Abstract
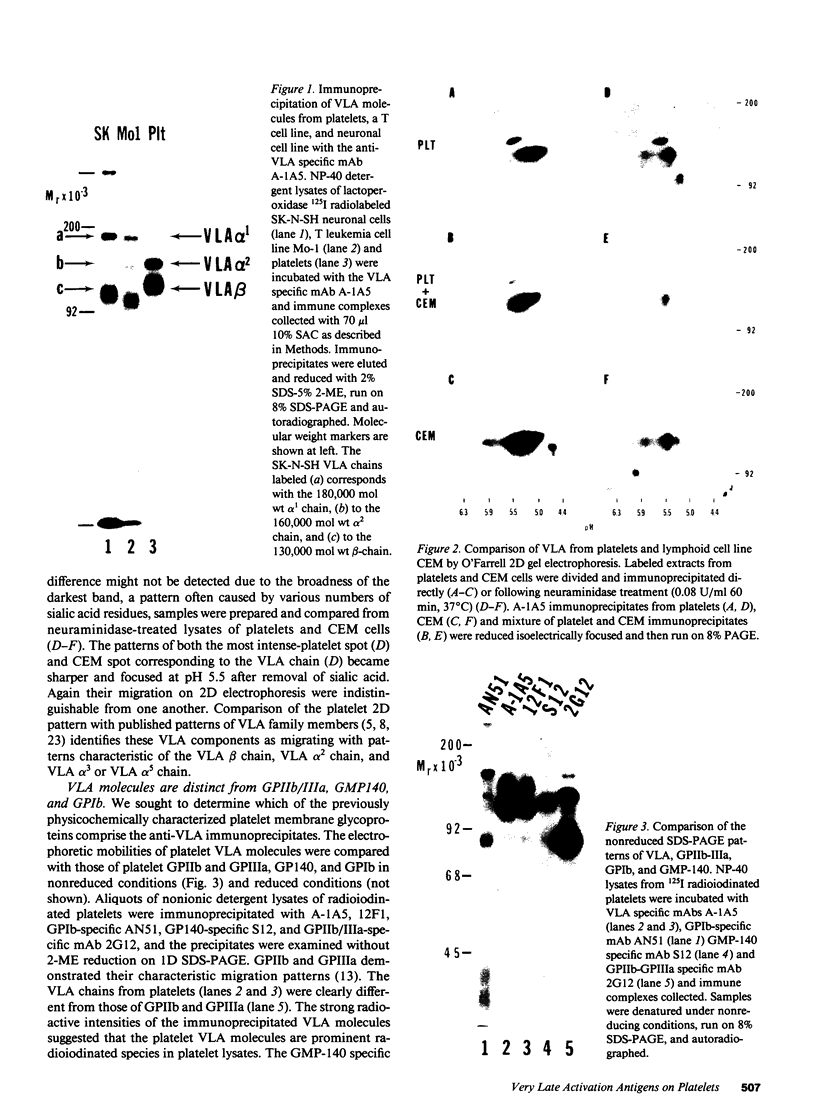
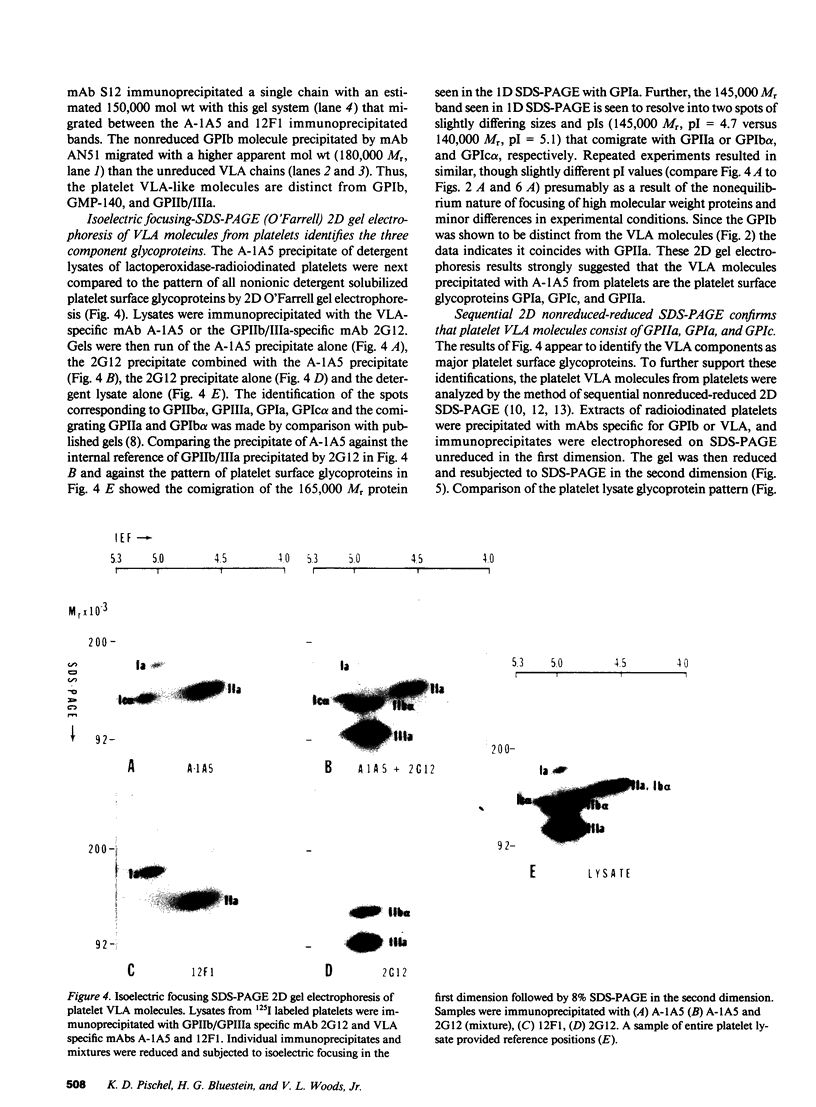
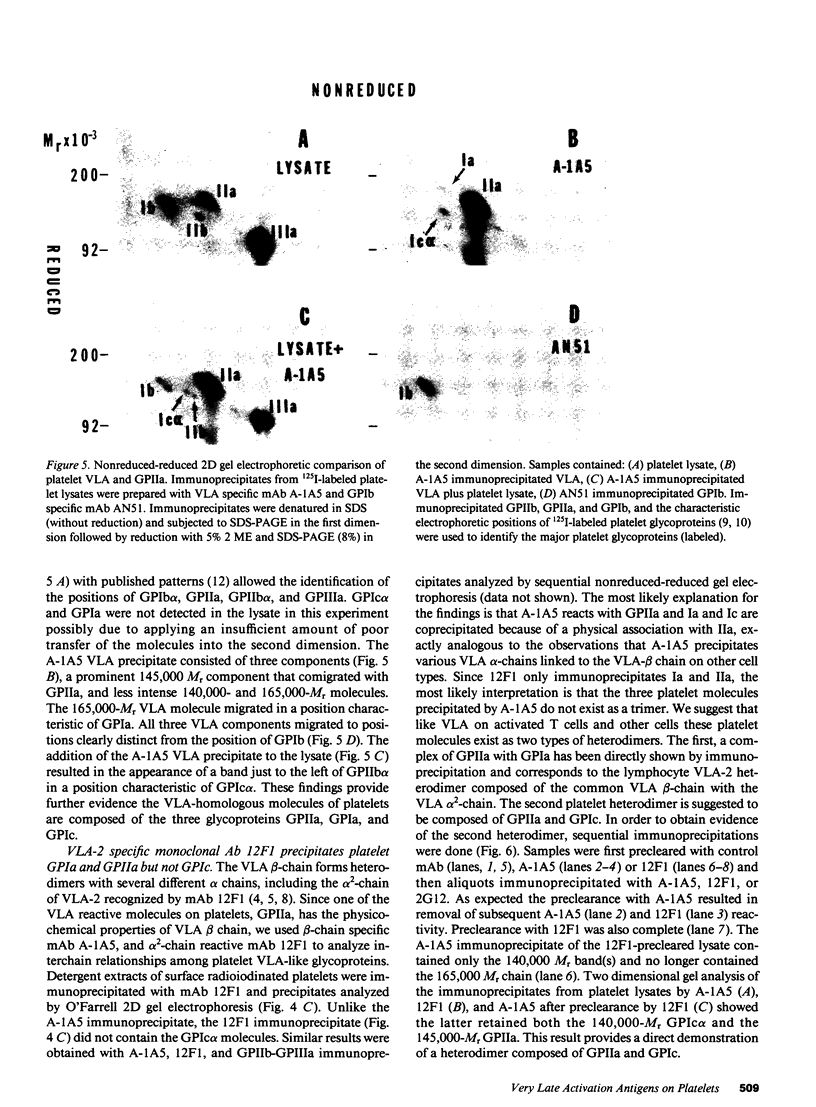
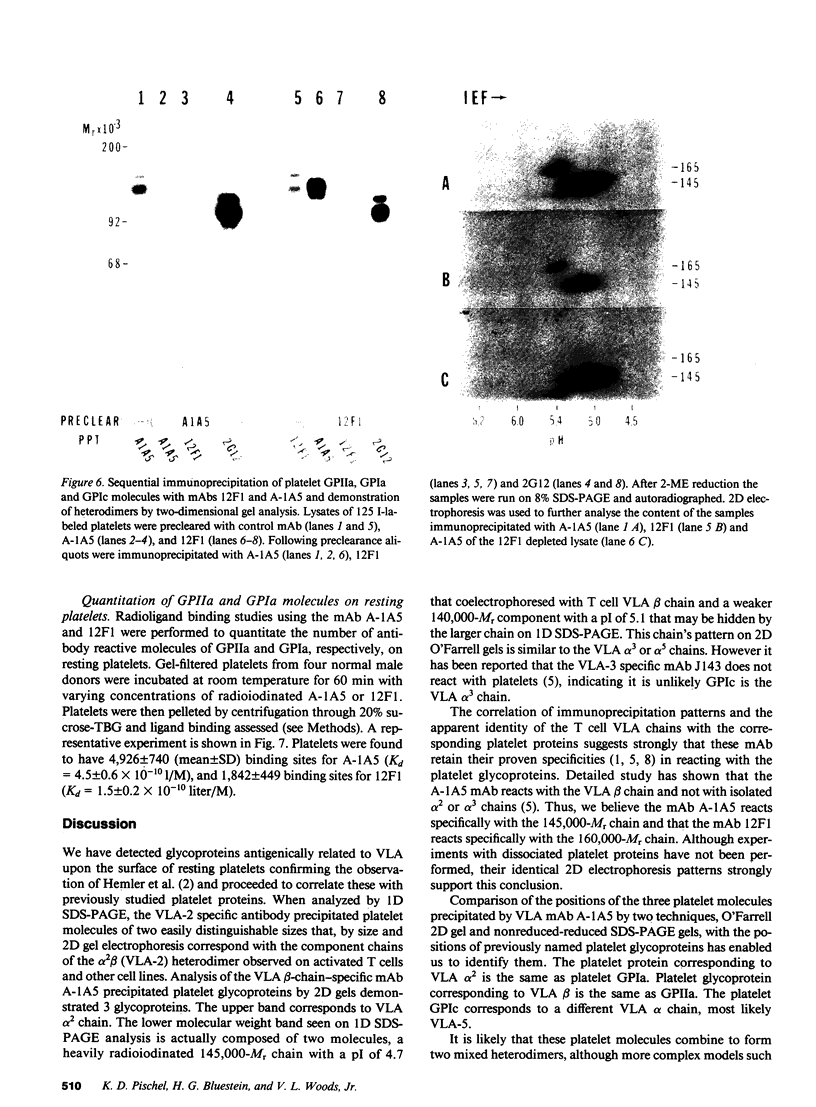
The very late activation antigens (VLA) are a subset of the superfamily of cell surface glycoproteins that serve as receptors from extracellular matrix proteins. One or more of the VLA heterodimers are present on T lymphocytes and most other cell types, including platelets. We have used VLA-specific monoclonal antibodies to isolate the reactive platelet membrane molecules. We have identified them as previously characterized platelet surface glycoproteins and have compared them with VLA molecules isolated from lymphocytes and other cells. Utilizing one-dimensional SDS-PAGE, two-dimensional O'Farrell gel electrophoresis, and nonreduced-reduced two-dimensional gel electrophoresis, we show that reduced VLA molecules of platelets are composed of three chains of molecular weights 165,000, 145,000, and 140,000 that possess the physicochemical properties of platelet glycoproteins GPIa, GPIc alpha, and GPIIa. GPIa corresponds to the VLA 165,000 alpha 2-chain, GPIIa corresponds to a 145,000 Mr VLA beta-chain, and GPIc alpha corresponds to a 140,000 Mr VLA alpha-chain. The polypeptide structure of VLA molecules on platelets and lymphocytes are very similar or identical. Platelet proteins GPIa and GPIIa exist as a mixed heterodimer in detergent lysates and correspond with the VLA-2 heterodimer found on activated T lymphocytes and other cell types. The platelet glycoproteins GPIIa and GPIc form a second mixed heterodimer. The mAb A-1A5, which binds to the VLA beta chain, binds to platelet GPIIa and precipitates both the GPIIa-GPIa and GPIIa-GPIc heterodimers, and binds to 4,926 +/- 740 sites per platelet. A VLA-2-specific mAb, 12F1, which binds to the VLA alpha 2-chain reacts with GPIa and immunoprecipitates only the GPIIa-GPIa heterodimer, and binds to 1,842 +/- 449 sites per platelet. The similarity of VLA chains and platelet GPIIa, GPIa, and GPIc molecules suggests that these molecules may have similar functions on various cell types.
Full text
PDF

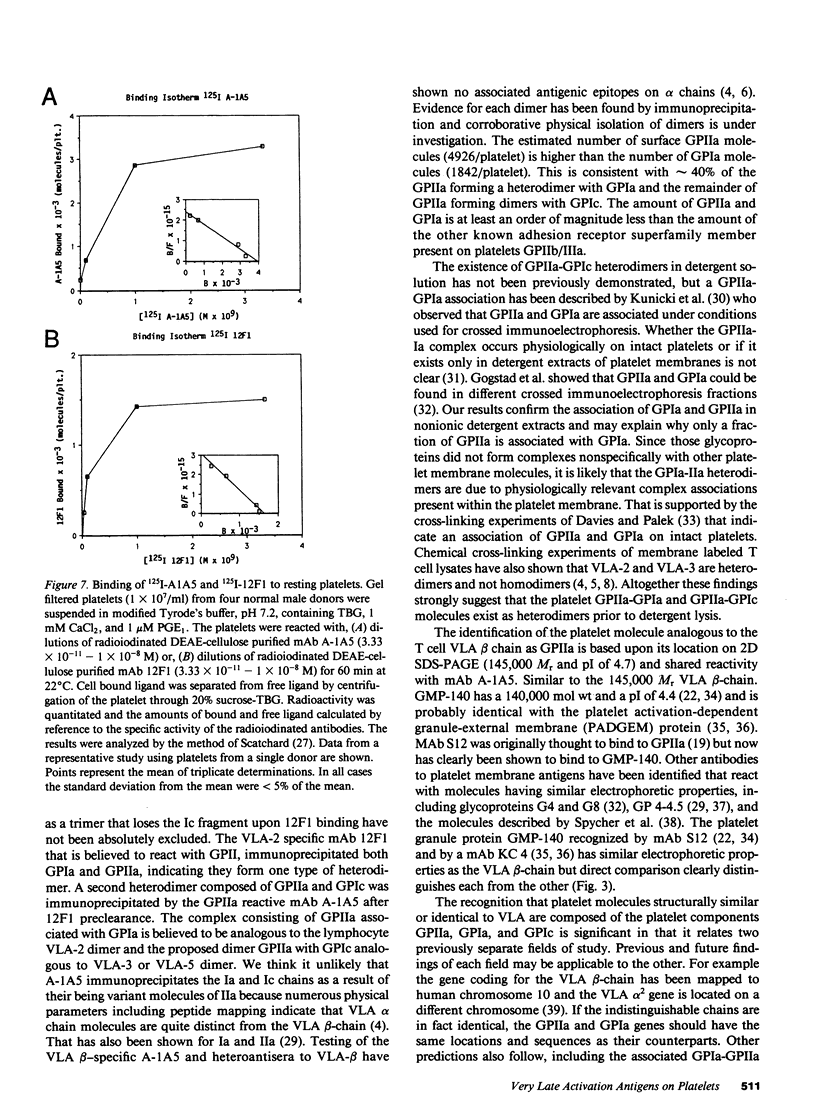


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baur S., Vitetta E. S., Sherr C. J., Schenkein I., Uhr J. W. Isolation of heavy and light chains of immunoglobulin from the surfaces of lymphoid cells. J Immunol. 1971 Apr;106(4):1133–1135. [PubMed] [Google Scholar]
- Bennett J. S., Hoxie J. A., Leitman S. F., Vilaire G., Cines D. B. Inhibition of fibrinogen binding to stimulated human platelets by a monoclonal antibody. Proc Natl Acad Sci U S A. 1983 May;80(9):2417–2421. doi: 10.1073/pnas.80.9.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman C. L., Yeo E. L., Wencel-Drake J. D., Furie B. C., Ginsberg M. H., Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986 Jul;78(1):130–137. doi: 10.1172/JCI112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Bluestein H. G. Neurocytotoxic antibodies in serum of patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3965–3969. doi: 10.1073/pnas.75.8.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson K. J., Capitanio A., Lüscher E. F. High resolution two-dimensional gel electrophoresis of the proteins and glycoproteins of human blood platelets and platelet membranes. Biochim Biophys Acta. 1979 May 3;553(1):11–24. doi: 10.1016/0005-2736(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Palek J. Platelet protein organization: analysis by treatment with membrane-permeable cross-linking reagents. Blood. 1982 Mar;59(3):502–513. [PubMed] [Google Scholar]
- Fradet Y., Cordon-Cardo C., Thomson T., Daly M. E., Whitmore W. F., Jr, Lloyd K. O., Melamed M. R., Old L. J. Cell surface antigens of human bladder cancer defined by mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Jan;81(1):224–228. doi: 10.1073/pnas.81.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. M., Hynes R. O. Interaction of fibronectin with its receptor on platelets. Cell. 1985 Sep;42(2):439–448. doi: 10.1016/0092-8674(85)90101-1. [DOI] [PubMed] [Google Scholar]
- Gogstad G. O., Krutnes M. B., Hetland O., Solum N. O. Comparison of protein and lipid composition of the human platelet alpha-granule membranes and glycerol lysis membranes. Biochim Biophys Acta. 1983 Aug 10;732(3):519–530. doi: 10.1016/0005-2736(83)90228-6. [DOI] [PubMed] [Google Scholar]
- Gogstad G. O., Krutnes M. B., Solum N. O. Calcium-binding proteins from human platelets. A study using crossed immunoelectrophoresis and 45Ca2+. Eur J Biochem. 1983 Jun 1;133(1):193–199. doi: 10.1111/j.1432-1033.1983.tb07447.x. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E., Jacobson J. G., Brenner M. B., Mann D., Strominger J. L. VLA-1: a T cell surface antigen which defines a novel late stage of human T cell activation. Eur J Immunol. 1985 May;15(5):502–508. doi: 10.1002/eji.1830150515. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Jacobson J. G., Strominger J. L. Biochemical characterization of VLA-1 and VLA-2. Cell surface heterodimers on activated T cells. J Biol Chem. 1985 Dec 5;260(28):15246–15252. [PubMed] [Google Scholar]
- Hemler M. E., Sanchez-Madrid F., Flotte T. J., Krensky A. M., Burakoff S. J., Bhan A. K., Springer T. A., Strominger J. L. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984 Jun;132(6):3011–3018. [PubMed] [Google Scholar]
- Hemler M. E., Ware C. F., Strominger J. L. Characterization of a novel differentiation antigen complex recognize by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983 Jul;131(1):334–340. [PubMed] [Google Scholar]
- Hsu-Lin S., Berman C. L., Furie B. C., August D., Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984 Jul 25;259(14):9121–9126. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kunicki T. J., Nurden A. T., Pidard D., Russell N. R., Caen J. P. Characterization of human platelet glycoprotein antigens giving rise to individual immunoprecipitates in crossed-immunoelectrophoresis. Blood. 1981 Dec;58(6):1190–1197. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marguerie G. A., Thomas-Maison N., Ginsberg M. H., Plow E. F. The platelet-fibrinogen interaction. Evidence for proximity of the A alpha chain of fibrinogen to platelet membrane glycoproteins IIb/III. Eur J Biochem. 1984 Feb 15;139(1):5–11. doi: 10.1111/j.1432-1033.1984.tb07968.x. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Martin M. N. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984 Aug 10;259(15):9799–9804. [PubMed] [Google Scholar]
- McGregor J. L., Brochier J., Wild F., Follea G., Trzeciak M. C., James E., Dechavanne M., McGregor L., Clemetson K. J. Monoclonal antibodies against platelet membrane glycoproteins. Characterization and effect on platelet function. Eur J Biochem. 1983 Mar 15;131(2):427–436. doi: 10.1111/j.1432-1033.1983.tb07281.x. [DOI] [PubMed] [Google Scholar]
- McGregor J. L., Clemetson K. J., James E., Capitanio A., Greenland T., Lüscher E. F., Dechavanne M. Glycoproteins of platelet membranes from Glanzmann's thrombasthenia. A comparison with normal using carbohydrate-specific or protein-specific labelling techniques and high-resolution two-dimensional gel electrophoresis. Eur J Biochem. 1981 May 15;116(2):379–388. doi: 10.1111/j.1432-1033.1981.tb05346.x. [DOI] [PubMed] [Google Scholar]
- McGregor J. L., Clemetson K. J., James E., Clezardin P., Dechavanne M., Lüscher E. F. Tryptic peptide map analysis of the major human blood platelet membrane glycoproteins separated by two-dimensional polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1982 Aug 12;689(3):513–522. doi: 10.1016/0005-2736(82)90309-1. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Rust N. A., Pilch J. R., Sochynsky R., Morton J., Mason D. Y., Ruan C., Tobelem G., Caen J. Monoclonal antibody to human platelet glycoprotein I. I. Immunological studies. Br J Haematol. 1981 Dec;49(4):501–509. doi: 10.1111/j.1365-2141.1981.tb07258.x. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis H. K., Sakariassen K. S., Houdijk W. P., Nievelstein P. F., Sixma J. J. Deficiency of platelet membrane glycoprotein Ia associated with a decreased platelet adhesion to subendothelium: a defect in platelet spreading. Blood. 1986 Sep;68(3):692–695. [PubMed] [Google Scholar]
- Nurden A. T., Dupuis D., Kunicki T. J., Caen J. P. Analysis of the glycoprotein and protein composition of Bernard-Soulier platelets by single and two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Clin Invest. 1981 May;67(5):1431–1440. doi: 10.1172/JCI110172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peters P. M., Kamarck M. E., Hemler M. E., Strominger J. L., Ruddle F. H. Genetic and biochemical characterization of human lymphocyte cell surface antigens. The A-1A5 and A-3A4 determinants. J Exp Med. 1984 May 1;159(5):1441–1454. doi: 10.1084/jem.159.5.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Pischel K. D., Bluestein H. G., Woods V. L., Jr Very late activation antigens (VLA) are human leukocyte-neuronal crossreactive cell surface antigens. J Exp Med. 1986 Aug 1;164(2):393–406. doi: 10.1084/jem.164.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischel K. D., Hemler M. E., Huang C., Bluestein H. G., Woods V. L., Jr Use of the monoclonal antibody 12F1 to characterize the differentiation antigen VLA-2. J Immunol. 1987 Jan 1;138(1):226–233. [PubMed] [Google Scholar]
- Pischel K. D., Little J. R. Shared chemical properties of different murine thymus-leukemia antigens. J Immunol. 1979 May;122(5):1821–1827. [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Bader R., de Marco L. Glanzmann thrombasthenia: deficient binding of von Willebrand factor to thrombin-stimulated platelets. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6038–6041. doi: 10.1073/pnas.79.19.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro S. A. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell. 1986 Sep 12;46(6):913–920. doi: 10.1016/0092-8674(86)90073-5. [DOI] [PubMed] [Google Scholar]
- Saxon A., Stevens R. H., Quan S. G., Golde D. W. Immunologic characterization of hairy cell leukemias in continuous culture. J Immunol. 1978 Mar;120(3):777–782. [PubMed] [Google Scholar]
- Sixma J. J., Schiphorst M. E. Identification of ectoproteins of human platelets. Combination of radioactive labelling and two-dimensional electrophoresis. Biochim Biophys Acta. 1980 Dec 2;603(1):70–83. doi: 10.1016/0005-2736(80)90392-2. [DOI] [PubMed] [Google Scholar]
- Spycher M. O., Clemetson K. J., Lüscher E. F. Surface labeling of human platelets by reductive methylation. Thromb Haemost. 1982 Oct 29;48(2):169–172. [PubMed] [Google Scholar]
- Stenberg P. E., McEver R. P., Shuman M. A., Jacques Y. V., Bainton D. F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985 Sep;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Huang C., Hemler M. E. Fibronectin receptor structures in the VLA family of heterodimers. Nature. 1987 Apr 9;326(6113):607–609. doi: 10.1038/326607a0. [DOI] [PubMed] [Google Scholar]
- Takada Y., Strominger J. L., Hemler M. E. The very late antigen family of heterodimers is part of a superfamily of molecules involved in adhesion and embryogenesis. Proc Natl Acad Sci U S A. 1987 May;84(10):3239–3243. doi: 10.1073/pnas.84.10.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods V. L., Jr, Oh E. H., Mason D., McMillan R. Autoantibodies against the platelet glycoprotein IIb/IIIa complex in patients with chronic ITP. Blood. 1984 Feb;63(2):368–375. [PubMed] [Google Scholar]
- Woods V. L., Jr, Wolff L. E., Keller D. M. Resting platelets contain a substantial centrally located pool of glycoprotein IIb-IIIa complex which may be accessible to some but not other extracellular proteins. J Biol Chem. 1986 Nov 15;261(32):15242–15251. [PubMed] [Google Scholar]









