Abstract
In both human and nonhuman primates the eyes are a highly salient feature of the face, conveying identity, emotion and attentional direction of conspecifics. Studies have indicated that the amygdala plays an important role in eye contact, and amygdala dysfunction may underlie social deficits in disorders such as autism through effects on eye contact. In the present study we compared the volume of the amygdala in 32 juvenile rhesus monkeys to visual fixation patterns in a social memory paradigm. Amygdala volume was determined from manual traces of structural MRIs and fixation patterns were assessed using eyetracking methodology. A significant positive relationship was found between amygdala volume and fixation on both the face and the eye region. Amygdala volume was also found to relate to habituation across multiple presentations of different photographs of the same individual monkeys indicating a role in social memory. These data provide an important linkage between structural variation of amygdala and previous demonstrations of function in a nonhuman primate model.
Keywords: Face Recognition, MRI, Social, Affiliative, Gaze
Previous studies have shown that the amygdala plays an important role in guiding behavioural responses in a social environment. Amygdala activation in brain imaging studies is frequently associated with social stimuli [3]; amygdala damage has a strong impact on social behavior [3–5]; and disorders of social behavior such as autism and social anxiety disorder are associated with aberrant amygdala functioning [6–8]. These studies indicate that although the amygdala is not necessary for perceptual processing of social stimuli it does appear to play an important role in recognition of emotional displays and in providing emotional salience in a social context [9].
In both human and nonhuman primates the face is among the most prominent of social stimuli. Faces are an important source of information in determining individual identity, emotional state, and intentions of other group members [10]. Within the face, the eyes appear to be a particularly powerful elicitor of amygdala activity, and studies have indicated that alterations in eye contact may be an important factor in social deficits that have been observed as a consequence of amygdala damage [3]. In fact, this work suggests that amygdala function relates specifically to an individual’s ability to attend to the eye region of the face [3]. Research has indicated that rhesus macaques, like humans engage in configural, holistic level processing of faces [11–12]. However, specific features within the face and in particular the eyes are also important means of identity and emotion identification [11–15]; and as with humans the eyes also appear to be particularly potent means of eliciting amygdala activity [3, 10]. Most research examining relations between amygdala activity and exposure to social stimuli considers group differences. It remains unclear if variation in amygdala structure or function among monkeys relates to variation in behaviors elicited by social stimuli.
In the present study we compared the relationship between amygdala volume and eye fixation patterns in a group of juvenile rhesus monkeys as they viewed unfamiliar monkey faces in a free viewing eyetracking task. Previous human studies have shown a significant positive relationship between amygdala volume and fixation on the eyes in an autistic population [17], and significant reductions in both amygdala volume and duration of eye contact in unaffected siblings of autistic individuals [18] using a similar eyetracking measure to the one used in the current study. Although studies indicated that amygdala abnormality is related to these disorders [19–21], direct evidence of the relationship between normative amygdala anatomy and fixation on eyes is lacking. Demonstration of such a relationship would be particularly useful in animal (especially primate) models. During our studies evaluating effects of early adverse rearing experience and long-term fluoxetine treatment on cognition and social behavior of rhesus monkeys, we found that amygdala volume was not affected by either rearing conditions or drug treatment, but was positively related to total time and number of fixation in the eye region and negatively related to the latency of eye fixation when viewing photographs of unfamiliar monkey faces.
Thirty-two male rhesus monkeys (Macaca mulatta) served as subjects in this study. All monkeys were born and reared in the NIH animal facility in Poolesville MD. Subjects were from four different birth year cohorts (8 per year), and were between 3–4 years of age at time of testing. The methods described here are part of a larger study examining the effects of long term fluoxetine treatment on development of monkeys with differential rearing experiences. Half of the monkeys from each birth year were reared under the standard peer rearing (PR) conditions described elsewhere [22], and half were reared under semi natural conditions with their mothers (MR). Between 1–2 years of age monkeys were transferred to indoor pair housing with a like reared partner. Between 2–3 years of age one member of each dyad was treated with 3 mg/kg fluoxetine daily for one year administered orally in a banana treat. All animal care and experimental procedures were approved by the National Institutes of Health (NIH) Animal Care and Use Committee.
As part of the protocol all monkeys underwent structural MRI scanning at four points during development: once prior to treatment, twice during treatment, and once after treatment. Monkeys were sedated with a ketamine (1 mg/kg)/xylazine (6 mg/kg) mixture delivered immediately prior to MRI and supplemented as needed. Images were acquired on a 3T GE magnet with a quadknee coil. During each image acquisition session five T1 weighted FSPGR volumes were generated using the following parameters (124 slices acquired in coronal plane, TR = 4.08s, TE = 1.66s, matrix = 256×256, FOV = 128 mm, slice thickness = 0.8 mm, flip angle = 90). In order to improve signal:noise ratio a single mean volume was computed from the five FSPGR series at each time point. The mean T1-weighted image of each subject was resampled to generate 0.43 mm isomorphic voxels and manually oriented to align the AC-PC axis.
Amygdala volume was determined by manual trace using methods described elsewhere [8]. Briefly, traces were first outlined in the coronal plane beginning at caudal most extent and proceding rostrally. Boundaries were then confirmed in both axial and sagittal planes. In caudal sections amygdala boundaries were the optic tract dorsomedially, the putamen and caudate dorsolaterally, the hippocampus ventrally and the lateral ventricle medially. In more rostral sections the ventromedial border is defined by the entorhinal and piriform cortices and the piriform cortex formed the rostral border at approximately the level of the anterior commisure. All tracing was done with Analyze 10.0 software by a single experimenter (EN, Cronbach’s alpha for intrarater reliability was 0.89). No differences in amygdala volume were found as a function of rearing, treatment, or interactions of these factors (all p > 0.2, see Supplement 1). For the purpose of the present study we report only the post-treatment amygdala volume which was closest in time to the eyetracking portion of the study (correlations with other time points are presented in Supplement 2). Since no laterality effect was found we collapsed across side and generated a single mean amygdala volume per subject. In order to assess specificity of amygdala findings we also obtained a measure of whole brain (WB) volume. WB volume was obtained with a semi-automated parcellation procedure contained in Analyze software. Automated parcellation was followed with manual refinements to exclude cerebellum, brainstem and spinal cord beneath the pons, and all other non-brain tissue contained in trace. The ratio of amygdala volume to whole brain volume was used to generate a metric of whole brain corrected (WBC) amygdala volume. All monkeys were between 3–4 years at time of scan and treated monkeys underwent a 2–3 month washout period prior to scanning.
Within 6 months after MRI acquisition all monkeys underwent eyetracking tests. The basic methods employed have been described in detail elsewhere [23]. Monkeys were placed in a primate chair with an attachable customized head restraint and tested in a sound attenuating chamber. Visual stimuli were presented on a 17 inch color monitor 60–70 cm in front of subjects. Habituation and initial training consisted of displaying non-test images to monkeys until they reliably fixated in the allotted time (at least one fixation in 10 secs) to at least 80 % of images. In the present experiment, the first cohort of 8 monkeys (2 monkeys in each 4 group) were initially tested using ASL eye tracking system (model 5000) (Applied Science Laboratories, Bedford, MA) and the other 24 were tested with the Tobii T60 Eye Tracker, with visual stimuli presented and data generated by a PC running software Tobii studio 2.0.6. Both systems used a 60 Hz infrared camera to detect fixations using data generated from pupil and corneal reflections.
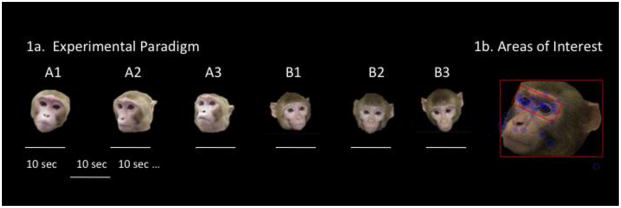
The test consisted of forty-eight 10 sec trials with a 10 sec blank screen ITI separating each trial. The stimuli for the task were 48 color photographs of 16 different monkey faces – 3 photographs of each monkey. Faces were all upright, forward or side oriented and had neutral facial expressions. Stimulus monkeys were of mixed age (all juvenile or young adult) and gender. Images were 720 × 540 pixels of faces on a black background. The 3 pictures of the same monkey were shown consecutively and then followed by another stimulus animal (A1, A2, A3, B1, B2, B3 etc - see Fig 1). This procedure was designed to probe both basic face orienting in monkeys by presenting a single face at a time; and because three images of the same stimulus individual were presented serially, it also enabled assessment of memory as monkeys devote greater attention to novel than familiar faces. Importantly, other than the initial habituation period, monkeys were not required to perform any task and so this paradigm was designed to probe spontaneous orienting behavior which may be altered by task demands [11].
Figure 1.
Three different photographs of the same monkey were presented sequentially for a 10 sec duration. The entire session consisted of 48 trials and 16 stimulus monkeys. Examples of AOIs drawn to capture fixations on the entire face and within the eye region of stimulus photographs.
Total fixation duration, frequency and latency to first fixation were calculated for two rectangular areas of interest (AOI): one for the whole face and one for the eyes (as shown in Fig 1B). We also calculated the proportion of face fixations which were in the eye region: eye fixations/face fixations (P_EYE). A fixation was defined as a gaze maintained within 0.5 degree of visual angle for at least 100 ms. Because of non-normality of data the duration and frequency of fixations on the eye region were subject to square root transformations. All other variables were normally distributed and untransformed raw data was used.
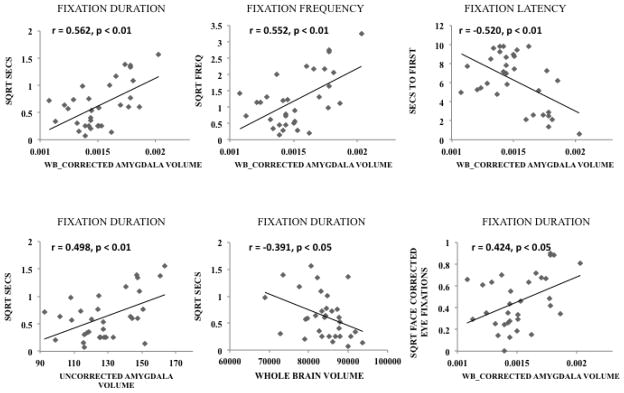
Pearson correlations were used to compare the relationship between amygdala volume and visual fixation patterns to first, second, and third presentation of each individual stimulus and the average of all three images across the sample. On several variables a significant correlation was found between amygdala volume and fixations on the face as a whole (see Table 1). However, these correlations were consistently stronger when we restricted our analysis to the eye region, and a significant correlation was found on mean values of face corrected eye contacts (P_EYE) (see Table 1 and Fig 2). These results indicate a direct and specific relationship between amygdala volume and tendency to fixate in the eye region.
Table 1.
| ORDER | DURATION | FREQUENCY | LATENCY | |
|---|---|---|---|---|
| FACE | FIRST | 0.404* | 0.497* | −0.342 |
| SECOND | 0.394* | 0.503** | −0.359* | |
| THIRD | 0.318 | 0.363* | −0.174 | |
| MEAN | 0.391* | 0.492* | −0.308 | |
| EYE | FIRST | 0.580** | 0.571** | −0.571** |
| SECOND | 0.502** | 0.501** | −0.453** | |
| THIRD | 0.540** | 0.533** | −0.420* | |
| MEAN | 0.562** | 0.552** | −0.520** | |
| P_EYE | MEAN | 0.424* | 0.438* | −0.117 |
Correlation coefficients (r values) between whole brain corrected amygdala volume (amygdala/whole brain) and fixation duration, frequency, and latency for the first, second and third presentations of different stimulus monkeys. Correlations for entire face are presented on the top half of the table and correlations restricted to the eye region are on the bottom. The final P_EYE row is a correlation of the corrected amygdala volume and the proportion of total face fixations that occurred in the eye region (eye/face). Across all face presentations, eye duration and frequency values were square root transformed.
p < 0.05;
p < 0.01
Figure 2.
The top three panels depict scatterplots of the duration, frequency, and latency to fixate in the eye region and WBC amygdala volume across all trials in this task. Duration and Frequency data were square root transformed to generate normal distributions. The bottom three panels depict fixation duration against raw amygdala volume (left), whole brain volume (middle) and face corrected fixations (P_eye) against WBC amygdala volume (right).
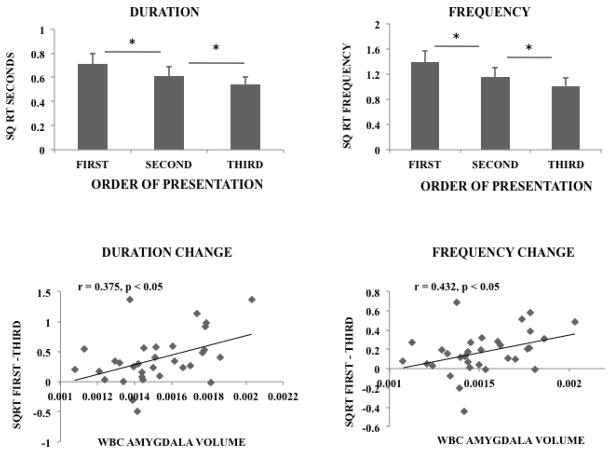
A secondary question in this study was the degree to which amygdala contributes to individual memory inferred from habituation to repeated presentations of the same stimulus individual. We tested this with a series of repeated measures ANOVAs using individual repetition as a within subjects variable. A highly significant effect of repetition was found for all three variables in the whole face fixation data (duration F(2, 62) = 13.6, p < 0.001; frequency F(2, 62) = 16.94, p < 0.001; latency F(2, 62) = 8.65, p < 0.001). Similarly, we found a highly significant effect of repetition on all three variables that were restricted to the eye region (duration F(2, 62) = 15.31, p < 0.001; frequency F(2, 62) = 21.45, p < 0.001; latency F(2, 62) = 8.67, p < 0.01). Follow up two tailed paired t tests revealed that for all but the first and second latency measure, the second presentation significantly differed from the first and the third differed significantly from the second indicating a graded degradation in responding (t(31) (duration t1–2 = 4.18, t2–3 = 2.58) (frequency t1–2 = 4.27, t2–3 = 3.57) (latency t1–2 = 1.26, t2–3 = 2.91), all but latency t1–2 p < 0.05).
These results indicate the formation of memory for different animals because they habituated to repeated presentations of the same individual even though the exact image differed. In order to estimate the degree to which amygdala volume contributed to this pattern we next used the same models but included WBC_amygdala volume as a covariate of interest. An interaction was found between corrected amygdala volume and face repeat for fixation frequency (F(2, 60) = 4.39, p < 0.05) but no significant interactions were found for latency (F(2, 60) = 2.43, p > 0.2) or duration (F(2, 60) = 1.28, p > 0.2). However, in the eye region, significant interaction between WBC amygdala volume and stimulus subject repetition was found for fixation duration (F(2, 60) = 4.37, p < 0.05) and fixation frequency (F(2, 60) = 6.50, p < 0.01). The fixation duration and frequency data across the three stimulus repetitions are depicted in the top half of Fig 3. The bottom half of Fig 3 is a scatterplot representing the change in fixation duration (first – third fixation durations) and the change in fixation frequency (first – third frequency) as a function of amygdala volume.
Figure 3.
The mean duration and frequency of fixations to the first, second and third presentations of each individual monkey in the series is depicted on the top panel. The change in these variables from first to third (first-third) is depicted as a function of WBC amygdala volume in scatterplots on the bottom panels. * p < 0.05
We conducted similar analyses on the face corrected (P_eye) data and found significant main effects of WBC amygdala volume on face corrected eye contacts for duration (F(1, 30) = 6.04, p < 0.05) and for frequency (F(1, 30) = 6.59, p < 0.05) of fixations but there were no significant interactions with repeated measure indicating that the role of the amygdala applies to face fixations but is not specific to eyes within the face.
These data reveal two important relationships between the amygdala and patterns of face fixation. One is that monkeys with larger amygdala tended to fixate in the eye region more than monkeys with small amygdala – this was true across all variables assessed and appeared to both the face as a whole and specifically to the eyes. Second, the data from the habituation tests revealed that not only does the amygdala appear to be important for face fixation generally but it may also play a role in individual memory. The memory function may be related to tendency to fixate on the eyes as previous studies have suggested the eyes are an important feature for memory and face recognition in human and nonhuman primates [14, 15]. Although the present data suggest that the relation between the amygdala and memory performance may not be as specific as eye contact more generally.
There is now a growing literature pointing to the amygdala as a key structure in mediating social behavior. However, the exact role the amygdala plays is not entirely clear as amygdala lesions do not abolish social behavior but rather change its character. It appears that the amygdala is principally involved in guiding behavior appropriately in a social context. The amygdala has long been thought to be a critical node in affective behavior. Beyond the role in mediating emotion affective responding, the anatomical interconnections of the amygdala with primary sensory and other medial temporal lobe structures also enables it to play a more general role in modulating cognitive processes like attention and memory. The present data suggest that the amygdala may play a particularly important role in directing attention to faces and to the eyes in particular - which are typically the most salient feature within the face. Human studies that have focused on patients with lesions or autistic syndromes have likewise implicated amygdala participation in eye contact [3, 9]. The current study provides data from non-human primates to support this view.
However while most studies have inferred amygdala function from pathology, lesion or intracellular recording data, there is also some indication that there may be a relationship between normal variation in amygdala volume and social function in a normative population. Two recent reports have found that amygdala volume is positively correlated with social network size in humans [24, 25]. The present findings are consistent with these reports. However, unlike these prior studies that focus on relatively global measures of social behavior, here we focus on a specific social behavior which may be either a consequence or a driver of behavioral variability. Important questions remain as to whether this finding will generalize to other primate populations or even humans. Nevertheless these data are an important first step in understanding the role of the amygdala in guiding social behavior under non-pathological conditions.
Supplementary Material
Highlights.
The size of the amygdala was significantly correlated with eye contact in rhesus monkeys
The size of the amygdala predicted memory formation for specific individuals
Amygdala was more strongly related to time looking in the eyes than the face
Acknowledgments
The work presented here was supported entirely by the intramural research program of the National Institutes of Health, NIMH. The authors wish to acknowledge the technical assistance and training in amygdala tracing provided by Julia Scott at the University of California – Davis. We also are indebted to the veterinary and animal care staff of the NIH animal facility in Poolesville and all of the staff at the NIMH primate core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Curr Biol. 2007;17:766–72. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 2.Rutishauser U, Tudusciuc O, Neumann D, Mamelak AN, Heller AC, Ross IB, et al. Single-Unit Responses Selective for Whole Faces in the Human Amygdala. Curr Biol. 2011 doi: 10.1016/j.cub.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in rhesus monkeys (Macaca mulatta): consistent pattern of behavior across different social contexts. Behav Neurosci. 2008;122:251–66. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:515–44. [PubMed] [Google Scholar]
- 6.Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65:1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau JY, Guyer AE, Tone EB, Jenness J, Parrish JM, Pine DS, et al. Neural responses to peer rejection in anxious adolescents: contributions from the amygdala-hippocampal comples. International Journal of Behavioral Development. 2011 doi: 10.1177/0165025411406854. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–7. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 10.Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG. Neural responses to facial expression and face identity in the monkey amygdala. J Neurophysiol. 2007;97:1671–83. doi: 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- 11.Dahl CD, Logothetis NK, Hoffman KL. Individuation and holistic processing of faces in rhesus monkeys. Proc Biol Sci. 2007;274:2069–76. doi: 10.1098/rspb.2007.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gothard KM, Brooks KN, Peterson MA. Multiple perceptual strategies used by macaque monkeys for face recognition. Anim Cogn. 2009;12:155–67. doi: 10.1007/s10071-008-0179-7. [DOI] [PubMed] [Google Scholar]
- 13.Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 14.McKelvie SJ. The role of eyes and mouth in the memory of a face. The American Journal of Psychology. 1976;89:311–23. [Google Scholar]
- 15.Parr LA, Winslow JT, Hopkins WD, de Waal FB. Recognizing facial cues: individual discrimination by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta) J Comp Psychol. 2000;114:47–60. doi: 10.1037/0735-7036.114.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spezio ML, Huang PY, Castelli F, Adolphs R. Amygdala damage impairs eye contact during conversations with real people. J Neurosci. 2007;27:3994–7. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, et al. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry. 2006;63:1417–28. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2007;61:512–20. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 20.Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–64. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 21.Amaral DG, Corbett BA. The amygdala, autism and anxiety. Novartis Found Symp. 2003;251:177–87. discussion 87–97, 281–97. [PubMed] [Google Scholar]
- 23.Machado CJ, Nelson EE. Eye-tracking with nonhuman primates is now more accessible than ever before. Am J Primatol. 2011;73:562–9. doi: 10.1002/ajp.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–4. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proc Biol Sci. 2011 doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.