Abstract
The orexin / hypocretin system is involved in several addiction-related behaviors. The present experiments examined the involvement of orexin in heroin reinforcement and relapse by administering the orexin 1 receptor antagonist SB-334867 prior to heroin self-administration or prior to cue- or heroin-induced reinstatement of extinguished heroin seeking in male Sprague Dawley rats. SB-334867 (30 mg/kg, i.p.) reduced heroin intake during self-administration under fixed ratio-1 (FR-1) and progressive ratio (PR) schedules. SB-334867 also attenuated reinstatement of heroin seeking elicited by cues, but not reinstatement elicited by a heroin prime. These results indicate that orexin antagonism reduces heroin self-administration, and they support a role for orexin in cue-triggered drug relapse.
Keywords: addiction, relapse, opiates, rats, reinstatement
Introduction
The orexin, or hypocretin, system has been implicated in drug reinforcement and relapse (Aston-Jones et al., 2010; Martin-Fardon et al., 2010; Sharf et al., 2010c; Kenny, 2011), in addition to its role in promoting arousal (Siegel, 2004; Sakurai, 2007). Signaling at orexin 1 receptors (OX1Rs), in particular, is involved in a variety of addiction-related behaviors. In a cocaine self-administration paradigm in rats, the OX1R antagonist SB-334867 reduced drug seeking triggered by drug-associated cues, contexts, and stressors (Boutrel et al., 2005; Smith et al., 2009; Smith et al., 2010). SB-334867 also reduced cue-induced reinstatement of seeking for other rewards, such as ethanol and sucrose, indicating a universal role for orexin in cue-elicited motivation across reward types (Lawrence et al., 2006; Cason et al., 2010; Jupp et al., 2011b).
Despite a consistent involvement of orexin in reward-seeking driven by external stimuli, the role of OX1R signaling in self-administration (or drug intake) appears to vary across reward types. Under fixed ratio (FR) schedules of self-administration, SB-334867 reduced intake of ethanol and nicotine, but had no effect on cocaine self-administration (Lawrence et al., 2006; Hollander et al., 2008; Richards et al., 2008; Smith et al., 2009; Espana et al., 2010; LeSage et al., 2010; Jupp et al., 2011a). However, under progressive ratio (PR) schedules of self-administration, which require increasing amounts of effort to obtain successive rewards, SB-334867 reduced responding for ethanol, nicotine, and cocaine (Hollander et al., 2008; Borgland et al., 2009; Espana et al., 2010; Jupp et al., 2011a). The role of OX1R signaling in food self-administration varies under FR and PR schedules, and depends heavily on the type of food reward (Hollander et al., 2008; Nair et al., 2008; Richards et al., 2008; Borgland et al., 2009; Cason et al., 2010; Choi et al., 2010; Espana et al., 2010; LeSage et al., 2010; Sharf et al., 2010b; Jupp et al., 2011a). The involvement of orexin signaling in opiate self-administration and relapse has not been explored to date.
Previous studies investigating the relationship between orexin and opiates found that OX1R signaling is involved in the acquisition and expression of a conditioned place preference for morphine (Harris et al., 2005; Narita et al., 2006; Harris et al., 2007; Sharf et al., 2010a). Additionally, orexin has been implicated in the expression of morphine withdrawal symptoms (Georgescu et al., 2003; Sharf et al., 2008). Here, we sought to investigate the role of OX1R signaling in heroin self-administration behaviors by evaluating the effects of SB-334867 on drug intake under FR and PR schedules, as well as reinstatement of extinguished heroin seeking elicited by cues or a heroin prime. Our results support a common role for OX1R signaling in cue-induced reinstatement of drug seeking, and indicate that SB-334867 reduces heroin self-administration under FR-1 and PR conditions.
Materials and Methods
Animals
Male Sprague Dawley rats (n=39; initial weight 250-300 g; Charles River, Raleigh, NC, USA) were pair-housed in a temperature- and humidity-controlled facility at the Medical University of South Carolina accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats were housed under a reversed 12-hr light/dark cycle (lights off at 6 a.m.), with ad libitum food and water access. All experiments were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina and conducted according to specifications of the National Institutes of Health as outlined in the Guide for the Care and Use of Laboratory Animals.
Catheter implantation
Following acclimation to the animal facility, rats were anesthetized with ketamine/xylazine, given a non-steroidal anti-inflammatory analgesic (meloxicam, 1 mg/kg), and implanted with chronic indwelling intravenous catheters, as previously described (Smith et al., 2009). Beginning three days after surgery, catheters were flushed once daily with 0.1 ml each of the antibiotic cefazolin (100 mg/ml) and heparin (100 U/ml). Self-administration sessions began after one week of recovery from surgery.
Self-administration - fixed ratio (FR)
Operant chambers were housed in sound-attenuating cubicles and controlled via MED-PC IV (Med-Associates, St. Albans, VT, USA). During 2-hr daily sessions, presses on an active lever resulted in a heroin infusion (FR-1; 50 μl via motorized pump) paired with tone and light cues (78 dB, 2900 Hz; white stimulus light above the active lever), followed by a 20-sec timeout. Presses on an inactive lever had no consequence. Rats were first given 2 sessions of FR-1 self-administration at a higher dose of heroin (0.04 mg/infusion), and then given 10-12 sessions at a lower dose (0.02 mg/infusion; ≥ 8 infusions per session). To evaluate the role of orexin signaling in established FR-1 self-administration, a subset of animals received SB-334867 (30 mg/kg, i.p.) 30 min prior to the tenth session of heroin self-administration (0.02 mg heroin/infusion), followed by two additional sessions of self-administration. Following FR-1 self-administration, separate groups of animals then were moved to PR self-administration or extinction/reinstatement.
Self-administration - progressive ratio (PR)
Following FR-1 self-administration, a subset of animals was placed on a PR schedule of reinforcement, in which successive heroin infusions (0.02 mg each) were earned at the completion of steps that required increasing ratios of lever presses, according to the logarithmic function [5e(step number × 0.2)] - 5 (Richardson & Roberts, 1996). However, to reduce sedation or satiation from excessive heroin exposure, some of the earlier PR steps were omitted (steps 2, 3, 5, and 6), so that a modified step sequence was used (1, 4, 7, 8, 9, 10…), which resulted in the following lever press ratio requirements (1, 6, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603…). All rats completed step 7, at a minimum, during testing. PR breakpoint was taken as the last step completed prior to a lapse of one hour with no infusions earned or the last step completed in five hours, whichever occurred first. Once rats showed stable daily PR self-administration, the role of orexin in PR responding was evaluated during three test sessions, for which SB-334867 (10 or 30 mg/kg, i.p.) or vehicle was given 30 min prior. Rats were assigned test order in a counterbalanced design, and were given additional PR self-administration sessions between testing to ensure a return to baseline responding.
Reinstatement
Following FR-1 self-administration, a subset of rats was given daily extinction sessions, during which lever presses had no consequence (no drug or cues). Some of these rats previously received SB-334867 on the tenth session of FR-1 self-administration; these animals had an additional two days of self-administration after SB-334867 treatment before extinction sessions commenced. Prior to reinstatement testing, rats were required to meet an extinction criterion of ≥ 25 active lever presses for two consecutive days, with at least seven extinction sessions prior to the first reinstatement session and two extinction sessions prior to subsequent reinstatement sessions. For cue-induced reinstatement, active lever presses resulted in presentation of tone and light cues in the same manner as during self-administration. For heroin-induced reinstatement, rats were injected with heroin (0.25 mg/kg, s.c.) immediately prior to an extinction session. To evaluate the role of orexin in reinstatement, SB-334867 (10 or 30 mg/kg, i.p.) was administered 30 min prior to reinstatement sessions. Each animal was given only two reinstatement sessions of each type (two cue, followed by two heroin prime), for which they were pretreated with only one dose of SB-334867 for one session and vehicle for the other session for each type of reinstatement in a counterbalanced order.
Drugs
Heroin HCl (National Institute on Drug Abuse, Rockville, MD, USA) was dissolved in 0.9% sterile saline. SB-334867 (generously donated by National Institute on Drug Abuse or by Eli Lilly, Indianapolis, IN, USA) was suspended in 2% dimethylsulfoxide and 10% 2-hydroxypropyl-β-cyclodextrin in sterile water, and administered at a volume of 4 ml/kg (i.p.) 30 min prior to testing. SB-334867 has 50-fold selectivity for OX1R over OX2R and 100-fold selectivity over approximately 50 other molecular targets (Porter et al., 2001; Smart et al., 2001), and has a half-life of approximately four hours in vivo (Ishii et al., 2005).
Data analyses
FR-1 and PR self-administration data were analyzed via repeated-measures one-way ANOVAs (within-subject design) with Tukey-Kramer post-hoc analyses. Reinstatement data were analyzed via paired t tests between vehicle and SB-334867 sessions (within-subject design). Reinstatement with vehicle pretreatment was not different across groups, so data were pooled into single bars for graphs (Figure 3); however, data were analyzed separately for groups as within-subject comparisons. Time course data were analyzed via mixed-model two-way ANOVAs with Bonferroni posttests. One animal was excluded from analyses of SB-334867 on FR-1 self-administration because active and inactive lever pressing was more than 3 SDs different from the group mean. Animals were excluded from reinstatement analyses if they showed ≤10 active lever presses on both reinstatement sessions (vehicle and SB-334867 sessions) or fewer active lever presses on the vehicle reinstatement as compared to the extinction session; this resulted in the exclusion of four animals from cue-induced reinstatement experiments and eight animals from heroin-induced reinstatement experiments. Three additional animals were excluded from heroin-induced reinstatement experiments because they made >200 active lever presses during reinstatement and were more than 2.5 SDs different from the group mean.
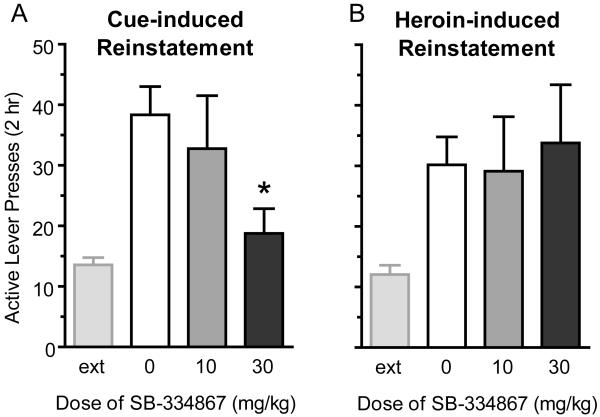
Figure 3.
Reinstatement elicited by cues, but not a heroin prime, is attenuated by the OX1R antagonist SB-334867 at 30 mg/kg. A) SB-334867 attenuated cue-induced reinstatement of extinguished heroin seeking at 30 mg/kg (n=13; *p<0.05), but not 10 mg/kg, (n=13), as compared to vehicle. Extinction (ext) levels of responding prior to reinstatement are also shown. B) SB-334867 had no significant effect on heroin-induced reinstatement when administered at 10 mg/kg (n=8) or 30 mg/kg (n=9).
Results
For all rats combined (n=39 total), the means (± SEM) for the last two days of FR-1 self-administration were 19.6 (±1.4) and 20.8 (±1.5) infusions (~ 1 mg/kg per day), and 72.3 (±10.7) and 87.8 (±17.8) active lever presses. There were no significant differences between groups for the last two days of self-administration. For animals that received extinction/reinstatement following FR-1 self-administration (n=29 total), the mean (± SEM) active lever presses for Sessions 1 and 7 of extinction were 84.9 (±9.9) and 18.0 (±2.4), respectively. All groups showed significant reinstatement triggered by cues or a heroin prime, as compared to extinction (p<0.05 each).
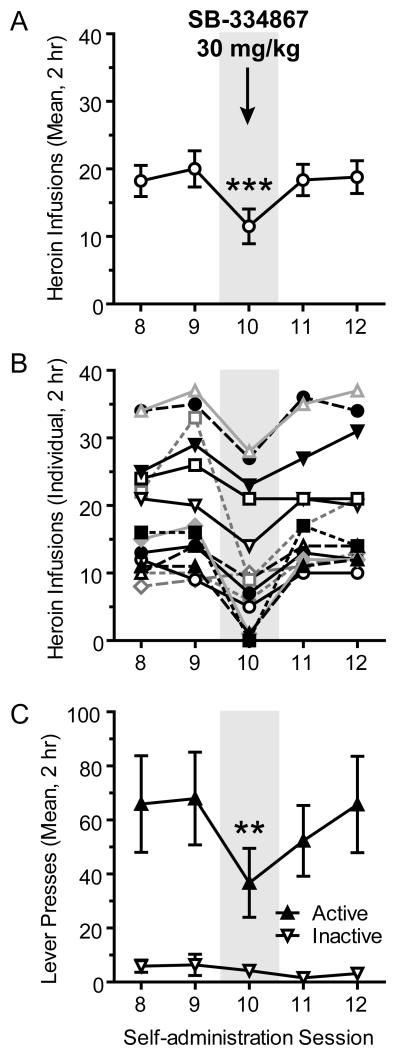
SB-334867 reduced heroin intake when administered prior to Session 10 of established FR-1 self-administration (n=14). SB-334867 (30 mg/kg) reduced the number of heroin infusions earned on Session 10, as compared to the two days before and two days after (F4,69=18.80; p<0.0001; Figure 1). Post-hoc analyses revealed that the number of infusions earned on Session 10 was different from all other days (p’s<0.001). SB-334867 also reduced active lever pressing (F4,69=5.37; p=0.0011), and post-hoc analyses revealed a difference on Session 10 as compared to Sessions 8, 9, and 12 (p<0.01 for each), but not Session 11. There was no effect on inactive lever pressing (F4,69=1.05; p=0.39).
Figure 1.
Heroin self-administration under a fixed ratio-1 (FR-1; 20-sec timeout) schedule of reinforcement is reduced by the OX1R antagonist SB-334867 at 30 mg/kg. A) When administered prior to the tenth session of self-administration, SB-334867 reduced the mean number of heroin infusions earned (0.02 mg/inf), as compared to the sessions before and after (n=14; ***p<0.001). B) Heroin intake for individual animals shows a consistent effect of SB-334867 among all subjects. C) SB-334867 also reduced active lever presses on Session 10 of self-administration, as compared to Sessions 8, 9, and 12 (**p<0.01), but had no effect on inactive lever presses.
SB-334867 also reduced heroin intake under a PR schedule of self-administration (n=10). When administered prior to PR self-administration, SB-334867 reduced the breakpoint, represented by the highest step completed (F2,27=3.85; p=0.041), and post-hoc analyses revealed a significant effect at the 30 mg/kg dose as compared to vehicle (p<0.05; Figure 2). Administration of vehicle alone had no effect on PR breakpoint, as compared to the PR session on the day before when no injection was given (t9=0.71; p=0.50; not shown). SB-334867 (30 mg/kg) also reduced the total number of infusions earned (F2,27=3.85; p=0.041; p<0.05); the number of infusions earned was different from the highest step completed due to the fact that some of the earlier steps were omitted by design (see Methods). There was no effect of SB-334867 on the time of the last infusion (F2,27=0.72; p=0.50), indicating that animals worked for a similar amount of time but earned fewer infusions overall. This parallels the slower rate of intake observed for FR-1 sessions following SB-334867 administration.
Figure 2.
Heroin self-administration under a progressive ratio (PR) schedule of reinforcement is reduced by the OX1R antagonist SB-334867 at 30 mg/kg, but not 10 mg/kg. A) SB-334867 reduced the breakpoint (or last step completed) for heroin PR self-administration (0.02 mg/inf) as compared to vehicle (n=10; *p<0.05). To reduce total heroin exposure, some early PR steps were omitted, so that a modified step sequence was used (1, 4, 7, 8, 9, 10…, as shown on left y-axis). Corresponding ratios for each step are shown on the right y-axis. B) SB-334867 did not significantly affect the time of the last infusion (when the breakpoint occurred), indicating a slower rate of intake overall.
When animals were tested for reinstatement of heroin seeking following extinction (n=29 total), we found that SB-334867 reduced reinstatement elicited by cues, but not that elicited by a heroin prime. For all reinstatement testing, each animal received only one dose of SB-334867 pretreatment, and this reinstatement session was compared to vehicle pretreatment in a within-subject design. Cue-induced reinstatement was attenuated by SB-334867 at 30 mg/kg (t12=2.96; p=0.01, significant after Bonferroni correction) but not 10 mg/kg (t12=0.06; p=0.95), as compared to vehicle (Figure 3). Due to significant results of 30 mg/kg on cue-induced reinstatement, we conducted additional analyses using a one-way repeated-measures ANOVA that compared the reinstatement sessions to extinction (F2,38=11.19; p<0.001); post-hoc analyses showed no significant difference between extinction and the reinstatement session with SB-334867 pretreatment, but did show a difference between vehicle and SB-334867 (p<0.01). In contrast to cue-induced reinstatement, SB-334867 had no effect on heroin-induced reinstatement at 10 mg/kg (t7=0.27; p=0.79) or 30 mg/kg (t8=0.51; p=0.63), as compared to vehicle. There were no significant effects of SB-334867 on inactive lever pressing.
Examination of the time courses for heroin self-administration and reinstatement (Figure 4) reveals that SB-334867 (30 mg/kg) reduced heroin intake consistently throughout the FR self-administration session, as compared to the control session (F1,52=43.61; p<0.0001). Post-hoc analyses revealed significant effects at all time points for FR (p<0.05). For PR self-administration (F1,45=10.31; p=0.002), post-hoc analyses showed significant differences between vehicle and SB-334867 at 60 and 120 min (p<0.05), when most of the infusions were earned. In effect, SB-334867 increased the time between heroin infusions (i.e., the inter-infusion interval) under both FR and PR schedules of reinforcement. Although analysis of cue-induced reinstatement found a significant difference between vehicle and SB-334867 sessions (F1,48=12.53; p=0.0009), post-hoc analyses did not reveal significant differences for the individual time points. Finally, analysis of heroin-induced reinstatement found no effect of SB-334867. In all time course analyses, there was a significant effect of time (p<0.05 for each).
Figure 4.
Time courses for the number of heroin infusions (A, B) or active lever presses (C, D) during A) fixed ratio-1 (FR-1) self-administration (n=14), B) progressive ratio (PR) self-administration (n=10), C) cue-induced reinstatement of extinguished heroin seeking (n=13), and D) heroin-induced reinstatement (n=9), following administration of SB-334867 (30 mg/kg) or vehicle. Significant overall effects are indicated by # (## p<0.01, ### p<0.001, n.s. = not significant), and significant post-hoc effects for individual time bins are indicated by * (*p<0.05, **p<0.01).
Discussion
We found that the OX1R antagonist SB-334867 reduced heroin self-administration under FR and PR schedules of reinforcement (Figures 1 and 2). SB-334867 decreased the number of heroin infusions consistently throughout the FR session, and at the beginning of the PR session when infusions were most frequent (Figure 4). We also found that SB-334867 (30 mg/kg) attenuated reinstatement of extinguished heroin seeking induced by cues, but had no significant effect on reinstatement elicited by a heroin prime (Figure 3). These results support a universal role for orexin signaling at OX1Rs in reward seeking elicited by conditioned cues, and indicate that pharmacotherapies aimed at OX1Rs might be beneficial for prevention of at least some types of heroin relapse.
Role of orexin in reinstatement
We found that orexin signaling at OX1Rs is necessary for cue-induced reinstatement of heroin seeking, which corroborates previous studies showing that orexin is involved in reward seeking elicited by conditioned stimuli. SB-334867 reduced cue- or context-induced reward seeking for cocaine, ethanol, and sucrose in the self-administration paradigm (Lawrence et al., 2006; Smith et al., 2009; Cason et al., 2010; Smith et al., 2010; Jupp et al., 2011b). Additionally, SB-334867 reduced preference for contextual cues associated with morphine or cocaine in the conditioned place preference paradigm (Harris et al., 2005; Sartor & Aston-Jones, 2010). This corresponds with observations that orexin neurons were Fos-activated after exposure to drug- and food-associated environments (Harris et al., 2005; Harris & Aston-Jones, 2006; Dayas et al., 2008). Importantly, orexin appears to be involved specifically in reward seeking triggered by external stimuli, and not in basic sensory processing of these stimuli, as indicated by the fact that SB-334867 did not affect learning about Pavlovian cues associated with cocaine or sucrose (Borgland et al., 2009; Smith et al., 2009).
In contrast to the cue-induced reinstatement findings, SB-334867 did not reduce reinstatement of heroin seeking elicited by a heroin prime, which mimics our results for cocaine seeking after a cocaine prime (Mahler et al., submitted). This lack of effect indicates that reductions in heroin self-administration or cue-induced reinstatement of heroin seeking caused by SB-334867 cannot be explained by general impairments in arousal or operant behavior, consistent with prior observations that SB-334867 is not sedating (Richards et al., 2008; Smith et al., 2009; Voorhees & Cunningham, 2011). Together with previous findings, these results show that orexin is involved specifically in drug seeking or relapse triggered by conditioned cues, but not by a drug prime.
Role of orexin in self-administration
We found that SB-334867 reduced heroin intake on FR and PR schedules of self-administration. One potential caveat to note is that a vehicle injection was not given prior to the control session for FR self-administration; however, given that we observed no effect of vehicle injection for PR self-administration, it is unlikely that it could account for the reductions in FR responding. Previous studies evaluating FR schedules of self-administration or home cage consumption found that SB-334867 reduced intake of nicotine and ethanol, but not cocaine (Lawrence et al., 2006; Hollander et al., 2008; Richards et al., 2008; Moorman & Aston-Jones, 2009; Smith et al., 2009; Espana et al., 2010; LeSage et al., 2010; Jupp et al., 2011a). However, studies using PR schedules showed that SB-334867 reduced self-administration for nicotine, ethanol, and cocaine (Hollander et al., 2008; Borgland et al., 2009; Espana et al., 2010; Jupp et al., 2011a). Taken together with the current heroin results, these studies indicate that SB-334867 does not have equal effects across drug types in FR self-administration, but that it does cause a consistent reduction in PR self-administration for drugs of abuse (but not necessarily PR self-administration for food; see Hollander et al., 2008; Borgland et al., 2009; Choi et al., 2010; Espana et al., 2010; Sharf et al., 2010b; Jupp et al., 2011a).
It is tempting to speculate, therefore, that SB-334867 reduces PR self-administration for all drug types due to a singular effect on high-effort motivation. However, analysis of the time course for SB-334867 effects on heroin self-administration indicates that this is not the case (Figure 4). SB-334867 reduced PR self-administration primarily in the first two hours of the session, when effort is lowest and the majority of infusions are earned. SB-334867 did not decrease the average number of infusions earned later in the PR session, which is likely due to a floor effect in responding and/or the half-life of SB-334867 (4 hours). Importantly, this resulted in a reduced breakpoint with SB-334867 without a significant change in the time to reach breakpoint (i.e., when the last infusion was earned). This contrasts with cocaine studies, which showed that reduced breakpoint following SB-334867 was due to an effect on the high-effort responding required later in the session, and that SB-334867 did not affect PR responding at the beginning of the session (Borgland et al., 2009; Espana et al., 2010). This indicates that different mechanisms underlie the effects of SB-334867 on PR self-administration for cocaine and heroin.
The time course data indicates that SB-334867 effects on PR heroin self-administration cannot be explained by reduced high-effort motivation. Rather, the reductions to PR self-administration appear to parallel the effects of SB-334867 on FR-1 self-administration. During FR-1 self-administration, animals still exhibited loading behavior (more infusions at the beginning of the session, as compared to later in the session), but showed lower intake throughout the entire session as compared to the control FR session. In other words, SB-334867 increased the time between heroin infusions (i.e., the inter-infusion interval) under both schedules of reinforcement, indicating that SB-334867 is acting via the same mechanism in both cases. Although FR and PR schedules are designed to measure different aspects of reinforcement, it is possible for both measures to be affected equally by a single factor.
For heroin, an increase in the inter-infusion interval following SB-334867 administration may indicate that the subjective effects of heroin have been altered. Numerous studies have investigated shifts in the inter-infusion interval, also known as the “trigger point,” “post-infusion pause,” or “satiety threshold,” and found, typically, that the response rate for a reward is inversely proportional to the unit dose (Pickens & Thompson, 1968; Gerber & Wise, 1989; Wise et al., 1995; Lynch et al., 1998; Tsibulsky & Norman, 1999; Panlilio et al., 2003). In other words, increasing the dose of a self-administered drug will result in a decreased response rate, or longer inter-infusion interval. This indicates that SB-334867 may increase the strength of the reinforcing or rewarding properties of each heroin infusion. The exact underlying mechanisms responsible for the change in heroin intake observed here may be difficult to pinpoint, however, because there are several factors that may contribute to such an effect (Lynch & Carroll, 2001).
Hypothesized role of orexin in addiction
Here, we found that SB-334867 reduced cue- but not heroin-induced reinstatement of heroin seeking. These results align with the hypothesis that orexin is involved in behaviors requiring glutamate-evoked increases in dopaminergic (DA) cell firing in ventral tegmental area (VTA) (Aston-Jones et al., 2009; Bonci & Borgland, 2009; Borgland et al., 2010). Glutamatergic signaling in VTA is likely necessary for cue-induced reinstatement of heroin seeking, as is the case for cue-induced reinstatement of cocaine seeking and context-induced reinstatement of heroin seeking (Bossert et al., 2004; Mahler et al., submitted). Recent data show that orexin signaling in VTA also is necessary for cue-induced reinstatement of cocaine seeking (James et al., 2011; Mahler et al., submitted). In contrast to a cocaine prime, where DA release occurs by actions directly at DA terminals, a heroin prime elicits DA release by activation of DA neural activity. This activation of DA neurons by heroin most likely stems from inhibition of GABA neurons leading to disinhibition, and not via increased VTA glutamate (Johnson & North, 1992); this may explain why orexin is not necessary for heroin-primed reinstatement. Notably, VTA and DA are necessary for heroin-induced reinstatement of drug seeking (Shaham & Stewart, 1996; LaLumiere & Kalivas, 2008; Rogers et al., 2008; See, 2009).
The current studies also found that SB-334867 reduced heroin self-administration. The exact role of DA in heroin reinforcement is complex and unresolved (see Xi & Stein, 2002b for review). However, given that the mechanism for DA cell activation by opiates is thought to be via GABA actions and not glutamate, we initially hypothesized that orexin antagonism would not affect heroin intake during self-administration. In fact, if orexin or glutamate is involved in heroin reinforcement, one might expect an increase in heroin intake (to compensate for the decreased effectiveness of heroin) when these transmitter systems are blocked (Xi & Stein, 2002a). Instead, we observed a decrease in heroin intake following SB-334867 administration. Therefore, the increased inter-infusion interval in our study after SB-334867 administration may be attributable to DA-independent mechanisms, such as a reduction in opiate withdrawal symptoms (Georgescu et al., 2003; Zhou et al., 2006) or slowed metabolism of heroin.
In conclusion, the current studies show that OX1R antagonism reduces opiate self-administration and reinstatement, indicating that the orexin system might be an important target for addiction pharmacotherapies. Our finding that SB-334867 reduced cue-induced reinstatement of heroin seeking supports a general role for orexin signaling in cue-triggered reward seeking.
Acknowledgments
This work was supported by National Institutes of Health grants R37-DA06214 and T32-DA007288, and conducted in an animal facility constructed with support from National Institutes of Health grant C06-RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
Abbreviations
- DA
dopamine
- FR
fixed ratio
- OX1R
orexin 1 receptor
- PR
progressive ratio
- VTA
ventral tegmental area
References
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Borgland S. Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacology. 2009;56(Suppl 1):107–111. doi: 10.1016/j.neuropharm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: Emerging players in addiction. Brain Res. 2010;1314:139–144. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber GJ, Wise RA. Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: a variable dose paradigm. Pharmacol Biochem Behav. 1989;32:527–531. doi: 10.1016/0091-3057(89)90192-5. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Jeffrey P, Summerfield S, Rodgers RJ. Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res. 2005;157:331–341. doi: 10.1016/j.bbr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14:684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 2011a;1391:54–59. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ. Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin receptors. Br J Pharmacol. 2011b;162:880–889. doi: 10.1111/j.1476-5381.2010.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ. Tobacco dependence, the insular cortex and the hypocretin connection. Pharmacol Biochem Behav. 2011;97:700–707. doi: 10.1016/j.pbb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, LaBounty LP, Carroll ME. A novel paradigm to investigate regulation of drug intake in rats self-administering cocaine or heroin intravenously. Exp Clin Psychopharmacol. 1998;6:22–31. doi: 10.1037//1064-1297.6.1.22. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. (submitted) Simultaneous orexin and glutamate neurotransmission in ventral tegmental area is required for cue-induced cocaine seeking [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacology (Berl) 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, Jewitt F, Jeffrey P, Austin N. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G. Lateral hypothalamic orexin/hypocretin afferents that are necessary for the expression of cocaine conditioned place preference; Neuroscience Meeting Planner Online, Program No. 67.5. Society for Neuroscience; San Diego, CA. 2010. [Google Scholar]
- See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology (Berl) 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Sharf R, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin mediates morphine place preference, but not morphine-induced hyperactivity or sensitization. Brain Res. 2010a;1317:24–32. doi: 10.1016/j.brainres.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010b;67:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Dileone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010c;1314:130–138. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC. SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Voorhees CM, Cunningham CL. Involvement of the orexin/hypocretin system in ethanol conditioned place preference. Psychopharmacology (Berl) 2011;214:805–818. doi: 10.1007/s00213-010-2082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Blockade of ionotropic glutamatergic transmission in the ventral tegmental area reduces heroin reinforcement in rat. Psychopharmacology (Berl) 2002a;164:144–150. doi: 10.1007/s00213-002-1190-3. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. GABAergic mechanisms of opiate reinforcement. Alcohol Alcohol. 2002b;37:485–494. doi: 10.1093/alcalc/37.5.485. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]