Abstract
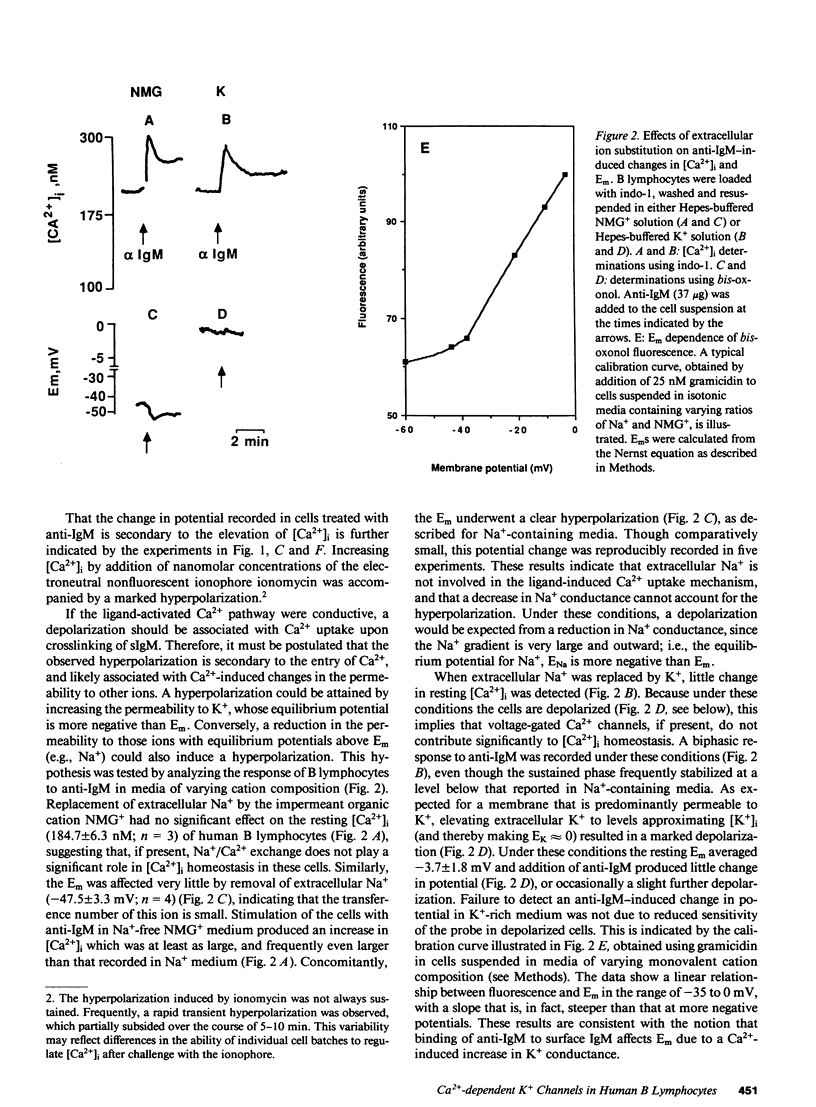
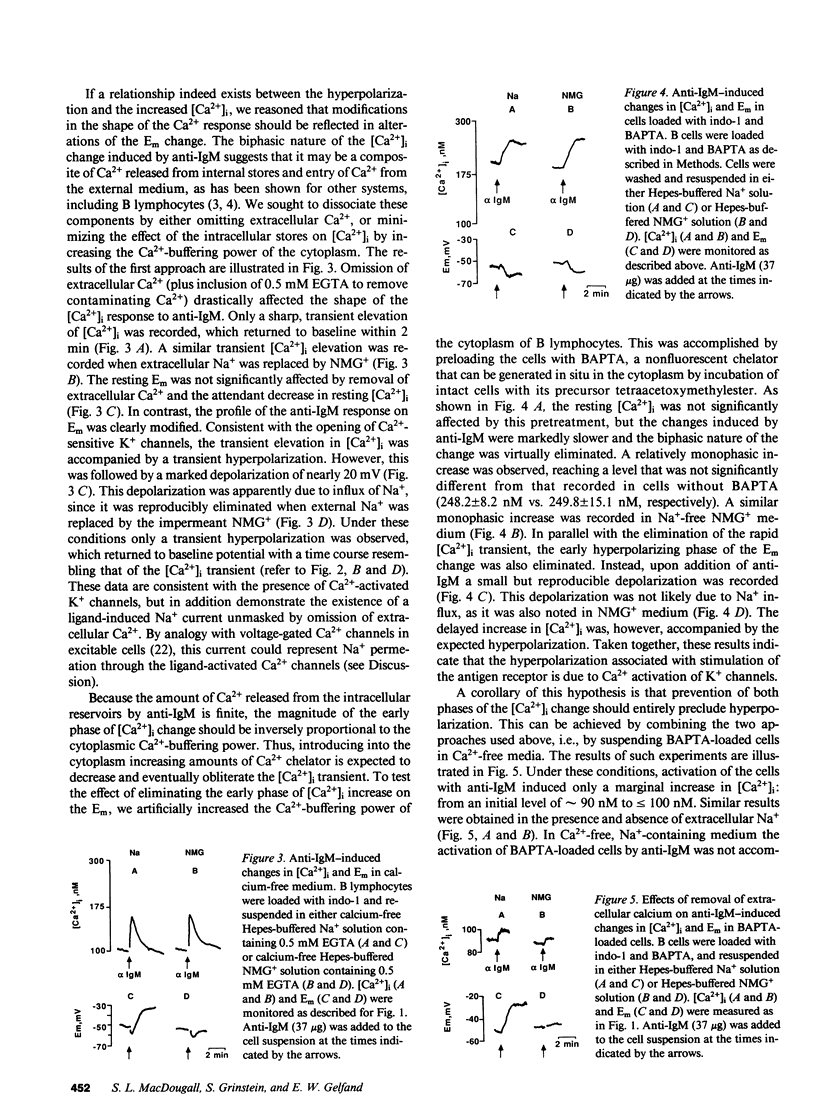
Many mammalian cell types exhibit Ca2+-dependent K+ channels, and activation of these channels by increasing intracellular calcium generally leads to a hyperpolarization of the plasma membrane. Their presence in B lymphocytes is as yet uncertain. Crosslinking Ig on the surface of B lymphocytes is known to increase the level of free cytoplasmic calcium ([Ca2+]i). However, rather than hyperpolarization, a depolarization has been reported to occur after treatment of B lymphocytes with anti-Ig. To determine if Ca2+-dependent K+ channels are present in B lymphocytes, and to examine the relationship between intracellular free calcium and membrane potential, we monitored [Ca2+]i by means of indo-1 and transmembrane potential using bis(1,3-diethylthiobarbituric)trimethine oxonol in human tonsillar B cells activated by anti-IgM. Treatment with anti-IgM induced a biphasic increase in [Ca2+]i and a simultaneous hyperpolarization. A similar hyperpolarization was induced by ionomycin, a Ca2+ ionophore. Delaying the development of the [Ca2+]i response by increasing the cytoplasmic Ca2+-buffering power delayed the hyperpolarization. Conversely, eliminating the sustained phase of the [Ca2+]i response by omission of external Ca2+ abolished the prolonged hyperpolarization. In fact, a sizable Na+-dependent depolarization was unmasked. This study demonstrates that in human B lymphocytes, Ca2+-dependent K+ channels can be activated by crosslinking of surface IgM. Moreover, it is likely that, by analogy with voltage-sensitive Ca2+ channels, Na+ can permeate through these ligand-gated Ca2+ "channels" in the absence of extracellular Ca2+.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Rigley K. P., Klaus G. G. Cross-linking of surface immunoglobulin on B lymphocytes induces both intracellular Ca2+ release and Ca2+ influx: analysis with indo-1. Biochem Biophys Res Commun. 1986 May 29;137(1):500–506. doi: 10.1016/0006-291x(86)91238-6. [DOI] [PubMed] [Google Scholar]
- Braun J., Sha'afi R. I., Unanue E. R. Crosslinking by ligands to surface immunoglobulin triggers mobilization of intracellular 45Ca2+ in B lymphocytes. J Cell Biol. 1979 Sep;82(3):755–766. doi: 10.1083/jcb.82.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung R. K., Grinstein S., Gelfand E. W. Volume regulation by human lymphocytes. Identification of differences between the two major lymphocyte subpopulations. J Clin Invest. 1982 Sep;70(3):632–638. doi: 10.1172/JCI110657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall K. M., Cambier J. C. B cell activation. VIII. Membrane immunoglobulins transduce signals via activation of phosphatidylinositol hydrolysis. J Immunol. 1984 Dec;133(6):3382–3386. [PubMed] [Google Scholar]
- Cooper M. D., Kearney J. F., Gathings W. E., Lawton A. R. Effects of anti-Ig antibodies on the development and differentiation of B cells. Immunol Rev. 1980;52:29–53. doi: 10.1111/j.1600-065x.1980.tb00329.x. [DOI] [PubMed] [Google Scholar]
- DeCoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984 Feb 2;307(5950):465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gulyaeva N. V., Konoshenko G. I., Mokhova E. N. Mitochondrial membrane potential in lymphocytes as monitored by fluorescent cation diS-C3-(5). Membr Biochem. 1985;6(1):19–32. doi: 10.3109/09687688509065440. [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Hawrylowicz C. M., Holman M., Keeler K. D. Activation and proliferation signals in mouse B cells. III. Intact (IGG) anti-immunoglobulin antibodies activate B cells but inhibit induction of DNA synthesis. Immunology. 1984 Dec;53(4):693–701. [PMC free article] [PubMed] [Google Scholar]
- LaBaer J., Tsien R. Y., Fahey K. A., DeFranco A. L. Stimulation of the antigen receptor on WEHI-231 B lymphoma cells results in a voltage-independent increase in cytoplasmic calcium. J Immunol. 1986 Sep 15;137(6):1836–1844. [PubMed] [Google Scholar]
- Latorre R., Coronado R., Vergara C. K+ channels gated by voltage and ions. Annu Rev Physiol. 1984;46:485–495. doi: 10.1146/annurev.ph.46.030184.002413. [DOI] [PubMed] [Google Scholar]
- Levi R., DeFelice L. J. Sodium-conducting channels in cardiac membranes in low calcium. Biophys J. 1986 Jul;50(1):5–9. doi: 10.1016/S0006-3495(86)83433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Deutsch C. K channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984 Feb 2;307(5950):468–471. doi: 10.1038/307468a0. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Cambier J. C. B cell activation. I. Anti-immunoglobulin-induced receptor cross-linking results in a decrease in the plasma membrane potential of murine B lymphocytes. J Exp Med. 1983 Jun 1;157(6):2073–2086. doi: 10.1084/jem.157.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom J. T., DiGiusto D. L., Cambier J. C. Single cell analysis of calcium mobilization in anti-immunoglobulin-stimulated B lymphocytes. J Immunol. 1986 Jan;136(1):54–57. [PubMed] [Google Scholar]
- Ransom J. T., Harris L. K., Cambier J. C. Anti-Ig induces release of inositol 1,4,5-trisphosphate, which mediates mobilization of intracellular Ca++ stores in B lymphocytes. J Immunol. 1986 Jul 15;137(2):708–714. [PubMed] [Google Scholar]
- Rink T. J., Deutsch C. Calcium-activated potassium channels in lymphocytes. Cell Calcium. 1983 Dec;4(5-6):463–473. doi: 10.1016/0143-4160(83)90022-2. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Montecucco C., Hesketh T. R., Tsien R. Y. Lymphocyte membrane potential assessed with fluorescent probes. Biochim Biophys Acta. 1980;595(1):15–30. doi: 10.1016/0005-2736(80)90243-6. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Passow H. Ca2+-activated K+ channels in erythrocytes and excitable cells. Annu Rev Physiol. 1983;45:359–374. doi: 10.1146/annurev.ph.45.030183.002043. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tatham P. E., O'Flynn K., Linch D. C. The relationship between mitogen-induced membrane potential changes and intracellular free calcium in human T-lymphocytes. Biochim Biophys Acta. 1986 Apr 14;856(2):202–211. doi: 10.1016/0005-2736(86)90029-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]


