Abstract
Growing evidence supports that mitochondrial calcium uptake is important for cell metabolism, signaling and survival. However, both the molecular nature of the mitochondrial Ca2+ transport sites and the calcium signals they respond to remained elusive. Recent RNA interference studies have identified new candidate proteins for Ca2+ uptake across the inner mitochondrial membrane, including LETM1, MCU, MICU1 and NCLX. The sensitivity of these factors to several drugs has been tested and in parallel, some new inhibitors of mitochondrial Ca2+ uptake have been described. This paper provides an update on the pharmacological aspects of the molecular mechanisms of the inner mitochondrial membrane Ca2+ transport.
Keywords: mitochondria, uniporter, ruthenium red, Ru360, CGP-37157, minocycline
1. Molecular mechanisms of mitochondrial calcium uptake and release
Calcium transport between the cytoplasm and the mitochondrial matrix involves the passage of Ca2+ across both the outer and inner mitochondrial membranes (OMM and IMM). The overall permeability of the OMM for Ca2+ is relatively high, presumably due to the abundant presence of the voltage dependent anion-selective channels (VDACs) that also offer high conductance for Ca2+ in their “closed” state. However, the IMM presents a tight barrier for Ca2+. Early studies with isolated mitochondria revealed a mechanism of Ca2+ uptake presented by passive transport of Ca2+ down its electrochemical gradient without coupling Ca2+ transport to the transport of another ion. Therefore, this mechanism was attributed to a Ca2+ uniporter (reviewed in (Gunter et al., 1994; Gunter and Pfeiffer, 1990)). It was also found that upon the reverse of the driving force the uniporter can also mediate Ca2+ efflux even though in energized mitochondria, Na+/Ca2+ or H+/Ca2+ exchange mediate the exit of Ca2+ (Gunter and Pfeiffer, 1990). Furthermore, under various mitochondrial stress conditions such as Ca2+ overload, formation of a large pore, the permeability transition pore (PTP) was observed that can also support Ca2+ release (Bernardi et al., 1998; Szalai et al., 1999). Moreover, the PTP has been shown to “flicker” or to open and close transiently under normal conditions associated with brief depolarization transients (Duchen et al., 1998), which may also provide a rapid mechanism for mitochondrial Ca2+ efflux (Barsukova et al., 2011; Ichas et al., 1994). The physiological relevance of this sophisticated and high capacity Ca2+ transport machinery was debated because of the requirement for supraphysiological [Ca2+] elevations (10 – 100 μM) to stimulate the uniporter-mediated Ca2+ uptake of isolated mitochondria. Thus, the subsequent demonstration of a mitochondrial matrix [Ca2+] ([Ca2+]m) rise and stimulation of the Ca2+ sensitive steps of oxidative metabolism in intact cells during calcium spikes and oscillations came as a major surprise (Hajnoczky et al., 1995; Rizzuto et al., 1994; Rizzuto et al., 1993). In the past 20 years, new clues were obtained as to how mitochondria sense the physiological calcium signals, including by strategic targeting of mitochondrial Ca2+ uptake sites close to the sites of ER Ca2+ release (IP3 receptors (IP3R) and ryanodine receptors) and plasma membrane Ca2+ entry sites (voltage-dependent Ca2+ channels and store-operated Ca2+ entry) (Csordas et al., 1999; Hoth et al., 1997; Korzeniowski et al., 2009; Rizzuto et al., 1998; Sanchez et al., 2001; Szalai et al., 2000). In several paradigms, mitochondrial Ca2+ uptake was also documented at physiological, submicromolar cytoplasmic [Ca2+] ([Ca2+]c) elevations (reviewed in (Gunter and Sheu, 2009; Spat et al., 2008)). A fundamental electrophysiology study has clarified that the Ca2+ uniport across the inner mitochondrial membrane (IMM) is mediated by a channel (MiCa) (Kirichok et al., 2004). However, within the “generic” Ca2+ uniport category, several distinct Ca2+ influx mechanisms have also been isolated. These include the rapid mode uptake (RaM) (Sparagna et al., 1995) and Ca2+ selective conductances (mCa1&2) (Michels et al., 2009), each displaying unique characteristics in Ca2+ affinity, kinetics and pharmacological properties (for review see (Ryu et al.)). For instance, RaM takes up Ca2+ approximately 1000 times faster than that via mitochondrial uniporter. It should be noted that different cell types have very distinct intracellular Ca2+ profiles with Ca2+ transients that last from a few tens or hundreds of milliseconds (e. g. neurons and cardiomyocytes) to a few tens or hundreds of seconds (e.g. hepatocytes and glia cells), mostly due to differential expressions of various Ca2+ transport proteins. It is conceivable that the IMM contains multiple entities of the Ca2+ uniport to decode distinct intracellular Ca2+ signaling in various cell types.
The first proposal for a mitochondrial Ca2+ channel with known molecular identity in the heart was the type 1 ryanodine receptor (Beutner et al., 2005). Evidence was also presented for the involvement of uncoupling protein 2/3 in the Ca2+ transfer across the IMM (Trenker et al., 2007).
Subsequently, in genome-wide siRNA screen studies, LETM1 was identified as a critical component in the [Ca2+]m signal (Jiang et al., 2009). However, LETM1 is an antiporter that other studies have identified as a K+/H+ exchanger (Dimmer et al., 2008; Nowikovsky et al., 2004). Currently, these candidates are still debated. While the majority of studies focused on the Ca2+ influx pathway, a strong candidate emerged for the Ca2+ efflux mediated by Na+/Ca2+ exchange. This protein, the NCLX (Palty et al., 2010) nicely reproduces the biochemical properties of a protein previously purified from mitochondria (Li et al., 1992) and uniquely displays Li+ promoted Ca2+ transport (Palty et al., 2004). Finally, during the past months some seminal studies uncovered the MCU protein that can form a Ca2+ channel in lipid bilayers (Baughman et al., 2011; De Stefani et al., 2011) and the MICU1, an EF hand containing, MCU binding protein that might act as an important regulator of the MCU channel (Perocchi et al., 2010). Although, additional components of the Ca2+ uniport might emerge and the H+/Ca2+ exchanger remains to be identified, the recently identified constituents of the IMM Ca2+ transport provide important means to dissect the mechanism and functional significance of the mitochondrial Ca2+ handling (Figure 1). The molecular composition, significance and pharmacology of the PTP have been extensively reviewed recently (Bernardi and Rasola, 2007; Halestrap, 2009), therefore the PTP will not be discussed in detail here.
Figure 1. Schematic presentation of the mitochondrial Ca2+ transport mechanisms and the candidate proteins.
The black arrows represent the directions of Ca2+ transport, whereas the red arrows depict the feed-back and feed-forward effects of Ca2+ on the transport mechanisms. The blue arrows link the IMM transport mechanisms to the molecular candidates. The currently favoured candidates are printed in bold. Abbreviations: UCP2/3, uncoupling protein 2/3; Cyp D, cyclophilin D. For the sake of simplicity both IP3R and RyRs are depicted at the ER/SR-mitochondrial interface. Based on ultrastructural studies the RyRs are localized outside the interface area in both cardiac and skeletal muscle (Franzini-Armstrong, 2007) but sufficiently close to the mitochondria to expose them to high [Ca2+]c (Garcia-Perez et al., 2008; Sharma et al., 2000).
2. Drugs affecting the IMM Ca2+ transport
The transport of Ca2+ across the IMM is determined by both the driving force and the capacity of transport sites. Therefore, any conditions that cause collapse of the mitochondrial membrane potential (ΔΨ m), the dominant component of the driving force, lead to suppression of the Ca2+ uptake. Protonophores (also referred as uncouplers: FCCP, CCCP, and 1799), respiratory complex inhibitors (rotenone and antimycin) lead to a decrease in the ΔΨm to interfere with Ca2+ uptake.
The Ca2+ uniport and the MiCa are effectively suppressed by ruthenium red and Ru360, a ruthenium derivative (Kirichok et al., 2004; Matlib et al., 1998). Although the use of ruthenium red or Ru360 is fairly straightforward in isolated mitochondria, the lack of permeation across the plasma membrane in many cell types is an important limitation of the application of these drugs in cell or tissue studies. Furthermore, ruthenium red binds to and inhibits a wide variety of Ca2+ and K+ channels presenting some limitations in complex systems (reviewed in (Hajnoczky et al., 2006)). For example, ruthenium red is a potent inhibitor of the ryanodine receptors so it affects both the endo/sarcoplasmic reticulum (ER/SR) and mitochondrial components of the cellular Ca2+ homeostasis. Ru360 shows specificity for the Ca2+ uniporter over ryanodine receptors, SERCA pump, sarcolemmal Na+/Ca2+ exchange, and L-type Ca2+ channel current in cardiac muscle (Matlib et al., 1998). Additional inhibitors of the Ca2+ uniport and MiCa include Mg2+, that exerts inhibition in physiological concentrations (Szanda et al., 2009), lanthanides, cardioactive drugs quinidine, alprenolol, propranolol, oxyfedrine, and tetracaine, the diuretic, ethacrynic acid, amiloride analogs and derivatives, and the antibiotic gentamicin (Kirichok et al., 2004; Vinogradov and Scarpa, 1973). Pharmacological activators of the mitochondrial Ca2+ uniport have also been described such as polyamines (Nicchitta and Williamson, 1984; Salvi and Toninello, 2004), the p38 MAP kinase inhibitor SB202190 (Montero et al., 2002), and a range of naturally occurring flavonoids (e.g. genistein, quercetin, kaempferol) (Montero et al., 2004).
Notably, the Ca2+ uniport exhibits allosteric positive control by Ca2+ (Csordas and Hajnoczky, 2003; Kroner, 1986a, b), which is inhibited by a variety of calmodulin inhibitors (Csordas and Hajnoczky, 2003). Among the recently identified molecules of mitochondrial Ca2+ uptake, the MCU has been shown to be sensitive to ruthenium red, Ru360 and gadolinium (Baughman et al., 2011; De Stefani et al., 2011). An interesting clue regarding the domain that might mediate the ruthenium sensitivity is the loss of Ru360 sensitivity in the S259A MCU mutant (Baughman et al., 2011). Reconstituted LETM1-mediated Ca2+ flux in liposomes was also abolished by ruthenium red or Ru360 and was partially inhibited (25%) by CGP-37157 (4 μM) (Jiang et al., 2009). Although experimental evidence is not available, based on the presence of the EF hands (though one is noncanonical), MICU1 might be a target of the calmodulin inhibitors. Thus, currently no drugs are available for effective and specific inhibition of the IMM Ca2+ uptake pathway in cellular systems. The genetic approach, including overexpression of dominant negative constructs (MCU-D260Q, E263Q double point mutant (De Stefani et al., 2011) and MCU E257A, D261A, E264A single point mutants (Baughman et al., 2011)) and silencing offer better options at this point.
Mitochondrial Ca2+ efflux was shown to be inhibited by benzodiazepine compounds (including a large group of neurotropic drogs such as clonazepam), CGP-37157, diltiazem, verapamil, bepridil, amiloride and amiloride analogues, which inhibit Ca2+ exchangers (Chiesi et al., 1987; Cox and Matlib, 1993). Diltiazem and CGP-37157 also interfere with several other Ca2+ transport mechanisms but 10 μM or less appears to suppress mitochondrial Ca2+ efflux without greatly altering other aspects of the intracellular calcium homeostasis. However, a point of discrepancy is that Neumann et al. showed less than 5 μM CGP-37157 to inhibit SERCA pumps and activate RyR (Neumann et al.), whereas Cox and Matlib reported that ≤10 μM CGP-37157 effectively inhibit the mitochondrial Na+/Ca2+ exchange without significantly inhibiting the plasma membrane Ca2+ channels, Na+/Ca2+ exchanger, Na+/K+ pump and SR Ca2+ pump (Cox and Matlib, 1993). Evidence has also been presented that the NCLX-mediated Ca2+ egress is also inhibited by CGP-37157 (5 μM: 50% inhibition, 7.5 μM: complete inhibition) (Palty et al., 2010). Although currently CGP-37157 is the sole effective and relatively specific drug to target the NCLX, it can be complemented with some potent molecular approaches (overexpression of a dominant-negative NCLX mutant, NCLX-S468T and silencing of NCLX by si/shRNA) (Palty et al., 2010).
3. Inhibition of the Ca2+ uniport by minocycline
Uncovering of the specific molecular components of the IMM Ca2+ transport will aid the design of new drugs to enhance the pharmacological approach. Recent studies of current drugs have also described some candidates for inhibition of the Ca2+ uptake, which may also turn out as useful tools to target the mitochondrial Ca2+ homeostasis. Minocycline, has received attention as a potential modulator of both the Ca2+ uniport and the PTP and also as a Ca2+ channel forming drug (Antonenko et al., 2010; Garcia-Martinez et al., 2010; Mansson et al.; Theruvath et al., 2008). To clarify the effect of minocycline on mitochondrial Ca2+ handling we measured cytoplasmic [Ca2+] ([Ca2+]c) and [Ca2+]m and the Δψm in permeabilized RBL-2H3 cells.
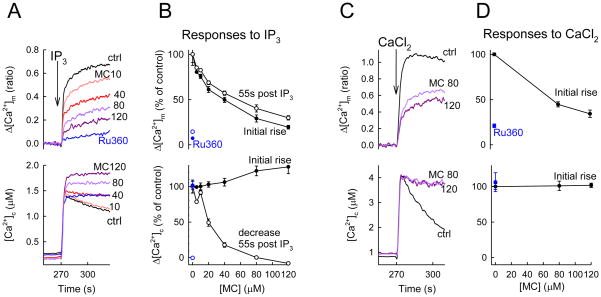
In simultaneous measurements of [Ca2+]c and [Ca2+]m, IP3-evoked a rapid rise in [Ca2+]c that was followed by a gradual decay (Figure 2A lower). Since IP3 was added together with thapsigargin, a SERCA inhibitor, the decay phase was unlikely due to ER Ca2+ uptake. Closely following the [Ca2+]c rise, the [Ca2+]m also displayed an increase followed by a plateau phase (Figure 2A upper). In the presence of Ru360, the [Ca2+]m increase and the decay phase of the [Ca2+]c rise were inhibited (Figure 2A), indicating that these changes reflected the uniporter-mediated Ca2+ uptake (additional evidence was presented in (Csordas et al., 1999)). When increasing concentrations of minocycline (10, 40, 80 and 120 μM) were included in the cytoplasmic buffer, both the IP3-induced [Ca2+]m increase and the decay of the [Ca2+]c rise were dose-dependently inhibited (Figure 2A,B). Half-maximal inhibition was attained when 20–40 μM minocycline was added (Figure 2B). These effects were not due to attenuation of the ER Ca2+ release since the IP3-induced rapid rise in [Ca2+]c was not suppressed by minocycline (Figure 2A lower). Furthermore, during bolus addition of CaCl2, the [Ca2+]m rise and the clearance of the [Ca2+]c increase were dose-dependently inhibited by minocycline (Figure 2CD).
Figure 2. Dose-dependent inhibition of the Ca2+ uniporter by minocycline (MC) in permeabilized RBL-2H3 cells.
[Ca2+]c and [Ca2+]m rises associated with IP3-induced Ca2+ release (A,B) or Ca2+ addition (CaCl2 10 μM, C,D) were recorded in suspensions of permeabilized cells using fura2FF/AM loaded to the mitochondria and rhod2 dissolved in the cytosolic buffer (for detailed description of the measurement see (Csordas and Hajnoczky, 2001, 2003; Csordas et al., 1999)). IP3 was applied in a saturating dose (8 μM). Analogue traces of IP3-induced Ca2+ responses with or without (ctrl, black trace) MC pretreatment (120s, in the indicated doses in μMs) are shown in A. For reference, the MCU was also inhibited by the specific blocker Ru360 (10 μM, blue traces). Only increases of [Ca2+]m are shown with the pre-stimulatory values shifted to zero because of a quenching artifact by MC (shown and explained in Figure 3). MC dose-inhibition curves for the [Ca2+]c and [Ca2+]m responses associated with IP3 stimulation are shown in B. The magnitude of the [Ca2+]m rise (upper) and the extent of [Ca2+]c clearance as the 55s post-peak decrease (lower) are shown. Analog traces and cumulated dose-inhibition correlation for 10 μM added Ca2+ are shown in C, D, respectively. Cumulated data are shown only for the initial rapid phase of the response, before the occurrence of significant depolarization (see Figure 3). For all, cumulative data are means±S.E. from 3 experiments.
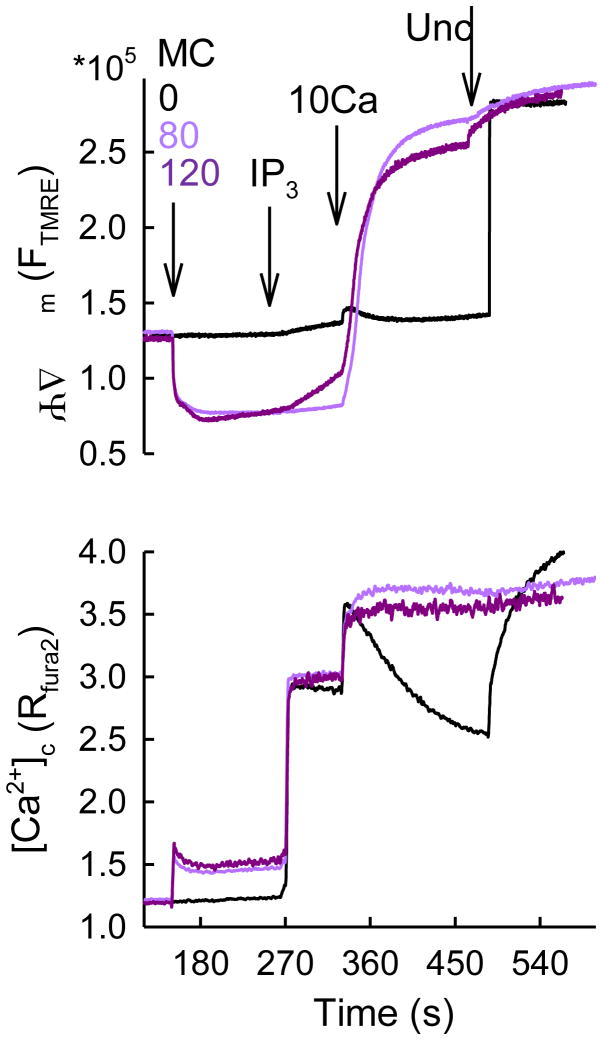
To test the possibility that the Ca2+ uniport was inhibited by minocycline because of a decrease in the driving force of the mitochondrial Ca2+ uptake, Δψm was monitored simultaneously with [Ca2+]c (Figure 3). Minocyclin by itself had a rapid, sustained hyperpolarizing effect possibly attributable to its ionophore nature. Up to 80 μM minocycline, the resting Δψm was maintained and no considerable depolarization was observed during IP3 addition (Figure 3). Thus, at ≤80 μM minocycline, inhibition of the propagation of IP3-induced Ca2+ release to the mitochondria would reflect inhibition of the uniporter activity. This concentration range of minocycline seems to be useful for selective inhibition of the uniporter. However, depolarization develops slowly at higher doses of minocycline upon IP3 stimulation or rapidly at either minocycline doses upon bolus Ca2+ addition, indicating that under those conditions the inhibition of Ca2+ uptake cannot be attributed to inhibition of the uniporter per se. Ca2+-dependent depolarizing effect of higher doses of minocycline was probably due to either activation of the PTP (Garcia-Martinez et al., 2010; Mansson et al.) or Ca2+-dependent channel formation by the drug itself (Antonenko et al., 2010). Since minocycline is membrane permeable, we also tested if the IP3-linked [Ca2+]m signal was sensitive to minocycline in intact cells. Unfortunately, no significant inhibition was observed when 80 μM minocycline was applied (n=3, not shown). In summary, minocycline appears to be a useful drug for inhibition of the uniporter in subcellular systems. However, it is less potent inhibitor than ruthenium red or Ru360 and elicits multiple changes in mitochondrial bioenergetics at ≥80μM concentration. Therefore, it remains a significant challenge for the field of mitochondrial Ca2+ that a selective inhibitor is still missing.
Figure 3. Effects of minocycline on the Δψm in permeabilized RBL-2H3 cells.
Simultaneous recordings of Δψm (TMRE) and [Ca2+]c (fura2) in suspensions of permeabilized RBL-2H3 cells are shown. TMRE was used in “dequenching mode” (depolarization increases the fluorescence). The cells were sequentially exposed to MC 0 (black) 80 (pink), 120 (purple) μM, IP3 (8 μM), CaCl2 (10 μM) and finally FCCP (Unc 10 μM) that completely dissipated Δψm. MC exerted quenching of fura2 inequally at the two excitation wavelengths (more at 380 nm), resulting in a Ca2+-independent rise in the ratio that also prevented proper translation of the fura2 signal to molar concentration of Ca2+. The rapid increase in fura2 ratio was followed by a gradual decrease. This decrease was absent in the presence of Tg, indicating that MC might exert some stimulatory effect on SERCAs. The rapid drop in TMRE fluorescence upon MC addition is likely to be a true hyperpolarization and not quench, since the fluorescence levels are equalized with the control after uncoupler addition.
Conclusions
Few drugs are available for effective and specific inhibition of the different IMM Ca2+ transport mechanisms. For inhibition of the uniporter, minocycline provides an alternative to ruthenium red and Ru360 in subcellular systems but the application of all these drugs is limited in intact cells. For selective inhibition of the Na+/Ca2+ exchanger, low concentrations of CGP-37157 can be used in both subcellular models and in intact cells. The recent advance in cloning of MCU and MCU1 provide new opportunity to identify crystal structure of these proteins so that structural-basis of drug design and discovery can be pursued. Moreover, with future elucidation in the regulation and posttranslational modification of these Ca2+ transport proteins, it will become feasible to design small molecules for inhibiting the signaling mechanisms in such regulations. Thus, identification of the specific molecules mediating the Ca2+ transport will provide a new opportunity to develop more specific and flexible pharmacological tools.
Highlights.
mitochondrial calcium uptake is important for cell metabolism, signaling and survival,
RNA interference studies have just identified candidate proteins for Ca2+ uptake,
the pharmacological aspects of the Ca2+ uptake mechanisms are reviewed.
Acknowledgments
This work was supported by a Thomas Jefferson Pilot Research Award to G.C. and by NIH grants (DK051526 and RC2AA019416) to G.H. P.V. was also supported by grants from the Hungarian Scientific Research Fund (OTKA NF-68563) and the Medical Research Council (ETT 494/2009) of Hungary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonenko YN, Rokitskaya TI, Cooper AJ, Krasnikov BF. Minocycline chelates Ca2+, binds to membranes, and depolarizes mitochondria by formation of Ca2+-dependent ion channels. J Bioenerg Biomembr. 2010;42:151–163. doi: 10.1007/s10863-010-9271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsukova A, Komarov A, Hajnoczky G, Bernardi P, Bourdette D, Forte M. Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons. Eur J Neurosci. 2011;33:831–842. doi: 10.1111/j.1460-9568.2010.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011 doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Colonna R, Costantini P, Eriksson O, Fontaine E, Ichas F, Massari S, Nicolli A, Petronilli V, Scorrano L. The mitochondrial permeability transition. Biofactors. 1998;8:273–281. doi: 10.1002/biof.5520080315. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: Transducer of excitation-metabolism coupling. Biochim Biophys Acta. 2005 doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Chiesi M, Rogg H, Eichenberger K, Gazzotti P, Carafoli E. Stereospecific action of diltiazem on the mitochondrial Na-Ca exchange system and on sarcolemmal Ca-channels. Biochem Pharmacol. 1987;36:2735–2740. doi: 10.1016/0006-2952(87)90257-7. [DOI] [PubMed] [Google Scholar]
- Cox DA, Matlib MA. Modulation of intramitochondrial free Ca2+ concentration by antagonists of Na(+)-Ca2+ exchange. Trends Pharmacol Sci. 1993;14:408–413. doi: 10.1016/0165-6147(93)90063-P. [DOI] [PubMed] [Google Scholar]
- Csordas G, Hajnoczky G. Sorting of calcium signals at the junctions of endoplasmic reticulum and mitochondria. Cell Calcium. 2001;29:249–262. doi: 10.1054/ceca.2000.0191. [DOI] [PubMed] [Google Scholar]
- Csordas G, Hajnoczky G. Plasticity of mitochondrial calcium signaling. J Biol Chem. 2003;278:42273–42282. doi: 10.1074/jbc.M305248200. [DOI] [PubMed] [Google Scholar]
- Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. Embo J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011 doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, Salviati L, Scorrano L. LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet. 2008;17:201–214. doi: 10.1093/hmg/ddm297. [DOI] [PubMed] [Google Scholar]
- Duchen MR, Leyssens A, Crompton M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol. 1998;142:975–988. doi: 10.1083/jcb.142.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C. ER-Mitochondria Communication. How Privileged? Physiology (Bethesda) 2007;22:261–268. doi: 10.1152/physiol.00017.2007. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez EM, Sanz-Blasco S, Karachitos A, Bandez MJ, Fernandez-Gomez FJ, Perez-Alvarez S, de Mera RM, Jordan MJ, Aguirre N, Galindo MF, Villalobos C, Navarro A, Kmita H, Jordan J. Mitochondria and calcium flux as targets of neuroprotection caused by minocycline in cerebellar granule cells. Biochem Pharmacol. 2010;79:239–250. doi: 10.1016/j.bcp.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez C, Hajnoczky G, Csordas G. Physical coupling supports the local Ca2+ transfer between sarcoplasmic reticulum subdomains and the mitochondria in heart muscle. J Biol Chem. 2008;283:32771–32780. doi: 10.1074/jbc.M803385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40:553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichas F, Jouaville LS, Sidash SS, Mazat JP, Holmuhamedov EL. Mitochondrial calcium spiking: a transduction mechanism based on calcium-induced permeability transition involved in cell calcium signalling. FEBS Lett. 1994;348:211–215. doi: 10.1016/0014-5793(94)00615-6. [DOI] [PubMed] [Google Scholar]
- Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- Korzeniowski MK, Szanda G, Balla T, Spat A. Store-operated Ca2+ influx and subplasmalemmal mitochondria. Cell Calcium. 2009;46:49–55. doi: 10.1016/j.ceca.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner H. “Allosteric regulation” of calcium-uptake in rat liver mitochondria. Biol Chem Hoppe Seyler. 1986a;367:483–493. doi: 10.1515/bchm3.1986.367.1.483. [DOI] [PubMed] [Google Scholar]
- Kroner H. Ca2+ ions, an allosteric activator of calcium uptake in rat liver mitochondria. Arch Biochem Biophys. 1986b;251:525–535. doi: 10.1016/0003-9861(86)90360-7. [DOI] [PubMed] [Google Scholar]
- Li W, Shariat-Madar Z, Powers M, Sun X, Lane RD, Garlid KD. Reconstitution, identification, purification, and immunological characterization of the 110-kDa Na+/Ca2+ antiporter from beef heart mitochondria. J Biol Chem. 1992;267:17983–17989. [PubMed] [Google Scholar]
- Mansson R, Morota S, Hansson MJ, Sonoda I, Yasuda Y, Shimazu M, Sugiura A, Yanagi S, Miura H, Uchino H, Elmer E. Minocycline sensitizes rodent and human liver mitochondria to the permeability transition: implications for toxicity in liver transplantation. Hepatology. 51:347–348. doi: 10.1002/hep.23465. author reply 349–350. [DOI] [PubMed] [Google Scholar]
- Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- Michels G, Khan IF, Endres-Becker J, Rottlaender D, Herzig S, Ruhparwar A, Wahlers T, Hoppe UC. Regulation of the Human Cardiac Mitochondrial Ca2+ Uptake by 2 Different Voltage-Gated Ca2+ Channels. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.835389. [DOI] [PubMed] [Google Scholar]
- Montero M, Lobaton CD, Hernandez-Sanmiguel E, Santodomingo J, Vay L, Moreno A, Alvarez J. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem J. 2004;384:19–24. doi: 10.1042/BJ20040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M, Lobaton CD, Moreno A, Alvarez J. A novel regulatory mechanism of the mitochondrial Ca2+ uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. Faseb J. 2002;16:1955–1957. doi: 10.1096/fj.02-0553fje. [DOI] [PubMed] [Google Scholar]
- Neumann JT, Diaz-Sylvester PL, Fleischer S, Copello JA. CGP-37157 inhibits the sarcoplasmic reticulum Ca(2)+ ATPase and activates ryanodine receptor channels in striated muscle. Mol Pharmacol. 79:141–147. doi: 10.1124/mol.110.067165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta CV, Williamson JR. Spermine. A regulator of mitochondrial calcium cycling. J Biol Chem. 1984;259:12978–12983. [PubMed] [Google Scholar]
- Nowikovsky K, Froschauer EM, Zsurka G, Samaj J, Reipert S, Kolisek M, Wiesenberger G, Schweyen RJ. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J Biol Chem. 2004;279:30307–30315. doi: 10.1074/jbc.M403607200. [DOI] [PubMed] [Google Scholar]
- Palty R, Ohana E, Hershfinkel M, Volokita M, Elgazar V, Beharier O, Silverman WF, Argaman M, Sekler I. Lithium-calcium exchange is mediated by a distinct potassium-independent sodium-calcium exchanger. J Biol Chem. 2004;279:25234–25240. doi: 10.1074/jbc.M401229200. [DOI] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Bastianutto C, Brini M, Murgia M, Pozzan T. Mitochondrial Ca2+ homeostasis in intact cells. J Cell Biol. 1994;126:1183–1194. doi: 10.1083/jcb.126.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Ryu SY, Beutner G, Dirksen RT, Kinnally KW, Sheu SS. Mitochondrial ryanodine receptors and other mitochondrial Ca2+ permeable channels. FEBS Lett. 584:1948–1955. doi: 10.1016/j.febslet.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi M, Toninello A. Effects of polyamines on mitochondrial Ca(2+) transport. Biochim Biophys Acta. 2004;1661:113–124. doi: 10.1016/j.bbamem.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Sanchez JA, Garcia MC, Sharma VK, Young KC, Matlib MA, Sheu SS. Mitochondria regulate inactivation of L-type Ca2+ channels in rat heart. J Physiol. 2001;536:387–396. doi: 10.1111/j.1469-7793.2001.0387c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- Spat A, Szanda G, Csordas G, Hajnoczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 2008;44:51–63. doi: 10.1016/j.ceca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanda G, Rajki A, Gallego-Sandin S, Garcia-Sancho J, Spat A. Effect of cytosolic Mg2+ on mitochondrial Ca2+ signaling. Pflugers Arch. 2009;457:941–954. doi: 10.1007/s00424-008-0551-0. [DOI] [PubMed] [Google Scholar]
- Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology. 2008;47:236–246. doi: 10.1002/hep.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov A, Scarpa A. The initial velocities of calcium uptake by rat liver mitochondria. J Biol Chem. 1973;248:5527–5531. [PubMed] [Google Scholar]