Abstract
Background
A critical need exists for reliable warning markers of in-hospital life-threatening arrhythmias. We employed a new quantitative method to track interlead heterogeneity of depolarization and repolarization to detect premonitory changes prior to ventricular tachycardia (VT) in hospitalized patients with acute decompensated heart failure.
Methods and Results
Ambulatory ECGs (leads V1, V5, and aVF) recorded before initiation of drug therapy from patients enrolled in the Prospective Randomized Evaluation of Cardiac Ectopy with Dobutamine or Nesiritide Therapy (PRECEDENT) trial were analyzed. R-wave and T-wave heterogeneity (RWH, TWH) were assessed by second central moment analysis and T-wave alternans (TWA) by Modified Moving Average analysis. Patients (N=44) studied included those (N = 22) with episodes of VT (≥4 beats at heart rates >100 beats/min) following ≥120 minutes of stable sinus rhythm and age- and sex-matched patients (N=22) without VT. TWA increased from 18.6±2.1μV (baseline, mean ± SEM) to 27.9±4.6μV in lead V5 at 15–30 minutes prior to VT (p<0.05) and remained elevated until the arrhythmia occurred. TWA results in V1 and aVF were similar. RWH and TWH were elevated from 164.1±33.1μV and 134.5±20.6μV (baseline) to 299.8±54.5μV and 239.2±37.0μV at 30–45 minutes prior to VT (p<0.05), respectively, preceding the crescendo in TWA by 15 minutes. Matched patients without VT did not display elevated RWH (185.5±29.4μV) or TWH (157.1±27.2μV) during the 24–hour period.
Conclusions
This is the first clinical demonstration of the potential utility of tracking depolarization and repolarization heterogeneity to detect crescendos in electrical instability that could forewarn of impending nonsustained ventricular tachycardia.
Clinical Trial Registration
Keywords: T-wave alternans, heterogeneity, depolarization, repolarization, tachycardia
Over one million patients are hospitalized for decompensated heart failure yearly among the population of more than 5 million Americans with heart failure.1 These individuals experience a high degree of ventricular ectopy and spontaneous ventricular arrhythmias. Sudden cardiac death constitutes a high proportion of deaths in this population (58% of New York Heart Association (NYHA) class III and 33% of NYHA IV patients).2,3 However, no standard electrocardiographic markers, including ventricular ectopy or arrhythmias, have proved to be reliable indicators of in-hospital life-threatening cardiac arrhythmias.
The objective of our investigation was to evaluate the potential clinical utility of a new method, second central moment analysis, for quantifying heterogeneity of depolarization (R-wave, RWH) and repolarization (T-wave, TWH) waveforms. The specific question addressed was whether changes in RWH and TWH could provide premonitory indications of increased cardiac electrical instability prior to onset of ventricular tachycardia (VT). The randomized, multicenter Prospective Randomized Evaluation of Cardiac Ectopy with Dobutamine or Nesiritide Therapy (PRECEDENT) trial enrolled 255 patients with previous diagnosis of NYHA Class III or IV congestive heart failure who were hospitalized with symptomatic, decompensated heart failure.4 We analyzed 24-hour ambulatory electrocardiograms (AECGs) recordings made in all patients immediately before randomization to treatment.
The rationale for combined analysis of RWH, TWH, and TWA is the close mechanistic linkage among these electrophysiologic entities.5,6 In the experimental laboratory, it was demonstrated that a progressive increase in TWH during acute myocardial ischemia precedes the development of both concordant and discordant TWA and complex forms culminating in ventricular fibrillation.7 TWA is an electrophysiologic phenomenon associated clinically with impending ventricular arrhythmias8 and an important marker of arrhythmia risk supported by extensive clinical evidence of its utility in stratifying risk for sudden cardiac death.9–12 Furthermore, this phenomenon may also be a trigger for arrhythmias by establishing steep repolarization gradients leading to reentry and wavebreak.5,6,13–15
Methods
The PRECEDENT trial enrolled 255 patients (age ≥18 years) who had a history of NYHA class III or IV congestive heart failure and had symptomatic, decompensated congestive heart failure for which inpatient, single-agent, intravenous therapy with either nesiritide or dobutamine (with or without diuretics) was deemed appropriate.4 Patients were either receiving no antiarrhythmic medications or else were receiving a stable dose of these drugs for at least 48 hours before starting study treatment. Oxygen, intravenous and oral diuretics, and all non-intravenous cardiac medications were permitted.
Exclusion criteria included recent acute myocardial infarction (≤48 hours before study entry); unstable angina or ongoing myocardial ischemia; cardiogenic shock; baseline systolic blood pressure consistently ≤85 mm Hg, or significant hemodynamic instability requiring immediate inotropic support, pressor support, or both; stroke within the past month; severe aortic stenosis; obstructive cardiomyopathy; and constrictive pericarditis. Patients were excluded if they had been treated for >4 hours with an intravenous vasoactive agent for the index episode of congestive heart failure. Patients were also excluded from the study if they could not tolerate a 24-hour baseline AECG period without intravenous vasoactive medications, could not tolerate the specified washout period for intravenous vasoactive medications received before the baseline AECG period, or both. Clinical characteristics of study participants are shown in Table 1.
Table 1.
Patient characteristics.
| Patients with VT (N=22) | Patients with no VT (Control, N=22) | Significance comparing patients with and without VT | |
|---|---|---|---|
| Males (n, %) | 15 (68.2%) | 16 (72.7%) | 0.632 |
| Age (years) | 53.8±12.7 | 57.2±8.1 | 0.848 |
| NYHA class | 0.375 | ||
| • III (n, %) | 12 (54.5%) | 14 (63.6%) | |
| • IV (n, %) | 10 (45.5%) | 8 (36.4%) | |
| CHF etiology | 0.002 | ||
| • Ischemic (n, %) | 8 (36.4%) | 15 (68.2%) | |
| • Idiopathic dilated cardiomyopathy (n, %) | 8 (36.4%) | 3 (13.6%) | |
| • Hypertensive (n, %) | 6 (27.3%) | 4 (18.2%) | |
| Diabetes | 9 (40.9%) | 12 (54.5%) | 0.199 |
| Prior MI | 12 (54.5%) | 14 (63.6%) | 0.375 |
| Ventricular premature contractions/hour for 0–120 min prior to VT | 21.3±9.0 | 22.1±7.8 | 0.45 |
All patients were monitored by AECG recording for the 24-hour period immediately before the start of the study drug (pre-randomization AECG tape). The 3-channel (leads V1, V5, and aVF) recordings were scanned with a commercially available AECG reader (Zymed model 2010, Philips Medical Systems, Andover, MA), archived on CDs, and then made available for analysis on a MARS-PC Holter Monitoring System (GE Medical Systems, Milwaukee WI). For TWA, the commercial software was used. For RWH and TWH, Matlab software was implemented according to our previous experimental study, in which extensive validation of the algorithms was performed.7 AECG tapes were labeled with a unique patient code and stripped of any other identifying information.
AECGs from 44 patients recorded during the pre-randomization phase of the PRECEDENT trial were analyzed, composed of the 22 patients who experienced a single bout of VT ≥4 beats at heart rates >100 bpm following 120 minutes of stable sinus rhythm and an age-and sex-matched group (N=22) without atrial fibrillation, VT, or other rhythm disturbances. In the latter group, TWA, RWH , and TWH analyses were performed for the entire 24-hour recordings. The Beth Israel Deaconess Medical Center Committee on Clinical Investigations certified the exempt status of this reanalysis of existing data from a completed clinical trial under exemption number 4 of the Code of Federal Regulations, 45 CFR 46.101(b).
RWH and TWH are continuous, noninvasive measures of spatial depolarization and repolarization heterogeneity, respectively, and complement the temporal heterogeneity information provided by TWA. Spatial heterogeneity throughout the entire R and T waveforms was assessed as previously described7 using second central moment analysis, a principle drawn from Newtonian mechanics. Essentially, a quantitative estimate is derived of splay (the second moment) about the mean morphology (the first moment) of the R and T waveforms. Using this analytical technique, heterogeneity is not unduly weighted by protracted termination or inflections in the waveforms, ST-segment changes, or presence of U waves, features that limit accurate dispersion measurement by conventional analyses. RWH and TWH were analyzed in AECG data from leads V1, V5 and aVF as the maximum square root of the second central moment, as depicted (Fig. 1). The ECGs were first corrected for intrinsic differences in the morphology of V1, V5, aVF by subtracting the baseline waveforms, which were obtained at 60–75 min before the arrhythmia. The heterogeneity waveform was computed as the square-root of the sum of the squares of the differences between the corrected waveform and the mean of the corrected waveforms. RWH is the maximum value of the heterogeneity waveform in the interval from the beginning of the Q wave to the end of the S wave. TWH was the maximum of the heterogeneity waveform in the interval between the J-point and the end of the T-wave. The analysis window began at 120 minutes prior to VT in patients with arrhythmia. In control patients wtthout arrhythmia, the entire 24-hour recording was analyzed. RWH and TWH maxima were computed for each 15-second interval, comparing waveforms in leads V1, V5, and aVF, and averaged over 15-minute epochs.
Figure 1.
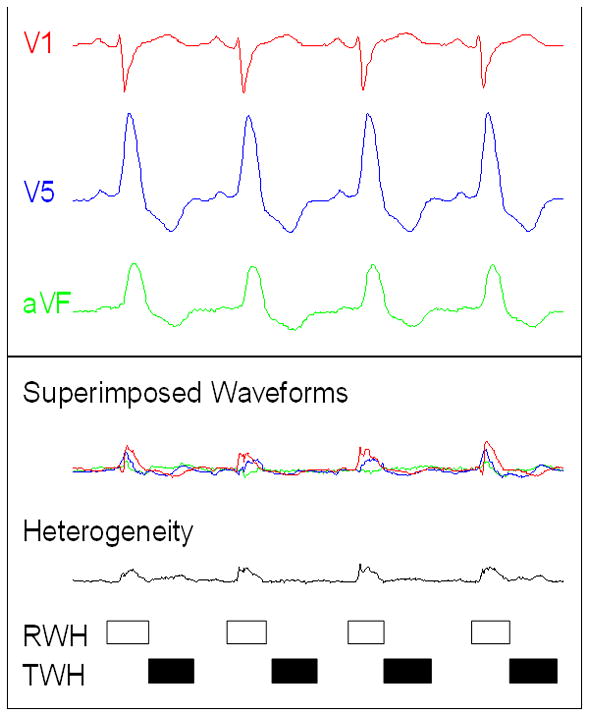
Depolarization and repolarization heterogeneity analysis. Flow chart for signal processing and computing of the second central moment calculation of R-wave heterogeneity (RWH) and T-wave heterogeneity (TWH). TOP: Electrocardiograms (ECGs) were simultaneously obtained from precordial leads V1, V5, and aVF of a representative PRECEDENT patient with decompensated heart failure who experienced ventricular tachycardia. ECGs were filtered to reduce high-frequency noise and to remove baseline wander. Ventricular and supraventricular premature beats as well as beats with a high noise level were removed. For each lead, the isoelectric level was made uniform. BOTTOM: The waveforms of successive beats were superimposed (Bn, Bn1, Bn2, etc.). Second central moment is a square function, because it is the computation of area around a central axis. The square root of the second central moment of simultaneous R waves [from beginning of Q wave to end of S wave, open box] and T waves [from J point to end of T wave (JT interval), black box] was computed from the superimposed waveforms to measure deviation across the entire waveform. The maximum square root of the second central moment was identified for each beat. An average RWH and TWH value was computed for each 15-s interval.
TWA magnitude was analyzed for 120 minutes prior to VT with the modified moving average (MMA) method (GE Healthcare, Milwaukee WI) in leads V1, V5, and aVF.16 Results were computed for each 15-second interval and averaged over 15-minute epochs. MMA computes TWA as the peak difference between odd and even beats in the beat stream at any point within the JT interval. Specifically, a stream of beats is divided into odd and even bins and the morphology of the beats in each bin is averaged over a few beats successively to create a moving average complex. TWA is computed as the maximum difference in amplitude between the odd-beat and the even-beat average complexes from the J point to the end of the T wave. This technique is based on the powerful noise-reduction principle of recursive averaging, achieves an excellent signal-to-noise ratio,17 is relatively tolerant of nonstationary data such as changing heart rates or motion artifact, and is independent of phase-shift perturbations.16 Respiration and motion artifacts have been further reduced by cubic alignment and other filters. These characteristics make MMA analysis suitable for use during AECG monitoring as it can quantify the effects of transient events such as surges in sympathetic nerve activity, which may occur reflexly or in response to behavioral stress and which exert a profound influence on arrhythmia vulnerability.
Statistics. RWH, TWH, and TWA levels were compared to baseline at 60–75 min prior to the onset of the arrhythmia in cases. RWH and TWH were analyzed during 24-hours in matched controls. Analysis of variance was employed with Tukey test for multiple comparisons (*p<0.05). Discrete patient characteristics were analyzed with Chi-square test. Age and rate of ventricular premature contractions were analyzed by Student’s t-test.
Results
Patient characteristics
The clinical characteristics of the 44 patients hospitalized with symptomatic decompensated heart failure, enrolled in the PRECEDENT trial, and included in this substudy are summarized in Table 1. Of the 255 subjects enrolled in the trial, we identified the 22 participants who had experienced VT episodes (≥4 beats at heart rates exceeding 100 bpm) following at least 120 minutes of stable sinus rhythm. These 22 VT events averaged 6.6±0.1 beats (mean ± SEM, range = 4 to 19 beats). Patients matched on age and sex who did not experience VT at the close of similarly quiescent periods (N=22) comprised the remainder of the study population. The clinical characteristics of the two groups did not differ significantly with respect to NYHA class, incidence of hypertensive etiology, diabetes, or prior MI. The patients who experienced VT following a 2-hour quiescent period had a lesser incidence of ischemic etiology of heart failure (8 vs. 15 of 22, p<0.002)
Changes in repolarization
R-Wave and T-Wave Heterogeneity
Patients experiencing VT exhibited marked increases in interlead RWH and TWH at 30–45 minutes prior to VT (Fig. 2), thus anticipating the development of TWA by 15 minutes. Maximum RWH across leads V1, V5, and aVF rose from 164.1±33.1 μV at baseline to 299.8±54.5 μV at 30–45 minutes prior to the arrhythmia (p<0.05). Meanwhile, maximum TWH across leads V1, V5, and aVF rose from 134.5±20.6 μV at baseline to 239.2±37.0 μV at 30–45 minutes prior to the arrhythmia (p<0.05). Just prior to VT, maximum RWH and TWH levels remained elevated, at 289.5±45.9 μV and 230.9±24.7 μV, respectively (p <0.05). Although the extent of change varied among patients, the crescendo pattern in ECG heterogeneity prior to NSVT was consistent (Pearson correlation coefficient for comparing RWH and TWH = 0.51, p = 0.01). In 20 (91%) of 22 patients, RWH or TWH remained elevated prior to onset of NSVT. In the remaining 2 cases, there was relatively minor fluctuation in these parameters. The consistency of the pattern is also indicated by the relatively small standard errors in the time course depicted in Fig. 2.
Figure 2.
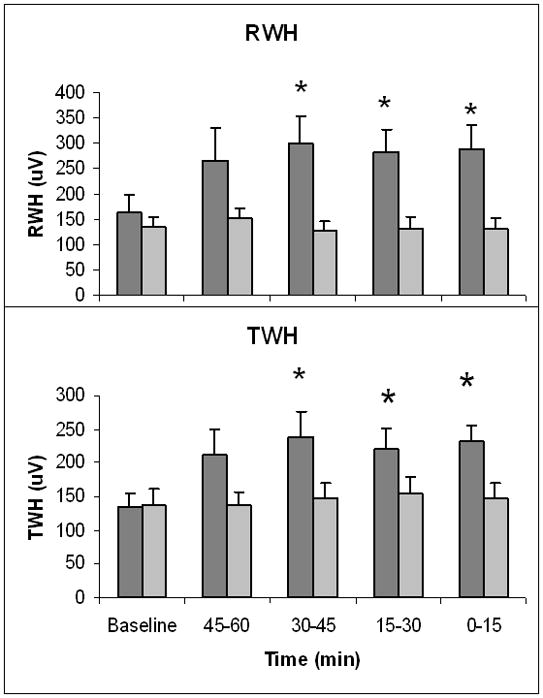
Increase in depolarization and repolarization heterogeneity prior to ventricular tachycardia. At 0–45 minutes prior to ventricular tachycardia (VT), R-wave heterogeneity (RWH) and T-wave heterogeneity (TWH) across leads V1, V5, and aVF were significantly increased above baseline in the 22 PRECEDENT patients with VT (dark grey bars) following a 2-hour quiescent period. Baseline was measured at 60–75 minutes before VT. PRECEDENT patients without VT (light grey bars) did not exhibit significant changes in RWH or TWH during a quiescent 120-minute observation period at a similar time of day (both N.S.).
Analysis of RWH and TWH in 15-second intervals across the entire 24-hour recordings demonstrated that these parameters were significantly higher among the cases prior to VT than at any time during the entire 24-hour period among the controls. Specifically, RWH prior to VT was higher than the 24-hour maximum of the controls (299.8±54.5 versus 185.5±29.4, p<0.05). In addition, TWH prior to VT was higher than the 24-hour maximum of the controls (239.2±37.0 versus 157.1±27.2, p<0.05).
Analysis of T-wave alternans
An example of visible TWA of 82μV in a patient who experienced VT is provided (Fig. 3). This patient exhibited increased levels of RWH and TWH that heralded the onset of TWA and VT.
Figure 3.
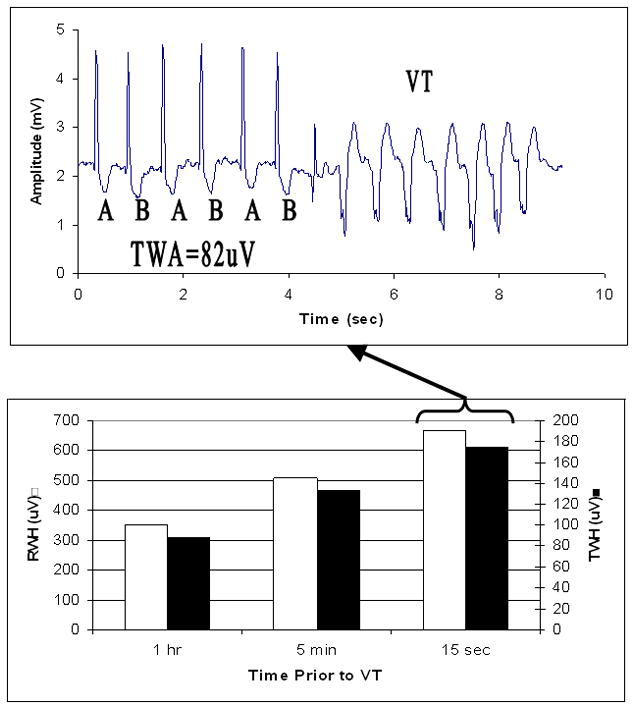
Crescendo in depolarization and repolarization heterogeneity culminating in T-wave alternans prior to ventricular tachycardia. Example of development of ventricular tachycardia (VT) heralded by crescendo in R-wave and T-wave heterogeneity (RWH, open box; TWH, black box) (lower panel) and T-wave alternans (TWA) (upper panel) in lead V5 prior to the arrhythmia in a patient with decompensated heart failure.
Significant increases in TWA levels in all three leads analyzed preceded the onset of VT. Elevated levels of TWA over baseline at 60–75 minutes were first evident at 15–30 minutes prior to the arrhythmia, namely, 24.2±3.9, 27.9±4.6, and 25.5±3.9μV in leads V1, V5, and aVF, respectively, and remained at high levels until VT occurred (Fig. 4) (p<0.05). The peak TWA levels for V1, V5, and aVF prior to VT were 29.2±3.8, 27.9±4.6, and 28.3±4.2μV, respectively, and were substantially higher than at baseline (p<0.05).
Figure 4.
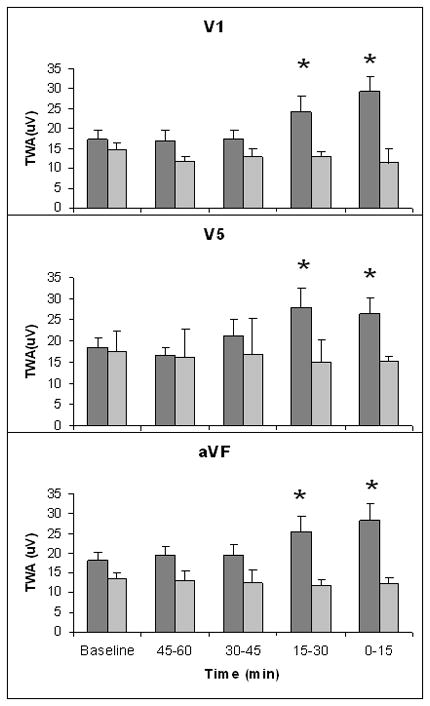
Increase in T-wave alternans prior to ventricular tachycardia. At 0–30 minutes preceding ventricular tachycardia (VT), T-wave alternans (TWA) was increased significantly above baseline in leads V1, V5, and aVF in the 22 PRECEDENT patients with VT (dark grey bars) following a 2-hour quiescent period. Baseline was determined at 60–75 minutes prior to VT. PRECEDENT patients without VT (light grey bars) did not exhibit significant changes in TWA in these leads during a quiescent 120-minute observation period at a similar time of day.
Heart rate
Heart rate was unchanged during the 2-hour observation period in patients who experienced VT, remaining in the range of 87.0±4.8 beats/min at baseline to 86.1±4.6 beats/min at 0–15 minutes prior to the arrhythmia (Fig. 5). Heart rates were similarly stable in patients without VT. As heart rate remained relatively constant, it did not provide warning of impending arrhythmia.
Figure 5.
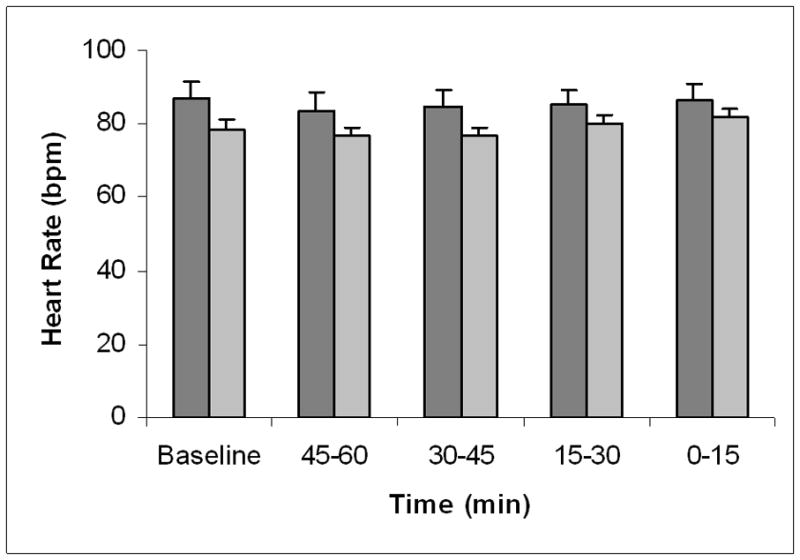
Time course of heart rate prior to ventricular tachycardia. Heart rates across the 120-minute observation period did not change either in patients with ventricular tachycardia (VT) (dark grey bars) or in patients without VT (light grey bars).
Discussion
This study demonstrates that combined monitoring of depolarization and repolarization heterogeneity together with TWA heralds the onset of nonsustained ventricular arrhythmias in hospitalized patients with decompensated heart failure. The rationale for the selection of these parameters was the extensive evidence linking these electrophysiologic entities to cardiac arrhythmogenesis under diverse experimental.5–7,13–15,18–21 and clinical conditions.22–24 The use of multiple leads permitted measurement of spatial as well as temporal heterogeneity, tracking the culmination in TWA and arrhythmia.
Previous studies
Extensive experimental studies point to a close linkage between repolarization heterogeneity, TWA, and ventricular tachyarrhythmias.5–7,13–15,18–21 Using isopotential maps in canines undergoing acute myocardial ischemia, Konta and coworkers were among the first to provide evidence that TWA occurs on a background of temporo-spatial heterogeneity of repolarization.13 Importantly, they found that discordant TWA, wherein repolarization is out-of-phase in neighboring regions, was highly profibrillatory. Subsequently, the importance of this observation was supported by a number of elegant optical mapping studies indicating that the occurrence of discordant TWA is not only a marker of arrhythmia risk but also a trigger, as this phenomenon sets the stage for unidirectional block, reentry, and wavebreak.5,15 We found in canines undergoing acute myocardial ischemia that TWH increased progressively prior to onset of ischemia-induced ventricular fibrillation in both epicardial and precordial leads.7 Importantly, the increase in TWH was associated with a parallel increase in the magnitude of TWA and the development of discordant TWA followed by more complex forms of oscillations that occurred a few seconds prior to onset of ventricular fibrillation. These observations suggested a close linkage between heterogeneity of repolarization and severity of concordant and discordant repolarization alternans.
The utility of TWA as a prognostic indicator in high-risk heart failure patients is well-established.12,24–38 Negative TWA test results are highly accurate in identifying individuals whose arrhythmic risk is low. However, when LVEF is severely depressed, the strength of TWA’s prediction of ventricular tachyarrhythmias by Spectral Method analysis may be lost.30,39 Sakaki, Ikeda and colleagues38 determined in patients with depressed left ventricular function that TWA by time-domain MMA analysis stratifies risk for cardiovascular and sudden death with hazard ratios of 17.1 and 22.6, respectively. Also using MMA-based TWA analysis, Stein and colleagues found that hospitalized post-MI patients with left ventricular dysfunction experienced significantly elevated levels of TWA that predicted the occurrence of sudden cardiac death and cardiovascular mortality during the 20±6 month followup.36 Moreover, these investigators illustrated the utility of QRS-aligned templates of superimposed electrocardiographic (ECG) complexes to verify TWA magnitude. Kodama and colleagues40 demonstrated in patients with chronic decompensated heart failure that TWA can be visible during rest, tachycardia, and dobutamine loading. The latter intervention provoked visible TWA in 10 (11%) of 94 patients, suggesting an association between mechanical and electrical alternans in heart failure patients.
TWA magnitude has also been found to parallel the increased short term risk of VT. Shusterman and colleagues demonstrated a significant increase in TWA as well as other electrophysiologic inhomogeneities prior to VT in the Electrophysiologic Study Versus Electrocardiographic Monitoring (ESVEM) trial.8 These findings are consistent with experimental studies in large animals, in which progressive increases in TWA magnitude were found to precede the onset of ventricular tachycardia and fibrillation.16,41,42
Chauhan and coworkers22 studied the interrelationship between repolarization heterogeneity and TWA in patients with cardiomyopathy using transvenous multi-electrode catheters placed along the apicobasal epicardial and endocardial surface of the ventricles. They found that patients exhibiting a positive TWA test and VT experienced heightened levels of repolarization heterogeneity. The authors proposed that the association between a positive TWA test and VT resulted from steep repolarization gradients, which provided the substrate for functional conduction block and reentry. Moreover, both spatiotemporal heterogeneity and discordant alternans were evident in patients with cardiomyopathy, and greater spatial distribution of intracardiac alternans was associated with alternans detected in precordial or limb leads.23
Present investigation
This investigation is consistent with the current literature indicating a close relationship between depolarization and repolarization heterogeneity, TWA, and nonsustained ventricular tachycardia in hospitalized patients with decompensated heart failure. The study breaks new ground in demonstrating that the electrophysiologic milieu of depolarization and repolarization heterogeneity sets the stage for heightened levels of TWA prior to onset of ventricular tachyarrhythmias. It is of interest that both RWH and TWH were significantly elevated in the 30–45 minute period prior to arrhythmias, preceding the appearance of TWA by ≥15 minutes. This short term indication of probable VT onset may be attributable to the fact that TWA is a more advanced and therefore delayed indicator of cardiac electrical instability than RWH or TWH.
Increased levels of TWH clearly preceded the development of TWA and transition to VT. This finding is consistent with our prior experimental studies using this methodology7 and other experimental studies.5,6,13–15,18–21 Our studies are also consistent with the clinical findings of Chauhan and coworkers22 that repolarization heterogeneity, TWA, and VT are closely linked. RWH and TWH establish an early background of heterogeneity of depolarization and repolarization for the subsequent development of macroscopic TWA, which may serve as a precipitating factor for VT by establishing steep repolarization gradients leading to unidirectional block and reentry. As the strength of correlation between RWH and TWH appears to be only moderate (Pearson correlation coefficient = 0.51, p = 0.01), these variables may differ in their relationship to arrhythmia onset depending on pathophysiologic changes in myocardial substrate among individual patients.
Role of heart rate
Heart rate remained relatively constant during the 120-minute observation period both in patients who experienced VT and in those who did not, indicating that the crescendo in RWH, TWH, and TWA occurred independently of alterations in chronotropy. This relative constancy in heart rate is consistent with the absence of major changes in autonomic balance prior to the arrhythmia. Had there been a significant increase in sympathetic tone and/or withdrawal of vagus nerve activity, then heart rate would have progressively increased. Conversely, the occurrence of bradycardia would have indicated a reciprocal autonomic pattern. As heterogeneity and TWA are influenced by heart rate,5,6,43 the absence of a change in heart rate indicates that the results were not confounded by alterations in chronotropic state. The progressive heart-rate independent changes in RWH, TWH, and TWA, which herald onset of VT, suggest although do not prove the possibility that intrinsic changes in the electrophysiologic milieu of the myocardial substrate underlie development of the arrhythmia.
Conclusions and Clinical Implications
The results of the present study suggest that concurrent monitoring of depolarization and repolarization heterogeneity in conjunction with T-wave alternans could potentially provide early warning of impending nonsustained ventricular tachycardia. It remains to be determined, however, whether ECG heterogeneity is predictive of sustained ventricular tachycardia. A prospective clinical study is needed to determine the predictive capacity of the combination of these parameters for life-threatening arrhythmias in hospitalized patients with decompensated heart failure.
Acknowledgments
The authors appreciate receiving advice through the Harvard Catalyst Biostatistics Program, grant number UL1 RR025758-Harvard Clinical and Translational Science Center from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Funding Sources: This work was supported by grants R21 HL085720 from the NIH, Center for Integration of Medicine and Innovative Technology (CIMIT), Boston MA, the American Heart Association Founders Chapter, and NIH Training Grant T32 HL0077374 to Beth Israel Deaconess Medical Center. General Electric Medical Systems, Inc., provided the MARS-PC Holter Monitoring System.
Abbreviations
- AECG
ambulatory electrocardiogram
- ECG
electrocardiogram
- ESVEM
Electrophysiologic Study Versus Electrocardiographic Monitoring trial
- MMA
Modified Moving Average
- NYHA
New York Heart Association
- PRECEDENT
Prospective Randomized Evaluation of Cardiac Ectopy with Dobutamine or Nesiritide Therapy
- RWH
R-wave heterogeneity
- TWA
T-wave alternans
- TWH
T-wave heterogeneity
- VT
ventricular tachycardia
Footnotes
Conflict of Interest Disclosures: RLV and BDN are inventors of the Modified Moving Average method for T-wave alternans analysis, with patent assigned to Beth Israel Deaconess Medical Center and licensed to GE Healthcare, Inc., and Medtronic, Inc. The other authors have no conflicts of interest relevant to this investigation.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics— 2011 Update. A Report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 3.MERIT-HF Investigators. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 4.Burger AJ, Horton DP, LeJemtel T, Ghali JK, Torre G, Dennish G, Koren M, Dinerman J, Silver M, Cheng ML, Elkayam U. Prospective randomized evaluation of cardiac ectopy with dobutamine or natrecor therapy. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002;144:1102–1108. doi: 10.1067/mhj.2002.125620. [DOI] [PubMed] [Google Scholar]
- 5.Cutler MJ, Rosenbaum DS. Explaining the clinical manifestations of T-wave alternans in patients at risk for sudden cardiac death. Heart Rhythm. 2009;6:S22–S28. doi: 10.1016/j.hrthm.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verrier RL, Kumar K, Nearing BD. Basis for sudden cardiac death prediction by T-wave alternans from an integrative physiology perspective. Heart Rhythm. 2009;6:416–422. doi: 10.1016/j.hrthm.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nearing BD, Verrier RL. Tracking heightened cardiac electrical instability by computing interlead heterogeneity of T-wave morphology. J Appl Physiol. 2003;95:2265–2272. doi: 10.1152/japplphysiol.00623.2003. [DOI] [PubMed] [Google Scholar]
- 8.Shusterman V, Goldberg A, London B. Upsurge in T-wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation. 2006;113:2880–2887. doi: 10.1161/CIRCULATIONAHA.105.607895. [DOI] [PubMed] [Google Scholar]
- 9.Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–281. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL American College of Cardiology/American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a Report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation. 2006;114:e385–e484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 11.Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, Maron BJ, Page RL, Passman RS, Siscovick D, Stevenson WG, Zipes DP. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: A Scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118:1497–1518. [PubMed] [Google Scholar]
- 12.Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner D, Hohnloser S, Ikeda T, Martinez JP, Narayan S, Nieminen T, Rosenbaum DS. Microvolt T-wave alternans: Physiologic basis, methods of measurement, and clinical utility. Consensus guideline by the International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol. 2011;58:1309–1324. doi: 10.1016/j.jacc.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konta T, Ikeda K, Yamaki M, Nakamura K, Honma K, Kubota I, Yasui S. Significance of discordant ST alternans in ventricular fibrillation. Circulation. 1990;82:2185–2189. doi: 10.1161/01.cir.82.6.2185. [DOI] [PubMed] [Google Scholar]
- 14.Tolkacheva EG, Anumonwo JM, Jalife J. Action potential duration restitution portraits of mammalian ventricular myocytes: role of calcium current. Biophys J. 2006;91:2735–2745. doi: 10.1529/biophysj.106.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss JN, Nivala M, Garfinkel A, Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res. 2011;108:98–112. doi: 10.1161/CIRCRESAHA.110.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nearing BD, Verrier RL. Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol. 2002;92:541–549. doi: 10.1152/japplphysiol.00592.2001. [DOI] [PubMed] [Google Scholar]
- 17.Martinez JP, Olmos S. Methodological principles of T wave alternans analysis: A unified framework. IEEE Trans Biomed Eng. 2005;52:599–613. doi: 10.1109/TBME.2005.844025. [DOI] [PubMed] [Google Scholar]
- 18.Adam DR, Smith JM, Akselrod S, Nyberg S, Powell AO, Cohen RJ. Fluctuations in T-wave morphology and susceptibility to ventricular fibrillation. J Electrocardiol. 1984;17:209–218. doi: 10.1016/s0022-0736(84)80057-6. [DOI] [PubMed] [Google Scholar]
- 19.Cordeiro JM, Malone JE, Di Diego JM, Scornik FS, Aistrup GL, Antzelevitch C, Wasserstrom JA. Cellular and subcellular alternans in the canine left ventricle. Am J Physiol Heart Circ Physiol. 2007;293:H3506–3516. doi: 10.1152/ajpheart.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aistrup GL, Shiferaw Y, Kapur S, Kadish AH, Wasserstrom JA. Mechanisms underlying the formation and dynamics of subcellular calcium alternans in the intact rat heart. Circ Res. 2009;104:639–649. doi: 10.1161/CIRCRESAHA.108.181909. [DOI] [PubMed] [Google Scholar]
- 21.Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, Laurita KR, Rosenbaum DS. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6:251–259. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauhan VS, Downar E, Nanthakumar K, Parker JD, Ross HJ, Chan W, Picton P. Increased ventricular repolarization heterogeneity in patients with ventricular arrhythmia vulnerability and cardiomyopathy: a human in vivo study. Am J Physiol Heart Circ Physiol. 2006;290:H79–86. doi: 10.1152/ajpheart.00648.2005. [DOI] [PubMed] [Google Scholar]
- 23.Selvaraj RJ, Picton P, Nanthakumar K, Mak S, Chauhan VS. Endocardial and epicardial repolarization alternans in human cardiomyopathy: Evidence for spatiotemporal heterogeneity and correlation with body surface T-wave alternans. J Am Coll Cardiol. 2007;49:338–346. doi: 10.1016/j.jacc.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 24.Narayan SM, Bayer JD, Lalani G, Trayanova NA. Action potential dynamics explain arrhythmic vulnerability in human heart failure: A clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol. 2008;52:1782–1792. doi: 10.1016/j.jacc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda T, Sakata T, Takami M, Kondo N, Tezuka N, Nakae T, Noro M, Enjoji Y, Abe R, Sugi K, Yamaguchi T. Combined assessment of T-wave alternans and late potentials used to predict arrhythmic events after myocardial infarction. A prospective study. J Am Coll Cardiol. 2000;35:722–730. doi: 10.1016/s0735-1097(99)00590-2. [DOI] [PubMed] [Google Scholar]
- 26.Klingenheben T, Zabel M, D'Agostino RB, Cohen RJ, Hohnloser SH. Predictive value of T-wave alternans for arrhythmic events in patients with congestive heart failure [letter] Lancet. 2000;356:651–652. doi: 10.1016/s0140-6736(00)02609-x. [DOI] [PubMed] [Google Scholar]
- 27.Klingenheben T, Ptaszynski P, Hohnloser SH. Quantitative assessment of microvolt T-wave alternans in patients with congestive heart failure. J Cardiovasc Electrophysiol. 2005;16:620–624. doi: 10.1111/j.1540-8167.2005.40708.x. [DOI] [PubMed] [Google Scholar]
- 28.Rashba EJ, Osman AF, MacMurdy K, Kirk MM, Sarang S, Peters RW, Shorofsky SR, Gold MR. Influence of QRS duration on the prognostic value of T wave alternans. J Cardiovasc Electrophysiol. 2002;13:770–775. doi: 10.1046/j.1540-8167.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- 29.Rashba EJ, Osman AF, MacMurdy K, Kirk MM, Sarang S, Peters RW, Shorofsky SR, Gold MR. Exercise is superior to pacing for T wave alternans measurement in subjects with chronic coronary artery disease and left ventricular dysfunction. J Cardiovasc Electrophysiol. 2002;13:845–850. doi: 10.1046/j.1540-8167.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- 30.Rashba EJ, Osman AF, Macmurdy K, Kirk MM, Sarang SE, Peters RW, Shorofsky SR, Gold MR. Enhanced detection of arrhythmia vulnerability using T wave alternans, left ventricular ejection fraction, and programmed ventricular stimulation: a prospective study in subjects with chronic ischemic heart disease. J Cardiovasc Electrophysiol. 2004;15:170–176. doi: 10.1046/j.1540-8167.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 31.Bloomfield DM, Steinman RC, Namerow PB, Parides M, Davidenko J, Kaufman ES, Shinn T, Curtis A, Fontaine J, Holmes D, Russo A, Tang C, Bigger JT., Jr Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885–1889. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 32.Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, Kaufman ES, Davidenko JM, Shinn TS, Fontaine JM. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–463. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Cantillon DJ, Stein KM, Markowitz SM, Mittal S, Shah BK, Morin DP, Zacks ES, Janik M, Ageno S, Mauer AC, Lerman BB, Iwai S. Predictive value of microvolt T-wave alternans in patients with left ventricular dysfunction. J Am Coll Cardiol. 2007;50:166–173. doi: 10.1016/j.jacc.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 34.Morin DP, Zacks ES, Mauer AC, Ageno S, Janik M, Markowitz SM, Mittal S, Iwai S, Shah BK, Lerman BB, Stein KM. Effect of bundle branch block on microvolt T-wave alternans and electrophysiologic testing in patients with ischemic cardiomyopathy. Heart Rhythm. 2007;4:904–912. doi: 10.1016/j.hrthm.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Salerno-Uriarte JA, De Ferrari GM, Klersy C, Pedretti RF, Tritto M, Sallusti L, Libero L, Pettinati G, Molon G, Curnis A, Occhetta E, Morandi F, Ferrero P, Accardi F ALPHA Study Group Investigators. Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: Results of the ALPHA study. J Am Coll Cardiol. 2007;50:1896–1904. doi: 10.1016/j.jacc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Stein PK, Sanghavi D, Domitrovich PP, Mackey RA, Deedwania P. Ambulatory ECG-based T-wave alternans predicts sudden cardiac death in high-risk post-MI patients with left ventricular dysfunction in the EPHESUS study. J Cardiovasc Electrophysiol. 2008;19:1037–1042. doi: 10.1111/j.1540-8167.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 37.Costantini O, Hohnloser SH, Kirk MM, Lerman BB, Baker JH, 2nd, Sethuraman B, Dettmer MM, Rosenbaum DS ABCD Trial Investigators. The ABCD (Alternans Before Cardioverter Defibrillator) trial: Strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol. 2009;53:471–479. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 38.Sakaki K, Ikeda T, Miwa Y, Miyakoshi M, Abe A, Tsukada T, Ishiguro H, Mera H, Yusu S, Yoshino H. Time-domain T-wave alternans measured from Holter electrocardiograms predicts cardiac mortality in patients with left ventricular dysfunction: A prospective study. Heart Rhythm. 2009;6:332–337. doi: 10.1016/j.hrthm.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Chow T, Kereiakes DJ, Onufer J, Woelfel A, Gursoy S, Peterson BJ, Brown ML, Pu W, Benditt DG MASTER Trial Investigators. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol. 2008;52:1607–1615. doi: 10.1016/j.jacc.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Kodama M, Kato K, Hirono S, Okura Y, Hanawa H, Yoshida T, Hayashi M, Tachikawa H, Kashimura T, Watanabe K, Aizawa Y. Linkage between mechanical and electrical alternans in patients with chronic heart failure. J Cardiovasc Electrophysiol. 2004;15:295–299. doi: 10.1046/j.1540-8167.2004.03016.x. [DOI] [PubMed] [Google Scholar]
- 41.Nearing BD, Huang AH, Verrier RL. Dynamic tracking of cardiac vulnerability by complex demodulation of the T-wave. Science. 1991;252:437–440. doi: 10.1126/science.2017682. [DOI] [PubMed] [Google Scholar]
- 42.Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia-induced vulnerability by precordial T-wave alternans analysis in dog and human. Cardiovasc Res. 1994;28:1440–1449. doi: 10.1093/cvr/28.9.1440. [DOI] [PubMed] [Google Scholar]
- 43.Verrier RL, Tan A. Heart rate, autonomic markers, and cardiac mortality. Heart Rhythm. 2009;6:S68–S75. doi: 10.1016/j.hrthm.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


