Abstract
Objective: In this study, we investigated the interrelationship between clinicopathologic findings and pre-B-cell leukemia transcription factor 2 (PBX2) expression in gingival squamous cell carcinoma (GSCC). Methods: Expression level of PBX2 was immunohistochemically examined in 66 GSCC subjects (30 men and 36 women) with ages ranging from 42 to 85 (median 64.5) years, in which staining intensity in tumor cells was categorized as either weaker (level 1) or equal to/stronger (level 2) than that in the endothelial cells. Results: PBX2 expression is correlated with valosin-containing protein (VCP) expression. Univariate and multivariate analyses revealed a high level of PBX2 expression to be a poor prognosticator for disease-free survival (DFS) and overall survival (OS), and PBX2 expression was an independent prognostic factor for both DFS and OS in GSCC. Conclusions: PBX2 expression level in GSCC is prognostic. PBX2 may be a useful marker to identify the potential for progression in GSCC.
Keywords: Gingival squamous cell carcinoma, Pre-B-cell leukemia transcription factor 2 (PBX2), Prognosis
1. Introduction
Squamous cell carcinoma (SCC) is the most common malignant neoplasm of the oral cavity (Randhawa et al., 2008). Worldwide, the annual incidence of new cases exceeds 300 000 (Lippman and Hong, 2001; Jemal et al., 2003). Despite advances in diagnosis and treatments during the past 40 years, the overall 5-year survival rates for oral and oropharyngeal SCC have only slightly improved and remain around 50% (Levi et al., 2005).
For gingival squamous cell carcinoma (GSCC), several prognostic factors have been proposed. Among them, tumor size and lymph node metastases are the main prognostic factors (Yokoo et al., 2002). Otherwise, tumor-node-metastasis (TNM) classification has been widely employed for the staging of GSCC (Sobin and Wittekind, 2002), but does not always predict clinical outcome accurately. Therefore, identification of additional prognostic factors for GSCC is necessary to establish appropriate treatment modalities for patients with GSCC.
We previously showed that a higher level expression of pre-B-cell leukemia transcription factor 2 (PBX2) is correlated with poor prognosis for patients with non-small cell lung cancer (NSCLC), gastric carcinoma (GC), and esophageal squamous cell carcinoma (ESCC) (Qiu et al., 2009; 2010). PBX2 expression was an independent prognostic indicator for GC and ESCC (Qiu et al., 2010). PBX2 protein, a main member of PBX family, is ubiquitously expressed in various tissues. PBX2 works as a cofactor with other proteins to regulate the expression of numbers of genes, thus inducing the execution of various cellular functions, such as anti-apoptosis, and inhibition of cell differentiation and proliferation (van Dijk et al., 1995). Experiments have shown that the knocked-down expression of PBX2 decreased in vitro colony formation and in vivo tumorigenic activities in cell lines of GC and ESCC. Increased apoptosis rate and decreased Bcl-2 expression were found in the knocked-down expression of PBX2 (Qiu et al., 2010).
The previous study also showed that the level of valosin-containing protein (VCP) expression, which is involved in invasion and metastasis of cancers, is correlated with metastatic potential in tumor cells and poor prognosis in many kinds of cancers, including GSCC (Yamamoto et al., 2003; 2004a; 2004b; 2004c). The PBX2 expression is correlated with VCP expression, which has been revealed in NSCLC cell lines and in the clinical samples. It has been shown that PBX enhanced the promoter activity of VCP gene through their recognition sites (Qiu et al., 2007). The PBX-VCP pathway may play an important role in regulating differentiation and proliferation of tumor cells (Qiu et al., 2009).
In the present study, the expression patterns of PBX2 and VCP proteins were evaluated by immunostaining in 66 subjects with GSCC who underwent curative surgery, and the association between VCP and PBX2, and its possible prognostic significance were analyzed in comparison with clinicopathological parameters and survival.
2. Subjects and methods
The experiment is performed as described by Yamamoto et al. (2004a).
2.1. Subjects and tissue samples
Tissue samples were obtained from 66 subjects who underwent curative resections for GSCC at the Division of Oral and Maxillofacial Diseases, Osaka University Dental Hospital and the Division of Otorhinolaryngology, Osaka University Hospital, Suita, Japan, during the period from February 1989 to March 1997. There were 30 males and 36 females with ages ranging from 42 to 85 (median 64.5) years. Tumors were located in the maxillary gingiva in 21 subjects and mandibular gingiva in 45 subjects. Samples obtained from the gingival lesions and dissected lymph nodes were fixed in 10% formalin (0.1 g/ml) and routinely processed for paraffin-embedding. Histologic sections cut at 4 μm were stained with hematoxylin and eosin (H&E) and immunoperoxidase procedures (avidin-biotin complex (ABC) method). Stained sections were reviewed independently by two investigators (Ying QIU and Eiichi MORII) to determine histological subtype and lymph node metastasis. Tumor stages were defined based on the pathologic tumor-node-metastasis (pTNM) classification (Sobin and Wittekind, 2002).
After surgery, all subjects received laboratory examination such as routine peripheral blood cell counts at 1–6-month intervals and chest X-ray at 6–12-month intervals. Adjuvant therapy was performed in 56 subjects, chemotherapy alone in 22 (pre-operatively in 5, post-operatively in 3 and combined pre- and post-operatively in 14), radiotherapy alone in 11 (post-operatively in 2, and combined pre- and post-operatively in 9), and combined chemo- and radiotherapy in 23 (post-operatively in 2, and combined pre- and post-operatively in 21). Cis-platinum (CDDP) and fluorouracil (5-FU) were the main chemotherapeutic agents in the present study. Follow-up period for survivors ranged from 2.7 to 176.7 (median 75.6) months.
2.2. Immunohistochemistry
For the detection of PBX2 and VCP, immunoperoxidase procedure (ABC method) was carried out on paraffin-embedded sections as previously described (Yamamoto et al., 2003). Briefly, antigen retrieval was carried out by heating the sections in 10 mmol/L citrate buffer for 5 min. Primary antibodies used were rabbit polyclonal anti-human PBX2 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse monoclonal anti-human VCP (1:3 000; Progen Biotechnik, Heidelberg, Germany). Sections were lightly counterstained by methyl green. Positive staining in endothelial cells was used as internal positive control. For negative controls, non-immunized mouse IgG serum (Vector Laboratories, Burlingame, CA, USA) was used as the primary antibody, and uniformly gave negative results. Stained sections were examined in a blind manner without any prior information of the clinical features of the subjects. Staining intensity in the cytoplasm of the tumor cells was evaluated in comparison to that of endothelial cells, and the staining intensity of tumor cells was categorized as equal to or stronger (level 2) and weaker (level 1) than that of endothelial cells. In total, there were four tumors with more than 50% representative section areas staining negative for PBX2. Thus, we also judged those samples as level 1 staining of PBX2.
2.3. Quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis of PBX2
Total RNA was extracted with GSCC using QuickExtract™ FFPE RNA extraction kit (Epicentre, Madison, USA) from slides in 20 GSCC cases of level 1 and level 2 expression PBX2, respectively, which was evaluated in immunohistochemistry staining. Complementary DNA (cDNA) was synthesized using oligo (dT) primers and SuperScript III reverse transcriptase (Invitrogen). Quantitative PCR was used to quantify mRNA expression of PBX2, using an ABI PRISM 7700 instrument (Applied Biosystems). RNA extracted from noncancerous sample in one subject was used as a standard. RT-PCR was carried out using TaqMan probe/primer sets specific for human PBX2. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference for gene amplification (Applied Biosystems).
2.4. Statistical analysis
Data for RT-PCR were expressed as mean±standard error (SE). The significance of differences of the mean values was determined by Student’s t-test. All experiments were performed in triplicate. Overall survival (OS) was measured from the date of surgery. Disease-free survival (DFS) was measured from the date of surgery to local recurrence of the disease, the occurrence of distant metastases, or death due to any cause. The data were analyzed using JMP software (SAS Institute Inc., Cary, NC, USA). The chi-square test and Fisher’s exact test were used to analyze the association between PBX2 expression measured by immunohistochemistry and clinicopathologic features of GSCC. Kaplan-Meier methods with the log-rank test were used to calculate survival rates and estimate differences in survival curves (Kaplan and Meier, 1958). The Cox (1972) proportional hazard regression model with a stepwise procedure was used to analyze the independent prognostic factors. P values of <0.05 were considered statistically significant.
3. Results
3.1. Histologic findings
Histologically, 36 tumors were classified as well-differentiated, 17 moderately-differentiated, and 13 poorly-differentiated GSCC. Tumor size was less than 2 cm (pT1) in 4 subjects, 2–4 cm (pT2) in 26 subjects, and more than 4 cm (pT3) in 19 subjects. Tumors in the remaining 17 subjects invaded into adjacent tissue (pT4). Dissected lymph nodes were histologically analyzed for the presence or absence of lymph node metastasis; 24 subjects had metastasis-positive nodes.
3.2. Relationship between PBX2 expression and clinicopathologic factors in GSCC
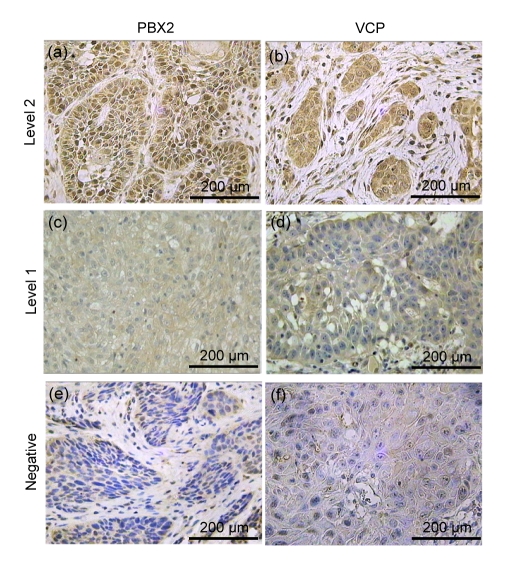
Twenty nine (43.9%) and thirty seven (56.1%) subjects showed level 1 and level 2 PBX2 cytoplasm expressions, respectively (Fig. 1). In comparison to GSCC with level 1 PBX2 expression, level 2 GSCC showed significantly advanced rates of pT and stage factors of pTNM classification (P<0.05). Subjects with tumors of level 2 PBX2 expression showed the higher expression level of VCP (Table 1).
Fig. 1.
Immunohistochemical findings
(a, b) Cancer cells showing a high level expression of PBX2 and VCP in GSCC; (c, d) Cancer cells with a low level expression of PBX2 and VCP; (e, f) Cancer cells with a negative expression of PBX2 and VCP in GSCC
Table 1.
Relationship between PBX2 expression and clinicopathologic factors of subjects with gingival cancer
| Clinicopathologic feature | n | Value# |
P | |
| PBX2 level 2 (n=37) | PBX2 level 1 (n=29) | |||
| Age (year) | 64.6±9.9 | 64.5±9.6 | NS | |
| Sex | ||||
| Female | 36 | 22 (61.1%) | 14 (38.9%) | NS |
| Male | 30 | 15 (50.0%) | 15 (50.0%) | |
| Tumor location | ||||
| Mandibular gingival | 45 | 24 (53.3%) | 21 (46.7%) | NS |
| Maxillary gingival | 21 | 13 (61.9%) | 8 (38.1%) | |
| Lymph node metastasis | ||||
| Present | 24 | 11 (45.8%) | 13 (54.2%) | NS |
| Absent | 42 | 26 (61.9%) | 16 (38.1%) | |
| Lymphatic invasion | ||||
| Present | 58 | 33 (56.7%) | 25 (43.3%) | NS |
| Absent | 8 | 4 (50.0%) | 4 (50.0%) | |
| T(pTNM) | ||||
| pT1 | 4 | 0 (0.0%) | 4 (100.0%) | <0.05* |
| pT2 | 26 | 12 (46.2%) | 14 (53.8%) | |
| pT3 | 19 | 12 (63.2%) | 7 (36.8%) | |
| pT4 | 17 | 13 (76.5%) | 4 (23.5%) | |
| Histologic differentiation | ||||
| Poorly | 13 | 8 (61.5%) | 5 (38.5%) | NS |
| Moderately | 17 | 10 (58.8%) | 7 (41.2%) | |
| Well | 36 | 24 (66.7%) | 12 (33.3%) | |
| Stage | ||||
| Stage 1 | 4 | 0 (0.0%) | 4 (100.0%) | <0.05** |
| Stage 2 | 22 | 10 (45.5%) | 12 (54.5%) | |
| Stage 3 | 23 | 14 (60.9%) | 9 (39.1%) | |
| Stage 4 | 17 | 4 (23.5%) | 13 (76.5%) | |
| Adjuvant therapy | ||||
| Not performed | 10 | 6 (60.0%) | 4 (40.0%) | NS |
| Performed | 56 | 31 (55.4%) | 25 (44.6%) | |
| VCP expression | ||||
| Level 2 | 37 | 26 (70.3%) | 11 (29.7%) | <0.05 |
| Level 1 | 29 | 11 (37.9%) | 18 (62.1%) | |
n: number of patients (total number is 66); pTNM: pathologic tumor-node-metastasis; PBX2: pre-B-cell leukemia transcription factor 2; VCP: valosin-containing protein; NS: not significant
Values are expressed as mean±SD or n(%)
pT1–3 vs. pT4
Stages 1–2 vs. Stages 3–4
3.3. PBX2 expression in GSCC
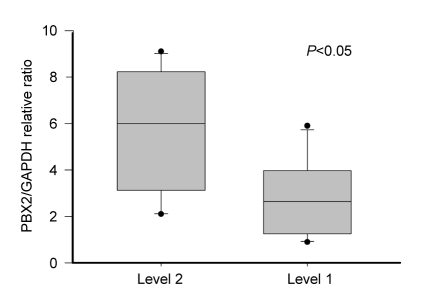
Quantitative RT-PCR analysis was performed in 20 GSCC with level 1 and level 2 expressions, respectively. Relative ratio of PBX2/β-actin expression in subjects with level 1 and level 2 expressions was 2.8±1.6 and 5.6±2.6 (mean±standard deviation (SD)), respectively (P<0.05; Fig. 2).
Fig. 2.
PBX2/GAPDH relative ratio of mRNA expression in GSCC subjects with PBX2 level 1 and level 2
Averages of mRNA expression with PBX2 level 1 showed ratios lower than those of level 2 (P<0.05). Values are expressed as mean±SD
3.4. Uni- and multivariate analyses of prognostic factors in GSCC patients
Five-year DFS and OS rates were 74.8% and 90.3%, respectively. Tumor recurrence was found in 20 subjects, local recurrence in 14 subjects, metastasis to cervical node in five subjects, and metastasis to skin and liver in one subject. Among these, seven subjects died due to the tumor.
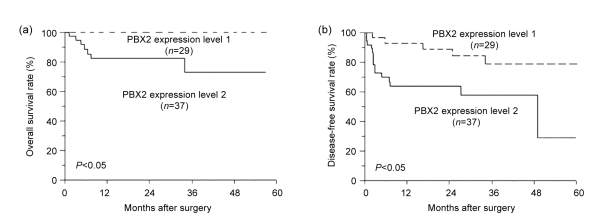
Subjects with level 1 PBX2 GSCC showed a significantly better 5-year OS rate than those with level 2 PBX2 GSCC (5-year DFS: 88.9% vs. 63.7%, P<0.05; 5-year OS: 100% vs. 82.1%, P<0.01) (Table 2 and Fig. 3). Univariate analysis revealed that expression of PBX2 and VCP, pT(TNM), and stage of pTNM were significant factors for both DFS and OS. Lymph node metastasis significantly affected DFS but not OS. Histologic differentiation affected OS but not DFS (Table 2). Multivariate analysis with factors found to be significant in the univariate analysis revealed that PBX2 expression was an independent prognostic factor for both of OS and DFS. Lymph node metastasis and pT classification were equally significant for DFS, and histologic differentiation was significant for OS (Table 3).
Table 2.
Univariate analyses of clinicopathologic factors for disease-free survival and overall survival of subjects with gingival cancer
| Factor | n | 5-year DFS rate |
5-year OS rate |
||
| Mean (%) | P | Mean (%) | P | ||
| PBX2 expression | |||||
| Level 2 | 37 | 63.7 | <0.05 | 82.1 | <0.05 |
| Level 1 | 29 | 88.9 | 100.0 | ||
| Age | |||||
| >65 years | 33 | 71.6 | NS | 96.6 | NS |
| ≤65 years | 33 | 78.8 | 84.3 | ||
| Sex | |||||
| Female | 36 | 71.1 | NS | 84.5 | NS |
| Male | 30 | 79.7 | 96.7 | ||
| Tumor location | |||||
| Mandibular gingival | 45 | 79.2 | NS | 88.3 | NS |
| Maxillary gingival | 21 | 59.1 | 95.0 | ||
| Lymph node metastasis | |||||
| Present | 24 | 50.9 | <0.05 | 82.5 | NS |
| Absent | 42 | 85.0 | 94.7 | ||
| Lymphatic invasion | |||||
| Present | 58 | 76.9 | NS | 90.9 | NS |
| Absent | 8 | 56.3 | 83.3 | ||
| T(pTNM) | |||||
| pT4 | 17 | 37.8 | <0.0001 | 67.9 | <0.05 |
| pT1–3 | 49 | 87.5 | 97.8 | ||
| Histologic differentiation | |||||
| Moderately+poorly | 30 | 66.7 | NS | 83.0 | <0.05 |
| Well | 36 | 81.8 | 96.8 | ||
| Stage | |||||
| 3+4 | 40 | 63.0 | <0.05 | 83.6 | <0.05 |
| 1+2 | 26 | 92.3 | 100.0 | ||
| VCP expression | |||||
| Level 2 | 37 | 58.9 | <0.05 | 81.9 | <0.05 |
| Level 1 | 29 | 93.3 | 100.0 | ||
n: number of patients (total number is 66); DFS: disease-free survival; OS: overall survival; pTNM: pathologic tumor-node-metastasis; PBX2: pre-B-cell leukemia transcription factor 2; VCP: valosin-containing protein; NS: not significant
Fig. 3.
Kaplan-Meier plots for overall survival (a) and disease-free survival (b) of subjects with levels 1 and 2 PBX2 GSCC
Significant difference was observed between the two groups
Table 3.
Multivariate analyses of clinicopathologic factors for disease-free survival and overall survival of subjects with gingival cancer
| Factor | χ2 | Relative risk | P |
| DFS | |||
| PBX2 expression | |||
| Level 1 vs. level 2 | 5.22 | 1.93 | <0.05 |
| T(pTNM) | |||
| pT1–3 vs. pT4 | 4.37 | 1.78 | <0.05 |
| Lymph node metastasis | |||
| Absent vs. present | 9.00 | 2.15 | <0.05 |
| VCP expression | |||
| Level 1 vs. level 2 | 1.60 | 1.58 | NS |
| Stage | |||
| 1+2 vs. 3+4 | 0.38 | 1.27 | NS |
| OS | |||
| PBX2 expression | |||
| Level 1 vs. level 2 | 5.35 | 3781.80 | <0.05 |
| T(pTNM) | |||
| pT1–3 vs. pT4 | 0.31 | 1.35 | NS |
| VCP expression | |||
| Level 1 vs. level 2 | 2.93 | 3082.10 | NS |
| Histologic differentiation | |||
| Well vs. moderately+poorly | 4.89 | 2.79 | <0.05 |
| Stage | |||
| 1+2 vs. 3+4 | 1.15 | 1713.50 | NS |
DFS: disease-free survival; OS: overall survival; pTNM: pathologic tumor-node-metastasis; PBX2: pre-B-cell leukemia transcription factor 2; VCP: valosin-containing protein; NS: not significant
3.5. Prognostic significance of PBX2 expression in grade
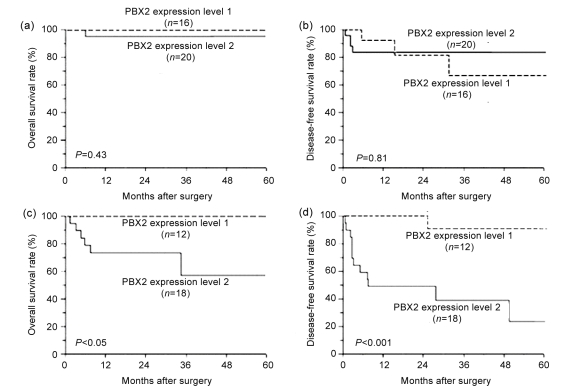
Prognostic significance of PBX2 expression was further analyzed according to the histologic differentiation. Subjects with good differentiation of GSCC showed favorable prognosis regardless of PBX2 expression status (Figs. 4a and 4b). However, at poor and moderate differentiations, subjects with level 1 PBX2 expression showed a better OS and DFS rates compared to level 2 patients (Figs. 4c and 4d; 5-year OS: 100% vs. 71.1%, P<0.05; 5-year DFS: 100% vs. 44.4%, P<0.001).
Fig. 4.
Overall and disease-free survival rates of subjects with levels 1 and 2 PBX2 GSCC
(a, b) There was no significant difference between PBX2 level 1 and level 2 subjects at good differentiation of GSCC; (c, d) Significant difference was observed between PBX2 level 1 and level 2 subjects at poor and moderate differentiations of GSCC
4. Discussion
GSCC grows very rapidly and typically invades nearby bone and tissue. The 5-year survival rate of GSCC patients is only 26% (Soo et al., 1988; Eicher et al., 1996). Standard treatment is still based on surgery and radiation therapy (Byers et al., 1981; Soo et al., 1988), but it does not change the low survival rates of GSCC.
Treatment failures can be attributed to multiple factors but remain difficult to predict because no reliable molecular marker is currently available for early detection or as an indicator of prognosis (Shaw et al., 2009). TNM staging is used as a standard method for prediction of prognosis of GSCC; however, prognosis is inconsistent even among patients in the same stage (Gomez et al., 2000). Therefore, there is an urgent need to seek an accurate marker of prognosis that might predict aggressive tumors.
In a previous study, the correlation between the high expression of PBX2 and poor prognosis was reported for several cancer tissues, including the lung, stomach, and esophagus (Qiu et al., 2009; 2010). PBX genes encode a family of three-amino-acid loop extension (TALE) homeodomain proteins that function as transcriptional regulators in numerous cell types (Mann and Affolter, 1998). PBX2 protein, a main member of the PBX family, works as a cofactor with other proteins, such as homeobox (HOX), and forms dimeric complexes with increasing DNA binding affinity and specificity (Longobardi and Blasi, 2003), thus, regulating proliferation and differentiation of normal and cancer cells with those proteins (Kamps et al., 1991; Phelan et al., 1995).
In our present study, to clarify whether PBX2 expression level could be used as a reliable prognostic factor for GSCC, the clinical implications of PBX2 immunostaining with prognosis were assessed. PBX2 expression level was examined in 20 subjects by combined quantitative RT-PCR and immunohistochemical analyses and in 46 subjects by immunohistochemistry alone. In the 20 subjects, there was a clear correlation in PBX2 expression between the protein (immunohistochemistry) and mRNA (RT-PCR) levels, which indicates the reliability of immunohistochemistry for evaluation of PBX2 expression. Among the clinicopathologic factors examined, high PBX2 expression was associated with increased pT classification and stage of TNM. pT and stage were prognostic for aggressive character of tumor growth and invasiveness of tumor. The correlation between PBX2 and pT/stage demonstrated that PBX2 may play a significant role in the development and invasion of GSCC.
In addition, high PBX2 expression was associated with high VCP expression. VCP is involved in the regulation of activation of nuclear factor kappa B (NFκB) (Naora et al., 2001; Takahashi et al., 2007), which is a transcription factor correlated with various cellular activities such as anti-apoptosis, and cell proliferation and invasion (Plowright et al., 2009). Univariate analysis revealed that expressions of VCP and PBX2 were significant factors for both DFS and OS. However, multivariate analysis showed that PBX2 expression, not VCP, was an independent prognostic factor for both of OS and DFS (Tables 2 and 3). It has been shown that VCP was an independent prognostic factor for both of OS and DFS by removing PBX2 factor (data not shown). This agrees with Yamamoto et al. (2004a)’s study. Therefore, PBX2 is a stronger prognostic factor than VCP in GSCC. In our previous study, it was shown that PBX2 works as a transcription factor enhancing VCP expression. PBX2 transactivated VCP promoter in NSCLC (Yamamoto et al., 2004b).
Prognostic significance of PBX2 expression was further analyzed according to the histological differentiation. At poor and moderate differentiations of GSCC, subjects with low PBX2 expression showed a better OS and DFS rates compared to subjects with high expression. Combination of PBX2 level and histological differentiation is a useful tool for prediction of prognosis for patients with GSCC.
5. Conclusions
PBX2 expression as determined by immunohistochemistry could be used as a new prognosticator for GSCC. This study shows that PBX2 expression level is useful for prediction of prognosis for GSCC patients. Furthermore, PBX2 may control expression of VCP. Further studies of the PBX2-VCP pathway are necessary to clarify the mechanism of cancer development. This, in turn, may be an effective treatment modality for GSCC.
Footnotes
Project (No. 30801382) supported by the National Natural Science Foundation of China
References
- 1.Byers RM, White D, Yue A. Squamous carcinoma of the oral cavity: choice of therapy. Curr Probl Cancer. 1981;6(5):1–27. doi: 10.1016/S0147-0272(81)80015-3. [DOI] [PubMed] [Google Scholar]
- 2.Cox DR. Regression models and life tables. J Roy Stat Soc. 1972;34(3):187–220. [Google Scholar]
- 3.Eicher SA, Clayman GL, Liu TJ, Shillitoe EJ, Storthz KA, Roth JA, Lotan R. Evaluation of topical gene therapy for head and neck squamous cell carcinoma in an organotypic model. Clin Cancer Res. 1996;2(10):1659–1664. [PubMed] [Google Scholar]
- 4.Gomez D, Faucher A, Picot V, Siberchicot F, Renaud-Salis JL, Bussières E, Pinsolle J. Outcome of squamous cell carcinoma of the gingiva: a follow-up study of 83 cases. J Craniomaxillofac Surg. 2000;28(6):331–335. doi: 10.1054/jcms.2000.0177. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics 2003. CA-Cancer J Clin. 2003;53(1):5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 6.Kamps MP, Look AT, Baltimore D. The human t(1; 19) translocation in pre-B ALL produces multiple nuclear E2A-Pbx1 fusion proteins with differing transforming potentials. Genes Dev. 1991;5(3):358–368. doi: 10.1101/gad.5.3.358. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan EL, Meier P. Non-parametric estimation for incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 8.Levi PA, Jr, Kim DM, Harsfield SL, Jacobson ER. Squamous cell carcinoma presenting as an endodontic-periodontic lesion. J Periodontol. 2005;76(10):1798–1804. doi: 10.1902/jop.2005.76.10.1798. [DOI] [PubMed] [Google Scholar]
- 9.Lippman SM, Hong WK. Molecular markers of the risk of oral cavity. N Engl J Med. 2001;344(17):1323–1326. doi: 10.1056/NEJM200104263441710. [DOI] [PubMed] [Google Scholar]
- 10.Longobardi E, Blasi F. Overexpression of PREP-1 in F9 teratocarcinoma cells leads to a functionally relevant increase of PBX-2 by preventing its degradation. J Biol Chem. 2003;278(40):39235–39241. doi: 10.1074/jbc.M304704200. [DOI] [PubMed] [Google Scholar]
- 11.Mann RS, Affolter M. Hox proteins meet more partners. Curr Opin Genet Dev. 1998;8(4):423–429. doi: 10.1016/S0959-437X(98)80113-5. [DOI] [PubMed] [Google Scholar]
- 12.Naora H, Yang YQ, Montz FJ, Seidman JD, Kurman RJ, Roden RB. A serologically identified tumor antigen encoded by a homeobox gene promotes growth of ovarian epithelial cells. PNAS. 2001;98(7):4060–4065. doi: 10.1073/pnas.071594398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phelan ML, Rambaldi I, Featherstone MS. Cooperative interactions between Hox and Pbx proteins mediated by a conserved peptide motif. Mol Cell Biol. 1995;15(8):3989–3997. doi: 10.1128/mcb.15.8.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plowright L, Harrington KJ, Pandha HS, Morgan R. HOX transcription factors are potential therapeutic targets in non-small-cell lung cancer (targeting HOX genes in lung cancer) Br J Cancer. 2009;100(3):470–475. doi: 10.1038/sj.bjc.6604857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Y, Tomita Y, Zhang BL, Nakamichi I, Morii E, Aozasa K. Pre-B-cell leukemia transcription factor 1 regulates expression of valosin-containing protein, a gene involved in cancer growth. Am J Pathol. 2007;170(1):152–159. doi: 10.2353/ajpath.2007.060722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu Y, Morii E, Tomita Y, Zhang BL, Matsumura A, Kitaichi M, Okumura M, Aozasa K. Prognostic significance of pre B cell leukemia transcription factor 2 (PBX2) expression in non-small cell lung carcinoma. Cancer Sci. 2009;100(7):1198–1209. doi: 10.1111/j.1349-7006.2009.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu Y, Song B, Zhao GF, Deng BY, Makino T, Tomita Y, Wang JC, Luo WJ, Doki Y, Aozasa K, et al. Expression level of pre B cell leukemia homeobox 2 (PBX2) correlates with poor prognosis of gastric adenocarcinoma and esophageal squamous cell carcinoma. Int J Oncol. 2010;36(3):651–663. doi: 10.3892/ijo_00000541. [DOI] [PubMed] [Google Scholar]
- 18.Randhawa T, Shameena PM, Sudha S, Nair RG. Squamous cell carcinoma of tongue in a 19-year-old female. Indian J Cancer. 2008;45(3):128–130. doi: 10.4103/0019-509X.44071. [DOI] [PubMed] [Google Scholar]
- 19.Shaw RJ, McGlashan G, Woolgar JA, Lowe D, Brown JS, Vaughan ED, Rogers SN. Prognostic importance of site in squamous cell carcinoma of the buccal mucosa. Br J Oral Maxillofac Surg. 2009;47(5):356–359. doi: 10.1016/j.bjoms.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Sobin LH, Wittekind CH. TNM Classification of Malignant Tumours. 6th Ed. New York, NY: Wiley-Liss; 2002. [Google Scholar]
- 21.Soo KC, Spiro RH, King W, Harvey W, Strong EW. Squamous carcinoma of the gums. Am J Surg. 1988;156(4):281–285. doi: 10.1016/S0002-9610(88)80292-7. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi O, Hamada J, Abe M, Hata S, Asano T, Takahashi Y, Tada M, Miyamoto M, Kondo S, Moriuchi T. Dysregulated expression of HOX and ParaHOX genes in human esophageal squamous cell carcinoma. Oncol Rep. 2007;17(4):753–760. [PubMed] [Google Scholar]
- 23.van Dijk MA, Peltenburg LT, Murre C. Hox gene products modulate the DNA binding activity of Pbx1 and Pbx2. Mech Dev. 1995;52(1):99–108. doi: 10.1016/0925-4773(95)00394-G. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S, Tomita Y, Hoshida Y, Takiguchi S, Fujiwara Y, Yasuda T, Yano M, Nakamori S, Sakon M, Monden M, et al. Expression level of valosin-containing protein is strongly associated with progression and prognosis of gastric carcinoma. J Clin Oncol. 2003;21(13):2537–2544. doi: 10.1200/JCO.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto S, Tomita Y, Hoshida Y, Toyosawa S, Inohara H, Kishino M, Kogo M, Nakazawa M, Murakami S, Iizuka N, et al. Expression level of valosin-containing protein (VCP) as a prognostic marker for gingival squamous cell carcinoma. Ann Oncol. 2004;15(9):1432–1438. doi: 10.1093/annonc/mdh354. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto S, Tomita Y, Hoshida Y, Iizuka N, Monden M, Yamamoto S, Iuchi K, Aozasa K. Expression level of valosin-containing protein (p97) is correlated with progression and prognosis of non-small-cell lung carcinoma. Ann Surg Oncol. 2004;11(7):697–704. doi: 10.1245/ASO.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto S, Tomita Y, Hoshida Y, Iizuka N, Kidogami S, Miyata H, Takiguchi S, Fujiwara Y, Yasuda T, Yano M, et al. Expression level of valosin-containing protein (p97) is associated with prognosis of esophageal carcinoma. Clin Cancer Res. 2004;10(16):5558–5565. doi: 10.1158/1078-0432.CCR-0723-03. [DOI] [PubMed] [Google Scholar]
- 28.Yokoo S, Umeda M, Komatsubara H, Shibuya Y, Komori T. Evaluation of T-classifications of upper gingival and hard palate carcinomas—a proposition for new criterion of T4. Oral Oncol. 2002;38(4):378–382. doi: 10.1016/S1368-8375(01)00077-X. [DOI] [PubMed] [Google Scholar]