Abstract
Self-assembled monolayers (SAMs) are widely used to confine proteins and cells to a pattern in order to study cellular processes and behavior. In order to fully explore some of these phenomena, it is necessary to control cell growth and confinement for several weeks. Here we present a simple method by which protein and cellular confinement to a pattern can be maintained for more than 35 days. This represents a significant increase in pattern stability compared to previous monolayer systems and is achieved by using an amide-linked glycol monomer on 50 Å titanium/100 Å gold-coated glass coverslips. In addition, this study provides insight into the method of SAM degradation and excludes interfacial mixing of the monomers and blooming of the adlayer as major mechanisms for SAM degradation.
Keywords: Self-Assembled Monolayer (SAM), patterned cell culture, monolayer stability, pattern fidelity, microcontact printing
Introduction
Classical systems for patterned cell culture, including self-assembled monolayers (SAMs) formed from alkanethiols on gold, have limited stability under cell culture conditions. Most systems are only stable for 5–7 days in cell culture,1,2 which significantly limits their use for the study of developmental events, in vitro disease models, and for long-term model systems for drug discovery.3–7 Monolayer instability has limited the use of patterned substrates to short-term cell culture experiments lasting only 1–2 days.1,2,8,9 Here we develop a system that is stable for over five weeks in culture and explore the mechanism of SAM degradation, which has been of some debate.
While the traditional ethylene glycol-terminated SAM monolayer (Figure 1a) is only stable for 5–7 days, a number of other systems with increased stability have been developed. These systems are typically based on sugar-terminated monomers and include the mannitol system developed by Mrksich and co-workers, which is stable for 25 days and the D+L gulitol racemic sugar system developed by Luk and co-workers which is stable for 23 days.2,10 Additionally, trichlorosilanes have been shown to form stable SAMs on glass for cell patterning,11 but the instability of these glycol monomers, which polymerize upon exposure to moisture, makes monolayer preparation notably more difficult than monolayer formation from alkanethiols on gold.
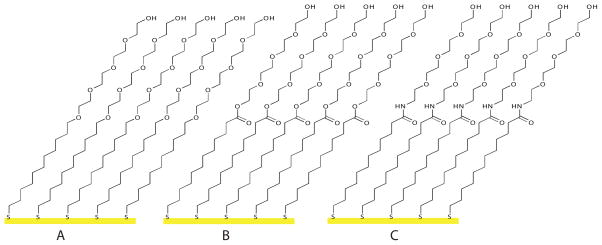
Figure 1.
Protein and cell resistant SAMs were created from a) ether-linked glycol thiol (1), b) ester-linked glycol thiol (2), and c) amide-linked glycol thiol (3)
SAMs formed from alkanethiols on gold have been hypothesized to deteriorate due to several intrinsic and environmental factors, including: interfacial mixing of the monomers, blooming of the adlayer, and oxidation of the thiol head group. Whitesides and coworkers showed that patterned bovine capillary endothelial cells lose confinement by growing into the interface of the pattern.1 This loss of confinement was attributed to mixing of the hexadecanethiol and glycol-terminated monomers at the edges of the pattern through thiol migration, resulting in poor glycol coverage. An additional factor that has been hypothesized to affect monolayer stability is blooming. In blooming, the metal adlayer, which is required for the formation of gold-coated glass, alloys with the gold resulting in disruption of the monolayer.12–15 Moreover, the optically transparent thin gold films used in cellular studies were expected to be highly prone to blooming because the gold layer is extremely thin (typically 100 to 250 Å). Another factor hypothesized to contribute to SAM degradation is oxidation of the gold-sulfur bond to a sulfonate, which is unable to form stable covalent bonds to gold. Sulfonate formation has been measured directly by x-ray photoelectron spectroscopy and indirectly through increased stability of SAMs in deoxygenated media.16,17
Here we demonstrate that we can dramatically increase patterned monolayer stability for cell culture by simply altering how the glycol moiety is attached to the alkanethiol (Figure 1). Cooper and Leggett previously reported that hydrogen bonding at the terminus of a SAM increased the stability of alkanethiol monomers to surface displacement.18 Also, the synthesis of a series of amide-linked glycol monomers and ester-linked glycol monomers have been reported and thermal stability of the SAMs was found to be dependant on the glycol-alkane chain linkage as evidenced by temperature-programmed desorption (TPD).19,20 However, ester and amide-linked glycol-terminated SAMs have not been studied under cell culture conditions. We demonstrate that ester and amide linkages greatly enhance patterned monolayer stability with the amide-linked monomer being stable on 100 Å gold for over five weeks in culture. The enhanced stability is due to the glycol-alkane chain linkage and not differences in van der Waal’s packing forces, since the monomers used in our study have the same number of methylene units. Additionally, using quantitative nanomechanical mapping (QNM), we demonstrate that there is no substantial inter-phase mixing for any of the glycol-terminated monolayers. By looking at a variety of gold thicknesses, we demonstrate that blooming does not substantially affect monolayer stability in cell culture. However, we observe significant differences in monolayer stability as a function of gold thickness, which can be attributed to gold topology.
Results and Discussion
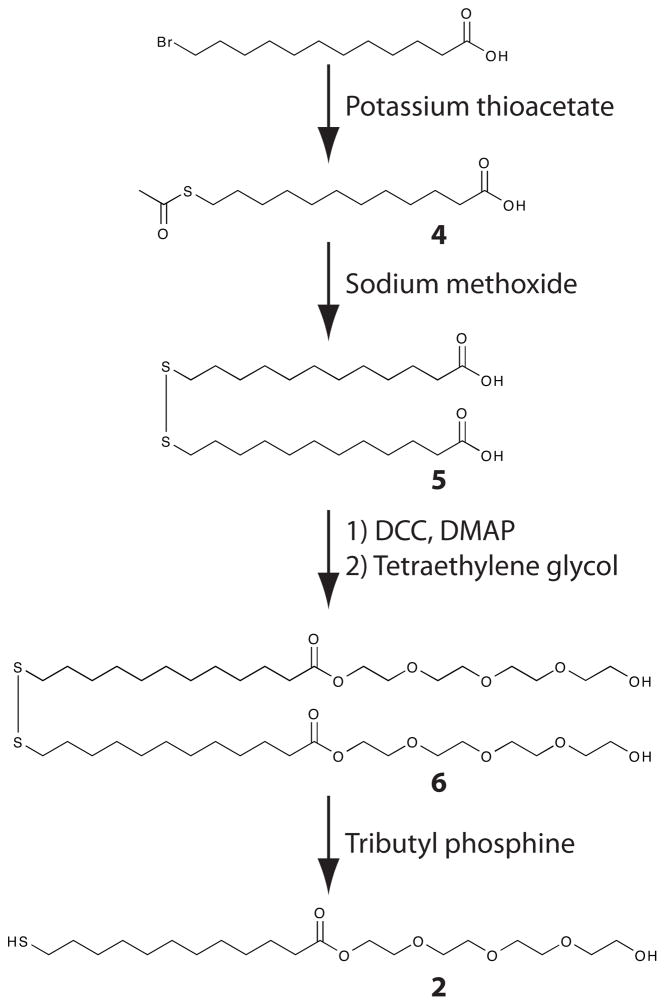
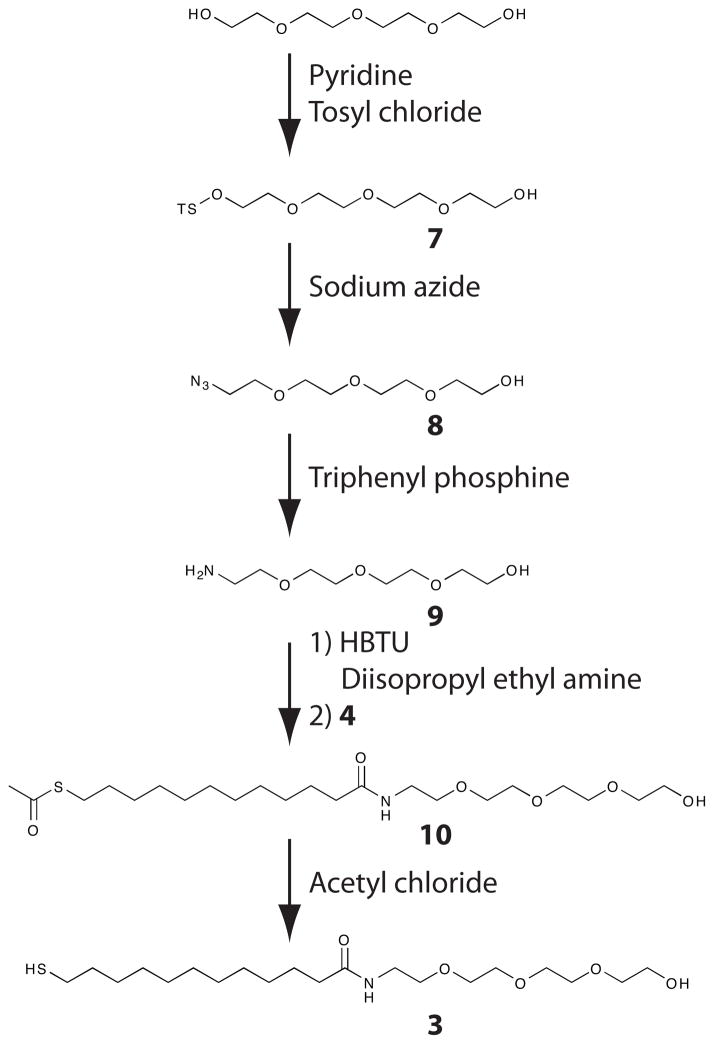
The monomers utilized in these studies vary only in the linkage between the alkane chain and glycol moiety to rule out differences in glycol ordering and structure as factors that affect protein resistance. Based on previous work, the tetraethylene glycol moiety should provide the necessary disorder in glycol structure to prevent protein and cell adhesion.21 The ether-linked monomer was synthesized as previously described.1,22,23 The synthesis of both the ester-linked and amide-linked monomers is straightforward from commercially available starting material (Schemes 1 and 2). These syntheses are not significantly more onerous than that of the ether-linked monomers. Synthetic details are provided in the supporting material.
Scheme 1.
Synthetic scheme for the synthesis of the ester-linked glycol-terminated thiol (2).
Scheme 2.
Synthetic scheme for the synthesis of the amide-linked glycol-terminated thiol (3).
Patterns for cell culture were prepared by microcontact printing circles of hexadecanethiol onto gold substrates of varying thicknesses, backfilling with glycol-terminated monomers and non-specifically adsorbing fibronectin onto the hexadecanethiol-coated region.24 Gold thicknesses ranging from 50 Å to 250 Å with a 50 Å titanium adhesion layer in all cases were examined. These thicknesses were compatible with inverted live-cell phase-contrast microscopy. Thicker metal substrates introduced a substantial neutral density filter into the microscope and were thus not well suited for inverted microscopy.
To determine pattern stability under cell culture conditions, chinese hamster ovary (CHO-K1) cells were seeded onto fibronectin-coated substrates. CHO-K1 cells were chosen because they rapidly reach confluence and after becoming confluent daughter cells can detach and reattach in defect sites formed on the surface. As a result, stability experiments carried out using CHO-K1 cells, as opposed to a more slowly growing fibroblast cell line, such as NIH-3T3 cells, most likely represent a worst-case scenario for pattern stability. This is important both for understanding the mechanism of pattern degradation and defining cell culture stability. It is possible that previous studies, which have employed slow growing fibroblasts, have over-estimated pattern stability.2,10
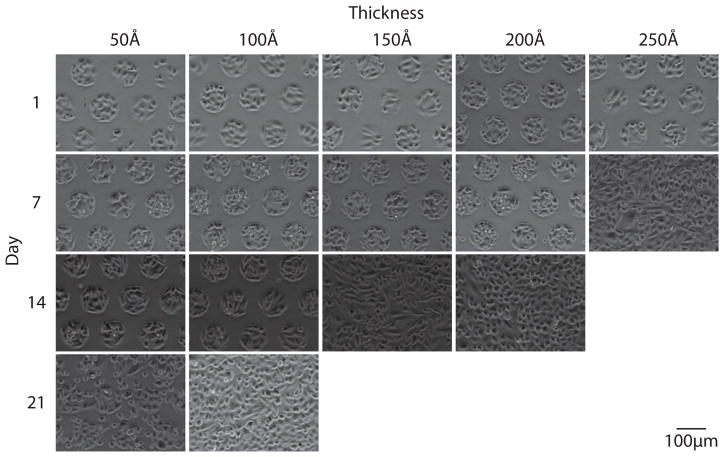
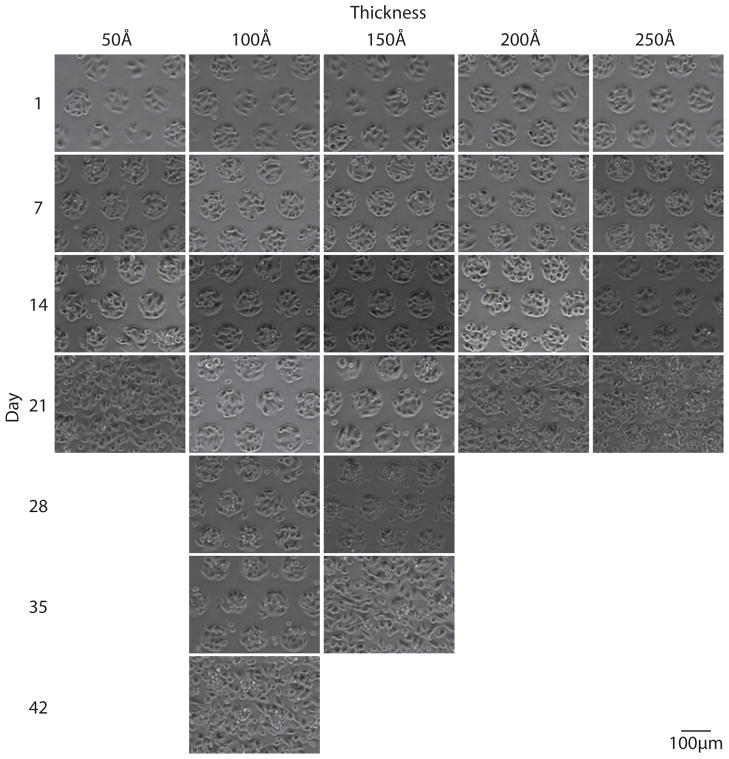
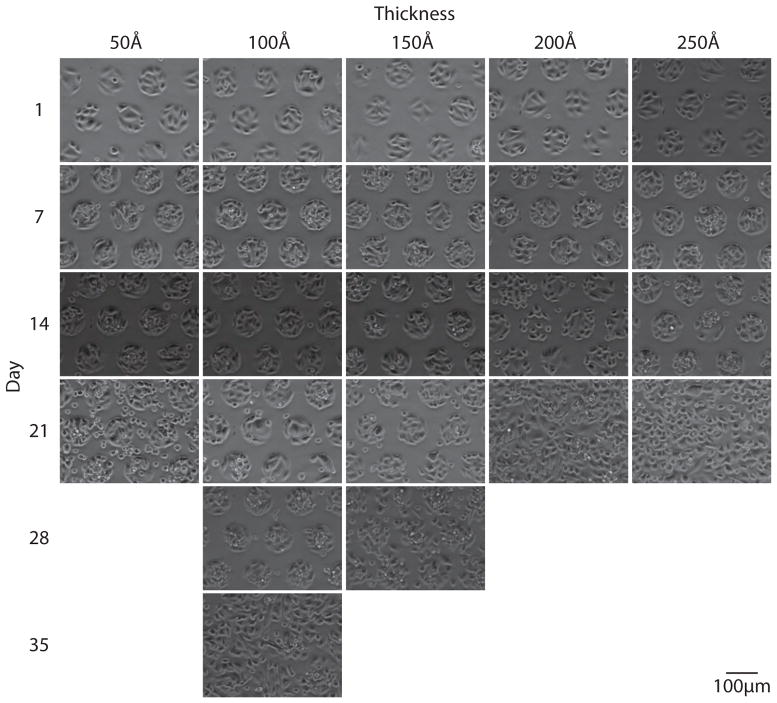
In order to monitor pattern integrity, substrates were imaged weekly until patterns reached approximately 50% confluence. Figures 2 through 4 show representative images of each gold thickness as a function of time for the three different glycol-terminated background monolayers (Figure 1). As is clearly seen in these images, pattern integrity is best maintained with the amide-linked monomer, followed by the ester-linked monomer, and finally the ether-linked monomer. This trend is in agreement with the thermal stabilities previously measured for these molecules.19,20
Figure 2.
Live-cell phase-contrast images acquired weekly for CHO-K1 cells grown on a 95 μm circles pattern with an ether-linked glycol (1) mono-layer background on varying gold thicknesses. Pattern stability is maintained for 14 days on 50 Å and 100 Å gold substrates.
Figure 4.
Live-cell phase-contrast images acquired weekly for CHO-K1 cells grown on a 95 μm circles pattern with an amide-linked glycol (3) mono-layer background on varying gold thicknesses. Pattern stability is maintained for 35 days on 100 Å gold substrates.
It is important to note that the synthetic method employed for the formation of the ester-linked monomer is critical to monolayer stability. In initial experiments conducted using the ester-linked monomer prepared with a trityl protecting group, rapid pattern degradation was observed for samples prepared with background ester-linked monolayers. This degradation was likely due to trace acid-terminated monomers produced during the trityl deprotection, which in turn catalyzed ester hydrolysis (data not shown). However, we were able to completely eliminate this instability by protecting the monomer as a disulfide as shown in scheme 1.
A clear trend in pattern fidelity is also observed as a function of gold thickness for thicknesses between 100 Å and 250 Å. Surprisingly, this trend is the opposite of what would be predicted if blooming played a major role in monolayer degradation. If blooming was important to pattern instability, one would expect alloying to occur more slowly as the gold thickness increased and thus pattern stability to increase with increasing gold thickness. Here we observe the opposite trend for gold thicknesses between 100 and 250 Å. However, blooming may contribute to degradation of the SAMs formed on 50 Å of gold, since none of the patterned SAMs at this thickness confine cells longer than 14 days.
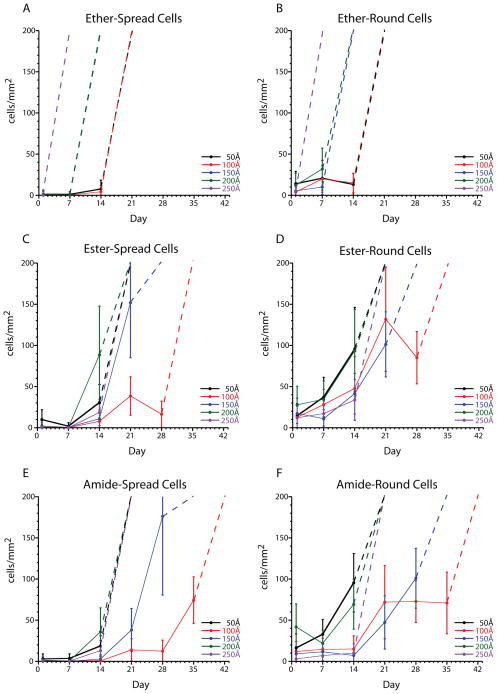
In order to quantitate the number of cells found in the background of the pattern, the number of spread (live, proliferating) and round (dead or weakly attached) cells growing outside the 95 μm circle pattern were determined from 42–49 images obtained from three independent samples at each condition. Figure 5 shows the number of round and spread cells for each glycol-terminated monolayer at each gold thickness. Substrates were considered confluent or partially confluent when the concentration of cells was greater than 200 cells/mm2 this is indicated in the figure by a dotted line going off scale. We have found that often round cells are easily removed by thorough rinsing of the substrate and are not indicative of monolayer degradation.
Figure 5.
Cell attachment as a function of monomer linkage, gold thickness, and time. Spread cells are indicative of loss of pattern stability (A, C, E). Round cells are often unattached or weakly attached to the substrate (B, D, F). Dashed lines represent confluent substrates and complete loss of pattern.
For the ether-linked monolayer, a low number of background cells is observed until confluency. This implies that degradation of the ether-linked monolayer is a rapid process. The deterioration of both the ester-linked and amide-linked monolayers is more gradual than for the ether-linked monolayer. As a result, it is likely that the formation of defect sites in the ether-linked monolayer results in fast deterioration of the SAM, whereas the ester and amide-linked SAMs are able to maintain confinement in the presence of defect sites.
An interesting finding in this study is the increased stability of 100 Å gold substrates compared to 150 Å gold substrates. Traditionally, little attention has been paid to the substrate thickness used in patterned cell studies with typical gold thickness ranging from 120 Å to 2000 Å.1,2,8–10,25 However, our data suggests that gold thickness is a critical parameter in stability with 100 Å gold substrates providing increased stability relative to thicker and thinner substrates. Additionally, 100 Å gold substrates are beneficial, compared to thicker gold substrates, in studies utilizing epifluorescence microscopy, since the gold substrate acts as a neutral density filter, decreasing the light that reaches the camera. Moreover, it is possible to use 50 Å titanium/50 Å gold coated coverlips for short experiments (on the order of one week), which should provide even better fluorescence signals.
While Whitesides and co-workers observed pattern degradation by loss of confinement at the interface of the hexadecanethiol region and the glycol region,1 we do not observe cells growing out from the pattern edges. Instead, we observe cells attaching and spreading throughout the background region during loss of confinement (Figures 2–4). Whitesides and co-workers’ observation of cells growing out from the pattern is likely a result of using slowly replicating fibroblast cells, which do not readily detach and reattach at background defect sites. Our observation is consistent with sulfur oxidation and monomer loss as opposed to interfacial mixing as the mechanism of background monolayer destruction.16,17,26,27 While oxidation of the glycol moiety has previously been discussed,2,28 this is likely not the mechanism at play here since solvent accessibility, and therefore oxygen exposure, to the glycol moieties should be similar.
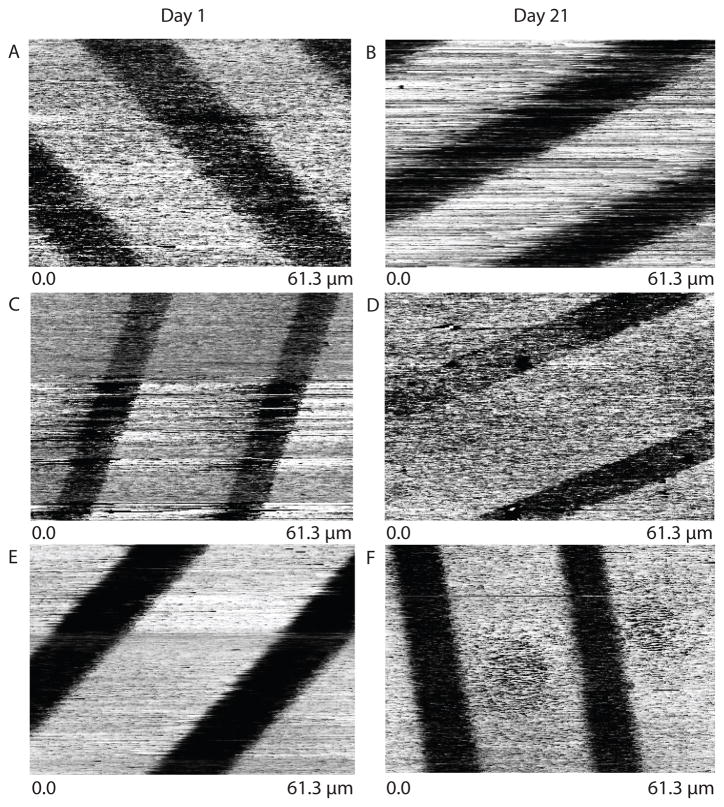
To further support our hypothesis that interfacial mixing is not a major contributor to glycol monolayer degradation, we examined interfacial mixing using scanning probe microscopy (SPM). While differences in hexadecanethiol versus glycol-terminated thiol regions of monolayers can be resolved in frictional force contact mode scanning probe microscopy,29 the observed height differences are likely artifactual. The observed height difference is likely due to significant differences in silicon tip adhesion between glycol-terminated and hexadecanethiol monomers. To examine interfacial mixing, we directly measured differences in tip adhesion as a function of time using QNM scanning probe microscopy (Figure 6). Samples for QNM analysis were prepared by microcontact printing 10 μm hexadecanethiol lines onto 150 Å gold-coated coverslips and backfilling with each of the glycol-terminated monomers. Force images were acquired weekly over three weeks for samples immersed in phosphate buffered saline at 37 °C. Despite significant differences of pattern fidelity in cell culture for some of these substrates, no significant changes were observed by force microscopy. If interfacial mixing were an important part of monolayer degradation, we would have expected to see a blurring of the glycol/hexadecanethiol monolayer interface with time and differences between the three glycol monomers. However, the glycol-hexadecanethiol interface appears sharp in all samples after 21 days. It is interesting to note that while significant differences in adhesion and other mechanical properties between the hexadecanethiol and glycol regions were observed for samples immersed in phosphate buffered saline and washed with distilled water prior to measurement, no differences in adhesion or mechanical properties were observed if samples had been immersed in cell culture media without fetal bovine serum prior to measurement. This was true even when care was taken to completely rinse the substrate with distilled water prior to bringing it through the air/water interface.
Figure 6.
Patterned substrate adhesion measured using QNM SPM. The wider lines are the glycol-terminated areas whereas the thinner lines are alkane-terminated. The ether-linked at day 1 (A) and day 21 (B), ester-linked at day 1 (C) and day 21 (D), and amide-linked at day 1 (E) and day 21 (F) do not show significant blurring of the pattern, indicating that interfacial mixing has not occurred.
To better understand the observed trend for gold thickness, we examine the roughness of the gold substrates using scanning probe microscopy in peak-force tapping mode (Figure 7). There are significant differences in appearance for the substrates with increasing roughness across the series from 50 Å to 250 Å. The change in roughness likely leads to a decrease in monolayer order, which in turn gives rise to the observed trend of decreasing stability with increasing gold thickness. Interestingly, the structure of the 50 Å substrate is very different from the other thicknesses and contains well-defined nanostructures. These nanostructures are a result of the underlying titanium-coated glass coverslip and explain the limited stability of glycol-terminated monolayers on 50 Å gold. The 100 Å and 150 Å gold substrates resemble each other and consist of soft rolling hills, which likely support well-ordered monolayers. In contrast, the 200 Å and 250 Å substrates contain sharper “peaks and “valleys”. As a result, it is not surprising that the 100 Å and 150 Å substrates provide the best stability. Moreover, the 100 Å substrate, which contains more “hills” than “valleys”, is most stable. The observation that gold topology greatly affects monolayer stability is to be expected in light of the observations that increased pattern stability could be achieved by varying the angle of electron beam evaporation.30 However, unlike variable angle deposition, thickness control provides a readily available method for stability control. All commercially available electron beam evaporators can easily control substrate thickness, however most evaporators are not equipped for angular deposition.
Figure 7.
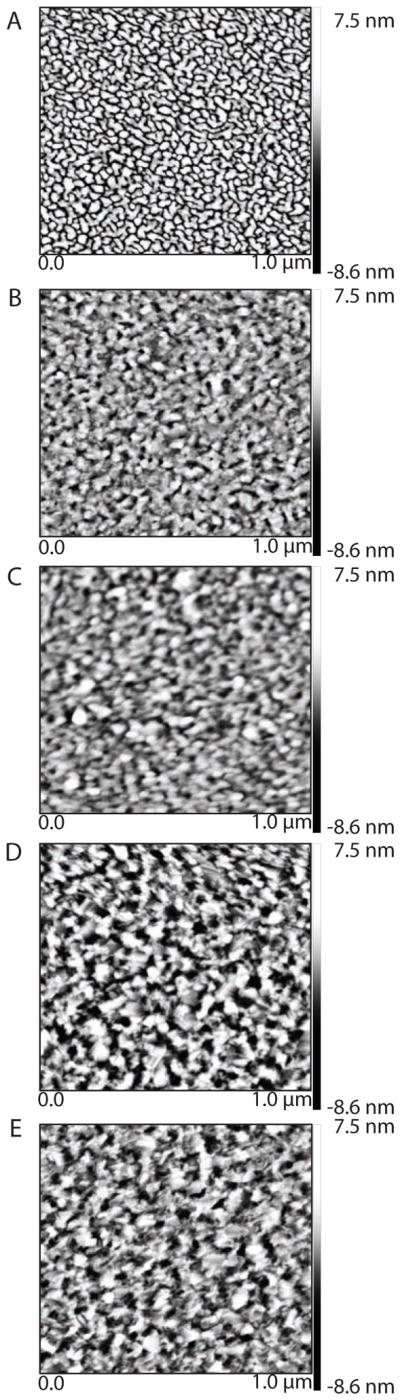
Scanning probe microscopy height images of gold substrates obtained in peak force tapping mode.
Conclusion
Patterned SAMs with amide-linked glycol background monolayers prepared on glass coverslips with 50 Å of titanium and 100 Å of gold allow for more than five weeks of high-fidelity patterned cell culture. This represents an enormous advancement in patterned cell culture substrate stability and will allow for long-term cell culture experiments. We have also found that gold thickness can be used to control gold nanotopology and, in turn, monolayer stability under cell culture conditions. Furthermore, loss of pattern fidelity in cell culture does not arise from blooming or interfacial mixing of the glycol monolayer with the hexadecanethiol monolayer and is therefore likely a result of sulfur oxidation and monolayer degradation.
Supplementary Material
Figure 3.
Live-cell phase-contrast images acquired weekly for CHO-K1 cells grown on a 95 μm circles pattern with an ester-linked glycol (2) mono-layer background on varying gold thicknesses. Pattern stability is maintained for 28 days on 100 Å gold substrates.
Acknowledgments
This work was funded by the National Institute of Mental Health (1R01MH085495). Support for the Mass Spectrometry Resource is provided by the National Institutes of Health and the National Center for Research Resources (2P41RR000954).
ABBREVIATIONS
- SAM
self-assembled monolayer
- QNM
quantitative nanomechanical mapping
- SPM
scanning probe microscopy
Footnotes
ASSOCIATED CONTENT
Supporting Information. Synthesis of monomers and experimental procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mrksich M, Dike L, Tien J, Ingber D, Whitesides G. Experimental Cell Research. 1997;235:305–313. doi: 10.1006/excr.1997.3668. [DOI] [PubMed] [Google Scholar]
- 2.Luk YY, Kato M, Mrksich M. Langmuir. 2000;16:9604–9608. [Google Scholar]
- 3.Davila JC, Rodriguez RJ, Melchert RB, Acosta D. Annual Review of Pharmacology and Toxicology. 1998;38:63–96. doi: 10.1146/annurev.pharmtox.38.1.63. [DOI] [PubMed] [Google Scholar]
- 4.Dwyer T, Earl D, Wang L. Journal of Neuroscience. 2008;28:6537–6538. doi: 10.1523/JNEUROSCI.1841-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberhuber G, Schwarzenhofer M, Vogelsang H. Digestive Diseases. 1998;16:341–4. doi: 10.1159/000016888. [DOI] [PubMed] [Google Scholar]
- 6.Ucuzian A, Greisler H. World Journal of Surgery. 2007;31:654–663. doi: 10.1007/s00268-006-0763-4. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Aschner M. Neurotoxicology. 2003;24:741–5. doi: 10.1016/S0161-813X(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 8.Cooper E, Wiggs R, Hutt D, Parker L, Leggett G, Parker T. Journal of Materials Chemistry. 1997;7:435–441. [Google Scholar]
- 9.Mrksich M, Whitesides G. Trends in Biotechnology. 1995;13:228–235. [Google Scholar]
- 10.Bandyopadhyay D, Prashar D, Luk YY. Chemical Communications. 2011;47:6165–6167. doi: 10.1039/c1cc10855g. [DOI] [PubMed] [Google Scholar]
- 11.Yanker D, Maurer J. Molecular Biosystems. 2008;4:502–504. doi: 10.1039/b801161c. [DOI] [PubMed] [Google Scholar]
- 12.George MA, Glaunsinger WS, Thundat T, Lindsay SM. Thin Solid Films. 1990;189:59–72. [Google Scholar]
- 13.Hampy R, Yost F, Ganyard F. Journal of Vacuum Science & Technology. 1979:25–30. [Google Scholar]
- 14.Love J, Estroff L, Kriebel J, Nuzzo R, Whitesides G. Chemical Reviews. 2005:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 15.Masahiro K, Noboru S. Journal of Materials Science. 1993:5088–5091. [Google Scholar]
- 16.Maciel J, Martins M, Barbosa M. Journal of Biomedical Materials Research Part A. 2010:833–843. doi: 10.1002/jbm.a.32746. [DOI] [PubMed] [Google Scholar]
- 17.Flynn NT, Tran TNT, Cima MJ, Langer R. Langmuir. 2003;19:10909–10915. [Google Scholar]
- 18.Cooper E, Leggett GJ. Langmuir. 1999;15:1024–1032. [Google Scholar]
- 19.Svedhem S, Hollander CA, Shi J, Konradsson P, Liedberg B, Svensson SCT. The Journal of Organic Chemistry. 2001;66:4494–4503. doi: 10.1021/jo0012290. [DOI] [PubMed] [Google Scholar]
- 20.Valiokas R, Ostblom M, Svedhem S, Svensson SCT, Liedberg B. The Journal of Physical Chemistry B. 2002;106:10401–10409. [Google Scholar]
- 21.Harder P, Grunze M, Dahint R, Whitesides GM, Laibinis PE. Journal of Physical Chemistry B. 1998;102:426–436. [Google Scholar]
- 22.Palegrosdemange C, Simon E, Prime K, Whitesides G. J Am Chem Soc. 1991:12–20. [Google Scholar]
- 23.Chen C, Mrksich M, Huang S, Whitesides G, Ingber D. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DM, LaFranzo NA, Maurer JA. Journal of Visualized Experiments. :e3164. doi: 10.3791/3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane R, Takayama S, Ostuni E, Ingber D, Whitesides G. Biomaterials. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 26.Cortes E, Rubert AA, Benitez G, Carro P, Vela ME, Salvarezza RC. Langmuir. 2009;25:5661–5666. doi: 10.1021/la804251a. [DOI] [PubMed] [Google Scholar]
- 27.Qin G, Cai C. Chemical Communications. 2009:5112–5114. doi: 10.1039/b911155g. [DOI] [PubMed] [Google Scholar]
- 28.Wieland B, Lancaster J, Hoaglund C, Holota P, Tornquist W. Langmuir. 1996;12:2594–2601. [Google Scholar]
- 29.Sasaki K, Koike Y, Azehara H, Hokari H, Fujihira M. Applied Physics A-Materials Science & Processing. 1998;66:S1275–S1277. [Google Scholar]
- 30.Simon K, Burton E, Han Y, Li J, Huang A, Luk Y. J Am Chem Soc. 2007;129:4892–4893. doi: 10.1021/ja0653472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.