Abstract
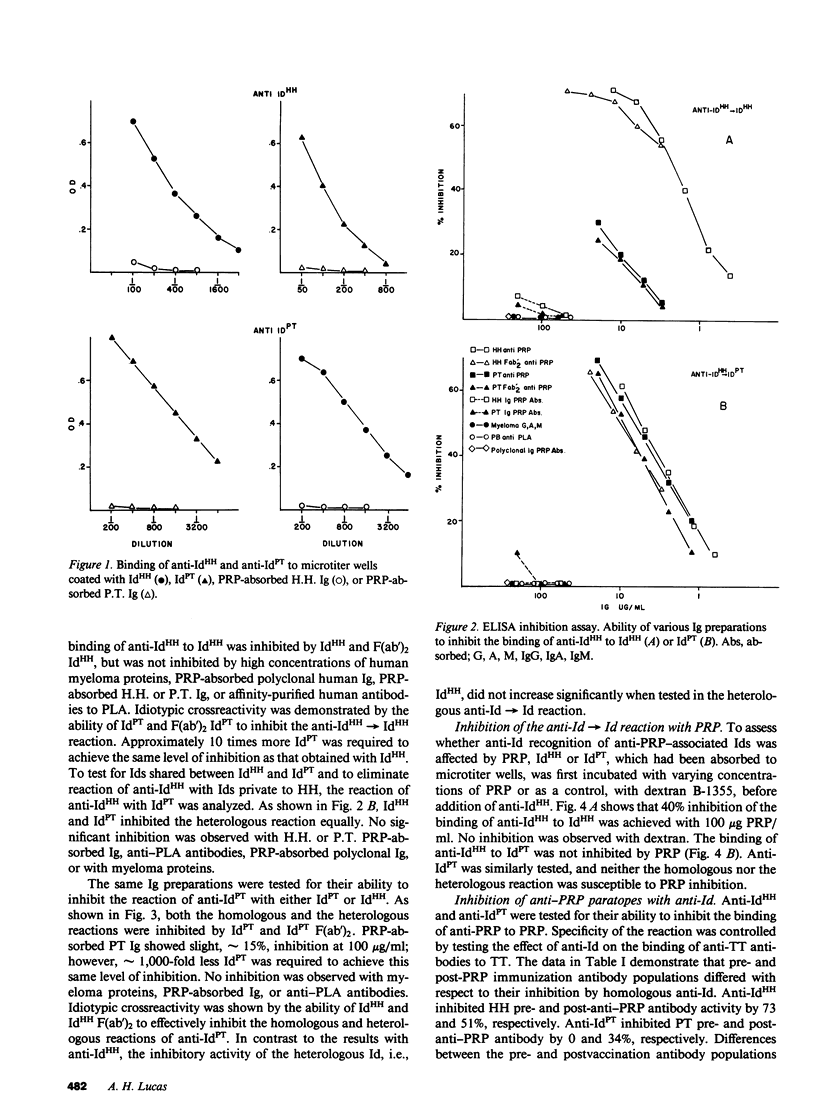
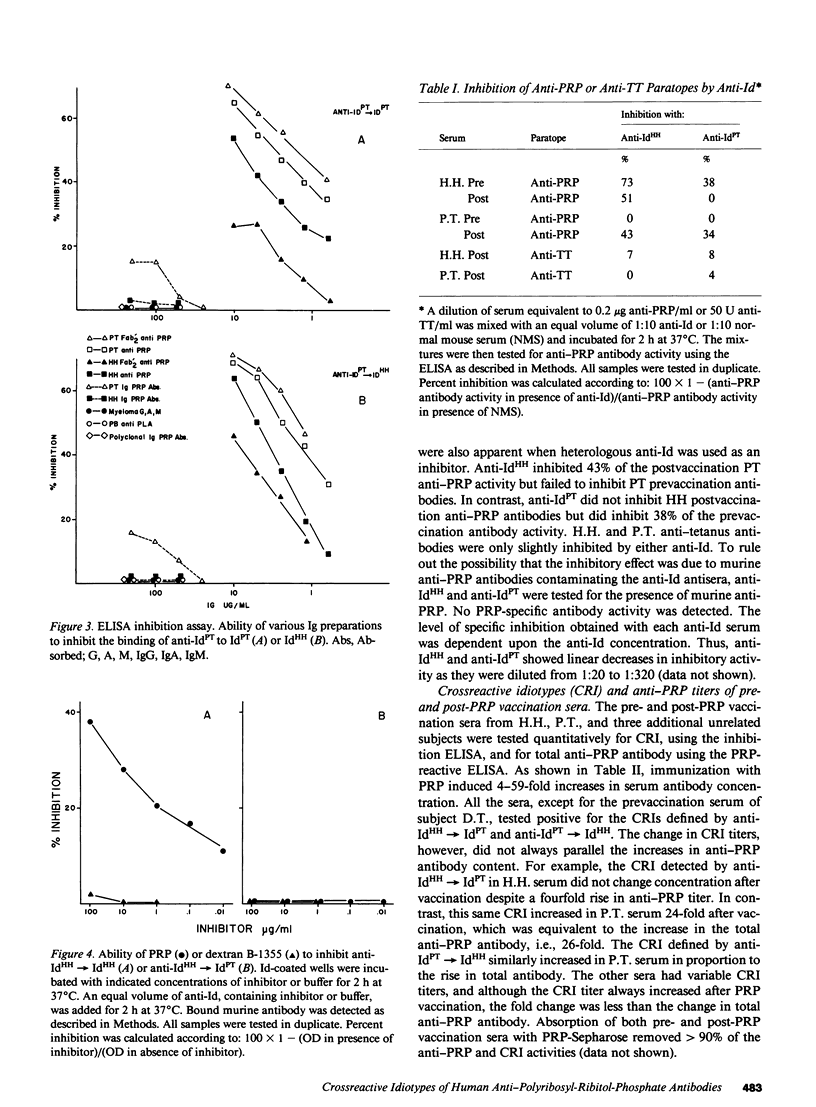
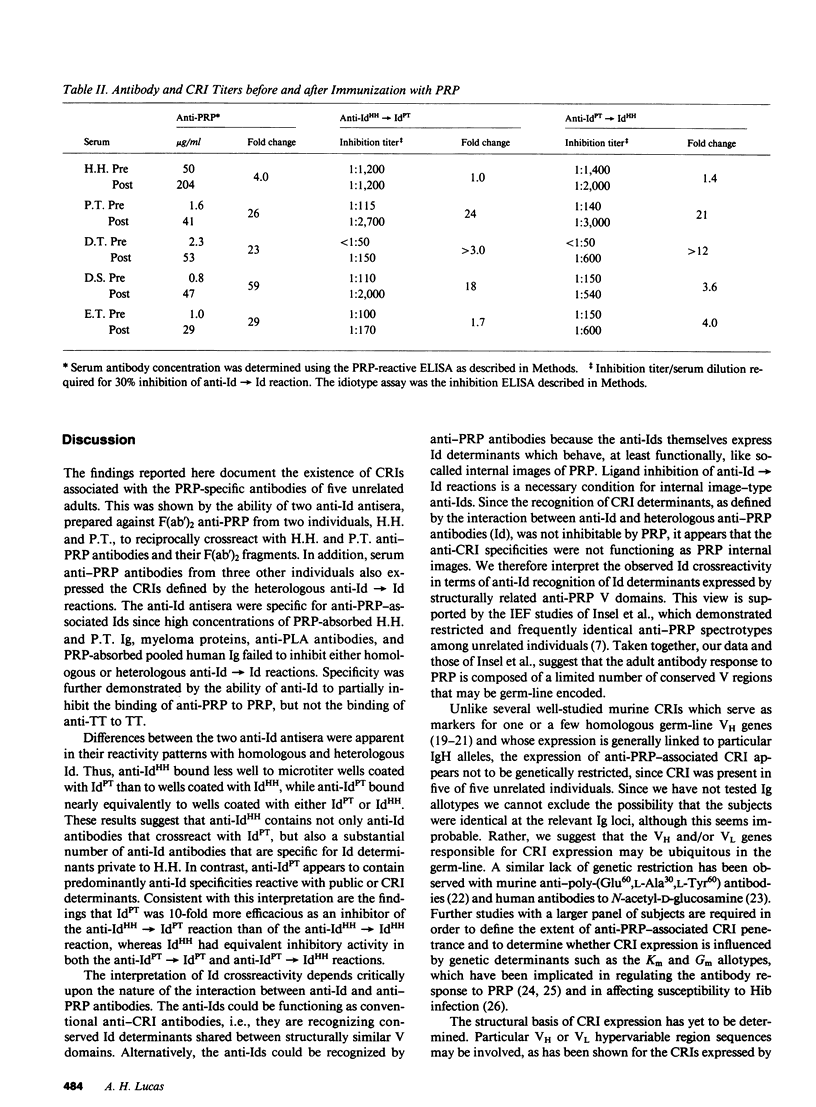
Human antibodies specific, for polyribosyl-ribitol-phosphate (PRP), the capsular polysaccharide of Hemophilus influenzae b, were studied using idiotypic analysis. Antisera were prepared against purified F(ab')2 anti-PRP from two unrelated adults, H.H. and P.T. After repeated absorption with IgG myeloma proteins and with PRP-absorbed normal human Ig and donor Ig, anti-idiotypic (anti-Id) sera were obtained that specifically reacted with anti-PRP antibodies. Anti-IdHH and anti-IdPT reciprocally crossreacted with H.H. and P.T. anti-PRP antibodies and F(ab')2 fragments, and also reacted with the serum anti-PRP antibodies from three additional adults unrelated to P.T. and H.H. Both anti-Id sera partially inhibited anti-PRP paratopes but not anti-tetanus toxoid paratopes. PRP did not inhibit anti-Id recognition of shared or crossreactive idiotypic (CRI) determinants. Naturally occurring and PRP immunization-induced anti-PRP antibodies expressed CRI. While CRI titer increased after immunization, the increase was usually less than the rise in total anti-PRP antibody. Quantitative differences in CRI expression were also apparent between natural and immunization-induced H.H. and P.T. anti-PRP antibodies as shown by their differential inhibitability by anti-Id. Our data demonstrate that anti-PRP antibodies from five unrelated adults express CRI determinants that are probably distant from the PRP combining site. Naturally occurring and immunization-induced anti-PRP antibodies share CRI and therefore appear to be clonally related, although immunization apparently induces the expression CRI-negative antibodies as well. These results, taken with previous studies showing restricted and identical anti-PRP isoelectric focusing spectrotypes in unrelated adults, suggest that some PRP-specific V domains are structurally conserved and probably germ-line encoded.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosino D. M., Barrus V. A., DeLange G. G., Siber G. R. Correlation of the Km(1) immunoglobulin allotype with anti-polysaccharide antibodies in Caucasian adults. J Clin Invest. 1986 Aug;78(2):361–365. doi: 10.1172/JCI112585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Peter G., Johnston R. B., Jr, Wetterlow L. H., Smith D. H. Immunization of humans with polyribophosphate, the capsular antigen of Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):39–44. doi: 10.1172/JCI106794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony B. F., Concepcion N. F., McGeary S. A., Ward J. I., Heiner D. C., Shapshak P., Insel R. A. Immunospecificity and quantitation of an enzyme-linked immunosorbent assay for group B streptococcal antibody. J Clin Microbiol. 1982 Aug;16(2):350–354. doi: 10.1128/jcm.16.2.350-354.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersch-Supan M. E., Agarwal S., White-Scharf M. E., Imanishi-Kari T. Heavy chain variable region. Multiple gene segments encode anti-4-(hydroxy-3-nitro-phenyl)acetyl idiotypic antibodies. J Exp Med. 1985 Jun 1;161(6):1272–1292. doi: 10.1084/jem.161.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. P., Fong S., Normansell D., Houghten R. A., Karras J. G., Vaughan J. H., Carson D. A. Delineation of a cross-reactive idiotype on human autoantibodies with antibody against a synthetic peptide. J Exp Med. 1984 May 1;159(5):1502–1511. doi: 10.1084/jem.159.5.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevinger B., Schilling J., Hood L., Davie J. M. Structural correlates of cross-reactive and individual idiotypic determinants on murine antibodies to alpha-(1 leads to 3) dextran. J Exp Med. 1980 May 1;151(5):1059–1070. doi: 10.1084/jem.151.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Greger B., Eichmann K. A cross-reacting human idiotype (B17) associated with antibodies to N-acetyl-D-glucosamine. Specificity, immunoglobulin class association, and distribution in the population. Eur J Immunol. 1983 Apr;13(4):273–278. doi: 10.1002/eji.1830130402. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Boies E., Squires J., Pandey J. P., Suarez B., Oldfather J., Rodey G. E. Interactive effect of genes associated with immunoglobulin allotypes and HLA specificities on susceptibility to Haemophilus influenzae disease. J Immunogenet. 1984 Jun-Aug;11(3-4):181–188. doi: 10.1111/j.1744-313x.1984.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Greenspan N. S., Davie J. M. Serologic and topographic characterization of idiotopes on murine monoclonal anti-streptococcal group A carbohydrate antibodies. J Immunol. 1985 Feb;134(2):1065–1072. [PubMed] [Google Scholar]
- Hiernaux J., Bona C., Baker P. J. Neonatal treatment with low doses of anti-idiotypic antibody leads to the expression of a silent clone. J Exp Med. 1981 Apr 1;153(4):1004–1008. doi: 10.1084/jem.153.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Anderson P. W. Oligosaccharide-protein conjugate vaccines induce and prime for oligoclonal IgG antibody responses to the Haemophilus influenzae b capsular polysaccharide in human infants. J Exp Med. 1986 Feb 1;163(2):262–269. doi: 10.1084/jem.163.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel R. A., Kittelberger A., Anderson P. Isoelectric focusing of human antibody to the Haemophilus influenzae b capsular polysaccharide: restricted and identical spectrotypes in adults. J Immunol. 1985 Oct;135(4):2810–2816. [PubMed] [Google Scholar]
- Ju S. T., Pierres M., Germain R. N., Benacerraf B., Dorf M. E. Idiotypic analysis of anti-GAT antibodies. VI. Identification and strain distribution of the GA-1 idiotype. J Immunol. 1979 Dec;123(6):2505–2510. [PubMed] [Google Scholar]
- Kennedy R. C., Melnick J. L., Dreesman G. R. Antibody to hepatitis B virus induced by injecting antibodies to the idiotype. Science. 1984 Mar 2;223(4639):930–931. doi: 10.1126/science.6198721. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Karanko V., Peltola H., Mäkelä P. H. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics. 1984 Nov;74(5):857–865. [PubMed] [Google Scholar]
- McNamara M. K., Ward R. E., Kohler H. Monoclonal idiotope vaccine against Streptococcus pneumoniae infection. Science. 1984 Dec 14;226(4680):1325–1326. doi: 10.1126/science.6505692. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Pandey J. P., Fudenberg H. H., Virella G., Kyong C. U., Loadholt C. B., Galbraith R. M., Gotschlich E. C., Parke J. C., Jr Association between immunoglobulin allotypes and immune responses to Haemophilus influenzae and Meningococcus polysaccharides. Lancet. 1979 Jan 27;1(8109):190–192. doi: 10.1016/s0140-6736(79)90584-1. [DOI] [PubMed] [Google Scholar]
- Reth M., Imanishi-Kari T., Rajewsky K. Analysis of the repertoire of anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti-idiotope antibodies. Eur J Immunol. 1979 Dec;9(12):1004–1013. doi: 10.1002/eji.1830091216. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Parke J. C., Jr, Schneerson R., Whisnant J. K. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res. 1973 Mar;7(3):103–110. doi: 10.1203/00006450-197303000-00001. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Tang F. L., Lucas A. H., Spiegelberg H. L., Tan E. M. IgG subclasses of anti-tetanus toxoid antibodies in adult and newborn normal subjects and in patients with systemic lupus erythematosus, Sjogren's syndrome, and drug-induced autoimmunity. J Immunol. 1986 Oct 15;137(8):2522–2527. [PubMed] [Google Scholar]
- Sacks D. L., Esser K. M., Sher A. Immunization of mice against African trypanosomiasis using anti-idiotypic antibodies. J Exp Med. 1982 Apr 1;155(4):1108–1119. doi: 10.1084/jem.155.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Gaulton G. N., McDade K. K., Fields B. N., Greene M. I. Syngeneic monoclonal antiidiotype can induce cellular immunity to reovirus. J Exp Med. 1984 Oct 1;160(4):1195–1205. doi: 10.1084/jem.160.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Huang S. Y., Gefter M. L. The genetic basis of antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983 Feb;13(2):123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- Stein K. E., Söderström T. Neonatal administration of idiotype or antiidiotype primes for protection against Escherichia coli K13 infection in mice. J Exp Med. 1984 Oct 1;160(4):1001–1011. doi: 10.1084/jem.160.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uytdehaag F. G., Osterhaus A. D. Induction of neutralizing antibody in mice against poliovirus type II with monoclonal anti-idiotypic antibody. J Immunol. 1985 Feb;134(2):1225–1229. [PubMed] [Google Scholar]



