Abstract
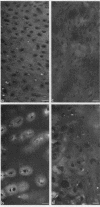
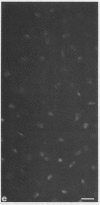
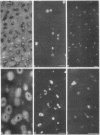
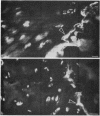
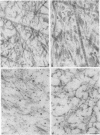
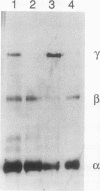
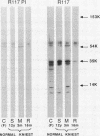
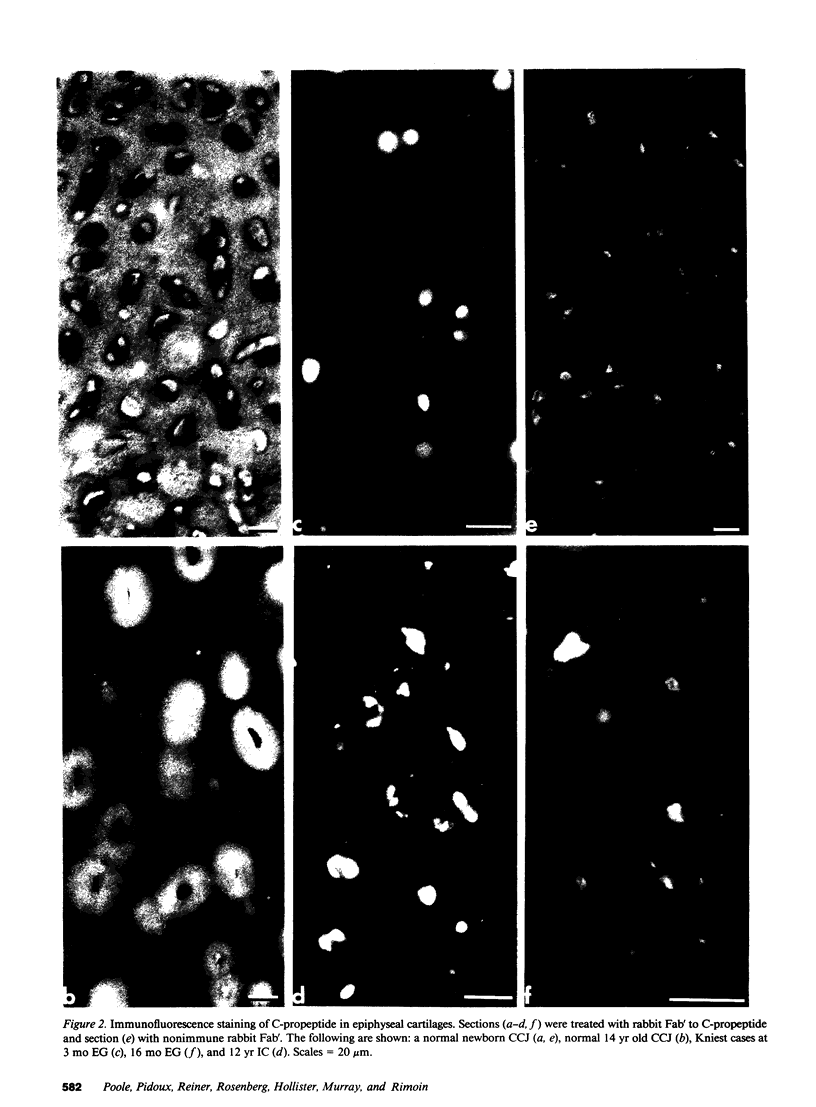
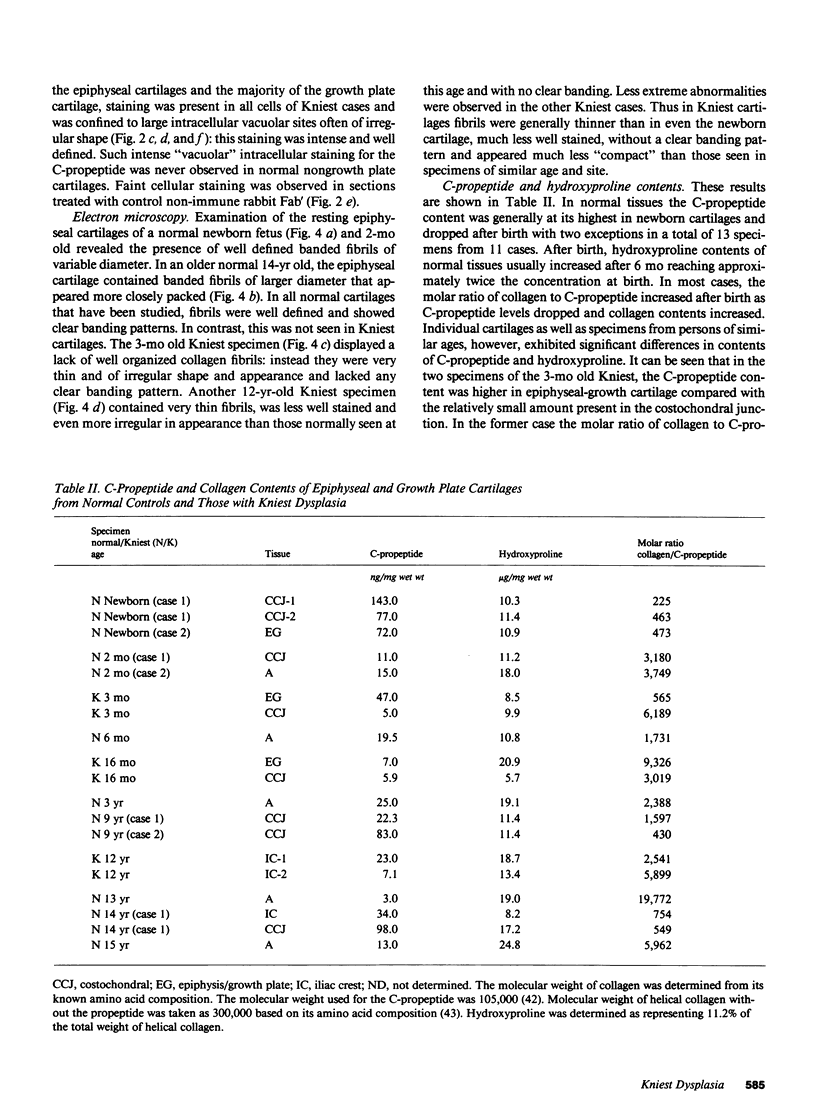
Epiphyseal and growth plate cartilages from four cases of Kniest dysplasia have been studied. In each case collagen fibril organization appeared abnormal by electron microscopy compared with age-matched normal cartilages: fibrils were much thinner, of irregular shape and did not exhibit the characteristic banding pattern. This was associated with the absence (compared with normal cartilage) of the C-propeptide of type II collagen (chondrocalcin) from the extracellular matrix of epiphyseal cartilages, although it was detected (as in normal cartilages) in the lower hypertrophic zone of the growth plate in association with calcifying cartilage. The C-propeptide was abnormally concentrated in intracellular vacuolar sites in Kniest cartilages and its total content was reduced in all cases but not in all cartilages. Moreover, it was not a part of the procollagen molecule. In contrast, type II collagen alpha-chain size was normal, indicating the formation of a triple helix. Also type II collagen content was normal and it was present in extracellular sites and only occasionally detected intracellularly. These observations suggest that the defect in Kniest dysplasia may result from the secretion of type II procollagen lacking the C-propeptide and abnormal fibril formation, and that the C-propeptide is normally required for fibril formation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruckner P., Eikenberry E. F., Prockop D. J. Formation of the triple helix of type I procollagen in cellulo. A kinetic model based on cis-trans isomerization of peptide bonds. Eur J Biochem. 1981 Sep 1;118(3):607–613. doi: 10.1111/j.1432-1033.1981.tb05562.x. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Holbrook K. A., Hall J. G., Bornstein P., Chandler J. W. A new variety of spondyloepiphyseal dysplasia characterized by punctate corneal dystrophy and abnormal dermal collagen fibrils. Hum Genet. 1978 Jan 19;40(2):157–169. doi: 10.1007/BF00272296. [DOI] [PubMed] [Google Scholar]
- Bächinger H. P., Fessler L. I., Timpl R., Fessler J. H. Chain assembly intermediate in the biosynthesis of type III procollagen in chick embryo blood vessels. J Biol Chem. 1981 Dec 25;256(24):13193–13199. [PubMed] [Google Scholar]
- Cheah K. S. Collagen genes and inherited connective tissue disease. Biochem J. 1985 Jul 15;229(2):287–303. doi: 10.1042/bj2290287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. U., Tang L. H., Johnson T. L., Pal S., Rosenberg L. C., Reiner A., Poole A. R. Isolation and characterization of a 35,000 molecular weight subunit fetal cartilage matrix protein. J Biol Chem. 1983 Jan 10;258(1):655–661. [PubMed] [Google Scholar]
- Davidson J. M., McEneany L. S., Bornstein P. Procollagen processing. Limited proteolysis of COOH-terminal extension peptides by a cathepsin-like protease secreted by tendon fibroblasts. Eur J Biochem. 1979 Oct 15;100(2):551–558. doi: 10.1111/j.1432-1033.1979.tb04201.x. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Upton M. P., Shapiro F. D., Wilkinson R. H., Vawter G. F. Nonexpression of cartilage type II collagen in a case of Langer-Saldino achondrogenesis. Am J Hum Genet. 1986 Jul;39(1):52–67. [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R., Olsen B. R., Timpl R., Perlish J. S., Lovelace O. Collagen fibril formation during embryogenesis. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3354–3358. doi: 10.1073/pnas.80.11.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R., Timpl R., Tuderman L., Raisher L., Wiestner M., Perlish J. S., Graves P. N. Ultrastructural identification of extension aminopropeptides of type I and III collagens in human skin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7360–7364. doi: 10.1073/pnas.78.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth D. L., Jr, Veis A. Cathepsin D-mediated processing of procollagen: lysosomal enzyme involvement in secretory processing of procollagen. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3302–3306. doi: 10.1073/pnas.81.11.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A., Reiner A., Poole A. R. The calcification of cartilage matrix in chondrocyte culture: studies of the C-propeptide of type II collagen (chondrocalcin). J Cell Biol. 1987 May;104(5):1435–1441. doi: 10.1083/jcb.104.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojima Y., van der Rest M., Prockop D. J. Type I procollagen carboxyl-terminal proteinase from chick embryo tendons. Purification and characterization. J Biol Chem. 1985 Dec 15;260(29):15996–16003. [PubMed] [Google Scholar]
- Hollister D. W., Byers P. H., Holbrook K. A. Genetic disorders of collagen metabolism. Adv Hum Genet. 1982;12:1–87. doi: 10.1007/978-1-4615-8315-8_1. [DOI] [PubMed] [Google Scholar]
- Hollister D. W., Sakai L. Y., Morris N. P., Shimono L. H., Burgeson R. E. Production and characterization of hybridoma antibody to native human type II collagen. Coll Relat Res. 1982;2(3):197–210. doi: 10.1016/s0174-173x(82)80014-9. [DOI] [PubMed] [Google Scholar]
- Horton W. A., Rimoin D. L. Kniest dysplasia. A histochemical study of the growth plate. Pediatr Res. 1979 Nov;13(11):1266–1270. doi: 10.1203/00006450-197911000-00012. [DOI] [PubMed] [Google Scholar]
- Lapière C. M., Lenaers A., Kohn L. D. Procollagen peptidase: an enzyme excising the coordination peptides of procollagen. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3054–3058. doi: 10.1073/pnas.68.12.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maumenee I. H., Traboulsi E. I. The ocular findings in Kniest dysplasia. Am J Ophthalmol. 1985 Jul 15;100(1):155–160. doi: 10.1016/s0002-9394(14)74998-0. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Lunde L. G. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(II) chain of bovine and human cartilage collagen. Biochemistry. 1973 Aug 14;12(17):3153–3159. doi: 10.1021/bi00741a003. [DOI] [PubMed] [Google Scholar]
- Miyahara M., Bruckner P., Helle O., Prockop D. J. Aggregation of a type I collagen precursor containing N-terminal propeptides. Coll Relat Res. 1983 Jul;3(4):279–293. doi: 10.1016/s0174-173x(83)80010-7. [DOI] [PubMed] [Google Scholar]
- Miyahara M., Njieha F. K., Prockop D. J. Formation of collagen fibrils in vitro by cleavage of procollagen with procollagen proteinases. J Biol Chem. 1982 Jul 25;257(14):8442–8448. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Peltonen L., Halila R., Ryhänen L. Enzymes converting procollagens to collagens. J Cell Biochem. 1985;28(1):15–21. doi: 10.1002/jcb.240280104. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Choi H., Rosenberg L. C. Association of an extracellular protein (chondrocalcin) with the calcification of cartilage in endochondral bone formation. J Cell Biol. 1984 Jan;98(1):54–65. doi: 10.1083/jcb.98.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Tang L. H., Choi H., Rosenberg L. Localization of proteoglycan monomer and link protein in the matrix of bovine articular cartilage: An immunohistochemical study. J Histochem Cytochem. 1980 Jul;28(7):621–635. doi: 10.1177/28.7.6156200. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (second of two parts). N Engl J Med. 1979 Jul 12;301(2):77–85. doi: 10.1056/NEJM197907123010204. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Rosenbloom J., Endo R., Harsch M. Termination of procollagen chain synthesis by puromycin. Evidence that assembly and secretion require a COOH-terminal extension. J Biol Chem. 1976 Apr 10;251(7):2070–2076. [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Schofield J. D., Uitto J., Prockop D. J. Formation of interchain disulfide bonds and helical structure during biosynthesis of procollagen by embryonic tendon cells. Biochemistry. 1974 Apr 23;13(9):1801–1806. doi: 10.1021/bi00706a004. [DOI] [PubMed] [Google Scholar]
- Siggers C. D., Rimoin D. L., Dorst J. P., Doty S. B., Williams B. R., Hollister D. W., Silberberg R., Cranley R. E., Kaufman R. L., McKusick V. A. The Kniest syndrome. Birth Defects Orig Artic Ser. 1974;10(9):193–208. [PubMed] [Google Scholar]
- Sillence D. O., Horton W. A., Rimoin D. L. Morphologic studies in the skeletal dysplasias. Am J Pathol. 1979 Sep;96(3):813–870. [PMC free article] [PubMed] [Google Scholar]
- Snowden J. M., Swann D. A. Vitreous structure. V. The morphology and thermal stability of vitreous collagen fibers and comparison to articular cartilage (type II) collagen. Invest Ophthalmol Vis Sci. 1980 Jun;19(6):610–618. [PubMed] [Google Scholar]
- Stanescu V., Stanescu R., Maroteaux P. Pathogenic mechanisms in osteochondrodysplasias. J Bone Joint Surg Am. 1984 Jul;66(6):817–836. doi: 10.2106/00004623-198466060-00002. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Sotman S. S. The chemical composition of bovine vitreous-humour collagen fibres. Biochem J. 1980 Mar 1;185(3):545–554. doi: 10.1042/bj1850545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Allan R. E., Polak K. L. Conversion of type II procollagen to collagen. Extracellular removal of the amino-terminal and carboxy-terminal extensions without a preferential sequence. Eur J Biochem. 1979 Aug 15;99(1):97–103. doi: 10.1111/j.1432-1033.1979.tb13236.x. [DOI] [PubMed] [Google Scholar]
- Uitto J., Prockop D. J. Biosynthesis of cartilage procollagen. Influence of chain association and hydroxylation of prolyl residues on the folding of the polypeptides into the triple-helical conformation. Biochemistry. 1974 Oct 22;13(22):4586–4591. doi: 10.1021/bi00719a018. [DOI] [PubMed] [Google Scholar]
- Van der Rest M., Rosenberg L. C., Olsen B. R., Poole A. R. Chondrocalcin is identical with the C-propeptide of type II procollagen. Biochem J. 1986 Aug 1;237(3):923–925. doi: 10.1042/bj2370923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K., von der Mark H., Timpl R., Trelstad R. L. Immunofluorescent localization of collagen types I, II, and III in the embryonic chick eye. Dev Biol. 1977 Aug;59(1):75–85. doi: 10.1016/0012-1606(77)90241-x. [DOI] [PubMed] [Google Scholar]