Abstract
Objective
Chronic stress has well-documented negative effects on hippocampal structure and function and has been suggested to contribute to age-related declines. In contrast, there is evidence that exercise has beneficial effects in older adults. The current investigation examined effects of lifetime stress on hippocampal volume and memory, the moderating role of stress on age effects and the moderating role of exercise on stress-related effects.
Method
Measures of lifetime stress, exercise engagement, MRI-based volumes and cognitive performance were obtained in a sample of healthy middle-aged and older adults.
Results
There was a significant negative influence of stress on hippocampal volume. In addition, exercise engagement moderated effects of lifetime stress on both hippocampal volume and memory. Specifically, lower exercise engagement individuals evidenced greater stress-related declines compared to high exercise individuals.
Conclusions
These novel findings suggest that benefits of exercise in later adulthood may extend to minimizing detrimental effects of stress on the hippocampus and memory.
Keywords: chronic stress, medial temporal, episodic memory, aerobic fitness, aging
Introduction
Cognitive and brain aging are characterized by considerable individual differences (Hertzog, 2008; Raz et al., 2010), which has prompted investigations into factors that may serve to contribute to decline or promote successful aging (Hertzog et al., 2009; Raz et al., 2009). Stress has long been suggested as a factor that contributes to cognitive and brain decline in aging (Landfield et al., 2007; Sapolsky et al., 1986; Sapolsky, 1999). Consistent with this hypothesis, stress targets similar brain structures and cognitive functions (e.g., memory) as advancing age (Balota et al., 2000; Raz & Rodrigue, 2006). Stress-related effects on the hippocampus have been emphasized as this structure is a target of stress hormones released by the hypothalamic-pituitary-adrenocortical (HPA) axis. The HPA axis is an integral component of brain regulation of the stress response, and the hippocampus has regulatory and feedback interactions with the HPA axis (Jankord & Herman, 2008). Both non-human animal and human evidence support an influence of stress on the hippocampus.
The non-human animal literature indicates that protracted elevations of glucocorticoids as a result of stress induce synaptic loss, reduced dendrite spines, decreased long-term potentiation and reduced neurogenesis in the hippocampus (McEwen, 2008; Radley & Morrison, 2005). Similarly, in the human literature, which has primarily examined stress-related conditions such as depression and post-traumatic stress disorder, there is evidence of reduced hippocampal volumes (Karl et al., 2006; Lorenzetti et al., 2009). Lastly, both stress and stress hormones have been associated with declines in memory performance in non-human animal and human investigations (Saura et al., 2003).
In support of the conceptualization that these stress-related effects on the hippocampus and memory may play a role in brain and cognitive aging, associations between stress hormones, hippocampal damage and/or memory impairment have been observed in aged rats (Issa et al., 1990; Landfield et al., 1978; Landfield et al., 2007; Sapolsky et al., 1986; Sapolsky, 1999). Furthermore, elevated cortisol, one stress hormone, has been consistently associated with poorer memory functioning (e.g., Carlson & Sherwin, 1999; Lupien et al., 1994; Lupien et al., 1998; Seeman et al., 1997) and reduced hippocampal volume (Lupien et al., 1998) in older adult humans. Importantly, these relationships have also been observed for self-report measures of stress in older adults. For example, perceived stress and reports of recent stressful life events are negatively related to memory performance (Neupert et al., 2006; Peavy et al., 2007; VonDras et al., 2005) and hippocampal volume (Gianaros et al., 2007). These findings converge on the possibility that chronic or repeated stress may lead to increased vulnerability to other toxic events such as those that may occur with aging (Sapolsky et al., 1986), thereby exacerbating the effects of aging for stressed individuals.
In comparison to the detrimental effects of stress, exercise engagement promotes benefits to brain structure and memory function including increased BDNF levels, neurogenesis and angiogenesis in the hippocampus and improved learning and memory in non-human animals (Hillman et al., 2008; Greenwood et al., 2009; van Praag, 2008). Beneficial effects of exercise on medial temporal structures, including the hippocampus, and memory functioning have also been observed for aged non-human animals and humans (Bugg & Head, 2010; Erickson et al., 2009; Gordon et al., 2008; Pereira et al., 2007; van Praag, 2008; van Praag et al., 2005; but see Madden et al., 1989; van Boxtel et al., 1997). Relatively few studies have examined the possibility that exercise may confer benefits by buffering against the influence of stress. Consistent with this possibility, some studies have demonstrated that exercise attenuates or reverses the negative effects of stress on hippocampal cell proliferation (Kannangara, Webber et al., 2009; Mello et al., 2009; Nakajima et al., 2010) even in aged animals (Kannangara, Lucero et al., 2009). However, others have observed that stress attenuates or delays exercise-related neurogenesis in the hippocampus (Leasure & Decker, 2009; Stranahan et al., 2006). Thus, further study is needed to assess whether exercise moderates the effects of lifetime stress on hippocampal structure and memory in adult humans.
The current cross-sectional investigation of the effects of stress in middle-aged and older adults had several goals. One goal was to examine the effects of lifetime stress on memory and hippocampal volume with the expectation that a higher occurrence of stressful events would be associated with lower memory performance and smaller hippocampal volumes. A second goal was to examine whether stress moderates the effects of age with the hypothesis that greater stress would be associated with steeper cross-sectional age-related decline in memory performance and hippocampal volume. Lastly, the current study assessed whether exercise engagement moderates the effects of lifetime stress with the hypothesis that greater exercise engagement would be associated with an attenuation of the negative effects of stress on memory performance and hippocampal volume. A novel component of the current study is that we examined the frequency of potential stressors over the lifespan, which represents an extension of past reports that examined self-reported stressful life events over a shorter time span (Neupert et al., 2006; Peavy et al., 2007; VonDras et al., 2005) or assessed self-reported perceived stress (Gianaros et al., 2007). In addition, we included the primary visual cortex as a control brain region and vocabulary as control cognitive domain to assess for the selectivity of the effects of stress and/or exercise engagement.
Materials and Methods
Participants
There were 59 older adult participants from a prior unpublished study in the lab with these individuals originally recruited from the St. Louis community through advertisements in local media (Community sample). For the Community sample, 51 of the 59 participants had an MRI scan. There were also 40 participants recruited from the Washington University Alzheimer’s Disease Research Center (ADRC). All 40 of these participants had an MRI scan as part of their participation in the ADRC. For the MRI analyses, the 91 participants’ data from the ADRC and Community samples were combined. Demographic information for the participants whose data contributed to the MRI analyses is presented in Table 1. Table 2 presents demographic information for participants whose data contributed to the cognitive analyses; all of these participants were from the Community sample.
Table 1.
Descriptive statistics for the MRI analyses.
| Low Exercise | High Exercise | |
|---|---|---|
| N | 44 | 44 |
| Age, years (mean (SD)) | 74 (9) | 71 (7) |
| Gender (F/M) | 19/25 | 24/20 |
| Education, years (mean (SD)) | 15.0 (2.8) | 15.7 (2.8) |
| History of Depression (−/+) | 38/6 | 43/1 |
| Geriatric Depression Scale | .96 (1.07) | .60 (.83) |
| Beck Depression Inventory-II | 2.8 (2.2) | 1.7 (2.3) |
| Hypertension (−/+) | 21/23 | 28/16 |
| Stress frequency | 13.2 (9.4) | 13.7 (9.2) |
| Exercise engagement, MET hrs/wk (mean (SD)) | .63 (.91) | 9.96 (6.97)** |
| Strenuous sports engagement, years (mean (SD)) | 2.55 (4.03) | 2.95 (4.07) |
| Hippocampus, cm2 (mean (SD)) | 7.3 (.69) | 7.3 (.72) |
| Primary visual cortex, cm2 (mean (SD)) | 3.5 (.73) | 3.5 (.69) |
The MRI sample consists of particpants combined across the ADRC and Community samples. The GDS was administered to the ADRC sample; The BDI-II was administered to the Community Sample. See text for details.
p<.05
p<.001
Table 2.
Descriptive statistics for the Cognitive analyses.
| Low Exercise | High Exercise | |
|---|---|---|
| N | 25 | 32 |
| Age, years (mean (SD)) | 73 (8) | 72 (8) |
| Gender (F/M) | 22/3 | 23/9 |
| Education, years (mean (SD)) | 14.7 (3.1) | 15.3 (2.7) |
| History of Depression (−/+) | 25/0 | 32/0 |
| Beck Depression Inventory-II | 3.0 (2.6) | 2.32 (2.8) |
| Hypertension (−/+) | 11/14 | 18/14 |
| Exercise Engagement, MET hrs/wk (mean (SD)) | .63 (.93) | 10.76 (7.8)* |
| Stress Frequency | 17.4 (10.7) | 17.7 (10.5) |
| Strenuous Sports Engagement, years (mean (SD)) | 2.7 (4.2) | 2.6 (3.9) |
| WASI Vocabulary | 66.2 (6.5) | 64.8 (7.1) |
| California Verbal Learning Test-II | 45.9 (8.5) | 44.4 (10.9) |
| Building Memory | 4.4 (2.1) | 3.7 (2.0) |
| Standardized memory composite | .05 (.77) | −.21 (.61) |
All of these participants were from the Community sample only. See text for details.
p<.001
The ADRC sample was screened for dementia based on the Clinical Dementia Rating scale (Clinical Dementia Rating=0; Morris, 1993). The Community sample was screened for gross cognitive functioning with the Short Blessed Test using a cut-off of 5 (Katzman et al., 1983; Morris et al., 1989). All participants were additionally screened for neurological (e.g., Parkinson’s disease, head injury, stroke), and major medical conditions (e.g., diabetes). Screening for the ADRC sample was conducted at or near the time of each assessment (see below for details of the timing of assessments) and screening for the Community sample was conducted at the time of the cognitive/MRI session. Participants consented to participation in accordance with guidelines of the Washington University Human Research Protection Office.
Lifetime Stress
Lifetime stress was estimated using the 32-item Cumulative Trauma Scale (CTS) (Kira et al., 2008). The CTS is based on a taxonomy of stressors including lifetime stress (e.g., repeated hassles), internal stressors (e.g., major medical conditions, pain), nature-made events (e.g., natural disasters), and made-made events, which includes person-made (e.g., motor vehicle accident, sexual abuse, racial discrimination) and socially-made (e.g., poverty). Thus, a wide range of potential stressors from across the lifespan is assessed in the measure (e.g., divorce, sexual assault, job loss, natural disasters, discrimination, illness, etc.).
Reliability and Validity
Reliability and validity of a short form (22 item) of the CTS measuring frequency of experiences were established in a sample of 499 individuals aged 12-79 (Kira et al., 2008). The scale demonstrated adequate internal consistency (Cronbach’s alpha=.85) and adequate convergent (i.e., positive associations with other trauma scales), divergent (negative associations with measures of adjustment and futuristic orientation) and predictive (i.e., positive associations with post-traumatic stress disorder, lifetime stress disorders and poor health) validity.
Procedure
The CTS was administered by telephone and participants indicated the frequency and impact of a list of 32 stressors. First, frequency with which a particular stressor was experienced was rated as never (0), once (1), twice (2), three times (3), or many times (4). Next, the stressor’s impact in terms of the degree to which it subsequently had a positive or negative influence on the participant’s life was rated on a 7-point Likert-type scale (i.e., extremely positive, very positive, somewhat positive, negative, somewhat negative, very negative, extremely negative). The current report focused on lifetime stress events and thus the independent variable used in analyses was the sum of the number of events across stressors (range=0-128) with higher values indicating greater frequency of stress events.
Exercise Engagement
Validity
A validated questionnaire assessing history of walking, running and jogging activity for the past 10 years was used to estimate exercise engagement (Bowles et al., 2004). The measure was significantly correlated with cardiorespiratory fitness measured via treadmill test in a sample of 5063 individuals aged 18-80 years. Stable correlations were observed between retrospective self-report of activity for a particular year and aerobic fitness for that year across the 10 1-year assessment periods, suggesting participants across the examined age range were capable of relatively accurate self-report over this extended time span. Although reliability was not directly assessed, validity is limited by reliability and thus the demonstrated validity can be considered indicative of adequate reliability (Bowles et al., 2004).
Procedure
The questionnaire was administered by telephone, and participants reported number of months/year, number of workouts/week, average number of miles/workout, and average time/mile for each year in which they engaged in an exercise program involving walking, running, or jogging for the preceding 10 years. A physical exercise engagement score was derived by estimating metabolic equivalent (MET) values using the compendium of physical activities, as described previously. The index of exercise engagement was average MET hours per week over the past 10 years. As a reference, an individual who followed the American Heart Association’s physical exercise recommendation for older adults (30 minutes of moderate exercise, 5 days/week) would score 7.5 MET-hours/week. Participants were categorized into low exercise and high exercise engagement groups based on the median value of 3.15 MET hrs/week for the sample.
Participants also reported the number of years, within the last 10 years, during which they participated in strenuous sports other than running, walking or jogging at least twice a week for 6 consecutive months. Examples included racquet sports, cycling, swimming, aerobic dance, or strenuous sports involving running such as basketball and soccer.
MR Acquisition
For all scans, cushions reduced head movement during scanning and a scout image was acquired first in order to center the field of view on the brain. For the ADRC sample (n=40), imaging was performed using a Siemens Vision 1.5T scanner and two to four T1-weighted sagittal MP-RAGE scans (TR = 9.7 ms, TE = 4 ms, flip angle = 10°, TI = 20 ms, 1 × 1 × 1.25 mm resolution) were acquired in each subject. For the Community sample (n=51), images were acquired using a Siemens 1.5T Sonata Scanner (Erlangen, Germany) and two T1-weighted sagittal MP-RAGE scans (TR = 1900ms, TE = 3.93 ms, flip angle=7°, TI=1100, 1 × 1 × 1.25mm resolution) were acquired in each subject.
Regional Volumetry
Hippocampal volume was obtained using Freesurfer software (Desikan et al., 2006; Fischl et al., 2002). During processing, each voxel is assigned a neuroanatomical label based on probabilistic information derived from a manually labeled training set, which included healthy young and older adults. Previous work indicates that this technique generates volumes with a high correspondence to manually generated volumes (Desikan et al., 2006; Fischl et al., 2002). As there were no hypotheses regarding laterality effects, volumes were summed across hemispheres. Total intracranial volume (ICV) was used to adjust hippocampal volumes for body size differences. The adjustment was performed via a formula based on the analyses of covariance approach: Adjusted volume = raw volume - (b × (ICV - mean ICV)), where b is the slope of the regression of the ROI volume on ICV (Jack et al., 1989; Mathalon et al., 1993). Adjusted hippocampal volume was used as the dependent variable in analysis.
Although there may be concerns with regard to biases in cross-scanner aggregation, there is evidence of reliability of Freesurfer-derived estimates of cortical thickness and volumes across scanner upgrades, different manufacturers, and number of MP-RAGE acquisitions, particularly when scanners have the same field strength (e.g., Fennema-Notestine et al., 2007; Han et al., 2006; Jovicich et al., 2009), and cross-scanner aggregation has been successfully used previously (e.g., Desikan et al., 2009; McEvoy et al., 2009; Storandt et al., 2009). An in-house comparison between the Sonata 1.5T and Vision 1.5T scanners in Freesurfer-derived hippocampal and primary visual cortex volumes yielded an average intraclass correlation of .80, indicating a strong correspondence between volumes derived from the two scanners.
Cognitive assessment
All 59 participants in the Community sample completed the cognitive measures: memory and vocabulary. We did not have equivalent cognitive data for the ADRC sample.
Memory
For the California Verbal Learning Test – II (Delis et al., 2000) participants were orally presented with a list of 16 categorizable grocery items, five times. The number of items correctly recalled across the five trials was the index of performance. The estimated test-retest reliability is .92 (Delis et al., 2000). For the Buildings memory test (Ekstrom et al., 1976), participants first studied a map of a fictitious urban location with landmark buildings. During the test phase participants were shown a blank map and tested on their memory for landmark locations in a multiple-choice format. The index of performance was number correct minus .25 points for false-positives. The estimated reliability is .80 (Ekstrom et al., 1976). A composite measure of memory was created by standardizing scores for each task and summing the standardized scores.
Vocabulary
The Vocabulary subtest from the Wechsler Abbreviated Scale of Intelligence (Wecshler et al., 1999) was administered and scored according to standard protocol. The raw score was the index of performance. The estimated split-half reliability is .94 (Wechsler et al., 1999).
Depression symptoms
Depression symptoms were measured with the Beck Depression Inventory-II (BDI-II; Beck et al., 1996) in the Community Sample and with the Geriatric Depression Scale (GDS; Yesavage et al., 1983) in the ADRC sample. The BDI-II has an estimated reliability of .92 (Beck et al., 1996) and the GDS has an estimated reliability of .94 (Yesavage et al., 1983). BDI-II scores range from 0 to 63 whereas scores on the GDS range from 0 to 15. While the specific item content and format differ between these two measures, they are strongly correlated (rs=.71 to .77; e.g, McCabe et al., 2006; von Hippel et al., 2008), show similar associations with cognitive measures (von Hippel et al., 2008) and show similar sensitivity to reductions in symptoms following cognitive-behavioral therapy (Laidlaw et al., 2008). Thus, there are indications that the two measures similarly estimate the underlying construct of depression symptomatology. In order to have an estimate of depressive symptoms for the MRI analyses (which were based on combined data from the ADRC and Community samples), scores on the respective depressive symptoms measure within each sample were standardized using a z-transformation to obtain an estimate of each individual’s relative ranking.
Timing of assessments
The MRI scan was conducted, on average, 1.62 years (SD = .85) prior to the exercise engagement assessment and 3.52 years (SD = 1.26) prior to the lifetime stress assessment. Thus, the exercise assessment captured exercise behavior preceding and during the time of the scan for all individuals. Similarly, the lifetime stress assessment captured stressful events preceding and during the time of the scan for all individuals. The lifetime stress assessment was subsequent to the exercise engagement assessment for 90% of the sample (M = 1.86 years; SD =.45). For the other 9 individuals stress assessment preceded exercise assessment by an average of just 5.5 months (SD = .13). Thus, any reported exercise behavior would have occurred almost exclusively during a time period for which stressful experiences were also captured.
The cognitive assessment was conducted, on average, 1.60 years (SD = .84) prior to the exercise engagement assessment and 2.65 years (SD = .32) prior to the lifetime stress assessment. Thus, the exercise assessment captured exercise behavior preceding and during the time of the cognitive assessment for all individuals. Similarly, the lifetime stress assessment captured stressful events preceding and during the time of the cognitive assessment for all individuals. The lifetime stress assessment was subsequent to the exercise engagement assessment for 83% of the sample (M = 1.41 years; SD = .17). For the other 10 individuals lifetime stress assessment preceded exercise assessment by an average of just 5.6 months (SD = .12). Thus, any reported exercise behavior would have occurred almost exclusively during a time period for which stressful experiences were also captured.
Data Analyses
Outliers and Missing Data
Univariate outliers were defined as values 3 standard deviations from the mean. Three individuals in the MRI analyses and two individuals in the Cognitive analyses had outlier data points. These outliers were removed from analyses to meet assumptions of regression analyses. Thus, the final sample was 88 for the MRI analyses and 57 for the Cognitive analyses (see Tables 1 and 2, respectively). Exercise engagement data were missing for four individuals, California Verbal Learning Test data were missing for four individuals, Building Memory data were missing for three individuals and Vocabulary data were missing for one individual representing .03 percent of the total data points. Missing data were replaced using a regression substitution imputation approach, which uses regression to predict the missing data point based on other variables. All other variables included in the regression model were used for the imputation (see below for details on model variables).
Covariates
Gender (coded as 0=male and 1=female), education (years), history of hypertension and standardized depression scores (combined across GDS and BDI-II for the MRI analyses; BDI-II only for the cognitive analyses) were included as covariates in all analyses. As there were individuals with a history of depression in the MRI analyses (but not the cognitive analyses), history of depression was an additional covariate in analyses of regional brain volumes.
Statistical Analyses
Four hierarchical regressions were conducted to address our primary questions regarding the main effects of lifetime stress, the moderating role of stress on age effects, and the moderating role of exercise on stress effects. The approach to investigating moderation followed the steps outlined by Baron and Kenny (Baron & Kenny, 1986). These steps included a hierarchical regression approach to examine the unique variance accounted for by the hypothesized two-way interactions above and beyond the hypothesized main effects. The dependent variables were hippocampal volume, primary visual cortex volume, memory performance or vocabulary performance. Exercise engagement status was coded as 0 or 1. The continuous age and lifetime stress predictors were standardized using a z-transformation in order to minimize multicollinearity with the interaction terms in the model. Interaction terms were created by multiplying the relevant variables.
In all analyses, the nuisance covariates were entered in the first step. Age was entered in the second step so that we could examine effects of the other predictors independent of age. The primary variable of interest, lifetime stress, was entered in the the next step and exercise engagement was entered in the fourth step. The two primary interactions of interest (age × lifetime stress; lifetime stress × exercise engagement) were entered in the next steps. The age × exercise engagement interaction was entered in the seventh step, and the 3-way interaction was entered in the last step. Cohen’s f2 was calculated as a measure of effect size.
Results
Regression Assumptions
Age, hippocampal volume, primary visual cortex volume, lifetime stress, memory and vocabulary were all normally distributed (all ps≥.09 for Kolmogorov-Smirnoff tests). For all models, error was normally distributed (all ps≥.21 Kolmogorov-Smirnoff tests). Examination of the plots of standardized residuals against standardized predicted values indicated that there was no homoscedasticity for any of the models (e.g., no systematic associations across the range of values). Homogeneity of variances was observed for the exercise groups in terms of the outcome variables (i.e., hippocampal volume, primary visual cortex volume, memory and vocabulary; all ps≥.44 for Levene’s Test for Equality of Variances). Based on the Durbin-Watson test there were independent observations (Durbin-Watson between 1.5 and 2.05 for all models). Lastly, there was not a problematic level of multicollinearity in any of the models (Variance Inflation Factor scores <4 with highest values for 3-way interaction).
Hippocampus
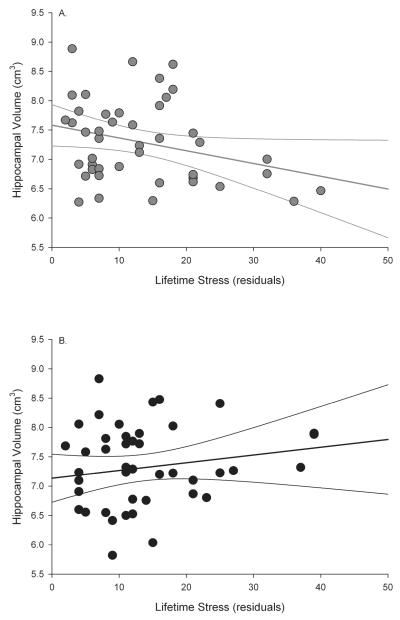
Age accounted for a significant amount of variance in hippocampal volume (ΔR2=.32, F(1,81)=42.20, ß =−.59, p<.001, f2=.52) with increasing age associated with smaller volumes. Lifetime stress also accounted for a significant amount of variance (ΔR2=.04, F(1,80)=5.01, =−.22, p<.05, f2=.06). Exercise engagement did not account for a significant amount of variance ß (ΔR2=.01, F(1,79)=.703, =−.09, ns, f2=.01). However, the lifetime stress × exercise engagement interaction did account for a significant amount ß of variance (ΔR2=.05, F(1,77)=7.83, =.36, p<.01, f2=.06), reflecting greater decline in hippocampal volume with increasing stress ß for the low exercise group (r(42)=−.29, p=.05) compared to the high exercise group (r(42)=.16, ns) (see Figure 1). Neither the 2-way interactions involving age (age × lifetime stress: ΔR2=.01, F(1,78)=.70, ß =.08, ns, f2=.01; age × exercise engagement: ΔR2=.02, F(1,76)=3.63, =−.21, ns, f2=.01) nor the age × lifetime stress × exercise engagement interaction (ΔR2=.00, F(1,75)=.00 ß, ß =−.01, ns, f2=.01) accounted for a significant amount of variance.
Figure 1.
Exercise engagement moderates stress effects on hippocampal volume. A) Low exercise group; B) High exercise group.
Additional models were tested to examine any potential influences of scanner type or the delays between assessments. It was not possible to enter all of the delays (i.e., scan-exercise; scan-stress; stress-exercise) in one model due to the strong correlations amongst the delay variables. The main effect of stress and the stress × exercise interaction remained significant when scanner type or delays between assessments were additionally controlled (all ps<.05). When individuals with missing data points were excluded, the main effect of stress was a non-significant trend (p=.056) and the stress × exercise interaction remained significant (p<.05).
Primary visual cortex
Neither the main effects of age, lifetime stress or exercise (all ps≥.42) nor any interactions (all ps≥.10) were significant for the primary visual cortex volumes. Results were similar with missing data excluded.
Memory performance
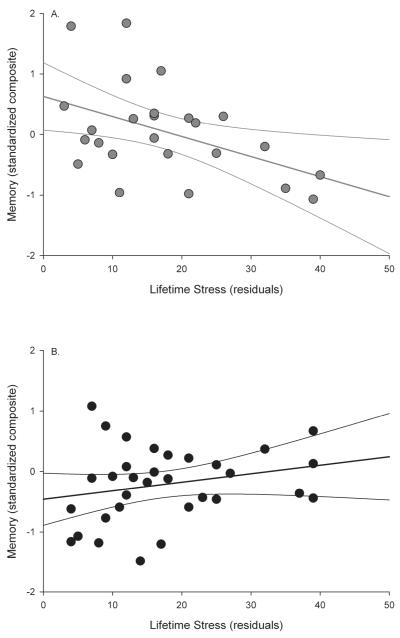
Neither age (ΔR2=.04, F(1,51)=2.37, =−.22, ns, f2=.05), lifetime stress (ΔR2=.01, ß F(1,50)=.80, ß =−.15, ns, f2=.02) nor exercise engagement (ΔR2=.05, F(1,49)=3.33, =−.25, ns, f2=.07) accounted for a significant amount of variance. However, the lifetime stress × exercise ß engagement interaction accounted for a significant amount of variance (ΔR2=.16, F(1, 47)=11.51, ß =.67, p<.01, f2=.13), which reflected a greater effect of stress on memory performance for those in the low exercise group (r(23)=−.46, p<.05) compared with those in the high exercise group (r(30)=.24, ns) (see Figure 2). Neither the 2-way interactions involving age (age × lifetime stress: ΔR2=.02, F(1, 48)=.94, =.15, ns, f2=.01; age × exercise engagement: ΔR2=.01, F(1,76)=.37, =.13, ns, f2=.01) nor the age × lifetime stress × exercise ß engagement ß interaction (ΔR2 =.01, F(1,45)=.87, ß =−.22, ns, f2=.01) accounted for a significant amount of variance. The stress × exercise interaction remained significant when delays between assessments were additionally controlled, and when individuals with missing data were excluded (all ps<.05).
Figure 2.
Exercise engagement moderates stress effects on memory performance. A) Low exercise group; B) High exercise group.
Vocabulary
Neither the main effects of age, lifetime stress or exercise (all ps≥.28) nor any interactions (all ps≥.15) were significant for vocabulary scores. Results were similar with missing data excluded.
Discussion
The present investigation examined the negative effects of lifetime stress and the moderating influence of exercise engagement on hippocampal volume and memory performance in healthy middle-aged and older adults. Considering the literature supporting exercise-related benefits on these variables, it was predicted that exercise engagement would moderate the effects of stress. In addition, it was expected that greater lifetime stress would be associated with greater cross-sectional age-related declines in these variables.
A significant negative influence of self-reported lifetime stress on hippocampal, but not primary visual cortex volume, was observed. A negative effect of stress on the hippocampus is consistent with the extensive literature on non-human animals (McEwen, 2008; Radley & Morrison, 2005), and stress-related psychiatric conditions (Karl et al., 2006; Lorenzetti et al., 2009), demonstrating the effects of chronic stress on the hippocampus. This finding also represents an extension of two past reports that observed associations of hippocampal volume with cortisol levels (Lupien et al., 1998) and with self-reported perceived stress (Gianaros et al., 2007) in psychiatrically normal older adult humans. Importantly, the effect of lifetime stress on the hippocampus (but not the primary visual cortex) was moderated by exercise engagement. Specifically, stress-related declines in hippocampal volume were greater for the low exercise group than for the high exercise group. This is a novel observation in humans, and the pattern coincides with reports that exercise may attenuate or reverse the negative effects of stress on hippocampal cell proliferation in non-human animals (Kannangara, Webber et al., 2009; Nakajima et al., 2010), including aged mice (Kannangara, Lucero et al., 2009). Our findings do, however, contrast with two additional non-human animal studies that observed stress-related attenuation of exercise-related neurogenesis in rats (Leasure & Decker, 2009; Stranahan et al., 2006). Discrepant findings across non-human animal studies may relate to the type of stressor, level of stress, species-related differences in perceptions of a particular stressor (e.g., social isolation), and/or animal care management protocols (Kannangara, Webber et al., 2009).
As expected, there was no association between lifetime stress and vocabulary. Although the influence of lifetime stress on memory performance was in the expected negative direction, a significant main effect was not observed. The absence of a main effect of stress on memory is inconsistent with previous reports of associations between cortisol (Carlson & Sherwin, 1999; Lupien et al., 1994; Lupien et al., 1998; Seeman et al., 1997) or self-reported stress during briefer intervals (Neupert et al., 2006; Peavy et al., 2007; VonDras et al., 2005) and memory in older adults. However, the absence of a main effect was observed in the context of a significant interaction between lifetime stress and exercise engagement for memory performance, but not vocabulary. This interaction reflected that stress-related reductions in memory were apparent, but only for the low exercise group and not the high exercise group. This pattern is consistent with recent reports of exercise attenuating stress effects on memory in non-human animals (Mello et al., 2009; Nakajima et al., 2010).
Collectively, the novel pattern of findings for both hippocampal volume and memory provide support for a buffering influence of exercise in the context of lifetime stress. In addition, current results are consistent with memory being in part a hippocampally-dependent function and post-hoc analysis revealed a significant association between hippocampal volume and memory performance in the subsample from the Community sample that had both measures (n=49; age-partialled r(42)=.30, p<.05). While the current report does not address mechanisms, exercise has been demonstrated to have multiple positive effects, including enhanced neurogenesis and angiogenesis, reduced levels of stress hormones, reduced HPA axis response to mild psychological stressors and reversal of corticosterone-induced HPA dysregulation (Hillman et al., 2008; Greenwood et al., 2009; Kannangara et al., 2009; van Praag, 2008; Droste et al., 2007; Kim et al., 2008), which may serve to protect hippocampal structure and memory functioning against the potentially detrimental effects of stress.
Notably, there were no significant interactions between stress and age for hippocampal volume or for memory performance. Thus, there was no support for the hypothesis that individuals who had experienced greater stress would experience greater declines with age. Much of the work that would suggest interactive effects of stress and age has focused on specific subfields of the hippocampus (e.g., dentate gyrus, CA3; Landfield et al., 2007; Sapolsky, 1999; McEwen, 2008) and thus it is conceivable that if specific subfields of the hippocampus were examined in humans, then interactive effects of stress and aging would be observed. Moreover, as the degree to which environmental events are considered as stressful depends in part on an individual’s perceptions, which may reflect multiple factors such as past experiences, personality characteristics, available coping resources and social support (McEwen, 2008; Lazarus & Folkman, 1984), future investigations might also include measurement of individual differences in cognitive appraisal processes and the factors associated with it. There is evidence of a down-regulation of negative emotions and improved ability to regulate emotions with age (e.g., Phillips et al., 2008; Scheibe & Carstensen, 2010), age differences in appraisal processes and coping strategies (e.g., Diehl et al., 1996), an influence of social support on cognitive functioning in older adults (Hertzog et al., 2009) and personality-related differences in brain structure in aging (e.g., Jackson et al., 2009). These age effects may serve to mitigate the experience of distress, and thereby stress-related effects on brain structure and cognition in older adults.
There are several potential limitations associated with the current study, including the retrospective self-report nature of the lifetime stress and exercise measurements. The measures of exercise and lifetime stress may be limited by the ability of healthy older adults to accurately recall and report their experiences over an extended time span and also by the use of phone administration as opposed to in-person administration. In addition, the cognitive status of the Community sample was not assessed at all time points and some individuals may have experienced cognitive decline in between measurements. These limitations could lead to either over- or under-estimation of stressful experiences or exercise behavior thereby adding noise to our measures and reducing the likelihood of finding significant effects of interest. Furthermore, the current self-report measure of exercise engagement is significantly but not perfectly related to cardiorespiratory fitness. However, the expected pattern of selective effects for the hippocampus and memory does provide some additional support for the validity of the self-report measures. In addition, although the low and high exercise groups did not differ significantly in years of participation in strenuous sports other than running, walking, and jogging, the exercise engagement measure did not include other potentially relevant occupational and leisure time physical activities or non-strenuous exercises. Another potential measurement limitation is that although Freesurfer provides automated estimates of hippocampal volume with strong correspondence to manually-derived estimates (Cherbuin et al., 2009; Fischl et al., 2002; Tae et al., 2008) it may overestimate volume (e.g., Cherbuin et al., 2009; Tae et al., 2008). However, automated measures show similar associations as manual measures with disease and cognitive outcomes (e.g., Cherbuin et al., 2009; Tae et al., 2008).
An additional limitation relates to the timing of the multiple assessments. For all individuals, the lifetime stress and exercise measurements captured experiences and behavior during the time of the MRI scan or cognitive assessment. In addition, for the large majority of participants the exercise assessment captured behavior during the time that stressful experiences would have been reported. For a small number of individuals there is a period of about 6 months on average during which exercise could have occurred subsequent to the occurrence of stressful experiences. If these individuals substantially increased their exercise subsequent to our measurement, this may have influenced the effects of stress and we would not have captured this. However, we did have an estimate of 9.5 years of exercise behavior that overlapped with stressful events and observed a significant interaction (even after controlling for the delay between these measurements).
Another consideration is that the current study aggregated stressors that might occur across an individual’s life. As stressors during development, adolescence or old age may lead to differential effects across brain regions (Lupien et al., 2009; Tottenham et al., 2020), future studies might make efforts to understand the influence of the developmental timing of stressors on multiple brain structures and cognitive domains in older adults. Stressful experiences can lead to the diagnosis of a stress-related disorder such as depression or to increased depression symptoms. While we attempted to control for this, the aggregation of two depression measures in the MRI analyses may not have fully captured depression symptoms leading to the potential conflation of stress experience and depression symptoms in these analyses. Lastly, interpretations of the current results are limited by the cross-sectional nature of the study, and it is conceivable that reduced hippocampal volumes or memory influenced exercise engagement or reports of lifetime stress. A recent randomized control trial observed that an aerobic exercise intervention was associated with increased hippocampal volume and improved memory (Erickson et al., 2011). Future longitudinal investigations of individual change in brain structure and function may incorporate individuals varying in stressful experiences to more directly assess interactions between stress and exercise.
Despite the limitations, the current findings of selective effects of stress on hippocampal volume and evidence that engagement in exercise may buffer against the detrimental effects of stress for the hippocampus and memory are suggestive that programmatic investigations of the influences of stress and exercise on cognitive and brain aging ares warranted. Finally, the current findings have implications for Alzheimer’s disease as hippocampal atrophy and memory decline are integral aspects of the disease (Barnes et al., 2009; Grober et al., 2008). Increasing evidence has suggested a link between stress and the development of Alzheimer’s disease (Csernansky et al., 2006; Li et al., 2010) with contrasting evidence suggesting physical exercise may have a beneficial effect on the disease (Honea et al., 2009; Liang et al., 2010; Rockwood & Middleton, 2007). Thus, examination of the interactions between stress and exercise in this population could provide important insights into approaches for prevention.
Acknowledgments
We thank Jennifer Travis and Marlisa Isom for assistance with this project. We thank the Clinical Core of the Washington University Alzheimer Disease Research Center for participant assessments and the Imaging Core for structural MRI data.
Funding This work was supported by the McDonnell Center for the Study of Higher Brain Function; and the National Institutes of Health (P50 AG05861, P01 AG03991 and P01 AG026276). Julie M. Bugg was supported by the National Institute of Aging (5T32AG00030).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
Financial Disclosures Dr. Head, Tara Singh and Dr. Julie M. Bugg reported no biomedical financial interests or potential conflicts of interest related to this work.
References
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy young and older adults. Handbook of Memory. In: Tulving E, Craik FIM, editors. Oxford University Press; 2000. pp. 395–410. [Google Scholar]
- Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, …Fox NC. A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiology of Aging. 2009;30:1711–1723. doi: 10.1016/j.neurobiolaging.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator distinction in social psychology research: Conceptual, strategic, and statistical consideration. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI II) Psychology Corporation; Texas: 1996. [Google Scholar]
- Bowles HR, FitzGerald SJ, Morrow JR, Jr., Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. American Journal of Epidemiology. 2004;160:279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.008. doi:10.1016/j.neurobiolaging.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB. Relationships among cortisol CRT, dehydroepiandrosterone-sulfate DHEAS, and memory in a longitudinal study of healthy elderly men and women. Neurobiology of Aging. 1999;20:315–324. doi: 10.1016/s0197-4580(99)00052-4. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Anstey KJ, Reglade-Meslin C, Sachdev PS. In vivo hippocampal measurement and memory: a comparison of manual tracing and automated segmentation in a large community-based sample. Public Libray of Science One. 2009;4:e5265. doi: 10.1371/journal.pone.0005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Dong H, Fagan AM, Wang L, Xiong C, Holtzman DM, Morris JC. Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. American Journal of Psychiatry. 2006;163:2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - II. The Psychological Corporation; New York: 2000. [Google Scholar]
- Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, …Fischl B. Alzheimer’s Disease Neuroimaging Initiative. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer’s disease. Brain. 2009;132:2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, …Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;311:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Diehl M, Coyle N, Labouvie-Vief G. Age and sex differences in strategies of coping and defense across the lifespan. Psychology and Aging. 1996;11:127–139. doi: 10.1037//0882-7974.11.1.127. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenal axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermen D. Manual for kit of factor referenced cognitive tests. Educational Testing Service; Princeton: 1976. [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, …Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Garnst C, Quinn BT, Pacheco J, Jernigan TL, Thal L, …Gollub RL. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5:235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, …Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. NeuroImage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, …Fabiani M. Neuroanatomical correlates of aging, cardiopulmonary fitness level and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus. 2009;19:988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction and intellectual decline in Alzheimer’s disease. Journal of the International Neuropsychological Society. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, …Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hertzog C. Theoretical approaches to the study of cognitive aging: An individual differences perspective. In: Hofer SM, Alwin DF, editors. Handbook of Cognitive Aging. Sage Publications; Thousand Oaks, CA: 2008. pp. 34–49. [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest. 2009;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer’s disease. Alzheimer Disease and Associated Disorders. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. Journal of Neuroscience. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Jackson J, Balota DA, Head D. Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.009. doi:10.1016/j.neurobiolaging.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, et al. MRI derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR, van Praag H. Running reduces stress and enhances cell genesis in aged mice. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.025. doi:10.1016/j.neurobiolaging.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara TS, Webber A, Gil-Mohapel J, Christie BR. Stress differentially regulates the effects of voluntary exercise on cell proliferation in the dentate gyrus of mice. Hippocampus. 2009;19:889–897. doi: 10.1002/hipo.20514. [DOI] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta analysis of structural brain abnormalities in PTSD. Neuroscience & Biobehavioral Reviews. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory concentration test of cognitive impairment. The American Journal of Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- Kim HG, Lim EY, Jung WR, Shin MK, Ann ES, Kim KL. Effects of treadmill exercise on hypoactivity of the hypothalamic-pituitary-adrenal axis induced by chronic administration of corticosterone in rats. Neuroscience Letters. 2008;434:46–40. doi: 10.1016/j.neulet.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Kira IA, Lewandowski L, Templin T, Ramaswamy V, Ozkan B, Mohanesh J. Measuring Lifetime Trauma Dose, Types, and Profiles Using a Development-Based Taxonomy of Traumas. Traumatology. 2008;14:62–87. [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamic-pituitary-adrenocortical function during acute and chronic stress. Annals of the New York Academy of Sciences. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw K, Davidson K, Toner H, Jackson G, Clark S, Law J, …Cross S. A randomised controlled trial of cognitive behavior theray vs treatment as usual in the treatment of mild to moderate late life depression. International Journal of Geriatric Psychiatry. 2008;23:843–850. doi: 10.1002/gps.1993. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Blalock EM, Chen K, Porter NM. A new glucocorticoid hypothesis of brain aging: implactions for Alzheimer’s disease. Current Alzheimer Research. 2007;4:205–212. doi: 10.2174/156720507780362083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Waymire JC, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978;202:1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. Springer Verlag; New York: 1984. [Google Scholar]
- Leasure JL, Decker L. Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus. 2009;19:907–912. doi: 10.1002/hipo.20563. [DOI] [PubMed] [Google Scholar]
- Li WZ, Li WP, Yao YY, Zhang W, Yin YY, Wu GC, Gong HL. Glucocorticoids increase impairments in learning and memory due to elevated amyloid precursor protein expression and neuronal apoptosis in 12-month old mice. European Journal of Pharmacology. 2010;6238:108–115. doi: 10.1016/j.ejphar.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, …Head D. Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Annals of Neurology. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. Journal of Affective Disorders. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, …Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. Journal of Neuroscience. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Blumenthal JA, Allen PA, Emery CF. Improving aerobic capacity in healthy older adults does not necessarily lead to improved cognitive performance. Psychology and Aging. 1989;4:307–320. doi: 10.1037//0882-7974.4.3.307. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Research. 1993;50:121–139. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- McCabe MP, Davison T, Mellor D, George K, Moore K, Ski C. Depression among older people with cognitive impairment: prevalence and detection. International Journal of Geriatric Psychiatry. 2006;21:633–644. doi: 10.1002/gps.1538. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Roddey JC, Hagler DJ, Jr, Holland D, Karow DS, …Dale AM. Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology. 2009;251:195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello PB, Benetti F, Cammarota M, Izquierdo I. Physical exercise can reverse the deficit in fear memory induced by maternal deprivation. Neurobiology of Learning and Memory. 2009;92:364–369. doi: 10.1016/j.nlm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology. 2008;4:33–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating CDR: current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, …Clark C, The Consortium to Establish a Registry for Alzheimer’s Disease CERAD. Part I Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Ohsawa I, Ohta S, Ohno M, Mikami T. Regular voluntary exercise cures stress-induced impairment of cognitive function and cell proliferation accompanied by increases in cerebral IGF-1 and GST activity in mice. Behavioural Brain Research. 2010;211:178–184. doi: 10.1016/j.bbr.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Neupert SD, Almeida DM, Mroczek DK, Spiro A. Daily stressors and memory failures in a naturalistic setting: findings from the VA Normative Aging Study. Psychology and Aging. 2006;21:424–429. doi: 10.1037/0882-7974.21.2.424. [DOI] [PubMed] [Google Scholar]
- Peavy GM, Lange KL, Salmon DP, Patterson TL, Goldman S, Gamst AC, …Galasko D. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biological Psychiatry. 2007;62:472–478. doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, …Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences. 2007;10413:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LH, Henry JD, Hosie JA, Milne AB. Effective regulation of the experience and expression of negative affect in old age. Journals of Gerontology. Series B. Psychological Sciences and Social Sciences. 2008;63B:P138–P145. doi: 10.1093/geronb/63.3.p138. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Research Reviews. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Middleton L. Physical activity and the maintenance of cognitive function. Alzheimer’s & Dementia. 2007;3:S38–44. doi: 10.1016/j.jalz.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress and their adverse neurological effects: relevance to aging. Experimental Gerontology. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sauro MD, Jorgensen RS, Pedlow CT. Stress, glucocorticoids, and memory: a meta-analytic review. Stress. 2003;6:235–245. doi: 10.1080/10253890310001616482. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. Journal of Clinical Endocrinology & Metabolism. 1997;82:2458–2465. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- Scheibe S, Carstensen LL. Emotional aging: recent findings and future trends. Journals of Gerontology. Series B. Psychological Sciences and Social Sciences. 2010;65B:135–144. doi: 10.1093/geronb/gbp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B. Archives of Neurology. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neuroscience. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2010;8:3–68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 50:569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- van Boxtel MP, Paas FG, Houx PH, Adam JJ, Teeken JC, Jolles J. Aerobic capacity and cognitive performance in a cross-sectional aging study. Medicine & Science in Sports & Exercise. 1997;29:1357–1365. doi: 10.1097/00005768-199710000-00013. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Medicine. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VonDras DD, Powless MR, Olson AK, Wheeler D, Snudden AL. Differential effects of everyday stress on the episodic memory test performances of young, mid-life, and older adults. Aging and Mental Health. 2005;9:60–70. doi: 10.1080/13607860412331323782. [DOI] [PubMed] [Google Scholar]
- von Hippel W, Vasey MW, Gonda T, Stern T. Executive function deficits, rumination and late-onset depressive symptoms in older adults. Cognitive Therapy and Research. 2008;32:474–487. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a Geriatric Depression Screening scale: A preliminary report. Journal of Psychiatry Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]