Abstract
Polyethylenimine (PEI) is a cationic polymer that is an efficient transfection reagent marred by high toxicity and a susceptibility to aggregate in the presence of serum. Dextran is a biodegradable natural polysaccharide that can be used to reduce the toxicity of PEI and increase its stability in the presence of serum. In this study, small branched PEI units (800/2000 Da) were attached to dextran (Dex; 15/100–200 kDa) to form dextran-polyethylenimine (Dex-PEI) conjugates. The Dex-PEI conjugates were then tested as a gene carrier in the model HEK293 cell line. Dex-PEI conjugates displayed significantly lower cytotoxicity than PEI (25k). Both Dex-PEI and PEI efficiently delivered firefly luciferase encoded plasmid DNA (pDNA) to the HEK293 cells. Dex-PEI resulted in moderately lower transfection efficiencies than PEI 25k when the transfection was carried out in media without serum for 4 h. However, in the presence of serum, which more accurately predicts the anticipated environment of non-viral vectors in vivo, Dex-PEI and unmodified PEI generated similar transfection efficiencies when incubated with the cells for 4 h. When the incubation time of the vectors was increased to 48 h, significantly higher transfection efficiencies were generated by Dex-PEI in comparison to PEI. Turbidity measurements showed that complexes formed between plasmid DNA and unmodified PEI were more susceptible to aggregation in serum-containing media than complexes formed from pDNA and Dex-PEI. Dex-PEI conjugates are therefore believed to have greater potential for translational applications because of lower cytotoxicity characteristics and improved stability in serum containing environments.
Keywords: Dextran, polyethylenimine, PEI, gene delivery, cationic polymer, transfection, plasmid DNA, pDNA, serum, complexes, nanoparticles, nanoplexes, gene delivery, non-viral
INTRODUCTION
Gene therapy is a promising technique for the treatment of life-threatening diseases such as cystic fibrosis (Knowles et al., 1995; Porteous et al., 1997; Zabner et al., 1993), adenosine deaminase deficiency (Onodera et al., 1999; Onodera and Sakiyama, 2000), HIV infection (Buchschacher and Wong-Staal, 2001) and cancer (Nabel et al., 1993; Rubin et al., 1997; Vogelzang et al., 1994). One major challenge associated with plasmid DNA based gene therapy is the rapid degradation of unprotected plasmid DNA (pDNA) when exposed to serum nucleases (Abbas et al., 2008). In addition, pDNA has a reduced ability to ferry through cell membranes into the cytoplasm independently due to its large size and net negative charge. Therefore, delivery vehicles such as cationic lipids or polymers are often utilized to improve the transfection efficiencies of pDNA. Cationic polymers such as polyethylenimine (PEI) can bind with pDNA electrostatically to form complexes which protect DNA from degradation in blood serum (Intra and Salem, 2008, 2011; Pearce et al., 2008). In addition, net positive surface charges achieved using appropriate nitrogen to phosphate (N/P) ratios can facilitate the uptake of complexes by cells.
The high efficiency of PEI in transfection has been reported to be linked to its buffering capacity and the associated proton sponge effect that occurs after endocytosis (Behr, 1997; Sonawane et al., 2003). However, PEI is toxic and susceptible to aggregation when administered in vivo, and it lacks degradability, which allows for potential accumulation in the body. PEI induces toxicity at the cellular and systemic levels. At the cellular level, the charge interactions between PEI and the cell membrane have been reported to induce apoptosis. At the systemic level, PEI-pDNA complexes aggregate following interaction with blood components such as serum that then results in capillary embolism (Ogris et al., 2003; Wightman et al., 2001). The cytotoxicity of PEI is dependent upon its concentration, architecture and molecular weight (Fischer et al., 1999; Fischer et al., 2002). In general, lower molecular weight (MW) PEI is less toxic than higher MW PEI. Therefore, reduced toxicity can be achieved when small size PEI units are assembled into larger degradable structures (Thomas et al., 2005). A previous study has described the conjugation of PEI 800 into 14–30k architectures using 1,3-butanediol diacrylate. The lowest cytotoxicity was observed with the conjugates that had the fastest ester hydrolysis rate (Forrest et al., 2003).
Dextran is a natural biodegradable polysaccharide. It can be slowly depolymerized by enzymes present in various organs such as the liver, spleen and kidney (Larsen, 1989; Rosenfeld and Lukomskaya, 1957). In vitro and in vivo experiments have shown that chemical modification may reduce the de-polymerization rate of dextran by enzymes (Vercauteren et al., 1990; Vercauteren et al., 1992). A number of studies have evaluated Dextran-PEI (Dex-PEI) conjugates as gene carriers in recent years (Azzam et al., 2002; Sun et al., 2008a; Sun et al., 2008b; Tseng et al., 2005; Tseng et al., 2004). The Dex-PEI conjugates used in these studies were constructed with relatively low Mw PEI (MW 600, 800) and dextran (MW<70–90k). These Dex-PEI conjugates showed reduced toxicity as well as reduced transgene efficiencies. However, grafting small dextran 1.5k to PEI 25k results in higher transfection efficiency and reduced toxicity at low conjugation degrees compared to PEI 25k alone (Tseng et al., 2004). Conjugation of the dextran to the PEI resulted in pegylation-like shielding effects and improved stability of the complexes in the presence of BSA. One potential challenge with this more efficacious construct was that the PEI component had an Mw (25K) that remained above the renal clearance cut-off and would potentially still have intrinsic toxicity once the dextran had degraded. As such, these studies prompted us to build on these previous findings and evaluate Dex-PEI conjugates that utilized PEI with MWs that were higher than the 600 to 800 Da PEI that had not previously enhanced transfection efficiencies and lower than the 25 kDa PEI that is expected to still have intrinsic toxicity. We also evaluated for the first time, whether the conjugation of dextran to PEI could be used to enhance transfection efficiencies when tested in serum containing media, which more accurately represents the environments we anticipate these systems would be exposed to when utilized in vivo.
MATERIALS AND METHODS
Materials
Dextran with an average MW 15k and 100–200k was obtained from USB (Cleveland, OH, USA). Sodium cyanoborohydride, PEI 800 and 2000 were purchased from Sigma-Aldrich (St Louis, MO, USA). Sodium periodate and PBS (phosphate buffer solution tablet) were supplied by Fluka (St Louis, MO, USA). Glycerin and glacial acetic acid were obtained from Fisher (Pittsburgh, PA, USA). PD-10 columns were purchased from Amersham (Piscataway, NJ, USA). MicroBCA Assay Kit and snake skin dialysis tubing with MWCO 10,000 were purchased from Pierce (Rockford, IL, USA). Agarose was purchased from Bio-Rad (Hercules, CA, USA). DMEM (Dulbecco’s Modi ed Eagle’s medium), trypsin and penicillin-streptomycin were obtained from Gibco (Carlsbad, CA, USA). FBS (Fetal Bovine Serum) was supplied by Atlanta Biologicals (Lawrenceville, GA, USA). MTS (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay Kit) was purchased from Promega (Madison, WI, USA). All other reagents were used as received at analytical grade. VR1255 plasmid DNA encoding luciferase was amplified and purified using a QIAGEN Giga plasmid purification kit (QIAGEN, CA, USA) according to manufacturer’s protocol.
Cell Culture
Human embryonic kidney cells (HEK293) and African green monkey kidney cells (COS7) were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The cells were maintained in DMEM supplemented with 10% FBS, streptomycin at 100 μg/ml, penicillin at 100 U/ml, and 4 mM L-glutamate at 37ºC in a humidified 5% CO2-containing atmosphere.
Synthesis of Dex-PEI
A modified method based on a previous study was followed to prepare the Dex-PEI conjugate polymer (Azzam et al., 2002). Dextran (178 mg, 1 mM) was dissolved in 2 ml HAc/NaAc buffer (0.1 N, pH 5.0) and equilibrated in ice/water bath. Sodium periodate (10/21 mg, 0.046/0.098 mM) was dissolved in 400 μl HAc/NaAc buffer and added to the dextran solution for 30-mins oxidation. The reaction was stopped by the addition of (100 μl, 1.35 mM) glycerin. An Amersham PD-10 column (Mr 5000) was used to separate oxidized dextran. PEI 165 or 330 mg was dissolved in 5 ml HAc/NaAc buffer, and the oxidized dextran was added to the PEI solution slowly. After overnight conjugation, NaCNBH3 (58 mg, 0.92 mM) was dissolved in 1 ml HAc/NaAc buffer and added to the reaction solution for a second overnight reduction. Dex-PEI was recovered after dialysis using Pierce tubing (MWCO 10,000) and lyophilization respectively.
1HNMR characterization of Dex-PEI
Dex-PEI samples were dissolved in D2O at 5 mg/ml, and 1HNMR spectra were recorded with a Fourier transform nuclear magnetic resonance spectrometer (Bruker AVANCE 300MHZ). The scanning number was set at 16.
Preparation of Dex-PEI/pDNA complexes
Plasmid (VR1255) encoding firefly luciferase was used to quantify the expression level of foreign genes in the host cells. All polymer/pDNA complexes were freshly prepared before use. Equal volumes of polymer solution and plasmid solution were mixed together with gentle vortexing, and incubated for 30 min at room temperature.
Agarose gel retardation assay on Dex-PEI/pDNA interaction
Agarose gel (0.8%) dyed with ethidium bromide (0.5 μg/ml) was used to investigate the particle condensation between Dex-PEI and pDNA. Samples of the complexes (1 μg pDNA/100 μl complexes) and corresponding amounts of polymers were prepared at N/P ratios of 5, 10 and 15. Complexes (10 μl) from each sample with appropriate amounts of 10× gel-loading buffer were loaded and electrophoresed on gel in TAE buffer at 80 mV for 45 min. The pDNA in the gel was visualized using a UV transilluminator (Spectroline TE-312S, Spectroline, Westbury, NY) and photographed.
Size and zeta potential measurement of the complexes
The size and zeta potential of polymer/pDNA complexes were measured using a Zetasizer (Malvern Nano ZS, Malvern, Southborough, MA). Particle samples (4 μg plasmid/800 μl complexes) at various N/P ratios were prepared with corresponding amounts of Dex-PEI and with PEI controls. The size measurements were performed at 25ºC using a 173º back scattering angle. The mean hydrodynamic diameter was calculated by cumulative analysis. The zeta potential values were determined based on the complexes electrophoretic mobility in the aqueous medium, using folded capillary cells designated to automatic mode.
Turbidity measurements to represent stability of complexes in serum
Complexes (50 μl) containing 5 μg pDNA and an appropriate amount of polymer based on the target N:P ratio were transferred to 96-well plates. 50 μl DMEM supplemented with 10% Fetal Bovine Serum (FBS) or deionized water were added to make up the volume to 100 μl. Changes in turbidity are represented by OD values measured with a UV/visible spectrophotometer (SpectraMAX Plus384, Molecular Device, Sunnyvale, CA) at 600 nm.
Cytotoxicity evaluation using the MTS assay
The MTS assay was used in HEK293 cells to evaluate the cytotoxicity of Dex-PEI polymers. The MTS assay was performed according to a protocol from Promega. HEK293 cells (104 cells/well) in 50 μl DMEM culture medium were seeded in 96-well plates 24h prior to using the MTS assay. Polymer stock solutions were also prepared in DMEM at 5–10 mg/ml and sterile filtered. Serial dilutions of the polymers were prepared using DMEM. Sample solutions (50 μl) were transferred to each well followed by 4h incubation. MTS solution (20 μl) was added to each well for 2h incubation. The absorbance readings representing viability were recorded at 490 nm using a spectrophotometer (SpectraMAX Plus384, Molecular Device). The relative cell viability was calculated using untreated cells as control.
Evaluation of luciferase expression
Luciferase gene transfection in serum-free culture media (4 h incubation)
The transfection efficiencies of Dex-PEI-pDNA complexes were quantified with HEK293 and COS7 cells by the level of firefly luciferase expression. HEK293 cells were seeded in a 24-well plate at 105/well and incubated at 37°C under 5% CO2 for 24h prior to transfection in 1 ml DMEM medium supplemented with 10% FBS. Dex-PEI complexes were prepared as described above. A volume of 500 μl DMEM (serum-free) was used to replace culture media immediately before transfection. Freshly prepared 100 μl complex-containing suspensions at various N/P ratios containing 1 μg pDNA were added to each well. The transfection media was removed and replaced by 1000 μl DMEM with 10% FBS after 4h. Cells were further incubated for 44h. The luciferase expression level of harvested cells was determined with Promega luciferase assay kit using a luminometer (Berthold Lumat LB 9507). Cells transfected with naked pDNA, PEI 800, PEI 2000 and PEI 25k were used as controls.
Luciferase gene transfection in serum-containing culture media (4 h incubation)
The method used for transfection experiments was the same as experiments carried out in serum-free media (4h incubation), with the exception that the transfection media was replaced with 500 μl DMEM with 10% FBS.
Luciferase gene transfection in serum-containing culture media (48 h incubation)
The method used for transfection experiments were similar to experiments carried out in serum-containing media for 4 h incubation with the exception that transfection media was not removed by 4h but left as the incubation media for 48h. Transfection experiments were also carried out using COS7 cells as a substitute for HEK293 cells under otherwise identical conditions.
Intracellular trafficking of complexes prepared with Alexa-labeled Dex-PEI
Alexa 568 succinimidyl ester was used to label PEI and Dex-PEI. The labeling protocol followed Invitrogen instructions. Amersham PD-10 columns were used to separate unreacted dye from PEI or Dex-PEI. Alexa 568 labeled PEI or DP3 was finally recovered by lyophilization. HEK293 cells were plated in 0.01% w/v poly-L-lysine coated 4 well chamber slides (Lab-Tak) at 5 × 104 cells/well and incubated overnight. The cells were incubated with 75 nM LysoTracker Green DND-26 (Invitrogen) containing DMEM for 1h prior to transfection to stain lysosomes and endosomes. Then cells were transfected with complexes made with Alexa 568 labeled DP3 and PEI 2000/25k carrying 0.5 μg pDNA (N/P=10) in fresh DMEM. At 2h post-transfection, the cells were washed with PBS and fixed with 4% paraformaldehyde, mounted with Vectashield Mounting Medium with DAPI for nucleus staining (Vector Laboratories, UK). The slides were covered with coverslips followed by 4°C storage in the dark before visualization under a multiphoton/confocal microscope (Bio-Rad Radience 2100MP) with 60x oil immersion lens.
Statistical analysis
All results were presented as mean ± standard deviation. Differences between groups were determined by ANOVA with a Tukey post-test analysis. Levels of significance were accepted at p < 0.05. Statistical analysis was performed using Prism 5 (Graphpad Software Inc, San Diego, CA)
RESULTS AND DISCUSSION
Dextran-PEI conjugates are synthesized and characterized by 1HNMR
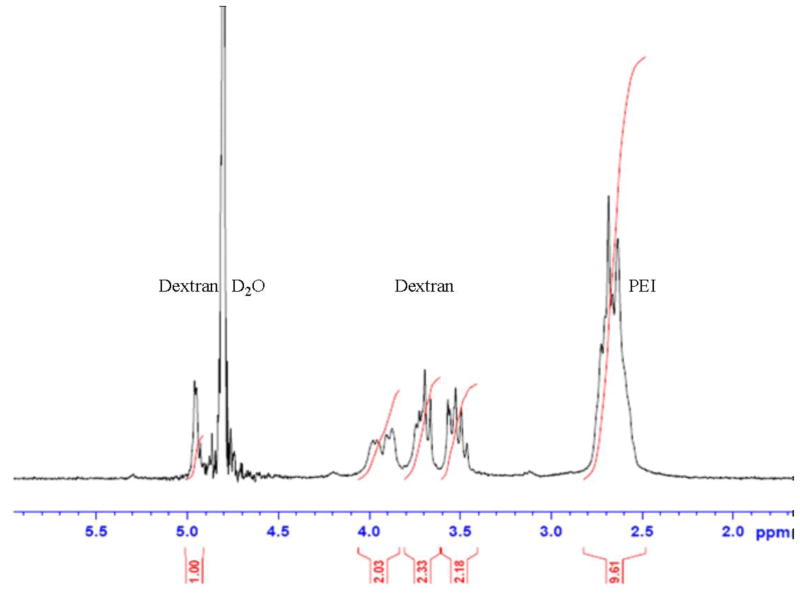
Oxidation followed with conjugation is a convenient method to prepare dextran-oligoamine conjugate structures (Azzam et al., 2002; Mehvar, 2000). The imine bond formed between oxidized dextran and PEI can be further reduced to amine. After this, the linkage becomes irreversible in aqueous solution. Figure 1 shows characteristic peaks of dextrose (dextran’s monomer) and PEI in the 1HNMR spectra of the conjugate polymer. The nitrogen normalized MW (N-Mw) of Dex-PEI was calculated based upon the peak area at 4.9 ppm for dextrose and 2.6 ppm for PEI, by the following equation:
Figure 1.
1HNMR spectra of DP4 (Bruker AVANCE III 300MHz): δ5.0-4.9 (C1-H, Dextrose), δ4.8 (H, H2O), δ4.1-3.4 (C2-C6-H, Dextrose), δ2.8-2.5 (PEI-H)
A lower N-MW value represents a higher amount of PEI in the Dex-PEI samples. Using the data in Table 1, the extent of PEI conjugation is correlated to the amount of sodium periodate added. Higher doses of sodium periodate result in a higher conjugation degree and lower N-MW. The MW of dextran used in the reaction also affects the results. Dextran 15k is not as efficient as dextran 100–200k for conjugation in the reaction, especially with PEI 800. While examining the reaction efficiency differences between PEI 800 and 2000, we observed that utilizing PEI 800 resulted in lower degrees of substitution in the conjugates. Amongst all the conjugates prepared, dextran(100–200k)-PEI(2000) (DP4) resulted in the highest degree of conjugation.
Table 1.
Characterization of Dex-PEIs (-l: low oxidation/substitution degree, 11 mg sodium periodate added; -h: high oxidation/substitution degree, 21 mg sodium periodate added; N/A: not applicable)
| sample | Dextran MW (kDa) | PEI MW (Da) | PEI wt % | N-MW | Ref code |
|---|---|---|---|---|---|
| D15P800-l | 15 | 800 | N/A | N/A | |
| D15P800-h | 15 | 800 | N/A | N/A | |
| D15P2000-l | 15 | 2000 | 16.195 | 266 | |
| D15P2000-h | 15 | 2000 | 27.497 | 156 | |
| D150P800-l | 100–200 | 800 | 6.5469 | 648 | DP1 |
| D150P800-h | 100–200 | 800 | 13.026 | 328 | DP2 |
| D150P2000-l | 100–200 | 2000 | 20.079 | 214 | DP3 |
| D150P2000-h | 100–200 | 2000 | 36.699 | 117 | DP4 |
Conjugating dextran to PEI significantly reduces toxicity when compared to PEI alone
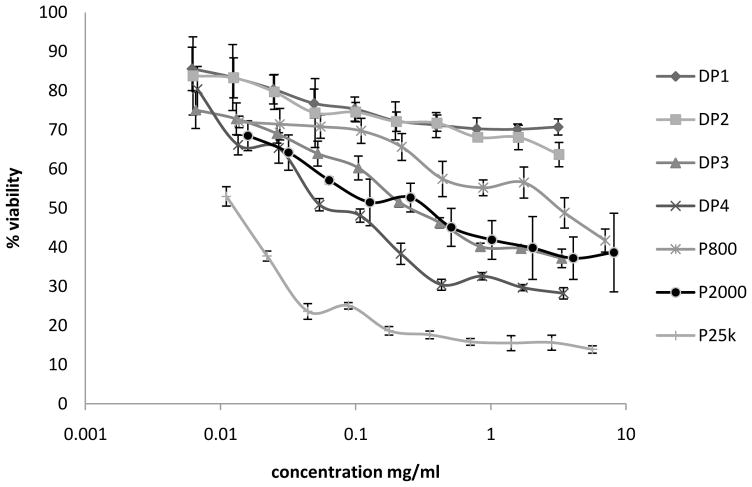
The MTS assay is a modified MTT assay which produces soluble formazan with λmax at 490 nm in living cells. HEK293 cells are exposed to polymer solutions at various concentrations for 4h, followed by 2h incubation with MTS solution. PEI 800, 2000 and 25k are used as controls. As seen in Figure 2, the general order of cytotoxicity is PEI 800 < PEI 2000 < PEI 25k in the range of concentrations tested. All Dex-PEI samples show a significantly reduced cytotoxicity when compared to PEI 25k. DP1 and DP2 show lower toxicity, even when compared to PEI 800. DP3 displays similar cytotoxicity profiles to PEI 2000. DP4 is slightly more toxic than PEI 2000, but significantly less toxic than PEI 25k. DP1/DP2 was synthesized using PEI 800, and DP3/DP4 was synthesized using PEI 2000. With equal molar concentrations of PEI, the constructs synthesized with PEI 2000 have approximate net 2.5 fold higher amounts of ethylenimine (EI) units relative to PEI 800. Therefore, the concentration of PEI in DP1/DP2 is significantly lower than DP3/DP4 when formed under similar reaction conditions (Table 1). The higher EI content of DP3/DP4 corresponds to higher toxicity when compared to DP1/DP2. This result is consistent with previous studies that have shown that grafting dextran to PEI can significantly reduce the toxicity of PEI (Tseng et al., 2004). Either grafting PEI to dextran or grafting dextran to PEI was capable of reducing cytotoxicity when compared to unmodified PEI. From the Dex-PEI constructs we have synthesized, the highest degree of conjugation was found in DP4, which was equivalent to one oxidation on every 19.4 dextrose monomers. This is a relatively low degree of oxidation, which is not expected to affect the enzymatic degradation or cytotoxicity profile of dextran.
Figure 2.
Cytotoxicity profiles of Dex-PEI (4h incubation with polymer solution and 2h incubation with MTS solution). HEK293 cell treated with Dex-PEIs and PEI controls in the concentration range: DP1: 0.00615–3.15 mg/ml; DP2: 0.00625–3.20 mg/ml; DP3: 0.00654–3.35 mg/ml; DP4: 0.00674–3.45 mg/ml; P800: 0.01372–7.03 mg/ml; P2000: 0.01592–8.15 mg/ml; P25k: 0.01104–5.65 mg/ml Cells were treated with polymers for 4h. Mean±SD, n=4
The surface charge and size of Dex-PEI-pDNA complexes is dependent of the Mw of PEI and the N:P ratio at which the complexes are prepared
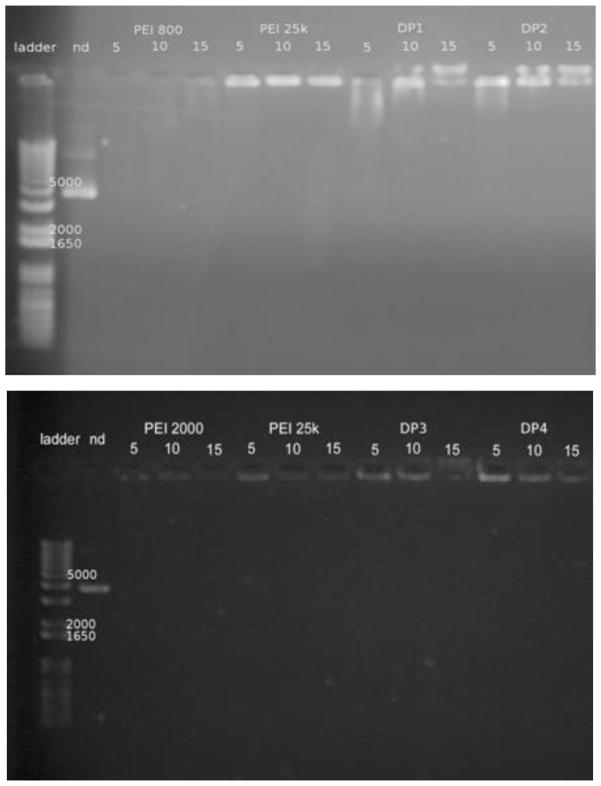
Effective condensation of pDNA by Dex-PEI into nanoparticles is critical for its successful application as a gene carrier. Condensation protects pDNA from enzymatic degradation and facilitates uptake of particles by cells. Using agarose gel retardation assays, Dex-PEI samples and PEI controls (800, 2000 and 25k) were evaluated for their ability to form complexes with pDNA at various N/P ratios. Figure 3a shows that PEI 800-based DP1 and DP2 exhibited poor complexation with pDNA at an N/P ratio of 5, but this improves as the N/P ratio increases above 10. In Figure 3b, DP3 and DP4, which are made from PEI 2000, fully retard migration of pDNA at all N/P ratios, suggesting more compact complexes are formed when compared to PEI-800 based conjugates. Complexes based on PEI 25k and PEI 2000 display limited migration of pDNA. In contrast, PEI 800 results in poor pDNA condensation. When comparing DP3/DP4 to PEI 25k, no significant differences in terms of complexation are found.
Figure 3.
a & 3b. Gel retardation assay. Agarose gel electrophoresis of Dex-PEI-pDNA complexes prepared at N/P ratios of 5, 10 and 15. (nd: naked pDNA)
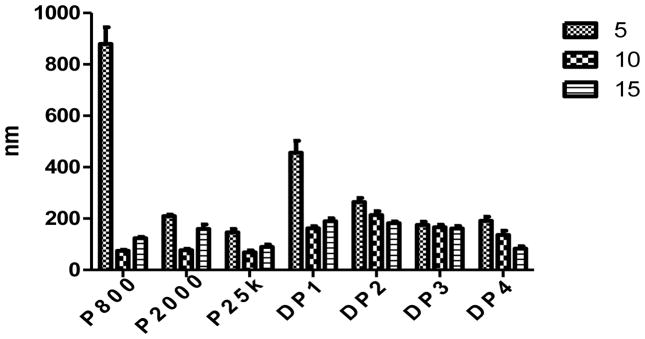
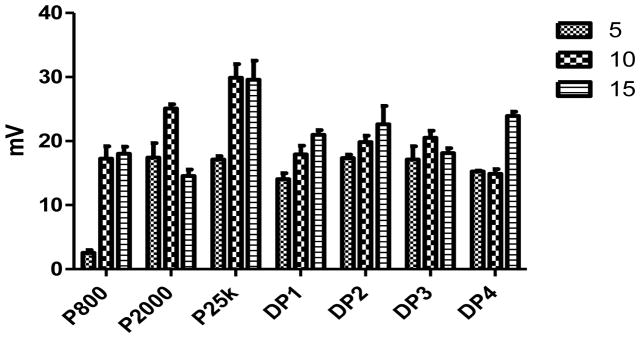
An appropriate size and net positive charge on the surface of the nanoparticles is also crucial for endocytosis of the complexes. Typically, nanoparticles should be less than 300 nm in size for efficient clathrin mediated endocytosis (Marsh and McMahon, 1999). Consistent with the gel retardation assay results, all Dex-PEI conjugates are able to form complexes with pDNA with net positive surface charges (Figure 4 & 5). PEI 800 is not as efficient as higher Mw PEIs in the formation of complexes with pDNA. At an N/P ratio of 5, PEI 800 forms significantly larger particles when compared to all other groups. Conjugating dextran to PEI 800 results in particles that are significantly smaller than PEI 800 alone when complexed with pDNA at an N/P ratio of 5. PEI 2000 complexed with pDNA generated particle sizes that are less than 210 nm when compared to particles generated with pDNA complexed with DP1 or DP2. Particle size can be correlated with the PEI wt% values presented in Table 1. DP3 and DP4 contain higher PEI content and less dextran relative to DP1 and DP2. The higher charge density associated with higher PEI content increases the charge interaction between the conjugates and the pDNA creating more compact particles. Decreased particle sizes are also observed as the N/P ratios increase, which also results in increasing the PEI concentration and charge interaction in the particles composition. At an N/P ratio of 15, particles formed using DP4 are as small as 83 nm and similar to 89 nm particles created by complexing pDNA with PEI 25k. The surface charge of the complexes is reversely correlated to increasing particle size. Increasing N/P ratios result in higher zeta potentials. Complexes formed with PEI 25k show the highest zeta potential of around 30 mV at an N/P ratio of 10 and 15. Dex-PEI/pDNA complexes exhibit lower values of 15–24 mV when compared to complexes prepared from pDNA and PEI 25k. The surface charge of Dex-PEI-pDNA complexes tend to range between 15–20 mV, which are equivalent to those generated by pDNA complexed with PEI 800 or PEI 2000 alone.
Figure 4.
Particle size of complexes formed by Dex-PEI conjugates and pDNA and PEI-pDNA controls at various N/P ratios. Mean+SD, n=3
Figure 5.
Zeta potential of complexes formed by Dex-PEI and pDNA and controls at various N/P ratios. Mean+SD, n=3
Turbidity measurements show that Dex-PEI-pDNA complexes are less susceptible to aggregation than PEI-pDNA complexes in the presence of serum
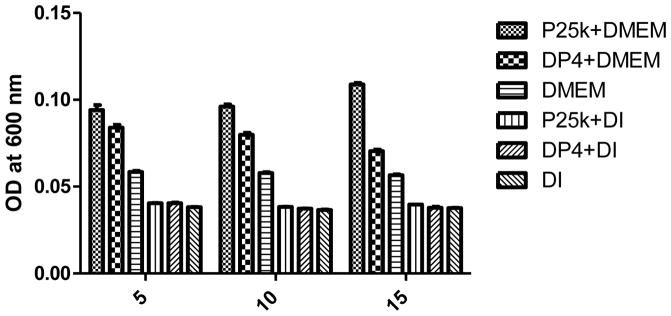
Turbidity is a commonly used method to detect aggregates or particles in the fluid phase. The turbidity index of a particulate sample will be reflected by the reduction of the incident beam intensity. This reduction is represented by OD or absorbance value changes measured using a UV-Vis spectrometer. The increase in turbidity is due to the increased average particle size when aggregation occurs. In Figure 6, particles formed with PEI 25k shows significantly higher turbidity in serum containing media than DP4 at N/P 5 (p < 0.001). As the N/P ratio increases, the turbidity of PEI 25k complexes also rises. In contrast, DP4’s turbidity decreases when the N/P ratio increases. This difference suggests that the presence of dextran in the polymer/pDNA complexes results in less aggregation and is consistent with the hypothesis that dextran chains provide stabilization, shielding and behavior similar to the properties created by pegylation (Tang et al., 2003).
Figure 6.
Turbidity of Dex-PEI/pDNA complexes in DMEM (with 10% FBS) at various N/P ratios. Mean+SD, n=6 (DI: deionized water)
Dex-PEI conjugates are less effective than PEI at transfecting cells in serum-free media
In an earlier examination of polysaccharide-oligoamine conjugates as a gene carrier (Azzam et al., 2002), dextran (20–500 kDa)-PEI 600 generated poor transfection efficiencies. Grafting of dextran (1500 and 10,000) to PEI (25k) greatly reduces its buffering capacity, cytotoxicity and transfection efficiency, and it was concluded that dextran grafting prevented pDNA from passing the cell membrane (Tseng et al., 2005; Tseng et al., 2004). However, the structure of the conjugate polymer in this study is different to previous conjugates because dextran is used as the core backbone instead of a grafting material. Two more recent studies bypassed oxidation and utilized spacer linking between dextran (15–25k and 60–90k) and PEI (800) (Sun et al., 2008a; Sun et al., 2008b). The dextran-PEI conjugate polymers showed lower toxicity and transfection efficiencies when compared to PEI 25k at the same N/P ratios. In these studies, however, the MW of PEI used was relatively low (600, 800). Higher MW materials can generally result in higher transfection efficiencies (Godbey et al., 1999). In our studies, PEI 2000 is a strong candidate for conjugation that still falls into the range of renal clearance.
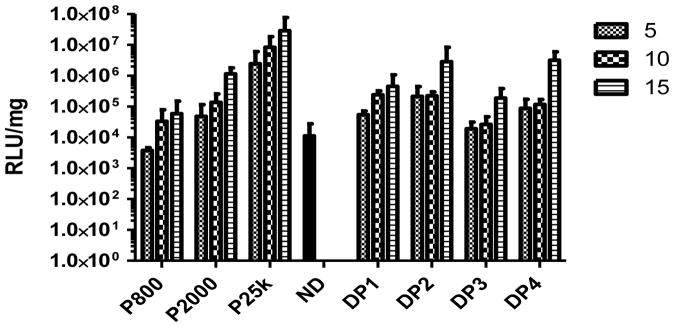
As shown in Figure 7, PEI 800 generates the lowest transfection efficiency, whereas PEI 25k generates the highest transfection efficiency. Naked pDNA exhibits similar transfection efficiency levels to those achieved using PEI 800 (p > 0.05). Increasing the N/P ratio at which complexes are prepared increases the luciferase expression level which is consistent with our previous observations (Intra and Salem, 2008). All of the dextran-PEI conjugates generate lower transfection efficiencies than PEI 25k. At an N/P ratio of 15, DP4 shows lower transfection efficiencies than PEI 25k, but this difference is not significant (p > 0.05). All DPs display similar transfection profiles regardless of the type of PEI used in the DP construct. These results are consistent with a number of previous reports (Azzam et al., 2002; Sun et al., 2008a; Sun et al., 2008b).
Figure 7.
Luciferase expression generated by delivery of pDNA using Dex-PEI conjugates and PEI controls prepared at various N/P ratios in HEK293 cells. Complexes were incubated with HEK293 cells for 4h in serum free media. The media containing complexes was then removed and cells were cultured in serum containing media for a further 44h before measurement of luciferase acitivity. Mean+SD, n=4 (ND: naked pDNA)
As mentioned earlier, one of the major obstacles to PEI based gene delivery is aggregation of complexes in the presence of serum, which can reduce activity and cause toxicity through subsequent capillary embolism. For this reason, we were particularly interested in how Dex-PEI would compare to PEI when tested for transfection in serum containing environments.
Dex-PEI conjugates are more effective at transfecting cell in serum containing media
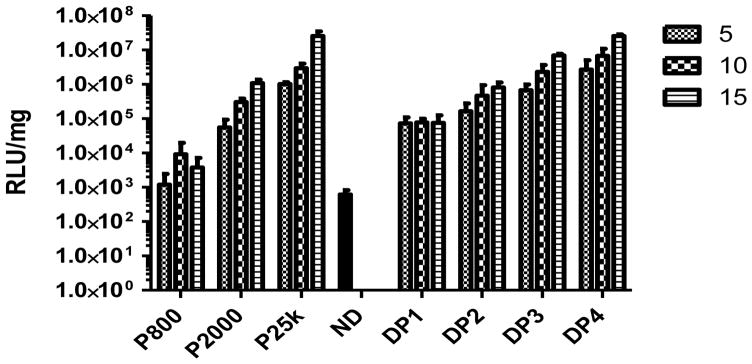
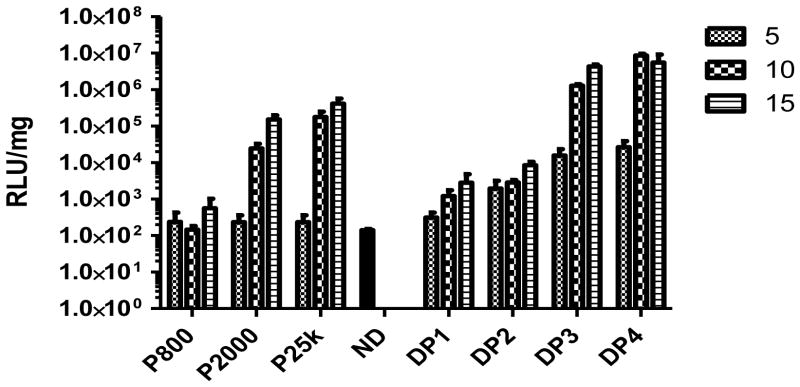
As suggested by results from the turbidity experiments, Dex-PEI-pDNA complexes display reduced particle aggregation in the presence of serum containing culture media when compared to PEI-pDNA complexes. We carried out transfection experiments in DMEM media containing 10% serum. Consistent with transfections carried out in serum free media, PEI 800 is not more efficient than naked pDNA (p > 0.05) at transfecting cells (Figure 8). PEI 25k shows the highest transfection efficiency at an N/P ratio of 15. In general, higher N/P ratios lead to higher transfection efficiencies. However, in contrast to experiments carried out in serum free-media, Dex-PEI conjugates show improved efficiencies of transfection relative to PEI when the experiment is carried out in serum containing media. The overall expression level of luciferase generated by Dex-PEIs follows the order DP1<DP2<DP3<DP4, and is correlated with decreasing N-MW values. DP4 generated high transfection efficiencies that are not significantly different to PEI 25k (p > 0.05).
Figure 8.
Luciferase expression generated by delivery of pDNA using Dex-PEI conjugates and PEI controls prepared at various N/P ratios in HEK293 cells. Complexes were incubated with HEK293 cells for 4h in serum containing media. The media containing complexes was then removed and cells were cultured for a further 44h in serum containing media before measurement of luciferase acitivity. Mean+SD, n=4 (ND: naked pDNA)
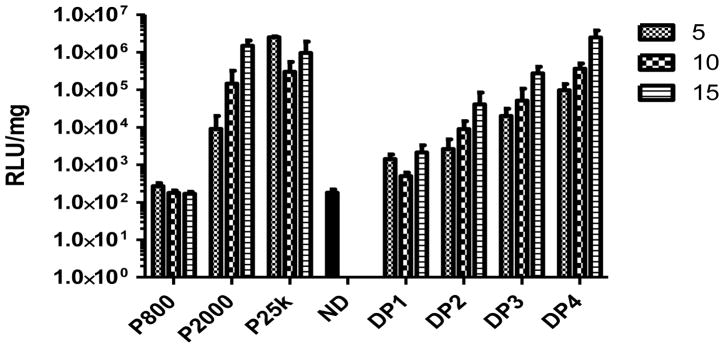
When the incubation (transfection) time length is extended to 48h, PEI 800 generates the lowest transfection efficiency when compared to all other vectors tested (Figure 9) and generates expression similar to naked pDNA (p > 0.05). The expression of luciferase generated by PEI 2000 was similar to that generated by PEI 25k, but PEI 25k has resulted in the death of more cells at the 48h time-point. Since PEI 2000 is significantly less toxic than PEI 25k, we hypothesize that the lower toxicity offsets lower transfection efficiencies to generate similar total luciferase expression levels. DP1 and DP2 generated relatively low transfection efficiencies when incubated with cells for 48h in serum containing media. This was presumably due to the relatively large, loose and unstable complexes formed between pDNA and DP1/DP2 (Figure 3 and 4) that would be expected to have lower cellular uptake efficiency and higher susceptibility to enzymatic degradation during transfection. However, DP3 and DP4 result in significantly higher transfection efficiencies than complexes prepared using pDNA and PEI 25k at an N/P ratio of 10 (p < 0.001) and 15 (p < 0.05) when incubated with HEK293 cells for 48h in serum containing media. Since the Dex-PEI conjugates generated superior transfections efficiencies when compared to PEI 25k at 48h incubation in serum containing media and equivalent transfection efficiencies to PEI 25k when incubated in serum containing media for 4h, we hypothesize that the improved cytotoxicity of Dex-PEI at 4h results in significantly enhanced transfection efficiencies by 48h. In addition, the significantly improved transfection efficiency of DP3/DP4 is also attributed to the increased stability of particles in serum containing media. Transfection experiments carried out in the COS7 cell line show that the results observed in HEK293 cells could be reproduced in alternative cell lines (Figure 10).
Figure 9.
Luciferase expression generated by delivery of pDNA using Dex-PEI conjugates and PEI controls prepared at various N/P ratios in HEK293 cells. Complexes were incubated with HEK293 cells for 48h in serum containing media. Mean+SD, n=4 (ND: naked pDNA)
Figure 10.
Luciferase expression generated by delivery of pDNA using Dex-PEI conjugates and PEI controls prepared at various N/P ratios in COS7 cells. Complexes were incubated with COS7 cells for 48h in serum containing media. Mean+SD, n=4 (ND: naked pDNA)
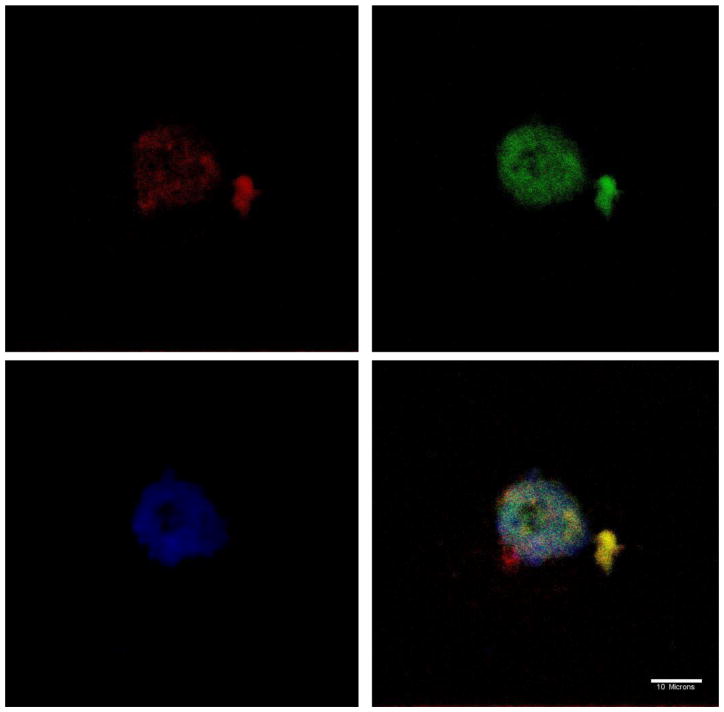
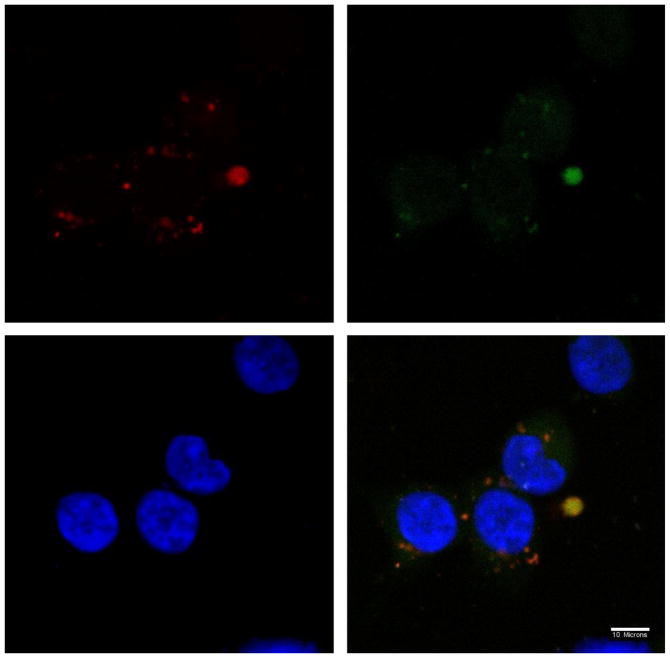
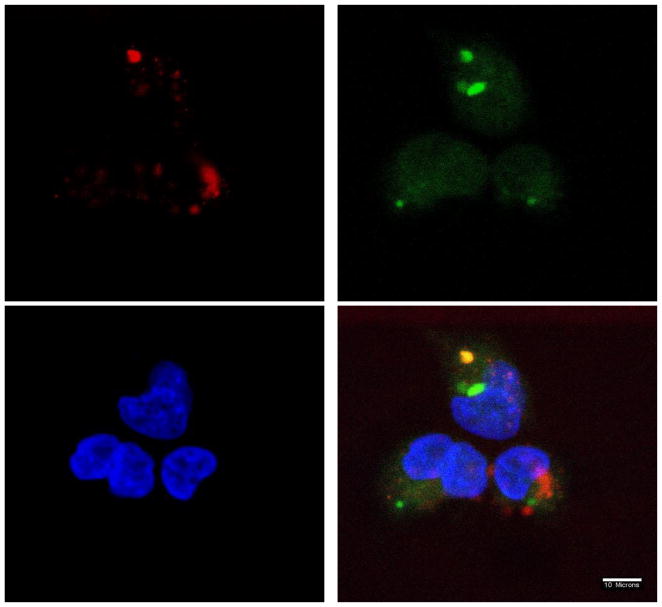
The intracellular fate of DP-pDNA complexes was visualized by confocal microscopy. Lysosomes and endosomes were stained with LysoTracker Green. The nucleus was stained with DAPI. Alexa 568 was used to label DP3 or PEI. As shown by confocal microscopy images in Figure 11, 12 and 13, complexes were internalized by cells at 2h post-transfection. The co-localization of PEI 25k and DP3 with lysosomes are represented as a yellow color in the images. DP3/pDNA complexes showed some level of dissociation with lysosomes. The relatively rapid color dissociation suggests a faster escape of DNA cargo from lysosomes into the cytoplasm, which could explain the high efficiency of Dex-PEI in transfection experiments.
Figure 11.
Confocal microscope images of the localization of PEI 2000-pDNA complexes prepared at an N/P ratio of 10 in HEK293 cells, 2h post-transfection (w/ serum). Nucleus stained with DAPI (blue); Lysosome stained with LysoTracker (green); Polymer stained with Alexa 568 (red)
Figure 12.
Confocal microscope images of the localization of PEI 25k-pDNA complexes prepared at an N/P ratio of 10 in HEK293 cells, 2h post-transfection (w/ serum). Nucleus stained with DAPI (blue); Lysosome stained with LysoTracker (green); Polymer stained with Alexa 568 (red)
Figure 13.
Confocal microscope images of the localization of DP3-DNA complexes prepared at an N/P ratio of 10 in HEK293 cells, 2h post-transfection (w/ serum). Nucleus stained with DAPI (blue); Lysosome stained with LysoTracker (green); Polymer stained with Alexa 568 (red)
In conclusion, we have synthesized a dextran-PEI conjugate that can generate transfection efficiencies that are equivalent to PEI alone when tested in physiologically relevant serum containing environments, whilst displaying significantly reduced toxicity. We anticipate that this construct should have significant potential for future in vivo studies and clinical applications.
Bullet-point summary of new findings for reviewers.
The two major new findings presented in this manuscript that we believe have not been reported before are:
This study reports on the synthesis and characterization of a dextran-PEI conjugate that utilizes an optimal Mw of PEI (2000) and dextran that has not been tested before.
This is the first study to show that dextran conjugation to PEI results in enhanced transfection efficiencies in physiologically relevant serum containing environments and to characterize the intracellular trafficking of these conjugates.
Acknowledgments
We gratefully acknowledge support from the American Cancer Society (RSG-09-015-01-CDD), the National Cancer Institute at the National Institutes of Health (1R21CA13345-01/ 1R21CA128414-01A2/UI Mayo Clinic Lymphoma SPORE), and the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas AO, Donovan MD, Salem AK. Formulating poly(lactide-co-glycolide) particles for plasmid DNA delivery. Journal of Pharmaceutical Sciences. 2008;97:2448–2461. doi: 10.1002/jps.21215. [DOI] [PubMed] [Google Scholar]
- Azzam T, Eliyahu H, Shapira L, Linial M, Barenholz Y, Domb AJ. Polysaccharide-oligoamine based conjugates for gene delivery. Journal of medicinal chemistry. 2002;45:1817–1824. doi: 10.1021/jm0105528. [DOI] [PubMed] [Google Scholar]
- Behr JP. The Proton Sponge: a Trick to Enter Cells the Viruses Did Not Exploit CHIMIA. 1997;51:34–36. [Google Scholar]
- Buchschacher GL, Jr, Wong-Staal F. Approaches to gene therapy for human immunodeficiency virus infection. Human gene therapy. 2001;12:1013–1019. doi: 10.1089/104303401750214249. [DOI] [PubMed] [Google Scholar]
- Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharmaceutical research. 1999;16:1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- Fischer D, von Harpe A, Kunath K, Petersen H, Li Y, Kissel T. Copolymers of ethylene imine and N-(2-hydroxyethyl)-ethylene imine as tools to study effects of polymer structure on physicochemical and biological properties of DNA complexes. Bioconjugate chemistry. 2002;13:1124–1133. doi: 10.1021/bc025550w. [DOI] [PubMed] [Google Scholar]
- Forrest ML, Koerber JT, Pack DW. A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjugate chemistry. 2003;14:934–940. doi: 10.1021/bc034014g. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res. 1999;45:268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Intra J, Salem AK. Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. Journal of Controlled Release. 2008;130:129–138. doi: 10.1016/j.jconrel.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intra J, Salem AK. Rational design, fabrication, characterization and in vitro testing of biodegradable microparticles that generate targeted and sustained transgene expression in HepG2 liver cells. Journal of Drug Targeting. 2011;19:393–408. doi: 10.3109/1061186X.2010.504263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Hohneker KW, Zhou Z, Olsen JC, Noah TL, Hu PC, Leigh MW, Engelhardt JF, Edwards LJ, Jones KR, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med. 1995;333:823–831. doi: 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- Larsen C. Dextran prodrugs -- structure and stability in relation to therapeutic activity. Advanced Drug Delivery Reviews. 1989;3:103–154. [Google Scholar]
- Marsh M, McMahon HT. Cell biology - The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Mehvar R. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J Control Release. 2000;69:1–25. doi: 10.1016/s0168-3659(00)00302-3. [DOI] [PubMed] [Google Scholar]
- Nabel GJ, Nabel EG, Yang ZY, Fox BA, Plautz GE, Gao X, Huang L, Shu S, Gordon D, Chang AE. Direct gene transfer with DNA-liposome complexes in melanoma: expression, biologic activity, and lack of toxicity in humans. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11307–11311. doi: 10.1073/pnas.90.23.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogris M, Walker G, Blessing T, Kircheis R, Wolschek M, Wagner E. Tumor-targeted gene therapy: strategies for the preparation of ligand-polyethylene glycol-polyethylenimine/DNA complexes. J Control Release. 2003;91:173–181. doi: 10.1016/s0168-3659(03)00230-x. [DOI] [PubMed] [Google Scholar]
- Onodera M, Nelson DM, Sakiyama Y, Candotti F, Blaese RM. Gene therapy for severe combined immunodeficiency caused by adenosine deaminase deficiency: improved retroviral vectors for clinical trials. Acta Haematol. 1999;101:89–96. doi: 10.1159/000040930. [DOI] [PubMed] [Google Scholar]
- Onodera M, Sakiyama Y. Adenosine deaminase deficiency as the first target disorder in gene therapy. Expert Opin Investig Drugs. 2000;9:543–549. doi: 10.1517/13543784.9.3.543. [DOI] [PubMed] [Google Scholar]
- Pearce ME, Mai HQ, Lee N, Larsen SC, Salem AK. Silicalite nanoparticles that promote transgene expression. Nanotechnology. 2008:19. doi: 10.1088/0957-4484/19/17/175103. [DOI] [PubMed] [Google Scholar]
- Porteous DJ, Dorin JR, McLachlan G, Davidson-Smith H, Davidson H, Stevenson BJ, Carothers AD, Wallace WA, Moralee S, Hoenes C, Kallmeyer G, Michaelis U, Naujoks K, Ho LP, Samways JM, Imrie M, Greening AP, Innes JA. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gene therapy. 1997;4:210–218. doi: 10.1038/sj.gt.3300390. [DOI] [PubMed] [Google Scholar]
- Rosenfeld EL, Lukomskaya IS. The splitting of dextran and isomaltose by animal tissues. Clinica chimica acta; international journal of clinical chemistry. 1957;2:105–114. doi: 10.1016/0009-8981(57)90090-6. [DOI] [PubMed] [Google Scholar]
- Rubin J, Galanis E, Pitot HC, Richardson RL, Burch PA, Charboneau JW, Reading CC, Lewis BD, Stahl S, Akporiaye ET, Harris DT. Phase I study of immunotherapy of hepatic metastases of colorectal carcinoma by direct gene transfer of an allogeneic histocompatibility antigen, HLA-B7. Gene therapy. 1997;4:419–425. doi: 10.1038/sj.gt.3300396. [DOI] [PubMed] [Google Scholar]
- Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. The Journal of biological chemistry. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- Sun YX, Xiao W, Cheng SX, Zhang XZ, Zhuo RX. Synthesis of (Dex-HMDI)-g-PEIs as effective and low cytotoxic nonviral gene vectors. J Control Release. 2008a;128:171–178. doi: 10.1016/j.jconrel.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Sun YX, Zhang XZ, Cheng H, Cheng SX, Zhuo RX. A low-toxic and efficient gene vector: carboxymethyl dextran-graft-polyethylenimine. Journal of biomedical materials research. 2008b;84:1102–1110. doi: 10.1002/jbm.a.31520. [DOI] [PubMed] [Google Scholar]
- Tang GP, Zeng JM, Gao SJ, Ma YX, Shi L, Li Y, Too HP, Wang S. Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: effects of PEGylation extent. Biomaterials. 2003;24:2351–2362. doi: 10.1016/s0142-9612(03)00029-2. [DOI] [PubMed] [Google Scholar]
- Thomas M, Ge Q, Lu JJ, Chen J, Klibanov AM. Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharmaceutical research. 2005;22:373–380. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng WC, Fang TY, Su LY, Tang CH. Dependence of transgene expression and the relative buffering capacity of dextran-grafted polyethylenimine. Molecular pharmaceutics. 2005;2:224–232. doi: 10.1021/mp050007t. [DOI] [PubMed] [Google Scholar]
- Tseng WC, Tang CH, Fang TY. The role of dextran conjugation in transfection mediated by dextran-grafted polyethylenimine. The journal of gene medicine. 2004;6:895–905. doi: 10.1002/jgm.572. [DOI] [PubMed] [Google Scholar]
- Vercauteren R, Bruneel D, Schacht E, Duncan R. Effect of the Chemical Modification of Dextran on the Degradation by Dextranase. Journal of Bioactive and Compatible Polymers. 1990;5:4–15. [Google Scholar]
- Vercauteren R, Schacht E, Duncan R. Effect of the Chemical Modification of Dextran on the Degradation by Rat Liver Lysosomal Enzymes. Journal of Bioactive and Compatible Polymers. 1992;7:346–357. [Google Scholar]
- Vogelzang NJ, Lestingi TM, Sudakoff G, Kradjian SA. Phase I study of immunotherapy of metastatic renal cell carcinoma by direct gene transfer into metastatic lesions. Human gene therapy. 1994;5:1357–1370. doi: 10.1089/hum.1994.5.11-1357. [DOI] [PubMed] [Google Scholar]
- Wightman L, Kircheis R, Rossler V, Carotta S, Ruzicka R, Kursa M, Wagner E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. The journal of gene medicine. 2001;3:362–372. doi: 10.1002/jgm.187. [DOI] [PubMed] [Google Scholar]
- Zabner J, Couture LA, Gregory RJ, Graham SM, Smith AE, Welsh MJ. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]