Introduction
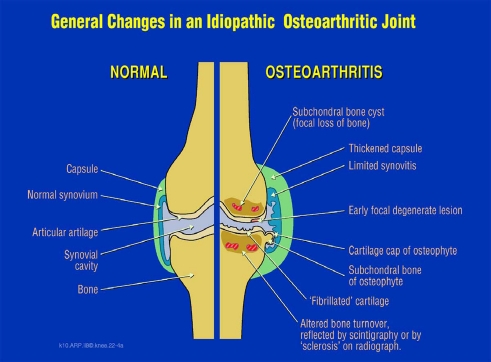
Osteoarthritis (OA) is a disease that causes pain and disability. It is usually slowly progressive, and cartilage degeneration with a loss of joint space (a common measure of progression) is often not continuous, as evidenced by numerous clinical trials and longitudinal studies. The emphasis of research and clinical trials has been on the degeneration of articular cartilage, being required with synovial fluid for the almost frictionless articulation and a key determinant, with the meniscus, of knee joint space on radiographic analysis. However, emerging evidence exists that all or almost all joint tissues are involved and/or affected by this degenerative process (Fig. 1).
Fig. 1.
Osteoarthritis is manifested by changes in all the tissues in the joint. Reprinted with permission from A.R. Poole, F. Guilak, S.B. Abramson, Etiopathogenesis of osteoarthritis in Osteoarthritis, Diagnosis and Medical management, 4th. edition, R.W. Moscowitz, R.D. Altman, M.C. Hochberg, J.A. Buckwalter, V.M.Goldberg, eds., Wolters Kluwer/Lippincott Williams and Wilkins, Philadelphia, pp. 27–49, 2007
OA Joint Pathology
Cartilage degeneration (see the article by Mary Goldring in this Symposium) involves a change in the chondrocyte phenotype called chondrocyte hypertrophy [14, 15], which in pathological situations can be induced by altered mechanical loading of chondrocytes [19]. This is normally seen in endochondral ossification in growth plates, fracture callous, and osteophyte formation, the latter being a feature of OA joint pathology. This hypertrophic state is characterized by abnormal cell behavior with increased resorption of collagen and proteoglycan in extracellular matrix, culminating in cell death [14, 15]. This change is associated in OA cartilage with onset of canonical Wnt signaling that is normally involved in cartilage and bone development [1, 12, 21]. Based on analytical information, such signaling changes may also partly explain the accompanying synovitis [1]. In view of the fact that the canonical Wnt signaling pathway is essential for normal osteoblast and chondrocyte maturation and SNPs of genes involved in this pathway exhibit associations with both OA and osteoporosis [12], abnormalities in this signaling pathway that are changed in cartilage, synovium, and bone in OA may help explain the pathology seen not only in articular cartilage but also in the synovium and in subchondral bone.
In OA, subchondral bone is characterized by changes that include bone “bruises” and cyst formation (seen with MRI [10, 11]) and increased bone turnover (seen by scintigraphy [3, 13]). These are all indicative of abnormal bone remodeling. They are also observed in conjunction with meniscal derangement [11]. These subchondral bone changes may result from a common denominator such as the canonical Wnt pathway or some other common pathway, the activation and/or regulation of which is changed in OA. Thus, it is important to think more of common denominators when considering pathology in different joint tissues, rather than considering each tissue alone as having independent and distinct pathology without a common cause. Changes in subchondral bone are thought by many to precede degenerative changes in articular cartilage. However, recent studies using MRI have come to the opposite conclusion [10]. Changes in menisci (see the article by David Hunter in this Symposium), seen with MRI, are common in older persons [5] and may precede those in articular cartilage as well as being closely associated with early subchondral bone changes [11] and synovial effusions [16] in OA.
Ligaments have received little attention in OA. However, histological examinations of macroscopically intact anterior cruciate ligaments at knee arthroplasty [4] and MRI analyses of collateral finger ligaments of patients with hand OA [17, 18] revealed common degenerative changes. The insertion sites into bone (entheses) of collateral ligaments also showed evidence of pathology as well as in the adjacent bone [17].
Synovitis, often of an innate immune variety probably caused by joint debris and calcium phosphate crystals that accumulate in OA in synovial fluid [18], is a well defined feature of knee OA (see the article by Carla Scanzello in this Symposium), being more pronounced in about 10% of the population. A mild synovitis is seen in a majority of patients. This may hasten joint damage through generation of proinflammatory cytokines and account, in part, for joint pain. Joint effusions normally associated with pronounced synovitis are also associated with meniscal damage [16].
Joint pain, which is the biggest problem for most patients with OA (see the article by Bruce Kidd in this Symposium) may also result from changes in nociception within subchondral bone and entheses as a consequence of altered loading within the joint. It is also associated with structural changes and synovitis [7]. The structural alterations may also involve articular cartilage. Although they are aneural, articular cartilage may cause indirect stimulation of sensory neurons and neuropathic pain based on evidence from recent biomarker analyses [8].
The infrapatellar fat pad of the knee is frequently a site of inflammation in OA with upregulation of chemokine expression [2, 9]. Whether this also contributes to knee pain and overall pathology is unclear.
Synovial fluid analyses provide an overall picture of joint degeneration in OA [6]. Marked increases occur in extracellular matrix components of cartilage and bone as well as of hyaluronan, (reflective of synovitis), proteinases, prostaglandins, cytokines and growth factors [8, 14, 15]. The presence of crystals of calcium phosphate is often associated with increased matrix calcification [20] as seen in chondrocyte hypertrophy. Proteonomic analyses of the multiple components are providing new insights into joint disease [6].
Summary
So, there can be little doubt that the whole joint is involved in OA, resulting in changes in function and proprioception and onset of altered nociception and pain that compound the problem. So, functional assessments of joint function should also be included in any measures of joint changes in OA. Many additional parameters might help in detecting and measuring disease onset and progression instead of relying on traditional measures of loss of and/or damage to articular cartilage. Most, if not all, joint tissues potentially offer important new opportunities as targets for therapeutic intervention.
Disclosures
The author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Blom AB, Brockbank SM, Lent PL, Beuningen HM, Guerts J, et al. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis. Prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60:501–12. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- 2.Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, Osch GJ, Offel JF, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage. 2010;18:876–82. doi: 10.1016/j.joca.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557–63. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas MJM, Hutchison JD, Sutherland AG. Anterior cruciate ligament integrity in osteoarthritis of the knee in patients undergoing total knee replacement. J Orthop Traumatol. 2010;11:149–154. doi: 10.1007/s10195-010-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med 11;359(11):1108–15, 2008 [DOI] [PMC free article] [PubMed]
- 6.Gobezie R, Kho A, Sarracino DA, Theornhill TS, Chase M, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther. 2007;9:R36. doi: 10.1186/ar2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishijima M, Watari T, Naito K, Kaneko H, Futami I et al. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Res Ther 14:13:R22, 2011 [DOI] [PMC free article] [PubMed]
- 9.Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, et al. Extended report: the infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851–7. doi: 10.1136/ard.2010.140046. [DOI] [PubMed] [Google Scholar]
- 10.Kothari A, Guermazi A, Chmiel JS, Dunlop D, Song J, et al. Within-subregion relationship between bone marrow lesions and subsequent cartilage loss in knee osteoarthritis. Arthritis Care Res. 2010;62:198–203. doi: 10.1002/acr.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo GH, Hunter DJ, Nevitt M, Lynch J, McAlindon TE, OAI Investigators Group Strong association of MRI meniscal derangement and bone marrow lesions in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009;17:743–7. doi: 10.1016/j.joca.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodewyckx L, Lories RJ. Wnt signaling in osteoarthritis and osteoporosis: what is the biological significance for the clinician. Curr Rheumatol Rep. 2009;11:23–30. doi: 10.1007/s11926-009-0004-6. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy C, Cushnaghan J, Dieppe P. The predictive role of scintigraphy in radiographic osteoarthritis of the hand. Osteoarthritis Cartilage. 1994;2:25–8. doi: 10.1016/S1063-4584(05)80003-2. [DOI] [PubMed] [Google Scholar]
- 14.Poole AR Cartilage in health and disease. In: Arthritis and Allied Conditions. A Textbook of Rheumatology, 15th Edition. Edited by W. Koopman, L.Moreland, Lippincott, Williams and Wilkins, Philadelphia, pp. 223–269, 2004.
- 15.Poole AR, Guilak F, Abramson SB. Etiopathogenesis of osteoarthritis. In:Osteoarthritis. Diagnosis and Medical/Surgical Management, 4th Edition. Edited by RW Moskowitz, RD Altman, MC Hochberg, JA Buckwalter, VM.Goldberg. Lippincott Williams & Wilkins, Philadelphia, pp.27-49, 2007.
- 16.Roemer FW, Guermazi A, Hunter DJ, Niu J, Zhang Y, Englund M, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage. 2008;17:748–53. doi: 10.1016/j.joca.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan AL, Grainger AJ, Tanner SF, Shelley DM, Pease C, et al. High-resolution magnetic resonance imaging for the assessment of hand osteoarthritis. Arthritis Rheum. 2005;52:2355–65. doi: 10.1002/art.21210. [DOI] [PubMed] [Google Scholar]
- 18.Tan AL, Toumi H, Benjamin M, Grainger AJ, Tanner SF, et al. Combined high-resolution imaging and histological examination to explore the role of ligaments and tendons in the phenotypic expression of early hand osteoarthritis. Ann Rheum Dis. 2006;65:1267–72. doi: 10.1136/ard.2005.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong M, Siegrist M, Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685–93. doi: 10.1016/S8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 20.Yavorskyy A, Hernandez-Santana A, McCarthy G, McMahon G. Detection of calcium phosphate crystals in the joint fluid of patients with osteoarthritis-analytical approaches and challenges. Analyst. 2008;133:302–18. doi: 10.1039/b716791a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu M, Tang D, Hao S, Chen M, Xie C, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Min Res. 2009;24:12–21. doi: 10.1359/jbmr.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]