Abstract
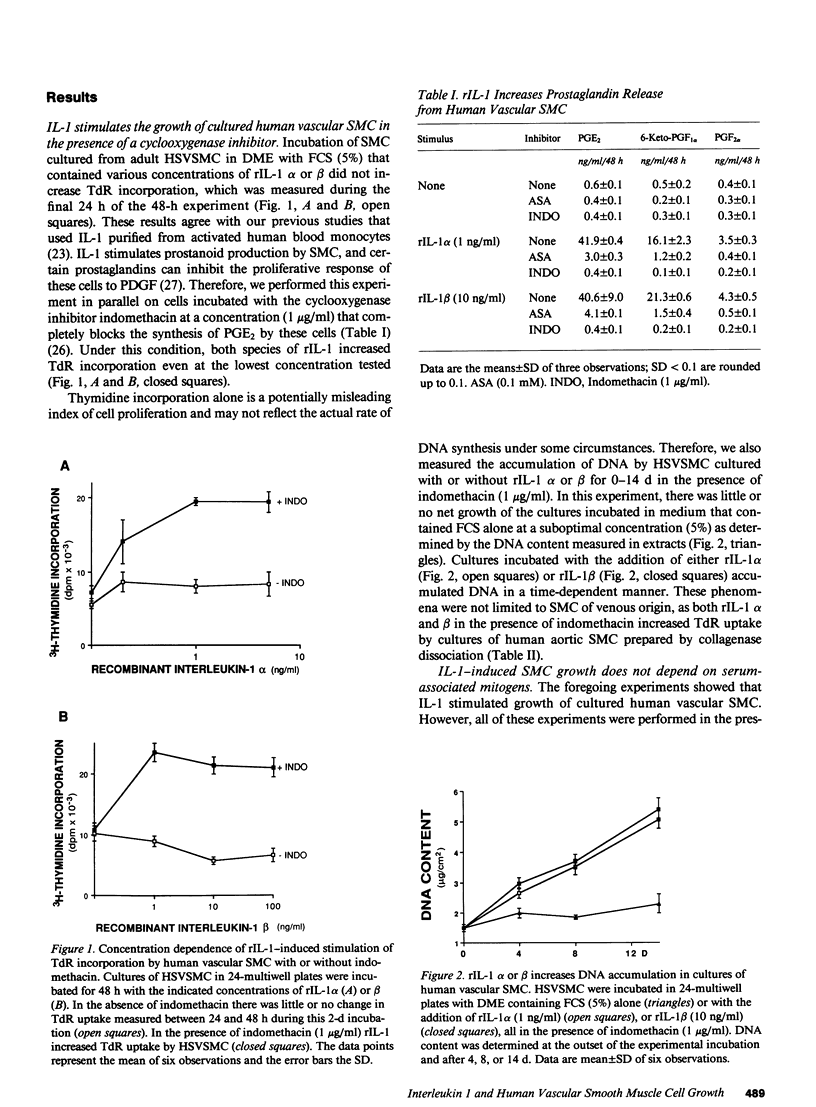
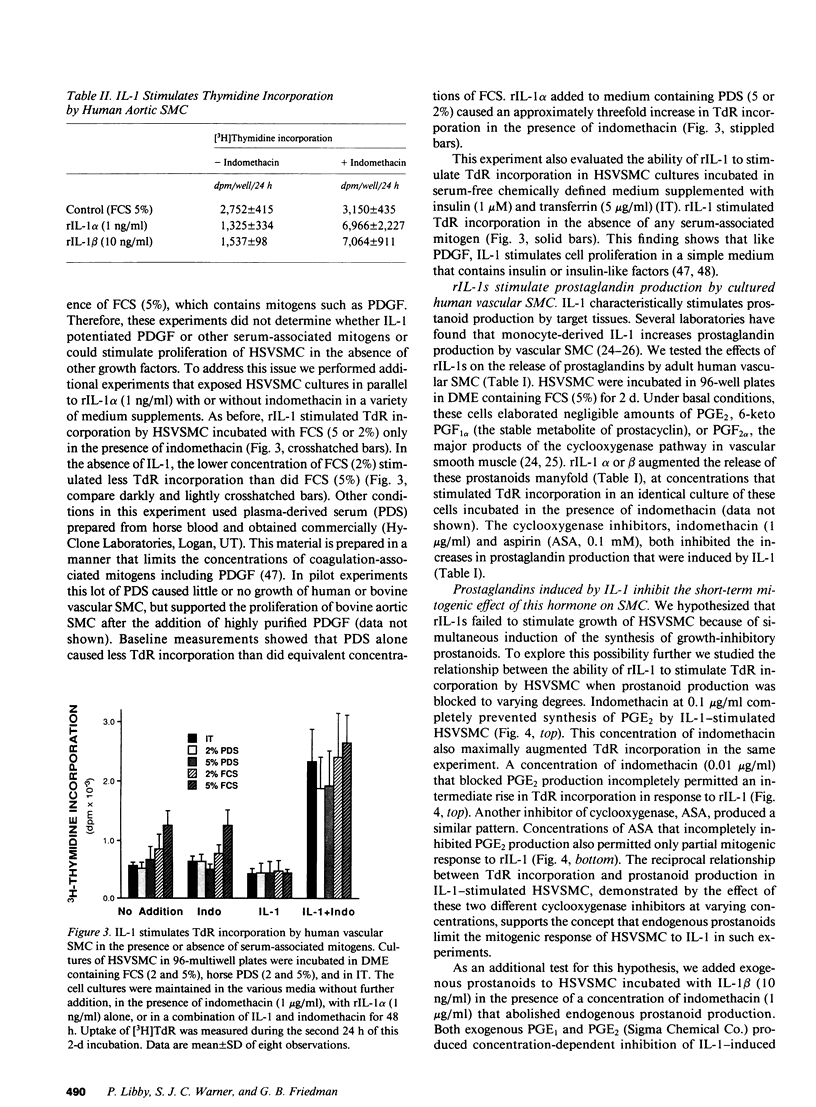
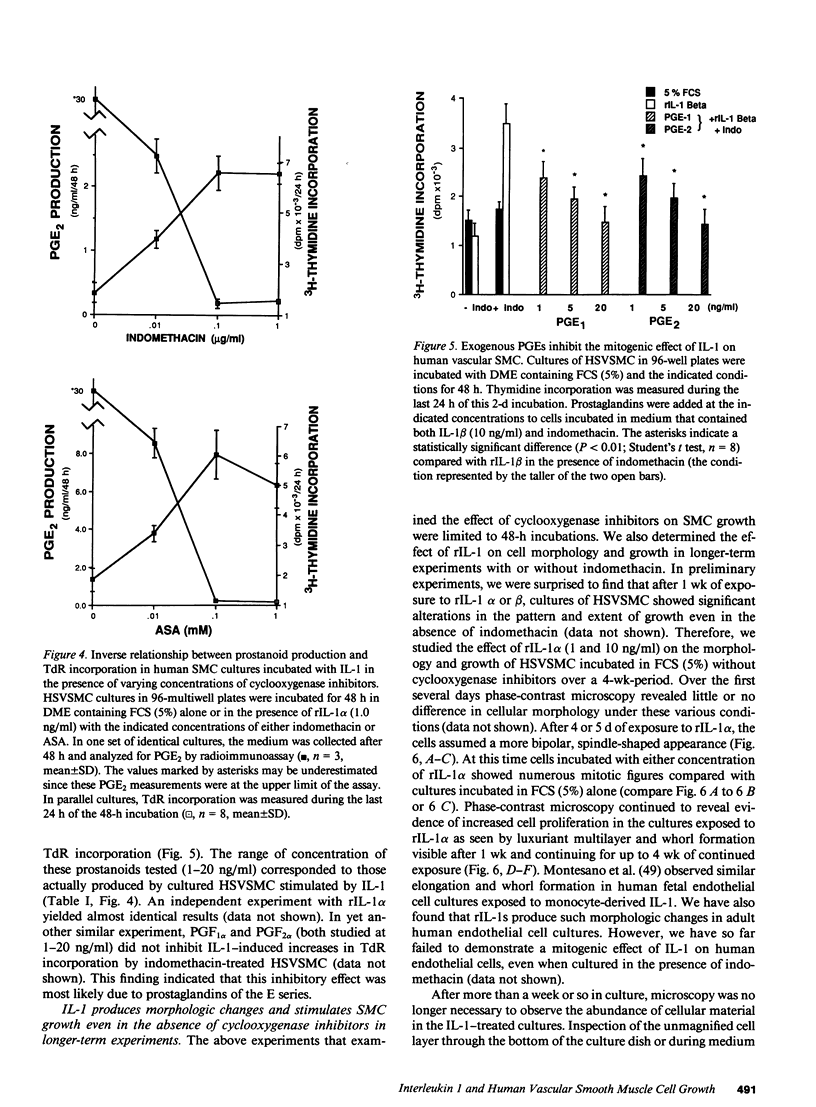
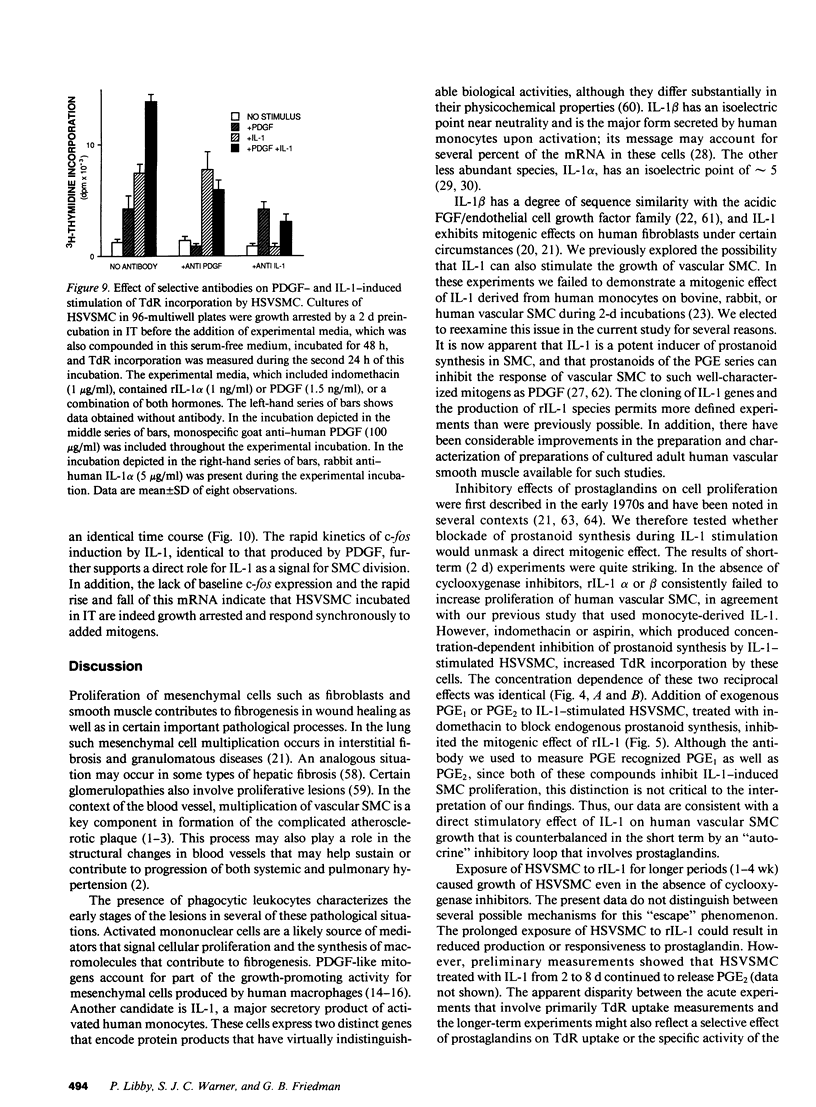
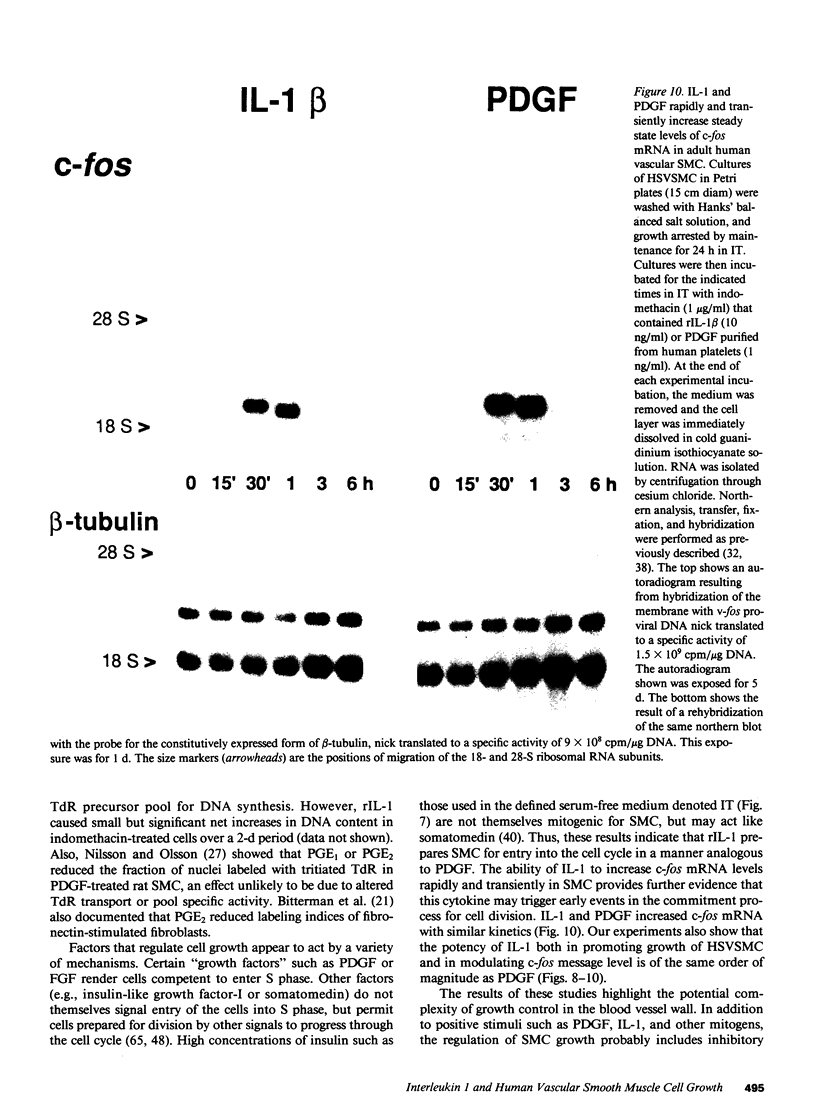
There is much interest in defining the signals that initiate abnormal proliferation of cells in a variety of states characterized by the presence of mononuclear phagocytes. Since IL-1 is a major secretory product of activated human monocytes we examined whether this cytokine can stimulate the growth of human vascular smooth muscle cells (SMC). Neither recombinant IL-1 (rIL-1) alpha (less than or equal to 5.0 ng/ml) nor beta (less than or equal to 100 ng/ml) stimulated SMC growth during 2-d incubations under usual conditions. IL-1 did stimulate SMC to produce prostanoids such as PGE1 or PGE2 that can inhibit SMC proliferation. When prostaglandin synthesis was inhibited by indomethacin or aspirin both rIL-1 alpha and beta (greater than or equal to 1 ng/ml) markedly increased SMC growth. In longer-term experiments (7-28 d) rIL-1 stimulated the growth of SMC even in the absence of cyclooxygenase inhibitors. The addition of exogenous PGE1 or PGE2 (but not PGF1 alpha, PGF2 alpha, PGI2) to indomethacin-treated SMC blocked their mitogenic response to rIL-1. Antibody to IL-1 (but not to platelet-derived growth factor [PDGF]) abolished the mitogenic response of SMC to rIL-1. Exposure of SMC to rIL-1 or PDGF caused rapid (maximal at 1 h) and transient (baseline by 3 h) expression of the c-fos proto-oncogene, determined by Northern analysis. We conclude that IL-1 is a potent mitogen for human SMC. Endogenous prostanoid production simultaneously induced by IL-1 appears to antagonize this growth-promoting effect in the short term (2 d) but not during more prolonged exposures. IL-1 produced by activated monocytes at sites of tissue inflammation or injury may thus mediate both positive and negative effects on SMC proliferation that are temporally distinct.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrightson C. R., Baenziger N. L., Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Wewers M. D., Rennard S. I., Adelberg S., Crystal R. G. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986 Mar;77(3):700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cotran R. S. Monocytes, proliferation, and glomerulonephritis. J Lab Clin Med. 1978 Dec;92(6):837–840. [PubMed] [Google Scholar]
- Coughlin S. R., Moskowitz M. A., Antoniades H. N., Levine L. Serotonin receptor-mediated stimulation of bovine smooth muscle cell prostacyclin synthesis and its modulation by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7134–7138. doi: 10.1073/pnas.78.11.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. An update on human interleukin-1: from molecular biology to clinical relevance. J Clin Immunol. 1985 Sep;5(5):287–297. doi: 10.1007/BF00918247. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Fowler S., Berberian P. A., Shio H., Goldfischer S., Wolinsky H. Characterization of cell populations isolated from aortas of rhesus monkeys with experimental atherosclerosis. Circ Res. 1980 Apr;46(4):520–530. doi: 10.1161/01.res.46.4.520. [DOI] [PubMed] [Google Scholar]
- Fowler S., Shio H., Haley N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab Invest. 1979 Oct;41(4):372–378. [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. M., Moskowitz M. A., Levine L. Inhibition of stimulated prostaglandin biosynthesis by retinoic acid in smooth muscle cells. Cancer Res. 1984 Jan;44(1):120–125. [PubMed] [Google Scholar]
- Gordon D., Schwartz S. M. Replication of arterial smooth muscle cells in hypertension and atherosclerosis. Am J Cardiol. 1987 Jan 23;59(2):44A–48A. doi: 10.1016/0002-9149(87)90175-5. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Chua A. O., Stern A. S., Hellmann C. P., Vitek M. P., DeChiara T. M., Benjamin W. R., Collier K. J., Dukovich M., Familletti P. C. Recombinant human interleukin 1 alpha: purification and biological characterization. J Immunol. 1986 Apr 1;136(7):2492–2497. [PubMed] [Google Scholar]
- Gunther S., Alexander R. W., Atkinson W. J., Gimbrone M. A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982 Feb;92(2):289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUST M. D., MORE R. H., MOVAT H. Z. The role of smooth muscle cells in the fibrogenesis of arteriosclerosis. Am J Pathol. 1960 Oct;37:377–389. [PMC free article] [PubMed] [Google Scholar]
- Huttner J. J., Gwebu E. T., Panganamala R. V., Milo G. E., Cornwell D. C., Sharma H. M., Geer J. C. Fatty acids and their prostaglandin derivatives: inhibitors of proliferation in aortic smooth muscle cells. Science. 1977 Jul 15;197(4300):289–291. doi: 10.1126/science.877555. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. Change in growth and morphology of fibroblasts by prostaglandins. J Natl Cancer Inst. 1971 Dec;47(6):1357–1364. [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Kindy M. S., Sonenshein G. E. Regulation of oncogene expression in cultured aortic smooth muscle cells. Post-transcriptional control of c-myc mRNA. J Biol Chem. 1986 Sep 25;261(27):12865–12868. [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Libby P., O'Brien K. V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J Cell Physiol. 1983 May;115(2):217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Wyler D. J., Janicka M. W., Dinarello C. A. Differential effects of human interleukin-1 on growth of human fibroblasts and vascular smooth muscle cells. Arteriosclerosis. 1985 Mar-Apr;5(2):186–191. doi: 10.1161/01.atv.5.2.186. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Sterzel R. B. Stimulation of rat mesangial cell proliferation by macrophage interleukin 1. J Immunol. 1983 Dec;131(6):2830–2836. [PubMed] [Google Scholar]
- Lovett D. H., Szamel M., Ryan J. L., Sterzel R. B., Gemsa D., Resch K. Interleukin 1 and the glomerular mesangium. I. Purification and characterization of a mesangial cell-derived autogrowth factor. J Immunol. 1986 May 15;136(10):3700–3705. [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Martin B. M., Gimbrone M. A., Jr, Unanue E. R., Cotran R. S. Stimulation of nonlymphoid mesenchymal cell proliferation by a macrophage-derived growth factor. J Immunol. 1981 Apr;126(4):1510–1515. [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L., Vassalli P. Human endothelial cell cultures: phenotypic modulation by leukocyte interleukins. J Cell Physiol. 1985 Mar;122(3):424–434. doi: 10.1002/jcp.1041220313. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Olsson A. G. Prostaglandin E1 inhibits DNA synthesis in arterial smooth muscle cells stimulated with platelet-derived growth factor. Atherosclerosis. 1984 Oct;53(1):77–82. doi: 10.1016/0021-9150(84)90107-2. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J. Antigen nonspecific lymphokines: an overview. Methods Enzymol. 1985;116:357–372. doi: 10.1016/s0076-6879(85)16028-3. [DOI] [PubMed] [Google Scholar]
- POOLE J. C., FLOREY H. W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J Pathol Bacteriol. 1958 Apr;75(2):245–251. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R., Nist C., Kariya B., Rivest M. J., Raines E., Callis J. Physiological quiescence in plasma-derived serum: influence of platelet-derived growth factor on cell growth in culture. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):497–508. doi: 10.1002/jcp.1040970325. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rossi V., Breviario F., Ghezzi P., Dejana E., Mantovani A. Prostacyclin synthesis induced in vascular cells by interleukin-1. Science. 1985 Jul 12;229(4709):174–176. doi: 10.1126/science.2409598. [DOI] [PubMed] [Google Scholar]
- Rupp E. A., Cameron P. M., Ranawat C. S., Schmidt J. A., Bayne E. K. Specific bioactivities of monocyte-derived interleukin 1 alpha and interleukin 1 beta are similar to each other on cultured murine thymocytes and on cultured human connective tissue cells. J Clin Invest. 1986 Sep;78(3):836–839. doi: 10.1172/JCI112649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Sejersen T., Betsholtz C., Sjölund M., Heldin C. H., Westermark B., Thyberg J. Rat skeletal myoblasts and arterial smooth muscle cells express the gene for the A chain but not the gene for the B chain (c-sis) of platelet-derived growth factor (PDGF) and produce a PDGF-like protein. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6844–6848. doi: 10.1073/pnas.83.18.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Giménez-Gallego G., DiSalvo J., Bennett C., Rodkey J., Fitzpatrick S. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6409–6413. doi: 10.1073/pnas.82.19.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A., Raines E., Kariya B., Rivest M. J., Ross R. Coordinate control of 3T3 cell proliferation by platelet-derived growth factor and plasma components. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2810–2814. doi: 10.1073/pnas.75.6.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. N., Bowen-Pope D. F., Ross R., Reidy M. A. Production of platelet-derived growth factor-like molecules by cultured arterial smooth muscle cells accompanies proliferation after arterial injury. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7311–7315. doi: 10.1073/pnas.83.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987 May 1;165(5):1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987 Sep 15;139(6):1911–1917. [PubMed] [Google Scholar]
- Werber H. I., Emancipator S. N., Tykocinski M. L., Sedor J. R. The interleukin 1 gene is expressed by rat glomerular mesangial cells and is augmented in immune complex glomerulonephritis. J Immunol. 1987 May 15;138(10):3207–3212. [PubMed] [Google Scholar]
- Wiley M. H., Feingold K. R., Grunfeld C., Quesney-Huneeus V., Wu J. M. Evidence for cAMP-independent inhibition of S-phase DNA synthesis by prostaglandins. J Biol Chem. 1983 Jan 10;258(1):491–496. [PubMed] [Google Scholar]
- Wyler D. J., Prakash S., Libby P. Mesenchymal target cell specificity of egg granuloma-derived fibroblast growth factor in schistosomiasis. J Infect Dis. 1987 Apr;155(4):728–736. doi: 10.1093/infdis/155.4.728. [DOI] [PubMed] [Google Scholar]
- van Straaten F., Müller R., Curran T., Van Beveren C., Verma I. M. Complete nucleotide sequence of a human c-onc gene: deduced amino acid sequence of the human c-fos protein. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3183–3187. doi: 10.1073/pnas.80.11.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]