motor cortex [6,155]. Apart from the work of Sanides [166–168], none of these studies have suggested a cytoarchitectural link between the anterior cingulate, premotor, and motor areas. In the present study we have discerned an underlying architectonic association between the anterior cingulate and dorsal motor areas. This viewpoint has also been supported by the gradual changing pattern of corticocortical connections of these areas as discussed below (Fig. 18).
Fig. 18.
Summary diagrams showing the cortical afferents to supracallosal areas 24a/b (A), mid-levels of M3 or area 24c and d (B), area 6DR (C), sulcal (s) area 6m (D), arm region of area 6m (MII) (E), area 6DC (F), the TMA (G) and medial area 4 (H). For abbreviations see Fig. 1, 3, 6 and 7.
Connectional Observations: Dorsal Proisocortical and Isocortical Areas of the Precentral and Premotor Regions
Anterior Cingulate Region
Proisocortical areas 24a and 24b are connected with other parts of the cingulate gyrus as well as with the cingulate motor areas, supplementary motor area, and pre-supplementary motor area Cases 1–4). Areas 24a and b are connected also with the medial and dorsal lateral parts of the premotor and precentral areas. Other connections of areas 24a and b are with various parts of lateral, orbital and medial prefrontal cortex, insula and the rostral part of the superior temporal gyrus and the multimodal areas of the superior temporal sulcus. Moreover, afferents to this proisocortical area arise from ventromedial temporal regions including the perirhinal and entorhinal cortex and the parahippocampal gyrus as well as CA1 and the subiculum of the hippocampus (Fig. 18A). Area 24c (Cases 5 and 6), has a basic pattern of connections similar to those of areas 24a and 24b. Area 24c receives widespread projections from the prefrontal cortex, the insula, the superior temporal gyrus as well as the multimodal area of the superior temporal sulcus. Area 24c has additional connections with the ventral part of area 6, dorsolateral parietal cortex as well as the medial parietal region. Compared to areas 24a and 24b, area 24c receives only limited afferents from ventral limbic areas (Fig. 18B).
A number of studies have investigated the corticocortical afferent connections of the anterior cingulate cortex using retrograde and anterograde tract traceing methodologies. Other studies have reported efferent projections to this area using anterograde methodology. These studies have shown that the anterior cingulate cortex receives projections from areas 10, 9, 46, and 47/12 of the lateral prefrontal cortex, and from areas 10m, 9m and 8Bm of the medial prefrontal cortex [1,4,8,20,46,56,79,106,124,142,150–152,192]. Our results also concur with previous observations [46,56,150] an anterior cingulate connection with area 45. The present findings also agree with the observed projections from orbitofrontal areas 10, 11, 12, 13, 14 and 25 to the anterior cingulate cortex [8,22,192]. Furthermore, our observations indicate that the anterior cingulate region located above the corpus callosum (Cases 2–4) is connected with the pre-SMA, MII, and 6DR in agreement with previous reports [21,79,94,101]. We also observed that area ProM projects to anterior cingulate cortex (Case 3). Additionally, we noted a weak projection to the anterior cingulate region (Cases 2–4) from the inferior parietal lobule as previously reported [23,56,148,164]. Our observations also indicate that the supplementary sensory area projects to the anterior cingulate region (cases 2–4). In terms of temporal lobe afferents, the present findings are in agreement with the reported lateral temporal lobe projection from the upper bank of the superior temporal sulcus (area TPO) as well as from the superior temporal gyrus (areas Pro, Ts1, Ts2 and Ts3) [1,4,8,73,127,149,161,165]. In addition, there is a ventral temporal lobe projection from areas 35, 28, TH, TL and TF [8,20,73,127,192]. In accord with other studies [5,65,162,192], we found hippocampal projections to the anterior cingulate region (Case 1) from CA1, prosubiculum, subiculum, presubiculum and parasubiculum. The present results also confirm anterior cingulate projections from insular areas Iag, Idg and Ig [1,8,108]. We observed anterior cingulated afferents from the parainsular areas Pro, PaI and ProA as well as SII and gustatory cortex as reported by Barbas et al., [8] and Romanski et al., [161]. Consistent with other studies, we found anterior cingulated afferents from the posterior cingulate cortex (area 23) [1,4,8,20,56,192] and retrosplenial cortex (areas 29 and 30) [56,72]. We should point out that Barbas and colleagues [8] reported a projection from area V2 to the perigenual cingulate region, whereas our findings suggest that area prostriata projects to the dorsal cingulate region (Cases 2–4). Finally, we observed projections from areas 9m, 32, M3 and M4 to all anterior cingulate regions (Cases 1–4) as previously reported [8,144].
Cingulate Motor Region (M3)
The present results show premotor (pre-SMA, MII, areas 6DR, 6DC, 6V, ProM, and 44) and motor cortex (MI) projections to M3 as reported in earlier investigations [6,52,87,101,177,180]. Regarding prefrontal connectivity, our observations are consistent with previous reports describing projections to M3 from areas 10 [124], 9 [9,124,152], 46 [7,9,90,151,172], 9/46 [52,113,180], 45 [46], 47/12 [9], 8BM [151], and 32 [144,152]. Our findings confirm previous observations of orbitofrontal (areas 25, 14, Pro, 13, 12 and 11) and paralimbic area (areas 24a, 24b, 32, 23a, 23b, M4, 29, 30, prostriata, 35, 28, TF and TH) projections to area M3 [114,116] and show further that hippocampal projections arise from the subiculum (Cases 5 and 6) and prosubiculum (Case 5). Furthermore, the present observations indicate that anterior temporal lobe projections to middle and rostral levels of M3 arise from areas Pro, Ts1 and Ts2, as reported by Petrides and Pandya [149]. In agreement with several other reports, we found parietal lobe connections of M3 with areas PG and PFG [23,164], PGop and PE [148], the postcentral gyrus [28,67], the medial parietal cortex [88,145] and adjacent area 31 [145]. Our findings show extensive M3 inputs from the cortex of the anterior and posterior parts of the cingulated sulcus as reported previously [52,180]. Finally, our connectional observations show that afferents to M3 arise from SII, PFop, Tpt, insular areas Iag, Idg and Ig, peri-insular areas paI, proA and paAc, retroinsular areas RI, reipt and reit, as well as areas TAa, TPO, PGa,IPa and MST of the STS.
Upper Bank of the Cingulate Sulcus
The pattern of corticocortical connections of area 6m of the cingulate sulcus has not been studied previously. Unlike the widespread connections of areas 24a, 24b and 24c, the projections to area 6m in the upper bank of the cingulate sulcus (Cases 7–8) are somewhat restricted, demonstrating a gradual change in the overall pattern of corticocortical connections paralleling the changes in cytoarchitecture. Thus, we observed that sulcal area 6m receives input from the dorsal part of the cingulate gyrus (area 24b), the cingulate motor areas (M3 and M4), and MII and the preSMA. It also receives projections from the dorsal premotor cortex and precentral motor cortex. Its other connections are derived from the dorsal somatosensory areas 3, 1 and 2 as well as areas PE, PEc and PEa of the superior parietal lobule, areas POa, PFop, and PGop of the inferior parietal lobule, and the insula. The sulcal component of area 6m receives input from the ventral premotor cortex (areas 6V and 44) and from ventral proisocortical area ProM (Fig. 18D). It seems that area 6m of the upper bank of the cingulate sulcus, like the rostral cingulate proisocortical areas, maintains connections with the cingulate proisocortex, cingulate motor areas, MII and the medial and dorsolateral premotor and precentral motor areas. Unlike the proisocortical cingulate region and cingulate motor areas, however, area 6m of the upper bank has substantial connections with the parietal lobe, only minimal prefrontal and temporal lobe connections, and no connections with the ventral limbic cortex.
The location and cortical connections of the transitional motor area (TMA) in the upper bank of the cingulate sulcus have not been investigated thus far. We found that TMA, located just ventral to the leg representation of area 4 (Case 9), receives even more restricted connections than the sulcal part of area 6m. Thus, TMA receives connections from M3, M4 and MII as well as the supplementary sensory area (SSA). It also receives projections from medial and dorsal areas 6 and 4. Other areas sending projections to TMA are the caudal insula, areas SII, PFop and PGop, and the superior parietal region (areas 3,1, 2, PE, PEc, PEa, and IPd) (Fig. 18G). Thus, TMA can be viewed as a step in the progression from the cingulate proisocortex to the medial and dorsal motor cortex (MI).
Dorsal Premotor Region (MII, 6DR and 6DC)
Compared to cingulate cortex connections, the premotor areas receive less widespread projections. Like TMA and the sulcal portion of area 6m, the medial portion of area 6 (supplementary motor area or MII) (Case 11) receives limited cortical connections compared to the cingulated proisocortical areas. These connections are from the dorsal cingulate region, cingulate motor areas and preSMA, and the dorsal portions of the premotor and precentral motor areas. MII also receives input from the medial (PEc and SSA) and lateral (PE, PEc, PEa, POa, IPd, PG, PGop, PFop and SII) parietal cortex, and the insula. MII has only minor prefrontal and ventral premotor connections (Fig. 18E).
Several studies have investigated the corticocortical connections of MII. Our data are in agreement with reports of frontal lobe projections from MI, areas 6DC (F2), 6DR (F7), 6V (F4/F5) and 44 [70, 92,94,106,113,184,198]. As previously reported by Luppino et al., [94], we noted projections to MII from the cingulate sulcus (M3, M4 and SSA), cingulate gyrus (areas 24b and 23b), superior parietal lobule (areas 2 or PC, PE and PEc), intraparietal sulcus (areas PEa and IPd), as well as the inferior parietal lobule (areas PG, PGop, PFop and SII). Furthermore, our findings confirm projections from the insula (Idg and Ig) and retroinsular area (Ri) [94], ProM [177], as well as prefrontal projections from areas 9/46d, 8B and 8Ad [9,90,106,113,172,198]. Finally, we found projections to MII from parietal area Opt, insular area Iag, and cingulate area 31 that have not been described previously.
The dorsal lateral premotor cortex (areas 6DR and 6DC) has connections with the rostral cingulate proisocortex, cingulate motor areas, and MII (Cases 12 and 13). In addition, it has connections with other parts of the medial and lateral premotor regions. In terms of motor cortex, area 6DC has connections with the dorsal and medial precentral motor cortex, whereas area 6DR has only minimal connections with the rostral part of the precentral motor cortex. By contrast, area 6DR has connections with prefrontal cortex and temporal lobe, whereas area 6DC has limited prefrontal afferents and no temporal lobe afferents. Projections to both 6DR and 6DC are derived from the medial and lateral posterior parietal cortex, with area 6DR input derived preferentially from the inferior parietal lobule, and area 6DC input from the superior parietal lobule. Both of these premotor areas receive minor projections from the insula (Fig. 18C, F). Thus, the dorsal premotor regions have connections with proisocortical cingulate motor areas on the one hand, and the isocortical MII, premotor, precentral and parietal regions on the other.
Our findings confirm frontal lobe afferents to area 6DR from MI, areas 6DC, 6V, ProM, 44, 45, 8A, 8B, 9, 46, MII, preSMA, 8Bm, and 9m [46, 79,96,177, 179, 200], including orbital area 47/12 [21,179]. We also noted a weak projection from obitofrontal areas Pro and 13 and from ventral temporal area TF that have not been reported. Our findings confirm cingulate input as shown previously from areas 24a, 24b, M3 and M4 [96,179], areas 23a, 23b and 31 [102,179], and retrosplenial areas 29 and 30 [72]. In terms of parietal afferents, our findings are in agreement with reports of projections to area 6DR from lateral parietal cortex including SII and area PFG; areas POa and IPd of the intraparietal sulcus [180]; and medial parietal areas PO (medial portion of area V6 of Galletti et al., 1995), PGm, and PEci (SSA) [102,180,183]. We found temporal lobe projections to area 6DR from the superior temporal gyrus (areas Ts2, Ts3, paAlt and Tpt) as reported previously [143] and our findings support substantial temporal lobe projections to area 6DR from the upper bank of the STS [98]. Finally, our observations agree with previously demonstrated insular projections from Iag, Idg and Ig [179], and suggest further, that peri-insular areas paI and proA, retroinsular areas Ri, reipt and reit project to area 6DR.
With respect to part of area 6DC in the vicinity of the precentral dimple, our findings support the existence of projections from MI and areas 6DR, 6Va, MII and the pre-SMA of the frontal lobe [35,47,80,96]. Our observations are in agreement with reports of a localized area 9/46d projection to area 6DC [35,47,96]. We also noted only few labeled cells in caudal prefrontal areas 8Ad and 8B. The present results confirm lateral parietal lobe projections of area 6DC with areas PEc, PE, 2, IPd and PEa [43,47,98,102,183], and medial parietal connections with areas PEc, PGm, PO, and SSA [102,183]. By contrast, we did not find a projection from the inferior parietal lobule as reported by Ghosh and Gattera [47] and Tanné-Gariépy et al. [183], which may be attributable to our more dorsally located injection site. In terms of temporal lobe projections, we noted a sparse projection from area Tpt of the superior temporal gyrus (STG), consistent with a projection to area 6DC from the posterior STG reported previously [143]. Our findings confirm projections from the cingulate sulcus as well as mid-levels of the cingulate gyrus (areas 24 and 23) as reported [80,47,96,35] including a projection from area 31 and the posterior part of area 23b [47,102]. Finally, the present observations suggest that limited projections to area 6DC arise from the insula (Iag, Idg and Ig), peri-insular cortex (SII, Ts2, paI and proA), as well as retroinsular areas reit and reipt which have not been previously reported.
Dorsal Precentral Motor Region
Even more restricted projections are received by medial and dorsolateral parts of area 4 (Cases 14 and 15). Thus, dorsal area 4 is connected with medial area 4 and vice versa. This area receives projections from the caudal parts of M3, M4 and MII as well as from the SSA. Other major afferents are derived from TMA and the dorsal premotor region as well as the dorsal and medial parts of areas 3, 1, 2 and PE (Fig. 18H).
The present results of frontal lobe projections to the hindlimb (HL) region of MI from areas 6DR, 6DC, and the caudal portion of MII are in agreement with previous reports [49,50,94,112,125]. In terms of cingulate sulcus labeling, our findings support projections from SSA and TSA, as well as a localized projection from the rostral cingulate motor cortex [49,50,94,112] followed by a label-free projection zone caudally, which in turn is followed by a dense distribution of labeled cells in the caudal cingulate motor cortex [50,112,125]. Our findings are in accord with reported parietal lobe projections from areas 3, 1 and 2 [49,50,67] and area PEa of the intraparietal sulcus [50] and suggest further that area PE projects to the hindlimb region of MI. However, we did not observe a projection from area POa of the intraparietal sulcus as shown by Hatanaka et al. [50]. Finally, within the Sylvian fissure, we noted projections from the insula, SII and retroinsular area as described previously [49,50].
Thus, it seems that the proisocortical areas of the rostral cingulate region have widespread cortical connections from prefrontal, parietotemporal, multimodal and limbic areas. By contrast, the medial and dorsal isocortical areas (areas 6m, TMA, 6DR and 6DC) have relatively less afferent connectivity (Fig. 18). The medial and dorsal portion of area 4 has the most restricted connections of the areas studied. Another interesting feature of this connectivity is that all regions, starting from proisocortical area 24 to area 4 (MI), maintain specific connections with MII, M3, M4 and, to some extent, SSA. Another area sending connections to all of these proisocortical and isocortical areas is dorsal premotor area 6 and the precentral motor cortex.
Tripartite Organization of the Dorsal Motor Trend
According to the present study, the dorsal motor region, starting from the rostral cingulated proisocortical areas, have specialized connections with motor and premotor as well as the supplementary motor, and cingulate motor areas. This pattern of connectivity resembles that of the dorsal part of the cortical somatosensory system as shown by Morecraft et al. [120]. In this earlier study it was suggested that the dorsal somatosensory areas are organized in a tripartite manner, that is, into root, core and belt lines. Furthermore, areas of the root, belt and core lines were found to be interconnected in particular ways. The present study shows that a similar connectional organization can be applied to the dorsal motor cortex, dorsal premotor cortex, cingulate motor cortex, and MII. Thus, in the dorsal motor trend, the cingulate motor areas (M3 and M4) and MII can be considered as root areas, whereas the dorsal premotor area 6 is considered as a belt area, and dorsal and medial area 4 is equivalent to a core region. The connectional observations reveal that the motor root areas are connected with root areas (MII, M3 and M4) as well as with the belt region (dorsal premotor areas 6DC and 6DR) and core region (dorsal precentral area 4). Likewise, the belt area is connected with belt (6DR and 6DC) and root areas (supplementary and cingulate motor areas) as well as with the core area (precentral area 4). Finally, the core region (dorsomedial area 4) is connected with a core region (lateral area 4) and with the adjacent root regions (cingulate motor areas and MII), as well as with a belt region (dorsal premotor area 6DC).
Thus we have suggested that the architectonic areas and the connections of the dorsal motor, premotor, and anterior cingulate regions are organized according to specific principles. Previous connectional studies of these areas did not propose the present type of schema, nonetheless their findings appear to support the concept of a tripartite organization. For example, studies of the connections of the cingulate proisocortical, cingulate motor, and supplementary motor areas have consistently shown connections between these regions and the lateral premotor and motor areas [2,4,35,79,94,101,112,114,135,144,170,193,197]. Likewise, dorsal premotor areas have been shown to be connected with cingulate motor areas and MII on the one hand, and with the primary motor area on the other [6,35,47,68,96,135,138,170]. Finally, the primary motor area has been shown to be connected with the lateral premotor areas as well as with MII and the cingulate motor areas [28,48–50,52,68,87,94,96,97,112,125,133,135,138,170,186,197]. Collectively, these observations are consistent with the notion of a tripartite organization of the dorsal motor system proposed here.
Structural Integration of a Cortical Somatosensorimotor System
Another interesting aspect of our study is that the dorsal precentral (core), premotor (belt) as well as cingulate and supplementary (root) motor areas are connected with dorsal somatosensory area 3 (core), areas 1, 2 and 5 (belt) as well as SSA and SII (root) of the postcentral region. It has been considered that somatosensory and somatomotor areas are integral parts of one system, that is, the somatosensorimotor system [202]. Therefore, it is not surprising that precentral, premotor and postcentral cortex are connectionally interrelated in a highly organized manner. Finally, we would like to point out that the dorsal motor trend arises from the cingulate proisocortex and leads to limb and trunk representations of the precentral motor cortex. Therefore the dorsal trend may significantly influence locomotion and exploratory activities. Our preliminary architectonic and connectional studies of the ventral premotor and motor areas suggest they are organized in a similar tripartite manner. It should be noted that the dorsal premotor and supplementary motor areas are connected with the ventral premotor cortex (belt) and SII as well as the insular areas (root). Although functional studies show that dorsal and ventral trend motor areas may have specialized and independent roles [61,132], interconnections that link both trends may provide a structural basis for complex, integrative motor functions.
Cortical Limbic-Motor Interactions
It has become increasingly clear that the motor activity emerging from the precentral gyrus requires that other cortical regions contribute in a meaningful way so that motor behavior becomes purposeful. A close relationship of the dorsal precentral and premotor areas with MII, cingulate motor areas (M3 and M4) and cingulate proisocortex suggests that behavioral constructs such as emotion, motivation, drive, will, planning, initiation, programming, and perhaps the comprehension of space and spatial memory are requisites for the proper guidance and execution of exploratory activity involving limbs and trunk. Such proposed functional relationships to motor activity emanating from the precentral motor areas remain to be further elucidated. However, it is reasonable to consider that activities such as locomotion, exploration and motivation require input from varied cortical sources. The fact that the dorsal frontal motor areas have substantial connections with cingulate proisocortical areas suggest that while motor areas on the convexity specialize in supporting voluntary motor functions, the cingulate areas may have an important role in providing the emotional, motivational and cognitive components of motor function [113–115,118,190]. In this context, it is notable that the cingulate motor cortex itself has been shown to be directly connected with subcortical forebrain, brainstem and spinal cord motor centers [10,17,34,63,78,95,105,117–119,123,128,181,190].
We recognize that a number of investigators who have focused on the rostral cingulate, premotor or motor regions have proposed alternative concepts of the overall organization of these brain regions. Furthermore, it is clear that physiological, connectional, chemoarchitectonic, myleoarchitectonic, pigmentarchitectonic, and receptor distribution studies, for example, have all provided important insight into the unique biological characteristics that distinguish these neighboring cortical regions. In the present report, however, we have attempted to identify some characteristics that underscore the structural continuity of the rostral cingulate area and the traditionally recognized motor and premotor areas of the frontal lobe. When these architectonic and connectional features are viewed as a whole, the sequential structural changes appear to link these brain regions into a single, coherent system. We have suggested that previously reported architectonic and connectional observations of the anterior cingulate and dorsal motor regions are consistent with such a proposal [134,139]. Furthermore, other evidence supporting a concept of this type has recently been reviewed by Hoshi and Tanji [61]. The present report includes new findings on the connections of the cortex forming the lower and upper banks of the cingulate sulcus, which collectively provide enhanced support for this proposal. We feel that such an integrative viewpoint has the potential to provide further insight into the cortical motor system in a way that adds to the developing concept of a dorsal cortical limbic-motor interface [114,115]. This interface is exemplified by the clinical observation that damage to the anterior cingulate cortex results in akinesia or a poverty of movement [3,84], whereas damage to the region of the supplementary motor cortex yields motor performance deficits [13,38,205].
CONCLUSIONS
In conclusion, the present study has used two approaches, the examination of cytoarchitectonic organization and the delineation of corticocortical connectional patterns, to show that the precentral and premotor areas are related to anterior cingulate cortex in a stepwise manner. We have further suggested that the dorsal motor, premotor, and the rostral cingulate motor areas as well as MII are organized into core, belt and root lines, respectively. The core line comprises primary motor cortex, whereas the belt consists of premotor areas. The root areas (M3, M4 and MII) are part of the paralimbic region. We have suggested that core areas are surrounded by root and belt areas. Although several studies have advanced different proposals regarding the organization of the sensorimotor system of the cerebral cortex, we have attempted to provide an underlying principle of organization for these areas based on the concurrent analysis of cytoarchitecture and connectivity. Using such a framework, future investigations can be devised that may lead to further insight into the coherent functional organization of the the cortical sensorimotor system and the developing concept of the cortical limbic-motor interface.
Highlights.
-
-
Cytoarchitecture and connections of the anterior cingulate and motor cortex were investigated.
-
-
Stepwise architectonic changes were paralleled by stepwise changes in cortical connections.
-
-
Widespread cingulate connections were followed by progressively more restricted connections.
-
-
These findings enhance our understanding of the primate cortical limbic- motor interface.
ACKNOWLEDGMENTS
Grant Sponsor: National Institutes of Health; Grant numbers: NS 046367 and NS 33003 (to RJM).
The authors would like to thank Dr. Edward Yeterian for valuable comments on the manuscript and Dr. Gary Van Hoesen for the many thoughtful discussions of this topic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Arikuni T, Sako H, Murata A. Ipsilateral connections of the anterior cingulate cortex with the frontal and medial temporal cortices in the macaque monkey. Neurosci. Res. 1994;21:19–39. doi: 10.1016/0168-0102(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 2.Arikuni T, Sakai M, Hamada I, Kubota K. Topographical projections from the prefrontal cortex to the post-arcuate area in the rhesus monkey, studied by retrograde axonal transport of horseradish peroxidase. Neurosci. Lett. 1980;19:155–160. doi: 10.1016/0304-3940(80)90187-1. [DOI] [PubMed] [Google Scholar]
- 3.Amyes EW, Nielsen JM. Clinicopathologic study of vascular lesions of the anterior cingulate region. Bull. Los Angeles Neurol. Soc. 1955;20:112–130. [PubMed] [Google Scholar]
- 4.Baleydier C, Mauguiere F. The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain. 1980;103:525–554. doi: 10.1093/brain/103.3.525. [DOI] [PubMed] [Google Scholar]
- 5.Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- 6.Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area-6) in the rhesus monkey. J. Comp. Neurol. 1987;256:211–228. doi: 10.1002/cne.902560203. [DOI] [PubMed] [Google Scholar]
- 7.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 8.Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J. Comp. Neurol. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Bates JF, Goldman-Rakic PS. Prefrontal connections of medial motor areas in the rhesus monkey. J. Comp. Neurol. 1993;336:211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- 10.Biber MP, Kneisley LW, LaVail JH. Cortical neurons projecting to the cervical and lumbar enlargements of the spinal cord in young adult rhesus monkeys. Exp. Neurol. 1978;59:492–508. doi: 10.1016/0014-4886(78)90240-6. [DOI] [PubMed] [Google Scholar]
- 11.Blackstad TW, Heimer L, Mugnaini E. Experimental neuroanatomy: general approaches and laboratory procedures. In: Heimer L, RoBards M, editors. Neuroanatomical Tract-tracing Methods. New York: Plenum Press; 1981. pp. 1–53. [Google Scholar]
- 12.Blatt GJ, Pandya DN, Rosene DL. Parcellation of cortical afferents to three distinct sectors in the parahippocampal gyrus of the rhesus monkey: an anatomical and neurophysiological study. J. Comp. Neurol. 2003;466:161–179. doi: 10.1002/cne.10866. [DOI] [PubMed] [Google Scholar]
- 13.Bleasel A, Comair Y, Lüders HO. Surgical ablations of the mesial frontal lobe in humans. In: Lüders HO, editor. Advances in Neurology: Supplementary Sensorimotor Area. Vol. 70. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 217–235. [PubMed] [Google Scholar]
- 14.Bonin G. Architecture of the precentral motor cortex and some adjacent areas. In: Bucy PC, editor. The Precentral Motor Cortex. Urbana, Illinois: The University of Illinois Press; 1949. pp. 7–82. [Google Scholar]
- 15.Bonin GV, Bailey P. The Neocortex of Macaca Mulatta. Urbana: University of Illinois Press; 1947. [Google Scholar]
- 16.Brodmann K. Beiträge zur histologischen lokalisation der grosshirnrinde: dritte mitteilung: die rindenfelder der niederen affen. J. Psychol. Neurol. 1905;4:177–226. [Google Scholar]
- 17.Burman K, Darian-Smith C, Darian-Smith I. Macaque red nucleus: origins of spinal and olivary projections and terminations of cortical inputs. J. Comp. Neurol. 2000;423:179–196. doi: 10.1002/1096-9861(20000724)423:2<179::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J. Comp. Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 21.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 22.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J. Comp. Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhry AM, Parkinson JA, Hinton EC, Owen AM, Roberts AC. Preference judgements involve a network of structures within frontal, cingulate and insula cortices. Eur. J. Neurosci. 2009;29:1047–1055. doi: 10.1111/j.1460-9568.2009.06646.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Scangos KW, Stuphorn V. Supplementary motor area exerts proactive and reactive control of arm movements. J. Neurosci. 2010;30:14657–14675. doi: 10.1523/JNEUROSCI.2669-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipolloni PB, Pandya DN. Connectional analysis of the ipsilateral and contralateral afferent neurons of the superior temporal region in the rhesus monkey. J. Comp. Neurol. 1989;281:567–585. doi: 10.1002/cne.902810407. [DOI] [PubMed] [Google Scholar]
- 27.Cipolloni PB, Pandya DN. Cortical connections of the frontoparietal opercular areas in the rhesus monkey. J. Comp. Neurol. 1999;403:431–458. [PubMed] [Google Scholar]
- 28.Darian-Smith C, Darian-Smith I, Burman K, Ratcliff N. Ipsilateral cortical projections to areas 3a, 3b, and 4 in the macaque monkey. J. Comp. Neurol. 1993;335:200–213. doi: 10.1002/cne.903350205. [DOI] [PubMed] [Google Scholar]
- 29.Deiber MP, Honda M, Ibañez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J. Neurophysiol. 1999;81:3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- 30.Denny-Brown D. Disintegration of motor function resulting from cerebral lesions. J. Nerv. Ment. Dis. 1950;112:1–45. [PubMed] [Google Scholar]
- 31.Denny-Brown D. The Cerebral Control of Movement. Springfield: Charles C. Thomas; 1996. [Google Scholar]
- 32.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 33.Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J. Comp. Neurol. 2003;462:382–399. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- 34.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J. Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Economo C, Koskinas GN. Die Cytoarchitektonik der Hirnrinde des erwachsenes Menschen. Wien Berlin: Springer; 1925. [Google Scholar]
- 37.Fabbri-Destrp M, Rizzolatti G. Mirror neurons and mirror systems in monkeys and humans. Physiol. 2008;23:171–179. doi: 10.1152/physiol.00004.2008. [DOI] [PubMed] [Google Scholar]
- 38.Fontaine D, Capelle L, Duffau H. Somatotopy of the supplementary motor area: evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurg. 2002;50:297–303. doi: 10.1097/00006123-200202000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Gabbott PLA, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J. Comp. Neurol. 1996;364:567–608. doi: 10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 40.Galaburda AM, Pandya DN. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J. Comp. Neurol. 1983;221:169–184. doi: 10.1002/cne.902210206. [DOI] [PubMed] [Google Scholar]
- 41.Galea MP, Darian-Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb. Cortex. 1994;4:166–194. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- 42.Galletti C, Battaglini PP, Fattori P. Eye position influence on the parieto-occipital area PO (V6) of the macaque monkey. Eur. J. Neurosci. 1995;7:2486–2501. doi: 10.1111/j.1460-9568.1995.tb01047.x. [DOI] [PubMed] [Google Scholar]
- 43.Gamberini M, Passarelli L, Fattori P, Zucchelli M, Bakola S, Luppino G, Galletti C. Cortical connections of the visuomotor parietooccipital area V6Ad of the macaque monkey. J. Comp. Neurol. 2009;513:622–642. doi: 10.1002/cne.21980. [DOI] [PubMed] [Google Scholar]
- 44.Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Functional organization of inferior area 6 in the macaque monkey. I. Somatotopy and the control of proximal movements. Exp. Brain Res. 1988;71:475–490. doi: 10.1007/BF00248741. [DOI] [PubMed] [Google Scholar]
- 45.Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Somatotopic representation in inferior area 6 of the macaque monkey. Brain Behav. Evol. 1989;33:118–121. doi: 10.1159/000115912. [DOI] [PubMed] [Google Scholar]
- 46.Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cereb. Cortex. 2009;20:141–168. doi: 10.1093/cercor/bhp087. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh S, Gattera R. A comparison of the ipsilateral cortical projections to the dorsal and ventral subdivisions of the macaque premotor cortex. Somatosens. Mot. Res. 1995;12:359–378. doi: 10.3109/08990229509093668. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh S, Brinkman C, Porter R. A quantitative study of the distribution of neurons projecting to the precentral motor cortex in the monkey (M. fascicularis) J. Comp. Neurol. 1987;259:424–444. doi: 10.1002/cne.902590309. [DOI] [PubMed] [Google Scholar]
- 49.Godschalk M, Lemon RN, Kuypers HG, Ronday HK. Cortical afferents and efferents of monkey postarcuate area: an anatomical and electrophysiological study. Exp. Brain Res. 1984;56:410–424. doi: 10.1007/BF00237982. [DOI] [PubMed] [Google Scholar]
- 50.Hatanaka N, Nambu A, Yamashita A, Takada M, Tokuno H. Somatotopic arrangement and corticocortical inputs of the hindlimb region of the primary motor cortex in the macaque monkey. Neurosci. Res. 2001;40:9–22. doi: 10.1016/s0168-0102(01)00210-3. [DOI] [PubMed] [Google Scholar]
- 51.Hatanaka N, Tokuno H, Nambu A, Inoue T, Takada M. Input-output organization of jaw movement-related areas in monkey frontal cortex. J. Comp. Neurol. 2005;492:401–425. doi: 10.1002/cne.20730. [DOI] [PubMed] [Google Scholar]
- 52.Hatanaka N, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Nambu A, Takada M. Thalamocortical and intracortical connections of monkey cingulate motor areas. J. Comp. Neurol. 2003;462:121–138. doi: 10.1002/cne.10720. [DOI] [PubMed] [Google Scholar]
- 53.He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J. Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hines M. Control of Movements by the Cerebral Cortex in Primates. Biol. Rev. 1943;18:1–31. [Google Scholar]
- 55.Hof PR, Nimchinsky EA. Regional distribution of neurofilament and calcium-binding proteins in the cingulate cortex of the macaque monkey. Cereb. Cortex. 1992;2:456–467. doi: 10.1093/cercor/2.6.456. [DOI] [PubMed] [Google Scholar]
- 56.Hof PR, Nimchinsky EA, Morrison JH. Neurochemical phenotype of corticocortical connections in the macaque monkey: quantitative analysis of a subset of neurofilament protein-immunoreactive projection neurons in frontal, parietal, temporal, and cingulate cortices. J. Comp. Neurol. 1995;362:109–133. doi: 10.1002/cne.903620107. [DOI] [PubMed] [Google Scholar]
- 57.Hof PR, Lüth H-J, Rogers JH, Celio MR. Calcium-binding proteins define subpopulations of interneurons in cingulate cortex. In: Vogt BA, Gabriel M, editors. Neurobiology of the Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Boston: Birkäuser; 1993. pp. 181–205. [Google Scholar]
- 58.Holstege G. Brainstem-spinal cord projections in the cat, related to control of head and axial movements. Rev. Oculomot. Res. 1988;2:431–470. [PubMed] [Google Scholar]
- 59.Hoshi E, Tanji J. Differential roles of neuronal activity in the supplementary and presupplementary motor areas: from information retrieval to motor planning and execution. J. Neurophysiol. 2004;92:3482–3499. doi: 10.1152/jn.00547.2004. [DOI] [PubMed] [Google Scholar]
- 60.Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J. Neurophysiol. 2006;95:3596–3616. doi: 10.1152/jn.01126.2005. [DOI] [PubMed] [Google Scholar]
- 61.Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr. Opin. Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Huntley GW, Jones EG. Relationship of intrinsic connections to forelimb movement representations in monkey motor cortex: a correlative anatomic and physiological study. J. Neurophysiol. 1991;66:390–413. doi: 10.1152/jn.1991.66.2.390. [DOI] [PubMed] [Google Scholar]
- 63.Hutchins KD, Martino AM, Strick PL. Corticospinal projections from the medial wall of the hemisphere. Exp. Brain Res. 1988;71:667–672. doi: 10.1007/BF00248761. [DOI] [PubMed] [Google Scholar]
- 64.Iacoboni M. Attention and sensorimotor integration: mapping the embodied mind. In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Systems. New York: Academic Press; 2000. pp. 463–490. [Google Scholar]
- 65.Insausti R, Muñoz M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis) Eur. J. Neurosci. 2001;14:435–451. doi: 10.1046/j.0953-816x.2001.01662.x. [DOI] [PubMed] [Google Scholar]
- 66.Jones EG, Burton H. Areal differences in the laminar distribution of thalamic afferents in the cortical fields of the insular, parietal and temporal regions of primates. J. Comp. Neurol. 1976;168:197–248. doi: 10.1002/cne.901680203. [DOI] [PubMed] [Google Scholar]
- 67.Jones EG, Powell TPS. Connexions of the somatic sensory cortex of the rhesus monkey. I. Ipsilateral cortical connections. Brain. 1969;92:477–502. doi: 10.1093/brain/92.3.477. [DOI] [PubMed] [Google Scholar]
- 68.Jones EG, Powell TPS. An anatomical study of converging sensory pathways in the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- 69.Jones EG, Coulter JD, Hendry SHC. Intracortical connectivity of architectonic fields in the somatic sensory, motor, and parietal cortex of monkeys. J. Comp. Neurol. 1978;181:291–348. doi: 10.1002/cne.901810206. [DOI] [PubMed] [Google Scholar]
- 70.Jürgens U. The efferent and afferent connections of the supplementary motor area. Brain Res. 1984;300:63–81. doi: 10.1016/0006-8993(84)91341-6. [DOI] [PubMed] [Google Scholar]
- 71.Kennard MA. Somatic Functions. In: Bucy PC, editor. The Precentral Motor Cortex. Urbana, Illinois: The University of Illinois Press; 1949. pp. 243–276. [Google Scholar]
- 72.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J. Comp. Neurol. 2007;502:810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- 73.Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- 74.Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- 75.Krubitzer L, Kaas J. Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Vis. Neurosci. 1990;5:165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- 76.Krubitzer L, Kaas J J. The evolution of the neocortex in mammals: how is phenotypic diversity generated? Curr. Opin. Neurobiol. 2005;15:444–453. doi: 10.1016/j.conb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J. Neurosci. 1995;15:3821–3839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J. Comp. Neurol. 1994;350:337–356. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- 79.Künzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in macaca fascicularis. Brain Behav. Evol. 1978;15:185–234. doi: 10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- 80.Kurata K. Corticocortical inputs to the dorsal and ventral aspects of the premotor cortex of macaque monkeys. Neurosci. Res. 1991;12:263–380. doi: 10.1016/0168-0102(91)90116-g. [DOI] [PubMed] [Google Scholar]
- 81.Kuypers HGJM. Anatomy of the descending motor pathways. In: Brooks VB, editor. Handbook of Physiology, Section I, The Nervous System, Vol. II, Motor Control, Part I. Bethesda MD: American Physiological Society; 1981. pp. 567–666. [Google Scholar]
- 82.Kuypers HG. A new look at the organization of the motor system. Prog. Brain Res. 1982;57:381–403. doi: 10.1016/S0079-6123(08)64138-2. [DOI] [PubMed] [Google Scholar]
- 83.Kuypers HG, Brinkman J. Precentral projections to different parts of the spinal intermediate zone in the rhesus monkey. Brain Res. 1970;24:29–48. doi: 10.1016/0006-8993(70)90272-6. [DOI] [PubMed] [Google Scholar]
- 84.Laplane D, Degos DJ, Baulac M, Gray F. Bilateral infarction of the anterior cingulate gyri and of the fornices. J. Neurol. Sci. 1981;51:289–300. doi: 10.1016/0022-510x(81)90107-6. [DOI] [PubMed] [Google Scholar]
- 85.Laskey AM. Acid thionin stain for Nissl bodies on frozen sections. Stain Technol. 1949;24:143. doi: 10.3109/10520294909139595. [DOI] [PubMed] [Google Scholar]
- 86.Lawrence DG, Kuypers HGJM. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 87.Leichnetz GR. Afferent and efferent connections of the dorsolateral precentral gyrus, (Area 4 hand/arm region) in the macaque monkey, with comparisons to area 8. J. Comp. Neurol. 1986;254:460–492. doi: 10.1002/cne.902540403. [DOI] [PubMed] [Google Scholar]
- 88.Leichnitz GR. Connections of the medial posterior parietal cortex (area 7m) in the monkey. Anat. Rec. 2001;263:215–236. doi: 10.1002/ar.1082. [DOI] [PubMed] [Google Scholar]
- 89.Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J. Psychol. Neurol. 1934;46:113–177. [Google Scholar]
- 90.Lu MT, Preston PB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J. Comp. Neurol. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- 91.Luppino G, Matelli M, Rizzolatti G. Patterns of cytochrome oxidase activity in the frontal agranular cortex of macaque monkey. Behav. Brain Res. 1985;18:125–137. doi: 10.1016/0166-4328(85)90068-3. [DOI] [PubMed] [Google Scholar]
- 92.Luppino G, Matelli M, Rizzolatti G. Cortico-cortical connections of two electrophysiologically identified arm representations in the mesial agranular frontal cortex. Exp. Brain Res. 1990;82:214–218. doi: 10.1007/BF00230855. [DOI] [PubMed] [Google Scholar]
- 93.Luppino G, Calzavara R, Rozzi S, Matelli M. Projections from the superior temporal sulcus to the agranular frontal cortex in the macaque. Eur. J. Neurosci. 2001;14:1035–1040. doi: 10.1046/j.0953-816x.2001.01734.x. [DOI] [PubMed] [Google Scholar]
- 94.Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J. Comp. Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- 95.Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticospinal projections from mesial frontal and cingulate areas in the monkey. Neuroreport. 1994;5:2545–2548. doi: 10.1097/00001756-199412000-00035. [DOI] [PubMed] [Google Scholar]
- 96.Luppino G, Rozzi S, Calzavara R, Matelli M. Prefrontal and agranular cingulate projections to the dorsal premotor areas F2 and F7 in the macaque monkey. Eur. J. Neurosci. 2003;17:559–578. doi: 10.1046/j.1460-9568.2003.02476.x. [DOI] [PubMed] [Google Scholar]
- 97.Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J. Comp. Neurol. 1991;311:463–482. doi: 10.1002/cne.903110403. [DOI] [PubMed] [Google Scholar]
- 98.Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb. Cortex. 2001;11:513–521. doi: 10.1093/cercor/11.6.513. [DOI] [PubMed] [Google Scholar]
- 99.Martino AM, Strick SL. Corticospinal projections originate from the arcuate premotor area. Brain Res. 1987;404:307–312. doi: 10.1016/0006-8993(87)91384-9. [DOI] [PubMed] [Google Scholar]
- 100.Matelli M, Luppino G, Rizzolatti G. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J. Comp. Neurol. 1991;311:445–462. doi: 10.1002/cne.903110402. [DOI] [PubMed] [Google Scholar]
- 101.Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J. Comp. Neurol. 1986;251:281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- 102.Matelli M, Govoni P, Galletti C, Kutz DF, Luppino G. Superior area 6 afferents from the superior parietal lobule in the macaque monkey. J. Comp. Neurol. 1998;402:327–352. [PubMed] [Google Scholar]
- 103.Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. J. Neurophysiol. 1992;68:653–662. doi: 10.1152/jn.1992.68.3.653. [DOI] [PubMed] [Google Scholar]
- 104.Mayberg HS, McGinnis S. Brain mapping: the applications. In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Systems. New York: Academic Press; 2000. pp. 491–522. [Google Scholar]
- 105.McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J. Neurosci. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McGuire PK, Bates JF, Goldman-Rakic PS. Interhemispheric integration: I. Symmetry and convergence of the cortical connections of the left and the right principal sulcus (PS) and left and right supplementary motor area (SMA) in the rhesus monkey. Cereb. Cortex. 1991;1:390–407. doi: 10.1093/cercor/1.5.390. [DOI] [PubMed] [Google Scholar]
- 107.McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, Pizzimenti MA, Rotella DL, Vanadurongvan T, Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J. Comp. Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mesulam MM, Mufson EJ. Insula of the old world monkey. III. Efferent cortical output and comments on function. J. Comp. Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 109.Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J. Comp. Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 110.Mitz AR, Wise SP. The somatotopic organization of the supplementary motor area: intracortical microstimulation mapping. J. Neurosci. 1987;7:1010–1021. doi: 10.1523/JNEUROSCI.07-04-01010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morecraft RJ, Tanji J. Cingulofrontal interactions and cingulate skeletomotor areas. In: Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford: Oxford University Press; 2009. pp. 113–144. [Google Scholar]
- 112.Morecraft RJ, Van Hoesen GW. Cingulate input to primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J. Comp. Neurol. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- 113.Morecraft RJ, Van Hoesen GW. Frontal granular cortex input to the cingulate (M3), supplementary (M2) and primary (MI) motor cortices in the rhesus monkey. J. Comp. Neurol. 1993;337:669–689. doi: 10.1002/cne.903370411. [DOI] [PubMed] [Google Scholar]
- 114.Morecraft RJ, Van Hoesen GW. Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res. Bull. 1998;45:209–232. doi: 10.1016/s0361-9230(97)00344-4. [DOI] [PubMed] [Google Scholar]
- 115.Morecraft RJ, Van Hoesen GW. Functional neuroanatomy of limbic structures and some relationships with prefrontal cortex. In: Fogel BS, Schiffer RD, Rao SM, editors. Neuropsychiatry. 2nd ed. Philadelphia: Williams and Wilkins; 2003. pp. 294–327. [Google Scholar]
- 116.Morecraft RJ, Rockland KS, Van Hoesen GW. Localization of area prostriata and its projection to the cingulate motor cortex in the rhesus monkey. Cereb. Cortex. 2000;10:192–203. doi: 10.1093/cercor/10.2.192. [DOI] [PubMed] [Google Scholar]
- 117.Morecraft RJ, Schroeder CM, Keifer J. Organization of face representation in the cingulate gyrus of the rhesus monkey. NeuroReport. 1996;7:1343–1348. doi: 10.1097/00001756-199605310-00002. [DOI] [PubMed] [Google Scholar]
- 118.Morecraft RJ, Louie JL, Herrick JL, Stilwell-Morecraft KS. Cortical innervation of the facial nucleus in the non-human primate: a new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain. 2001;124:176–208. doi: 10.1093/brain/124.1.176. [DOI] [PubMed] [Google Scholar]
- 119.Morecraft RJ, Louie JL, Schroeder CM, Avramov K. Segregated parallel inputs to the brachial spinal cord from the cingulate motor cortex in the monkey. Neuroreport. 1997;8:3933–3938. [PubMed] [Google Scholar]
- 120.Morecraft RJ, Cipolloni PB, Stilwell-Morecraft KS, Gedney MT, Pandya DN. Cytoarchitecture and cortical connections of the posterior cingulate and adjacent somatosensory fields in the rhesus monkey. J. Comp. Neurol. 2004;469:37–69. doi: 10.1002/cne.10980. [DOI] [PubMed] [Google Scholar]
- 121.Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Dvanajscak Z, Ge J, Schneider P. Localization of arm representation in the cerebral peduncle of the non-human primate. J. Comp. Neurol. 2007;504:149–167. doi: 10.1002/cne.21438. [DOI] [PubMed] [Google Scholar]
- 122.Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, Van Hoesen GW. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J. Comp. Neurol. 2007;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- 123.Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, Schoolfield MW. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125:176–198. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- 124.Morris R, Petrides M, Pandya DN. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J. Comp. Neurol. 1999;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 125.Muakkassa KF, Strick PL. Frontal lobe inputs to the primary motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain Res. 1979;177:176–182. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- 126.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 127.Muñoz M, Insausti R. Cortical efferents of the entorhinal cortex and adjacent parahippocampal region in the monkey (macaca fascicularis) Eur. J. Neurosci. 2005;22:1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- 128.Murray EA, Coulter JD. Organization of corticospinal neurons in the monkey. J. Comp. Neurol. 1981;195:339–365. doi: 10.1002/cne.901950212. [DOI] [PubMed] [Google Scholar]
- 129.Murray EA, Coulter JD. Supplementary sensory area. The medial parietal cortex in monkey. In: Woolsey CN, editor. Cortical Sensory Organization, Vol. I, Multiple Somatic Sensory Areas. Clifton: Humana Press; 1981. pp. 167–195. [Google Scholar]
- 130.Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J. Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- 131.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 132.Nakayama Y, Yamagata T, Tanji J, Hoshi E. Transformation of a virtual action plan into a motor plan in the premotor cortex. J. Neurosci. 2008;28:10287–10297. doi: 10.1523/JNEUROSCI.2372-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nimchinsky EA, Hof PR, Young WG, Morrison JH. Neurochemical, morphologic, and laminar characterization of cortical projection neurons in the cingulate motor areas of the macaque monkey. J. Comp. Neurol. 1996;374:136–160. doi: 10.1002/(SICI)1096-9861(19961007)374:1<136::AID-CNE10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 134.Pandya DN. General discussion 2. In: Bock G, O’Connor M, Marsh J, editors. Motor Areas of the Cerebral Cortex: Ciba Foundation Symposium 132. New York: John Wiley & Sons; 1987. pp. 165–170. [Google Scholar]
- 135.Pandya DN, Kuypers HGJM. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- 136.Pandya DN, Sanides F. Architectonic parcellation of the temporal operculum in rhesus monkey, and its projection pattern. Z. Anat. Entwicklungsgesch. 1973;139:127–161. doi: 10.1007/BF00523634. [DOI] [PubMed] [Google Scholar]
- 137.Pandya DN, Seltzer B. Intrinsic connections and architectonics of the posterior parietal cortex in the rhesus monkey. J. Comp. Neurol. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- 138.Pandya DN, Vignolo LA. Intra- and interhemispheric projections of the precentral, premotor and arcuate areas in the rhesus monkey. Brain Res. 1971;26:217–233. [PubMed] [Google Scholar]
- 139.Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Cerebral Cortex, Vol. 4, Association and Auditory Cortices. New York: Plenum Press; 1985. pp. 3–61. [Google Scholar]
- 140.Pandya DN, Yeterian EH. Prefrontal cortex in relation to other cortical areas in rhesus monkey: architecture and connections. In: Uylings HBM, Van Eden CG, De Bruin JPC, Corner MA, Feenstra MGP, editors. The Prefrontal Cortex: Its Structure, Function and Pathology, Progress in Brain Research. Vol. 85. Amsterdam: Elsevier; 1990. pp. 63–94. [DOI] [PubMed] [Google Scholar]
- 141.Pandya DN, Yeterian EH. Architecture and connections of cerebral cortex: implications for brain evolution and function. In: Scheibel AB, Adam F, editors. Neurobiology of Higher Cognitive Function. New York: The Guilford Press; 1990. pp. 53–84. [Google Scholar]
- 142.Pandya DN, Dye P, Butters N. Efferent cortico-cortical projections of the prefrontal cortex in the rhesus monkey. Brain Res. 1971;31:35–46. doi: 10.1016/0006-8993(71)90632-9. [DOI] [PubMed] [Google Scholar]
- 143.Pandya DN, Hallett M, Mukherjee SK. Intra- and interhemispheric connections of the neocortical auditory system in the rhesus monkey. Brain Res. 1969;14:49–65. doi: 10.1016/0006-8993(69)90030-4. [DOI] [PubMed] [Google Scholar]
- 144.Pandya DN, Van Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the monkey. Exp. Brain Res. 1981;42:319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- 145.Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteriomedial cortex in the macaque. Proc. Natl. Acad. Sci. USA. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Paxinos G, Huang X-F, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. New York: Academic Press; 2000. [Google Scholar]
- 147.Peters A. An introduction to neuronal fine structure. Med. Biol. Illus. 1968;818:103–109. [PubMed] [Google Scholar]
- 148.Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J. Comp. Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- 149.Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region of the rhesus monkey. J. Comp. Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- 150.Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 151.Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J. Comp. Neurol. 2006;498:227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- 152.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J. Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Picard N, Strick PL. Motor areas on the medial wall: a review of their location and functional activation. Cereb. Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 154.Pons TP, Kaas JH. Corticocortical connections of area 2 of somatosensory cortex in macaque monkeys: a correlative anatomical and electrophysiological study. J. Comp. Neurol. 1986;248:313–335. doi: 10.1002/cne.902480303. [DOI] [PubMed] [Google Scholar]
- 155.Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate macaca. J. Comp. Neurol. 1991;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- 156.Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc. Natl. Acad. Sci. USA. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- 158.Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp. Brain Res. 1988;71:491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- 159.Roberts TS, Akert AK. Insular and opercular cortex and its thalamic projection in macaca mulatta. Schweitz. Arch. Neurol. Neurochir. Psychiatr. 1963;92:1–43. [PubMed] [Google Scholar]
- 160.Roland PE, Larsen B, Lassen NA, Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J. Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- 161.Romanski LM, Bates JF, Goldman-Rakic PS. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1999;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 162.Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of the cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- 163.Rosene DL, Van Hoesen GW. The hippocampal formation of the primate brain: a review of some comparative aspects of cytoarchitecture and connections. In: Jones EG, Peters A, editors. Cerebral Cortex, Vol. 6, Further Aspects of Cortical Function, including Hippocampus. New York: Plenum Press; 1987. pp. 345–456. [Google Scholar]
- 164.Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb. Cortex. 2006;16:1389–1417. doi: 10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- 165.Saleem KS, Kondo H, Price PL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J. Comp. Neurol. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- 166.Sanides F. The architecture of the cortical taste nerve areas in the squirrel monkey (Saimiri sciureus) and their relationships to insular, sensorimotor and prefrontal regions. Brain Res. 1968;8:97–124. doi: 10.1016/0006-8993(68)90174-1. [DOI] [PubMed] [Google Scholar]
- 167.Sanides F. Comparative architectonics of the neocortex of mammals and their evolutionary interpretation. Ann. N.Y. Acad. Sci. 1969;167:404–423. [Google Scholar]
- 168.Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: Noback CR, Montagna W, editors. The Primate Brain, Advances in Primatology. Vol. 1. New York: Appleton-Century-Crofts; 1972. pp. 137–208. [Google Scholar]
- 169.Sarkissov SA, Filimonoff IN, Kononowa EP, Preobraschenskaji IS, Kukuew LA. Atlas of the Cytoarchitectonics of the Human Cerebral Cortex. Moscow: Medgiz; 1955. [Google Scholar]
- 170.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford: Oxford University Press; 2006. [Google Scholar]
- 171.Schmidt L, Cléry-Melin ML, Lafargue G, Valabrègue R, Fossati P, Dubois B, Pessiglione M. Get aroused and be stronger: emotional facilitation of physical effort in the human brain. J. Neurosci. 2009;29:9450–9457. doi: 10.1523/JNEUROSCI.1951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J. Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- 174.Seltzer B, Pandya DN. Posterior cingulate and retrosplenial cortex connections of the caudal superior temporal region in the rhesus monkey. Exp. Brain Res. 2009;195:325–334. doi: 10.1007/s00221-009-1795-4. [DOI] [PubMed] [Google Scholar]
- 175.Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- 176.Shima K, Aya K, Mushiake H, Inase M, Aizawa H, Tanji J. Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J. Neurophysiol. 1991;65:188–202. doi: 10.1152/jn.1991.65.2.188. [DOI] [PubMed] [Google Scholar]
- 177.Simonyan K, Jürgens U. Cortico-cortical projections of the motor cortical larynx area in the rhesus monkey. Brain Res. 2002;949:23–31. doi: 10.1016/s0006-8993(02)02960-8. [DOI] [PubMed] [Google Scholar]
- 178.Stepniewska I, Preuss TM, Kaas JH. Architectonics, somatotopic organization, and ipsilateral cortical connections of the primary motor area (MI) of owl monkeys. J. Comp. Neurol. 1993;330:238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- 179.Tachibana Y, Nambu A, Hatanaka N, Miyachi S, Takada M. Input-output organization of the rostral part of the dorsal premotor cortex, with special reference to its corticostriatal projection. Neurosci. Res. 2004;48:45–57. doi: 10.1016/j.neures.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 180.Takada M, Nambu A, Hatanaka N, Tachibana Y, Miyachi S, Taira M, Inase M. Organization of prefrontal outflow toward frontal motor-related areas in macaque monkeys. Eur. J. Neurosci. 2004;19:3328–3342. doi: 10.1111/j.0953-816X.2004.03425.x. [DOI] [PubMed] [Google Scholar]
- 181.Takada M, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Hatanaka N, Nambu A. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur. J. Neurosci. 2001;14:1633–1650. doi: 10.1046/j.0953-816x.2001.01789.x. [DOI] [PubMed] [Google Scholar]
- 182.Tanji J. The supplementary motor area in the cerebral cortex. Neurosci. Res. 1994;19:251–268. doi: 10.1016/0168-0102(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 183.Tanné-Gariépy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp. Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- 184.Tokuno H, Inase M. Direct projections from the ventral premotor cortex to the hindlimb region of the supplementary motor area in the macaque monkey. Neurosci. Lett. 1994;171:159–162. doi: 10.1016/0304-3940(94)90629-7. [DOI] [PubMed] [Google Scholar]
- 185.Tokuno H, Tanji J. Input organization of distal and proximal forelimb areas in the monkey primary motor cortex: a retrograde double labeling study. J. Comp. Neurol. 1993;333:199–209. doi: 10.1002/cne.903330206. [DOI] [PubMed] [Google Scholar]
- 186.Tokuno H, Takada M, Nambu A, Inase M. Reevaluation of ipsilateral corticocortical inputs to the orofacial region of the primary motor cortex in the macaque monkey. J. Comp. Neurol. 1997;389:34–48. doi: 10.1002/(sici)1096-9861(19971208)389:1<34::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 187.Tower SS. Pyramidal lesion in the monkey. Brain. 1940;63:36–90. [Google Scholar]
- 188.Tower SS. The pyramidal tract. In: Bucy PC, editor. The Precentral Motor Cortex. Urbana, Illinois: The University of Illinois Press; 1949. pp. 149–172. [Google Scholar]
- 189.Uhl F, Kornhuber AW, Wartberger P, Lindinger G, Lang W, Deecke L. Supplementary motor area in spatial coordination of bilateral movements: a new aspect to 'the SMA debate'? Electroencephalogr. Clin. Neurophysiol. 1996;101:469–477. doi: 10.1016/s0013-4694(96)93577-8. [DOI] [PubMed] [Google Scholar]
- 190.Van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Vogt BA BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Boston: Birkhäuser; 1993. pp. 249–284. [Google Scholar]
- 191.Vogt BA, Pandya DN. Cortico-cortical connections of somatic sensory cortex (areas 3, 1 and 2) in the rhesus monkey. J. Comp. Neurol. 1978;177:179–191. doi: 10.1002/cne.901770202. [DOI] [PubMed] [Google Scholar]
- 192.Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 193.Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J. Comp. Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- 194.Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204:205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- 195.Vogt BA, Vogt L, Farber NB, Bush G. Architecture and neurocytology of the monkey cingulate gyrus. J. Comp. Neurol. 2005;485:218–239. doi: 10.1002/cne.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang Y, Matsuzaka Y, Shima K, Tanji J. Cingulate cortical cells projecting to monkey frontal eye field and primary motor cortex. Neuroreport. 2004;15:1559–1563. doi: 10.1097/01.wnr.0000133300.62031.9b. [DOI] [PubMed] [Google Scholar]
- 197.Wang Y, Shima K, Sawamura H, Tanji J. Spatial distribution of cingulate cells projecting to the primary, supplementary, and pre-supplementary motor areas: a retrograde multiple labeling study in the macaque monkey. Neurosci. Res. 2001;39:39–49. doi: 10.1016/s0168-0102(00)00198-x. [DOI] [PubMed] [Google Scholar]
- 198.Wiesendanger M, Wiesendanger R. The supplementary motor area in the light of recent investigations. Exp. Brain Res. Suppl. 1984;9:382–392. [Google Scholar]
- 199.Wiesendanger M, Rouiller EM, Kazennikov O, Perrig S. Is the supplementary motor area a bilaterally organized system? In: Lüders HO, editor. Advances in Neurology, Vol. 70, Supplementary Sensorimotor Area. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 85–93. [PubMed] [Google Scholar]
- 200.Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computation. Ann. Rev. Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- 201.Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blümcke I. NeuN: a useful neuronal marker for diagnostic histopathology. J. Histochem. Cytochem. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- 202.Woolsey CN. Organization of sensory and motor areas. In: Harlow HF, Woolsey CN, editors. Biological and Biochemical Bases of Behavior. Madison, Wisconsin: University of Wisconsin Press; 1958. pp. 63–81. [Google Scholar]
- 203.Woolsey CN, Settlage PH, Meyer DR, Sencer W, Pinto Hamuy T, Travis AM. Patterns of localization in precentral and "supplementary" motor areas and their relation to the concept of a premotor area. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- 204.Yeterian EH, Pandya DN. Fiber pathways and cortical connections of the preoccipital areas in rhesus monkey. J. Comp. Neurol. 2010;518:3725–3751. doi: 10.1002/cne.22420. [DOI] [PubMed] [Google Scholar]
- 205.Zentner J, Hufnagel A, Pechstein U, Wolf HK, Schramm J. Functional results after resective procedures involving the supplementary motor area. J. Neurosurg. 1996;85:542–549. doi: 10.3171/jns.1996.85.4.0542. [DOI] [PubMed] [Google Scholar]
- 206.Zilles K, Schlaug G, Matelli M, Luppino G, Schleicher A, Qü M, Dabringhaus A, Seitz R, Roland PE. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J. Anat. 1995;187:515–537. [PMC free article] [PubMed] [Google Scholar]