Abstract
Matricellular proteins play diverse roles in modulating cell behavior by engaging specific cell surface receptors and interacting with extracellular matrix proteins, secreted enzymes, and growth factors. Studies of such interactions involving thrombospondin-1 have revealed several physiological functions and roles in the pathogenesis of injury responses and cancer, but the relatively mild phenotypes of mice lacking thrombospondin-1 suggested that thrombospondin-1 would not be a central player that could be exploited therapeutically. Recent research focusing on signaling through its receptor CD47, however, has uncovered more critical roles for thrombospondin-1 in acute regulation of cardiovascular dynamics, hemostasis, immunity, and mitochondrial homeostasis. Several of these functions are mediated by potent and redundant inhibition of the canonical nitric oxide pathway. Conversely, elevated tissue thrombospondin-1 levels in major chronic diseases of aging may account for the deficient nitric oxide signaling that characterizes these diseases, and experimental therapeutics targeting CD47 show promise for treating such chronic diseases as well as acute stress conditions that are associated with elevated thrombospondin-1 expression.
Keywords: Thrombospondin-1, nitric oxide, blood pressure, ischemia, CD47, cGMP
1. Introduction
Thrombospondin-1 (TSP1) is a matricellular protein that is transiently expressed in extracellular matrix and modulates cell function in a context-specific manner by engaging cell surface receptors and other components of the extracellular matrix (Bornstein, 1995; Roberts and Lau, 2011). TSP1 circulates at ~100 pM levels in plasma, and platelet α-granules represent a major preformed storage pool that can rapidly increase extracellular concentrations of the protein at sites of injury. Studies reviewed here comparing vascular responses of wild type (WT) and TSP1 null mice show that endogenous levels of TSP1 in vascular tissues and circulating in plasma serve important physiological and pathophysiological functions.
1.1. TSP1 and the CD47 receptor on vascular cells
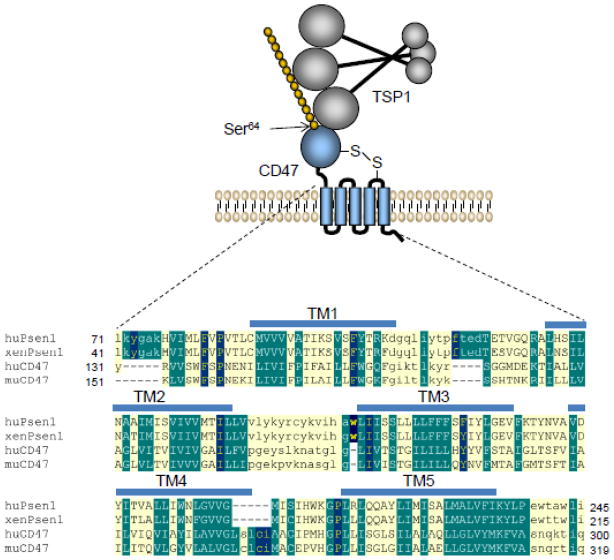
Several excellent reviews broadly describe the structure and interactions of TSP1 (Carlson et al., 2008; Elzie and Murphy-Ullrich, 2004; Mosher and Adams, 2012; Murphy-Ullrich and Iozzo, 2012; Risher and Eroglu, 2012; Sweetwyne and Murphy-Ullrich, 2012; Tan and Lawler, 2009; Murphy-Ullrich and Iozzo, 2012) , so we focus here on its interactions with the receptor CD47. CD47 is an integral membrane protein containing an extracellular IgV domain, a domain that spans the membrane 5 times that is probably derived from a presenilin transmembrane domain (Watanabe et al., 2010), and a short variably spliced C-terminal cytoplasmic tail (Frazier et al., 2010) (Fig. 1). CD47 is universally expressed by vascular and circulating blood cells of land-dwelling vertebrates.
Fig. 1. The TSP1 receptor CD47.
A model for the interaction of TSP1 with the heparan sulfate modified isoform of CD47 is presented. Modification of CD47 at Ser64 is required for inhibitory TSP1 signaling in T cells (Kaur et al., 2011). A long range disulfide bond links the extracellular IgV domain (blue) to the extracellular loop between the transmembrane segments TM4 and TM5 and is required for CD47 signaling (Rebres et al., 2001). BLINK Blast analysis of human CD47 identified multiple presenilins that show significant homology with its transmembrane domain. The lower panel shows alignment of transmembrane domains of xenopus and human presenilin-1 with those of human and mouse CD47. CD47 and presenilin-1 sequences were aligned using the Segment Pair Overlap algorithm of MACAW. Capital letters indicate aligned blocks, and shading indicates conserved residues.
TSP1 engages CD47 via its C-terminal domain (Frazier et al., 2010). CD47 was originally identified as a TSP1 receptor based on its binding to an immobilized peptide derived from this domain of TSP1 that contains a VVM motif (Gao et al., 1996). Although this motif is conserved in other mammalian TSPs, subsequent studies showed CD47 binding to be relatively specific for TSP1 (Isenberg et al., 2009a). Furthermore, the two VVM motifs in the C-terminal domain of TSP1 are not exposed to solvent (Kvansakul et al., 2004). Thus, the residues on TSP1 that interact with CD47 remain unclear. Furthermore, post-translational modification of CD47 with a glycosaminoglycan chain at Ser64 of CD47 is necessary for TSP1 to inhibit CD47 signaling in T cells (Kaur et al., 2011). This modification is prevalent throughout vascular cells, implying that TSP1 signaling through CD47 generally involves interactions with both protein and glycosaminoglycan determinants (Fig. 1).
1.2. CD47 regulation of NO/cGMP signaling
Nitric oxide (NO) plays a central role in cardiovascular physiology to promote arterial dilation and as an anti-thrombotic and anti-inflammatory agent (Ignarro, 2002). Loss of NO synthesis or responsiveness in the cardiovascular system is characteristic of chronic diseases including atherosclerosis, peripheral vascular disease, hypertension, sickle cell disease, and Alzheimer’s disease (Chirkov and Horowitz, 2007). Thus, drugs that enhance NO signaling have been developed to improve blood flow, tissue perfusion, and promote angiogenesis in ischemic tissues (Fraisse and Wessel, 2010; Schade et al., 2010; Schmidt et al., 2009). Organonitrates clearly benefit cardiac function (Nossaman et al., 2010), but efforts to use NO-generating drugs or cGMP phosphodiesterase inhibitors to reverse chronic conditions of NO-insufficiency have shown limited benefit. This raises the question of whether signaling downstream of NO is blocked in these chronic vascular diseases.
Endothelium is the primary source of NO in blood vessels. NO produced by endothelial nitric oxide synthase (eNOS, NOS3) (Dudzinski and Michel, 2007) provides a paracrine signal that controls VSMC contractility to regulate blood flow, blood pressure, and tissue perfusion (Stauss et al., 1999). NO activates its primary signaling target soluble guanylyl cyclase (sGC) in VSMC by binding to its prosthetic heme iron, which allosterically accelerates cGMP production several hundred-fold (Arnold et al., 1977; Stone and Marletta, 1994). cGMP activates cGMP dependent kinase-1 (cGK1), which phosphorylates and inhibits myosin light chain kinase and initiates smooth muscle relaxation by allowing dephosphorylation of myosin light chain (MLC-2) (Lincoln et al., 2001). NO/cGMP signaling stimulates angiogenesis by enhancing endothelial cell chemotaxis and proliferation (Papapetropoulos et al., 1997) and limits platelet activation through cGK1 dependent phosphorylation of targets that limit activation of the platelet integrin αIIbβ3 (Butt et al., 1994; Danielewski et al., 2005; Li et al., 2006b).
TSP1 at picomolar concentrations inhibits NO-stimulated activation of sGC in endothelial cells, VSMC, platelets, and T cells (Isenberg et al., 2005b; Isenberg et al., 2008e; Isenberg et al., 2006b; Ramanathan et al., 2011). This activity is lost in CD47 null but not in CD36 null cells and is mimicked by certain ligands of CD47 (Isenberg et al., 2006a). Ligation of CD36 also inhibits sGC activation, but only at higher concentrations of TSP1 and only in cells that express CD47. Tissue cGMP levels are higher in TSP1 and CD47 null mice. Therefore, physiological levels of TSP1 significantly limit NO/cGMP signaling, and CD47 is the primary and necessary receptor to mediate this inhibitory activity. TSP1 also inhibits sGC activation by heme-independent chemical activators (Miller et al., 2010a). TSP1 signaling through CD47 prevents sGC activation by increasing cytoplasmic calcium levels in Jurkat T cells (Ramanathan et al., 2011). CD47 regulates calcium signaling in several other cell types, which regulates eNOS as well as sGC (Bauer et al., 2010; Frazier et al., 2010; Ramanathan et al., 2011).
1.3. Upstream regulation of NO signaling by TSP1
In addition to inhibiting sGC, TSP1 controls NO synthesis (Fig. 2). The activity of eNOS is controlled by mechanical stimuli and by circulating factors such as VEGF and acetylcholine (Dudzinski and Michel, 2007). VEGF signaling through VEGFR2 on endothelial cells activates phosphoinositide 3-kinase, which stimulates Akt activation. Akt phosphorylates eNOS at the activating site Ser1177 (Dimmeler et al., 2000). Simultaneously, activated VEGFR2 recruits Src and induces Ca2+ signaling, which further activate eNOS (Duval et al., 2007; Gelinas et al., 2002). TSP1 inhibits VEGF signaling via several mechanisms. TSP1 binds to VEGF and prevents VEGFR2 signaling either by competing with extracellular VEGF for binding to cell surface proteoglycans or by promoting VEGF clearance via low density lipoprotein receptor-related protein-1 (LRP1/CD91) (Greenaway et al., 2007; Gupta et al., 1999). Second, TSP1 binding to its receptor CD36 modulates VEGFR2 via a complex involving CD36, the TSP1-binding integrin α6β1, and VEGFR2 (Zhang et al., 2009). Third, CD47 is a proximal binding partner of VEGFR2, and TSP1 binding to CD47 disrupts this complex and inhibits VEGFR2 activation (Kaur et al., 2010; Kaur and Roberts, 2011). Endothelial cells lacking CD47 are resistant to inhibition of VEGFR2 activation by physiological concentrations of TSP1, indicating that this is the dominant pathway under physiological conditions. The other two pathways may become significant under conditions where TSP1 levels are elevated. Downstream activation of Akt phosphorylation was blocked by engaging CD47, consistent with a previous report of increased Akt activation in retinas from TSP1 null mice (Sun et al., 2009). Because CD47 interaction with VEGFR2 globally inhibits VEGF signaling, effects of VEGF signaling on cell motility and survival that are NO-independent can also be inhibited by TSP1 via CD47 (Fig. 2).
Fig. 2. CD47-mediated regulation of vascular cell signaling by TSP1.
Binding of TSP1 to CD47 has a global effect upon the canonical NO pathway. In endothelial cells, TSP1 inhibits VEGFR2 activation through its ability to disrupt the constitutive interactions of CD47 with VEGFR2 (Kaur et al., 2010). This CD47 signal limits Akt-mediated phosphorylation of eNOS at Ser1177 and alters endothelial cell calcium transients to prevent calmodulin-mediated activation of eNOS, thereby redundantly suppressing NO production stimulated by VEGF or acetylcholine. CD47 ligation also controls VEGFR2 signaling through the PLCγ pathway and the TSAd adapter to Src that mediate NO-independent endothelial cell growth, migration, and permeability responses. The TSP1/CD47 signal also inhibits heme-dependent and hem-independent activation of the NO cellular target sGC in all vascular cells and T cells (Miller et al., 2010a). TSP1/CD47 signaling independently inhibits the cGMP-stimulated activation of the cGMP target cGK-1. Thus, the TSP1/CD47 axis is a global inhibitor of endogenous and exogenous NO, NO-pro-drugs such as nitrite (Isenberg et al., 2009d), and cGMP promoting drugs such as synthetic activators of sCG (YC-1, BAY 41-2272; (Miller et al., 2010a) and cGMP phosphodiesterase inhibitors (e.g. sildenafil).
Acetylcholine-mediated activation of eNOS in endothelial cells is also inhibited by TSP1 and its CD47-binding domain in a Ca2+-dependent manner (Bauer et al., 2010). Exposure of endothelium in WT and TSP1 null arteries but not in CD47 null vessels to soluble TSP1 at physiological concentrations inhibited acetylcholine-stimulated vessel relaxation (Bauer et al., 2010). Conversely, TSP1 potentiated phenylephrine-stimulated vasoconstriction in WT and TSP1 null arteries. In mice lacking TSP1, acetylcholine resulted in a greater decrease in blood pressure compared to WT controls.
1.4. Downstream NO/cGMP target regulation by TSP1
cGK can be activated independent of NO by cell permeable analogs of cGMP, and TSP1 inhibits such activation of cGK in endothelial cells, platelets, and VSMC (Isenberg et al., 2005b; Isenberg et al., 2008e; Isenberg et al., 2006b). This may involve post-translational modification of cGK because inhibition persisted in lysates from TSP1-treated platelets (Isenberg et al., 2008e). Therefore, TSP1 redundantly targets the NO/cGMP signaling cascade and can have direct and indirect effects on vascular cell responses (Fig. 2).
1.5. CD47 cross talk with other TSP1 receptors
CD47 was first isolated based on its co-purification with αvβ3 integrin, and subsequent studies have revealed specific association of CD47 with additional integrins including α2β1, α4β1, α6β1, and the platelet integrin αIIbβ3 (Frazier et al., 2010). TSP1 engaging CD47 can induce activation of these integrins. The integrins αvβ3, α6β1, and α4β1 also serve as TSP1 receptors on vascular cells. TSP1 engaging each of these integrins results in proangiogenic signaling in endothelial cells (Calzada et al., 2004). Proliferation and migration of VSMC under normal and hyperglycemic conditions is regulated by TSP1 in a αvβ3-dependent manner, and VSMC proliferation was enhanced by ligation of CD47 (Panchatcharam et al., 2010; Isenberg et al., 2005a). Further research is needed to clarify how CD47 modulates TSP1 signaling via these integrins.
CD47 may also participate in vascular signaling via the TSP1 receptor CD36. CD47 is necessary for inhibition of NO signaling by CD36-binding fragments of TSP1 and by the CD36 ligand β-amyloid (Isenberg et al., 2006a; Miller et al., 2010b). This may involve the co-association of CD36 and CD47 with the integrin α6β1, which was first documented in neural cells (Bamberger et al., 2003).
Evidence in T cells suggests that CD47 also regulates the internalization of TSP1 via calreticulin/LRP1 (Li et al., 2006a). Therefore, CD47 may more broadly regulate TSP1 signaling via all of its receptors by directing its removal from the extracellular space.
1.6. Additional targets of CD47 signaling
cAMP levels in VSMC are in part regulated by cross-talk with cGMP via cGMP-inhibitable cAMP phosphodiesterases, which provides one mechanism for CD47 signaling to control intracellular cAMP levels (Yao et al., 2010). In VSMC and endothelial-free arteries, TSP1 also inhibited adenylate cyclase activation and cAMP accumulation independent of cGMP-mediated cross talk. CD47 ligation also controls cAMP levels by regulation of adenylate cyclase via heterotrimeric G proteins (Frazier et al., 1999).
Based on yeast two-hybrid studies, the short cytoplasmic tail of CD47 interacts with cytoplasmic proteins including PLIC1 and Bcl-2/adenovirus E1B 19-kDa interacting protein (BNIP3) (Frazier et al., 2010). Ligation of CD47 on T cells by TSP1 modulates the interaction between CD47 and BNIP3, which regulates apoptosis by translocating to mitochondria and interacting with Bcl-2 and Bcl-X(L) (Lamy et al., 2007). Reduced T cell apoptosis in TSP1 and CD47 null mice suggested that TSP1 triggers apoptosis via this pathway (Lamy et al., 2007), but TSP1 signaling via CD47 is only implied by the apoptotic activity of extreme concentrations of the modified TSP1 peptide 4N1K (Lamy et al., 2003). CD47 ligation can also stimulate apoptosis by modulation of Fas signaling (Manna et al., 2005), and down-regulation of Drp1 reduced caspase-independent death of leukemic cells induced by soluble TSP1 and a CD47 antibody (Bras et al., 2007). Therefore, TSP1 clearly regulates T cell survival via CD47, but the relative contributions of these three signaling mechanisms remains unclear.
2. Cardiovascular functions of TSP1/CD47 signaling
2.1. Acute effects of TSP1 on tissue perfusion and blood pressure
NO controls regional blood flow by relaxing resistance arteries. Functional MRI of healthy TSP1 null mice showed a greater increase in skeletal muscle blood flow in response to NO within 5 minutes (Isenberg et al., 2007a). Thus, pre-existing levels of TSP1 in the arterial wall or blood constantly limit NO-mediated vasodilation and blood flow. Treatment of isolated pre-contracted VSMCs with TSP1 completely inhibited NO-stimulated relaxation and dephosphorylation of MLC-2. Subsequent studies confirmed that this activity in VSMC is mediated via CD47 (Isenberg et al., 2007b).
Plasma levels of TSP1 in healthy people only partially inhibit eNOS and sGC activation in endothelium. However, plasma TSP1 is known to increase significantly in various disease conditions (Lin et al., 2003; Rico et al., 2010; Smadja et al., 2011). Furthermore, TSP1 levels in the matrix surrounding arterial VSMC increase in atherosclerosis (Riessen et al., 1998; Roth et al., 1998). Thus, direct inhibition of VSMC NO signaling and indirect inhibition via limiting endothelial NO synthesis are CD47-dependent targets that can control vascular tone. Additional TSP1 receptors are present on vascular cells, some of which can oppose these CD47-dependent signals if TSP1 levels are sufficiently elevated (Stenina and Plow, 2008).
Mice lacking or under-expressing eNOS exhibit spontaneous and diet-induced hypertension (Cook et al., 2004). Conversely, inhibiting NO in animals and human subjects raises blood pressure (Broere et al., 1998; Kung et al., 1995). Resting TSP1 null and WT mice had similar mean arterial pressure, but pulse pressure was low in the null mice, suggesting that endogenous TSP1 has a hypertensive activity (Isenberg et al., 2009c). At rest, CD47 null mice are hypotensive with lower mean systolic and diastolic pressures, consistent with enhanced NO signaling and vasodilation. Furthermore, TSP1 and CD47 null mice showed greater decreases in mean arterial blood pressure on treatment with an NO donor or eNOS activator (Bauer et al., 2010; Isenberg et al., 2009c).
Autonomic innervation modulates arterial tone and can be temporarily blocked with hexamethonium. A dose of hexamethonium that induces moderate hypotension in WT mice elicited profound hypotension and death in TSP1 null (Isenberg et al., 2009c) and CD47 null mice (Isenberg, unpublished). These phenotypes of the null mice reveal critical hypertensive roles of TSP1 and CD47 for maintaining central blood pressure under stress. This function is reinforced by evidence that TSP1 has an acute hypertensive activity. Intravenous treatment of WT and TSP1 null mice with TSP1 resulted in increased blood pressure (Bauer et al., 2010). A TSP1 “mimicking” antibody and TSP1 peptides that bind to CD47 similarly increased blood pressure. This hypertensive activity of TSP1 may involve regulation of eNOS activity and antagonism of the hypotensive activity of circulating VEGF (Curwen et al., 2008).
2.2. Effects of TSP1 and CD47 on cardiac dynamics
Several studies have implicated TSP1 in cardiac disease and remodeling, but the TSP1 receptors involved remain unclear (Schellings et al., 2009). The role of NO/cGMP signaling in the heart is also controversial (Garcia-Dorado et al., 2009; Gonzalez et al., 2008). However, cAMP is a potent mediator of both cardiac response and vascular tone (Zaccolo, 2009), and TSP1 and CD47 null cardiac muscle shows increased cAMP levels compared to WT mice (Isenberg et al., 2009c). Cardiac output and ejection fraction increased more in the TSP1 and CD47 null mice in response to systemic vasodilation by an NO donor (Isenberg et al., 2009c). It is unclear, however, whether these enhanced responses are due to loss of TSP1/CD47 signaling in the heart versus in peripheral resistance arteries.
2.3. Platelet hemostasis
NO is a physiological inhibitor of platelet activation (Radomski et al., 1990). In the presence of physiological levels of the NOS substrate arginine or its product NO, platelets from TSP1 null mice were resistant to aggregation induced by thrombin (Isenberg et al., 2008e). Conversely, exogenous TSP1 and CD47 ligands enhanced aggregation under these conditions. Therefore, TSP1 released from platelets provides positive feedback to reinforce platelet aggregation by overcoming the anti-thrombostatic activity of physiological NO concentrations. Thus, the role of elevated TSP1 in wounds may involve two acute activities in addition to long term regulation of angiogenesis and vascular remodeling: 1) TSP1 is a potent vasoconstrictor that initially limits bleeding but can be counterproductive for tissue survival under ischemic stress, and 2) TSP1 is an autocrine factor released by platelets that promotes hemostasis. These insights may also enable us to better understand why TSP1 is down-regulated in many cancers (Isenberg et al., 2009b). Because TSP1 acutely limits tissue perfusion, its expression may be a disadvantage to a growing tumor (Isenberg et al., 2009b). Loss of TSP1 expression may also decrease the thrombogenic potential of the tumor vasculature by maximizing the anti-thrombotic activity of NO produced by the tumor and its stroma.
2.4. Mitochondrial biogenesis
NO/cGMP signaling is a major regulator of mitochondrial biogenesis (Haas et al., 2009; Nisoli and Carruba, 2006). This suggested that mitochondrial homeostasis might be altered in TSP1 and CD47 null mice. Electron microscopy studies revealed increased number and size of mitochondria in skeletal muscle from TSP1 null mice (Frazier et al., 2011). Subsequent work identified similar increases in skeletal muscle mitochondria and in several NO-sensitive regulators of mitochondrial biogenesis in CD47 null mice. Consistent with their increased muscle mitochondrial densities, CD47 null mice exhibit increased exercise endurance and decreased oxygen consumption (Frazier et al., 2011). These studies demonstrate that TSP1/CD47 regulation of physiological NO/cGMP signaling extends beyond the cardiovascular system and has broad metabolic implications.
3. Pathophysiological functions and therapeutic applications
3.1. Functions in injury and stress responses
TSP1/CD47 signaling plays important roles in tumor and wound angiogenesis, fixed ischemic injuries, and ischemia/reperfusion injuries, and readers are referred to recent reviews (Frazier et al., 2010; Isenberg et al., 2008a; Isenberg et al., 2009b; Roberts and Lau, 2011). Elevated plasma TSP1 levels correlate positively with cardiovascular disease (Smadja et al., 2011), suggesting that TSP1 is a biomarker for and potentiator of vascular dysfunction. Recent reports define roles for plasma and tissue TSP1 in promoting pulmonary arterial hypertension (Kaiser et al., 2010; Ochoa et al., 2011; Ochoa et al., 2010). TSP1 null mice exposed to chronic hypoxia exhibited less increase in pulmonary arterial pressure compared to WT mice (Ochoa et al., 2010). Conversely, low plasma TSP1 in human subjects is associated with hypotension (Liu et al., 2008). Although the role of CD47 as a TSP1 receptor in these chronic diseases remains to be established, the TSP1 levels reported should limit NO/cGMP signaling via this receptor.
3.2 Therapeutic opportunities
To date, three approaches have been demonstrated to disrupt the TSP1/CD47 axis in animal disease models. TSP1 can be sequestered or prevented from binding to CD47 using certain TSP1 or CD47 antibodies. The first therapeutic use of a TSP1 antibody was to prevent neointima formation and accelerate re-endothelialization in balloon injured rat carotid arteries (Chen et al., 1999). The TSP1 antibody used, C6.7, was subsequently shown to inhibit TSP1 interactions with CD47 and increase proliferation of CNS endothelial cells to a similar degree as a CD47 blocking antibody (Myers et al., 2011). The TSP1 antibody A6.1 blocks the ability of TSP1 to inhibit NO-stimulated cGMP accumulation in vitro and enhanced survival of ischemic skin flaps in mice and miniature pigs (Isenberg et al., 2007b; Isenberg et al., 2008d). The same TSP1 antibody is radioprotective for human endothelial cells (Maxhimer et al., 2009b), suggesting that it may have therapeutic activity in humans. An anti-mouse CD47 antibody protects animals from ischemic and IR injuries (Isenberg et al., 2008b; Isenberg et al., 2008c; Isenberg et al., 2007b). An anti-rat CD47 antibody similarly protected against IR injury in rats when employed pre- or post- reperfusion (Maxhimer et al., 2009a).
Antisense approaches can be used to temporarily suppress CD47 expression. This approach prevents both TSP1 and SIRPα signaling through CD47 and so may have effects that extend beyond NO/cGMP signaling. Suppression of CD47 expression using a CD47 antisense morpholino protected mice from ischemic and radiation injuries (Isenberg et al., 2008c; Isenberg et al., 2007b; Maxhimer et al., 2009b). The morpholino enhanced survival of ischemic skin flaps in pigs, which better model human ischemic injuries (Isenberg et al., 2008d).
The above results are encouraging for the continued preclinical development of CD47-targeted drugs for treating ischemic injuries, radioprotection, and cancer. These drugs may also be beneficial for treating chronic diseases that are associated with NO insufficiency and elevated TSP1 levels.
Fig. 3. Physiological and pathological functions of TSP1/CD47 signaling.
At physiological levels of TSP1, signaling through CD47 limits NO/cGMP signaling to support blood pressure, limit tissue perfusion, promote platelet hemostasis, and control mitochondrial biogenesis. Acute or chronic elevation of TSP1 levels in circulation or in tissues contributes to a deficit in NO/cGMP signaling associated with fixed ischemic injuries, ischemia/reperfusion, and several chronic diseases of aging. TSP1/CD47 signaling through cGMP-independent pathways also plays significant roles in some adaptive responses to stress, which involve signaling through additional TSP1 receptors including integrins, CD36, and calreticulin/LRP1.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute, NIH to DDR and K22CA128616, R01 HL-108954 (NIH), American Heart Association grant 11BGIA7210001, the Vascular Medicine Institute, the Institute for Transfusion Medicine, and the Hemophilia Center of Western PA to JSI.
Abbreviations
- 4N1K
KRFYVVMWKK
- BNIP3
Bcl-2/adenovirus E1B 19-kDa interacting protein
- cGK
cGMP-dependent protein kinase
- IR
ischemia/reperfusion
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- L-NAME
L-NG-nitroarginine methyl ester
- LRP1
low density lipoprotein receptor-related protein-1
- MLC-2
myosin light chain-2
- sGC
soluble guanylate cyclase
- SIRPα
signal regulatory protein-α
- TSP
thrombospondin
- VEGFR2
vascular endothelial growth factor receptor-2
- VSMC
vascular smooth muscle cell
Footnotes
Disclosures
JSI is chair of the scientific advisory boards of Vasculox, Inc. (St. Louis, MO) and Radiation Control Technologies, Inc. (Rockville, MD)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3':5'-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res. 2010;88:471–481. doi: 10.1093/cvr/cvq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras M, Yuste VJ, Roue G, Barbier S, Sancho P, Virely C, Rubio M, Baudet S, Esquerda JE, Merle-Beral H, Sarfati M, Susin SA. Drp1 mediates caspase-independent type III cell death in normal and leukemic cells. Mol Cell Biol. 2007;27:7073–7088. doi: 10.1128/MCB.02116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broere A, Van Den Meiracker AH, Boomsma F, Derkx FH, Veld AJ, Schalekamp MA. Human renal and systemic hemodynamic, natriuretic, and neurohumoral responses to different doses of L-NAME. Am J Physiol. 1998;275:F870–877. doi: 10.1152/ajprenal.1998.275.6.F870. [DOI] [PubMed] [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- Calzada MJ, Zhou L, Sipes JM, Zhang J, Krutzsch HC, Iruela-Arispe ML, Annis DS, Mosher DF, Roberts DD. α4β1 integrin mediates selective endothelial cell responses to thrombospondins in vitro and modulates angiogenesis in vivo. Circ Res. 2004;94:462–470. doi: 10.1161/01.RES.0000115555.05668.93. [DOI] [PubMed] [Google Scholar]
- Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci. 2008;65:672–686. doi: 10.1007/s00018-007-7484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Asahara T, Krasinski K, Witzenbichler B, Yang J, Magner M, Kearney M, Frazier WA, Isner JM, Andres V. Antibody blockade of thrombospondin accelerates reendothelialization and reduces neointima formation in balloon-injured rat carotid artery. Circulation. 1999;100:849–854. doi: 10.1161/01.cir.100.8.849. [DOI] [PubMed] [Google Scholar]
- Chirkov YY, Horowitz JD. Impaired tissue responsiveness to organic nitrates and nitric oxide: a new therapeutic frontier? Pharmacol Ther. 2007;116:287–305. doi: 10.1016/j.pharmthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Cook S, Hugli O, Egli M, Menard B, Thalmann S, Sartori C, Perrin C, Nicod P, Thorens B, Vollenweider P, Scherrer U, Burcelin R. Partial gene deletion of endothelial nitric oxide synthase predisposes to exaggerated high-fat diet-induced insulin resistance and arterial hypertension. Diabetes. 2004;53:2067–2072. doi: 10.2337/diabetes.53.8.2067. [DOI] [PubMed] [Google Scholar]
- Curwen JO, Musgrove HL, Kendrew J, Richmond GH, Ogilvie DJ, Wedge SR. Inhibition of vascular endothelial growth factor-a signaling induces hypertension: examining the effect of cediranib (recentin; AZD2171) treatment on blood pressure in rat and the use of concomitant antihypertensive therapy. Clin Cancer Res. 2008;14:3124–3131. doi: 10.1158/1078-0432.CCR-07-4783. [DOI] [PubMed] [Google Scholar]
- Danielewski O, Schultess J, Smolenski A. The NO/cGMP pathway inhibits Rap 1 activation in human platelets via cGMP-dependent protein kinase I. Thromb Haemost. 2005;93:319–325. doi: 10.1160/TH04-09-0582. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Dernbach E, Zeiher AM. Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett. 2000;477:258–262. doi: 10.1016/s0014-5793(00)01657-4. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval M, Le Boeuf F, Huot J, Gratton JP. Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Mol Biol Cell. 2007;18:4659–4668. doi: 10.1091/mbc.E07-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzie CA, Murphy-Ullrich JE. The N-terminus of thrombospondin: the domain stands apart. Int J Biochem Cell Biol. 2004;36:1090–1101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Fraisse A, Wessel DL. Acute pulmonary hypertension in infants and children: cGMP-related drugs. Pediatr Crit Care Med. 2010;11:S37–40. doi: 10.1097/PCC.0b013e3181c8e6e9. [DOI] [PubMed] [Google Scholar]
- Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, Abu-Asab MS, Tsokos M, Roberts DD, Frazier WA. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol. 2011;30:154–161. doi: 10.1016/j.matbio.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier WA, Gao A-G, Dimitry J, Chung J, Brown EJ, Lindberg FP, Linder ME. The thrombospondin receptor integrin-associated protein (CD47) functionally couples to heterotrimeric Gi. J Biol Chem. 1999;274:8554–8560. doi: 10.1074/jbc.274.13.8554. [DOI] [PubMed] [Google Scholar]
- Frazier WA, Isenberg JS, Kaur S, Roberts DD. CD47, Nature Signaling Gateway 2010 [Google Scholar]
- Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- Garcia-Dorado D, Agullo L, Sartorio CL, Ruiz-Meana M. Myocardial protection against reperfusion injury: the cGMP pathway. Thromb Haemost. 2009;101:635–642. [PubMed] [Google Scholar]
- Gelinas DS, Bernatchez PN, Rollin S, Bazan NG, Sirois MG. Immediate and delayed VEGF-mediated NO synthesis in endothelial cells: role of PI3K, PKC and PLC pathways. Br J Pharmacol. 2002;137:1021–1030. doi: 10.1038/sj.bjp.0704956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DR, Fernandez IC, Ordenes PP, Treuer AV, Eller G, Boric MP. Differential role of S-nitrosylation and the NO-cGMP-PKG pathway in cardiac contractility. Nitric Oxide. 2008;18:157–167. doi: 10.1016/j.niox.2007.09.086. [DOI] [PubMed] [Google Scholar]
- Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1) J Cell Physiol. 2007;210:807–818. doi: 10.1002/jcp.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3:147–158. doi: 10.1023/a:1009018702832. [DOI] [PubMed] [Google Scholar]
- Haas B, Mayer P, Jennissen K, Scholz D, Diaz MB, Bloch W, Herzig S, Fassler R, Pfeifer A. Protein kinase G controls brown fat cell differentiation and mitochondrial biogenesis. Sci Signal. 2009;2:ra78. doi: 10.1126/scisignal.2000511. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- Isenberg J, Calzada M, Zhou L, Guo N, Lawler J, Wang X, Frazier W, Roberts D. Endogenous thrombospondin-1 is not necessary for proliferation but is permissive for vascular smooth muscle cell responses to platelet-derived growth factor. Matrix Biol. 2005a;24:110–123. doi: 10.1016/j.matbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem. 2009a;284:1116–1125. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Frazier WA, Krishna MC, Wink DA, Roberts DD. Enhancing cardiovascular dynamics by inhibition of thrombospondin-1/CD47 signaling. Curr Drug Targets. 2008a;9:833–841. doi: 10.2174/138945008785909338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007a;109:1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009b;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD. Treatment of ischemia/reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery. 2008b;144:752–761. doi: 10.1016/j.surg.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, Frazier WA, Roberts DD. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg. 2008c;247:180–190. doi: 10.1097/SLA.0b013e31815685dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009c;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006a;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A. 2005b;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007b;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Maxhimer JB, Smedley J, Frazier WA, Roberts DD. Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: implications for human disease. Ann Surg. 2008d;247:860–868. doi: 10.1097/SLA.0b013e31816c4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008e;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Shiva S, Gladwin M. Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation. Nitric Oxide. 2009d;21:52–62. doi: 10.1016/j.niox.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006b;71:785–793. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Frantz C, Bals R, Böhm M, Wilkens H. Thrombospondin-1 as a biomarker in pulmonary hypertension. Chest. 2010;138:893A. [Google Scholar]
- Kaur S, Kuznetsova SA, Pendrak ML, Sipes JM, Romeo MJ, Li Z, Zhang L, Roberts DD. Identification of CD47 and amyloid precursor-like protein-2 as the major heparan sulfate proteoglycans on T lymphocytes and this isoform of CD47 as the signaling receptor for thrombospondin-1. J Biol Chem. 2011;286:14991–15002. doi: 10.1074/jbc.M110.179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits vascular endothelial growth factor receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010 doi: 10.1074/jbc.M1110.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Roberts DD. CD47 applies the brakes to angiogenesis via vascular endothelial growth factor receptor-2. Cell Cycle. 2011;10:10–12. doi: 10.4161/cc.10.1.14324. [DOI] [PubMed] [Google Scholar]
- Kung CF, Moreau P, Takase H, Luscher TF. L-NAME hypertension alters endothelial and smooth muscle function in rat aorta. Prevention by trandolapril and verapamil. Hypertension. 1995;26:744–751. doi: 10.1161/01.hyp.26.5.744. [DOI] [PubMed] [Google Scholar]
- Kvansakul M, Adams JC, Hohenester E. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. Embo J. 2004;23:1223–1233. doi: 10.1038/sj.emboj.7600166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol. 2007;178:5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- Lamy L, Ticchioni M, Rouquette-Jazdanian AK, Samson M, Deckert M, Greenberg AH, Bernard A. CD47 and the 19 kDa interacting protein-3 (BNIP3) in T cell apoptosis. J Biol Chem. 2003;278:23915–23921. doi: 10.1074/jbc.M301869200. [DOI] [PubMed] [Google Scholar]
- Li SS, Liu Z, Uzunel M, Sundqvist KG. Endogenous thrombospondin-1 is a cell surface ligand for regulation of integrin dependent T lymphocyte adhesion. Blood. 2006a doi: 10.1182/blood-2006-04-016832. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin alphaIIb beta3. Blood. 2006b;107:965–972. doi: 10.1182/blood-2005-03-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.str.0000047100.84604.ba. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–1430. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhao Q, Han Q, Gao M, Zhang N. Serum thrombospondin-1 is altered in patients with hemorrhagic fever with renal syndrome. J Med Virol. 2008;80:1799–1803. doi: 10.1002/jmv.21270. [DOI] [PubMed] [Google Scholar]
- Manna PP, Dimitry J, Oldenborg PA, Frazier WA. CD47 augments Fas/CD95-mediated apoptosis. J Biol Chem. 2005;280:29637–29644. doi: 10.1074/jbc.M500922200. [DOI] [PubMed] [Google Scholar]
- Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1-CD47 blockade following ischemia reperfusion injury is tissue protective. Plast Reconstr Surg. 2009a;124:1880–1889. doi: 10.1097/PRS.0b013e3181bceec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, DeGraff WG, Tsokos M, Wink DA, Isenberg JS, DRD Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009b;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Isenberg JS, Roberts DD. Thrombospondin-1 is an inhibitor of pharmacological activation of soluble guanylate cyclase. Br J Pharmacol. 2010a;159:1542–1547. doi: 10.1111/j.1476-5381.2009.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Isenberg JS, Shih HB, Wang Y, Roberts DD. Amyloid-beta Inhibits No-cGMP Signaling in a CD36- and CD47-Dependent Manner. PLoS One. 2010b;5:e15686. doi: 10.1371/journal.pone.0015686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF, Adams JC. Adhesion-modulating/matricellular ECM protein families: a structural, functional and evolutionary appraisal. Matrix Biology. 2012 doi: 10.1016/j.matbio.2012.01.003. In press. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Iozzo RV. Thematic Mini-Review Series: Thrombospondins in physiology and disease, new tricks for an old dog. Matrix Biology. 2012 doi: 10.1016/j.matbio.2012.01.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SA, DeVries WH, Andres KR, Gruenthal MJ, Benton RL, Hoying JB, Hagg T, Whittemore SR. CD47 knockout mice exhibit improved recovery from spinal cord injury. Neurobiol Dis. 2011;42:21–34. doi: 10.1016/j.nbd.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- Nossaman VE, Nossaman BD, Kadowitz PJ. Nitrates and nitrites in the treatment of ischemic cardiac disease. Cardiol Rev. 2010;18:190–197. doi: 10.1097/CRD.0b013e3181c8e14a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa CD, Fouty BW, Hales CA. Thrombospondin-1, endothelium and systemic vascular tone. Future Cardiol. 2011;7:169–172. doi: 10.2217/fca.11.7. [DOI] [PubMed] [Google Scholar]
- Ochoa CD, Yu L, Al-Ansari E, Hales CA, Quinn DA. Thrombospondin-1 null mice are resistant to hypoxia-induced pulmonary hypertension. J Cardiothorac Surg. 2010;5:32. doi: 10.1186/1749-8090-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchatcharam M, Miriyala S, Yang F, Leitges M, Chrzanowska-Wodnicka M, Quilliam LA, Anaya P, Morris AJ, Smyth SS. Enhanced proliferation and migration of vascular smooth muscle cells in response to vascular injury under hyperglycemic conditions is controlled by beta3 integrin signaling. Int J Biochem Cell Biol. 2010;42:965–974. doi: 10.1016/j.biocel.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci U S A. 1990;87:5193–5197. doi: 10.1073/pnas.87.13.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S, Mazzalupo S, Boitano S, Montfort WR. Thrombospondin-1 and angiotensin II inhibit soluble guanylyl cyclase through an increase in intracellular calcium concentration. Biochemistry. 2011;50:7787–7799. doi: 10.1021/bi201060c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebres RA, Vaz LE, Green JM, Brown EJ. Normal ligand binding and signaling by CD47 (integrin-associated protein) requires a long range disulfide bond between the extracellular and membrane spanning domains. J Biol Chem. 2001;13:13. doi: 10.1074/jbc.M106107200. [DOI] [PubMed] [Google Scholar]
- Rico MC, Rough JJ, Del Carpio-Cano FE, Kunapuli SP, DeLa Cadena RA. The axis of thrombospondin-1, transforming growth factor beta and connective tissue growth factor: an emerging therapeutic target in rheumatoid arthritis. Curr Vasc Pharmacol. 2010;8:338–343. doi: 10.2174/157016110791112296. [DOI] [PubMed] [Google Scholar]
- Riessen R, Kearney M, Lawler J, Isner JM. Immunolocalization of thrombospondin-1 in human atherosclerotic and restenotic arteries. Am Heart J. 1998;135:357–364. doi: 10.1016/s0002-8703(98)70105-x. [DOI] [PubMed] [Google Scholar]
- Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biology. 2012 doi: 10.1016/j.matbio.2012.01.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DD, Lau LF. Matricellular proteins. In: Mecham RP, editor. The extracellular matrix: an overview. Springer-Verlag; Berlin Heidelberg: 2011. pp. 369–413. [Google Scholar]
- Roth JJ, Gahtan V, Brown JL, Gerhard C, Swami VK, Rothman VL, Tulenko TN, Tuszynski GP. Thrombospondin-1 is elevated with both intimal hyperplasia and hypercholesterolemia. J Surg Res. 1998;74:11–16. doi: 10.1006/jsre.1997.5209. [DOI] [PubMed] [Google Scholar]
- Schade D, Kotthaus J, Clement B. Modulating the NO generating system from a medicinal chemistry perspective: current trends and therapeutic options in cardiovascular disease. Pharmacol Ther. 2010;126:279–300. doi: 10.1016/j.pharmthera.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Schellings MW, van Almen GC, Sage EH, Heymans S. Thrombospondins in the heart: potential functions in cardiac remodeling. J Cell Commun Signal. 2009;3:201–213. doi: 10.1007/s12079-009-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HH, Schmidt PM, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol. 2009;191:309–339. doi: 10.1007/978-3-540-68964-5_14. [DOI] [PubMed] [Google Scholar]
- Smadja DM, d'Audigier C, Bieche I, Evrard S, Mauge L, Dias JV, Labreuche J, Laurendeau I, Marsac B, Dizier B, Wagner-Ballon O, Boisson-Vidal C, Morandi V, Duong-Van-Huyen JP, Bruneval P, Dignat-George F, Emmerich J, Gaussem P. Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol. 2011;31:551–559. doi: 10.1161/ATVBAHA.110.220624. [DOI] [PubMed] [Google Scholar]
- Stauss HM, Godecke A, Mrowka R, Schrader J, Persson PB. Enhanced blood pressure variability in eNOS knockout mice. Hypertension. 1999;33:1359–1363. doi: 10.1161/01.hyp.33.6.1359. [DOI] [PubMed] [Google Scholar]
- Stenina OI, Plow EF. Counterbalancing forces: what is thrombospondin-1 doing in atherosclerotic lesions? Circ Res. 2008;103:1053–1055. doi: 10.1161/CIRCRESAHA.108.188870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- Sun J, Hopkins BD, Tsujikawa K, Perruzzi C, Adini I, Swerlick R, Bornstein P, Lawler J, Benjamin LE. Thrombospondin-1 modulates VEGF-A-mediated Akt signaling and capillary survival in the developing retina. Am J Physiol Heart Circ Physiol. 2009;296:H1344–1351. doi: 10.1152/ajpheart.01246.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biology. 2012 doi: 10.1016/j.matbio.2012.01.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K, Lawler J. The interaction of Thrombospondins with extracellular matrix proteins. J Cell Commun Signal. 2009;3:177–187. doi: 10.1007/s12079-009-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Image I, II, Takagi S, Image I, II, Tominaga A, Image Image I, Tomita T, Image I, II, Iwatsubo T, Image I. Functional analysis of the transmembrane domains of presenilin 1: participation of transmembrane domains 2 and 6 in the formation of initial substrate-binding site of gamma-secretase. J Biol Chem. 2010;285:19738–19746. doi: 10.1074/jbc.M110.101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Roberts DD, Isenberg JS. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res. 2010 doi: 10.1016/j.phrs.2010.1010.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M. cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br J Pharmacol. 2009;158:50–60. doi: 10.1111/j.1476-5381.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kazerounian S, Duquette M, Perruzzi C, Nagy JA, Dvorak HF, Parangi S, Lawler J. Thrombospondin-1 modulates vascular endothelial growth factor activity at the receptor level. FASEB J. 2009;23:3368–3376. doi: 10.1096/fj.09-131649. [DOI] [PMC free article] [PubMed] [Google Scholar]