Abstract
Paracetamol (PCM) overdose can cause nephrotoxicity with oxidative stress as one of the possible mechanisms mediating the event. In this study, the effects of ethyl acetate extract of Zingiber zerumbet rhizome [200 mg per kg of body weight (mg/kg) and 400 mg/kg] on PCM-induced nephrotoxicity were examined. Rats were divided into five groups containing 10 rats each. The control group received distilled water while other groups were treated with extract alone (400 mg/kg), PCM alone (750 mg/kg), 750 mg/kg PCM+200 mg/kg extract (PCM+ 200-extract), and 750 mg/kg PCM+400 mg/kg extract (PCM+400-extract), respectively, for seven consecutive days. The Z. zerumbet extract was given intraperitoneally concurrent with oral administration of PCM. Treatment with Z. zerumbet extract at doses of 200 and 400 mg/kg prevented the PCM-induced nephrotoxicity and oxidative impairments of the kidney, as evidenced by a significantly reduced (P<0.05) level of plasma creatinine, plasma and renal malondialdehyde (MDA), plasma protein carbonyl, and renal advanced oxidation protein product (AOPP). Furthermore, both doses were also able to induce a significant increment (P<0.05) of plasma and renal levels of glutathione (GSH) and plasma superoxide dismutase (SOD) activity. The nephroprotective effects of Z. zerumbet extract were confirmed by a reduced intensity of renal cellular damage, as evidenced by histological findings. Moreover, Z. zerumbet extract administered at 400 mg/kg was found to show greater protective effects than that at 200 mg/kg. In conclusion, ethyl acetate extract of Z. zerumbet rhizome has a protective role against PCM-induced nephrotoxicity and the process is probably mediated through its antioxidant properties.
Keywords: Zingiber zerumbet, Antioxidant, Oxidative stress, Nephrotoxicity, Paracetamol
1. Introduction
Paracetamol (PCM) or acetaminophen was first discovered in 1889 and is a widely used nonprescriptive analgesic and antipyretic agent (Brown, 1968). PCM toxicity is one of the major causes of poisoning worldwide (Gunnell et al., 2000), and its overdose is commonly associated with hepatic (Nelson, 1995) and renal damages (Placke et al., 1987). PCM toxicity is mediated by the activity of its reactive metabolite known as N-acetyl-p-benzoquinoneimine (NAPQI), which is detoxified by intracellular glutathione (GSH) (Borne, 1995). Therefore, an overdose of PCM will saturate the conjugation pathways of GSH and cause depletion of cellular GSH. This subsequently led to a reduced capacity of GSH to detoxify NAPQI. Increased level of NAPQI mediates oxidative damage, and thus enhances cellular injuries and organ dysfunction, including renal damage (Hart et al., 1994).
Although the occurrence of nephrotoxicity is less common than that of hepatotoxicity as reported by Blakely and McDonald (1995), a number of studies have documented that PCM-induced renal tubular damage and acute renal failure can occur even in the absence of liver damage (Carpenter and Mudge, 1981), and can be the primary manifestation of PCM toxicity (Boutis and Shannon, 2001). As PCM overdose is shown to mediate severe renal damage, which can be life-threatening, antidotes or treatments that could offer maximum protection against PCM-induced renal injury are therefore required. To date, N-acetyl-cysteine (NAC) has been used clinically for the treatment of PCM toxicity (Dilger and Baker, 2007).
Current evidence suggests that PCM-induced hepatorenal injury is mediated by oxidative damage (Das et al., 2010; Kheradpezhouh et al., 2010). Therefore, the alternative treatments for PCM toxicity could be achieved using a natural compound with antioxidant activity. Zingiber zerumbet (L.) Smith, or locally known as lempoyang or wild ginger, belonging to the Zingiberaceae family (Saadiah and Halijah, 1995) has been shown to possess a number of biological activities, including anti-cancer (Huang et al., 2005; Rashid et al., 2005; Sharifah Sakinah et al., 2007; Abdul et al., 2008), anti-inflammatory (Jaganath and Ng, 2000; Somchit and Shukriyah, 2003), antimicrobial (Abdul et al., 2008; Kaderi et al., 2010), and antioxidant properties (Ruslay et al., 2007). Z. zerumbet has been shown to contain flavonoid compounds that exhibit the antioxidant properties (Pietta, 2000), and the ethyl acetate extract of the plants has been shown to exhibit strong antioxidant activities (Ruslay et al., 2007). In a recent study, zerumbone, which is the active compound of the Z. zerumbet rhizome, has been shown to protect against cisplatin-induced renal dysfunction by preventing lipid peroxidation and preserving antioxidant (Ibrahim et al., 2010).
In this study, we determined the potential protective effects and antioxidant activities of ethyl acetate extract of the Z. zerumbet rhizome against PCM-induced nephrotoxicity. The effects were determined by measuring the levels of plasma creatinine (indicator of renal function), endogenous antioxidants [GSH and superoxide dismutase (SOD)], and oxidative stress markers [malondialdehyde (MDA), advanced oxidation protein product (AOPP), and protein carbonylation], and by histological change analysis.
2. Materials and methods
2.1. Plant materials
Fresh rhizomes (7 kg) of Z. zerumbet were collected from Temerloh, Pahang, Malaysia and authenticated by a plant taxonomist at Department of Botany, Faculty of Science and Technology, Universiti Kebangsaan Malaysia (UKM), and were deposited as a voucher specimen at the herbarium of UKM, Bangi, Selangor, Malaysia. The specimen was cleaned and chopped into small pieces and then air-dried at room temperature for three days.
2.2. Plant extraction
The air-dried rhizomes of Z. zerumbet were sequentially soaked at room temperature in n-hexane, ethyl acetate, and then methanol for 72 h. The resultant extracts were filtered and evaporated to dryness in vacuo to yield crude extracts of hexane, ethyl acetate, and methanol. All the crude extracts were stored at 4 °C until tested for bioassay. Prior to use, the Z. zerumbet ethyl acetate extract was dissolved in dimethyl sulfoxide (DMSO) and diluted in phosphate buffer saline (PBS; pH 7.4).
2.3. Experimental protocol
All procedures involving the use of laboratory animals were reviewed and approved by the Animal Ethics Committee of UKM. Fifty male Sprague-Dawley rats (230–250 g) were obtained from the UKM Animal Resource Unit. The animals were housed in a controlled environment with room temperature and a 12-h light-dark cycle. Animals were fed mouse pellet and fresh water ad libitum for a week prior to experiments. Rats were randomly divided into five groups containing 10 animals each and all treatments were given daily for seven days. PCM was administered orally, while Z. zerumbet extracts at 200 and 400 mg per kg of body weight (mg/kg) were delivered intraperitoneally concurrent with PCM administration. The selected doses of Z. zerumbet extract were based on Hemabarathy et al. (2009) who showed the hepatoprotective effects of aqueous extracts of Alpina Galanga (Zingiberaceae family) on the PCM-induced hepatotoxicity. Rats in Group I served as the control group and were administered distilled water only. Groups II received 400 mg/kg Z. zerumbet extract alone, while Group III received 750 mg/kg PCM alone. In Group IV, rats were treated with 750 mg/kg PCM and 200 mg/kg Z. zerumbet extract. Meanwhile, rats in Group V were treated with 750 mg/kg PCM and 400 mg/kg Z. zerumbet extract. On Day 8, all animals were weighed and anaesthetized with diethyl ether.
2.4. Sample preparation
Blood was collected via cardiac puncture using a 10 ml syringe and 25G needle after 24 h of last treatment. Blood was collected in EDTA tubes and plasma was obtained after centrifugation at 3 000 r/min for 10 min at 4 °C. Plasma sample was stored at −20 °C until further analysis. The kidney was obtained after the rat was sacrificed, washed in ice-cold 11.5 g/L KCl to remove blood, and quickly blotted and weighed on digital scale (B303 Mettler-Toledo, Switzerland). For histological studies, the kidney was preserved in 10% formalin while for biochemical analysis; the kidney was minced into small pieces and homogenate with 11.5 g/L KCl in 4 ml/g ratio using homogenizers (Ultra Turrax T25).
2.5. Biochemical analysis
Creatinine level was determined according to Jaffe’s reaction, based on creatinine reaction with picric acid in alkaline solution to form a red solution measured spectrophotometrically at 520 nm (Slot, 1965). The level of creatinine was extrapolated from the standard graph and the values were then stated as milligram per deciliter (mg/dl).
SOD activity was assayed according to the method as described by Beyer and Fridovich (1987) based on the reaction of SOD with nitrotetrazolium blue chloride (NBT·2HCl), Triton-X 100 and riboflavin and the absorbance was measured with a spectrophotometer at 560 nm. One unit (1 U) of SOD was defined as the amount of SOD required for 50% inhibition of NBT·2HCl reduction.
GSH was quantified using 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) according to the protocols as described by Ellman (1959). The reaction between GSH and DTNB formed a yellow-colored complex that was measured spectrophotometrically at 420 nm.
MDA was determined based on the production of thiobarbituric acid reactive substances (TBARS), the absorbance of which was measured spectrophotometrically at 532 nm (Esterbauer and Cheeseman, 1990). The concentration of TBARS was determined from the extrapolated standard curve of serially diluted 1,1,3,3-tetraethoxypropane (TEP). The final concentration of MDA was then expressed in nmol/g protein.
Protein carbonyls were estimated according to the procedure as described by Levine et al. (1990) based on the reaction of carbonyl compounds with 2,4-dinitrophenyl hydrazine (2,4-DNPH) for 1 h and precipitations with 20% trichloroacetic acid (TCA, 0.2 kg/L). The released carbonyl compounds were measured by spectrophotometer at 380 nm. The amounts of carbonyls are calculated based on the total amount of protein contents in each sample.
AOPP of renal homogenate was measured according to Witko-Sarsat et al. (1996). Renal homogenate was centrifuged at 2 500 r/min for 10 min at 40 °C. The supernatant was then added to a reaction mixture containing 50% acetic acid and 1.16 mol/L potassium iodide (KI) in PBS solution. The absorbance was recorded at 340 nm and the concentration of AOPP was determined from the extrapolated standard curve of serially diluted AOPP standard solution using 500 μmol/L chloramines stock.
2.6. Histological analysis
Examination of renal histology was performed according to routine histology techniques. Briefly, after the animal was sacrificed, the kidney was harvested, rinsed in normal saline, and sectioned into small pieces. The sectioned tissue was then fixed in 10% formalin, dehydrated in stepwise with increasing concentration of ethanol solution (50% to 100%), and embedded in paraffin. Using a microtome, tissue sections of 4-µm thickness were produced, fixed overnight on the slide, subsequently stained with hematoxylin and eosin (H&E), and were then observed under a light microscope (Olympus BX41, Japan).
2.7. Statistical analysis
Statistical analysis was done using SPSS version 18.0. Data were analyzed using Shapiro-Wilk normality test and one way analysis of variance (ANOVA) was used for comparison between groups followed by post-hoc Tukey test. Data were expressed as mean±standard deviation (SD) and P<0.05 showed statistically significance.
3. Results
3.1. Effects of Z. zerumbet extract on body weight, kidney weight, and food and water intake
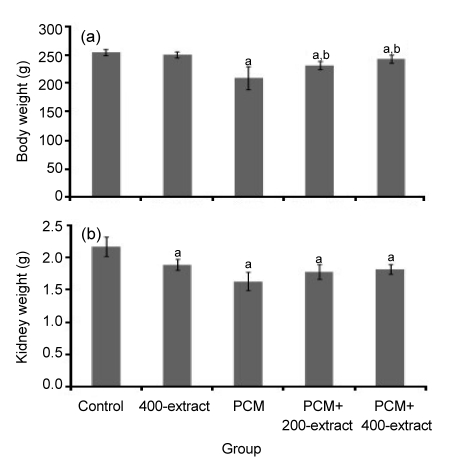
In all experimental groups, no mortality was observed during the period of the study. Reduced water intake and food consumption, as well as reduced body weight, were observed in PCM group as compared to control (Fig. 1a). In contrast, groups receiving PCM concurrent with administration of either 200 or 400 mg/kg of Z. zerumbet extract weighed significantly higher (P<0.05) than PCM group. Meanwhile, no significant alteration of body weight was recorded from rats treated with 400 mg/kg of extract only. Furthermore, the kidney weight was significantly lower (P<0.05) in all treated groups as compared to the control, and the reduction was markedly noticeable in the PCM group (Fig. 1b).
Fig. 1.
Effects of ethyl acetate extract of Z. zerumbet on body weight (a) and kidney weight (b) of rats after seven days of treatments
Each column represents mean±SD (n=10). a P<0.05 versus control group; b P<0.05 versus PCM group. 400-extract, PCM, PCM+200-extract, and PCM+400-extract groups were treated with 400 mg/kg extract alone, 750 mg/kg PCM alone, 750 mg/kg PCM+200 mg/kg extract, and 750 mg/kg PCM+400 mg/kg extract, respectively
3.2. Effect of Z. zerumbet extract on creatinine level
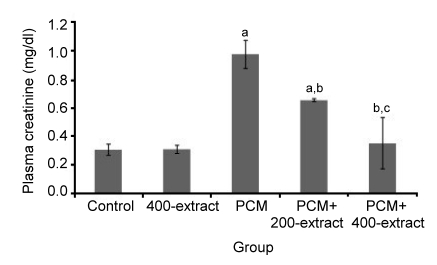
A significantly increased (P<0.05) level of plasma creatinine was observed in plasma samples of the PCM group (Fig. 2). However, supplementation with Z. zerumbet extract at 200 and 400 mg/kg significantly prevented (P<0.05) further elevations of creatinine, with obvious effects observed in the PCM+400 mg/kg extract group. There were no nephrotoxic effects observed in rats administered 400 mg/kg of the Z. zerumbet extract alone, as evidenced by no significant difference in plasma creatinine levels as compared to the control group.
Fig. 2.
Effects of ethyl acetate extract of Z. zerumbet on plasma creatinine in rats after seven days of treatments
Each column represents mean±SD (n=10). a P<0.05 versus control group; b P<0.05 versus PCM group; c P<0.05 versus PCM+200-extract group
3.3. Effect of Z. zerumbet extract on antioxidant status
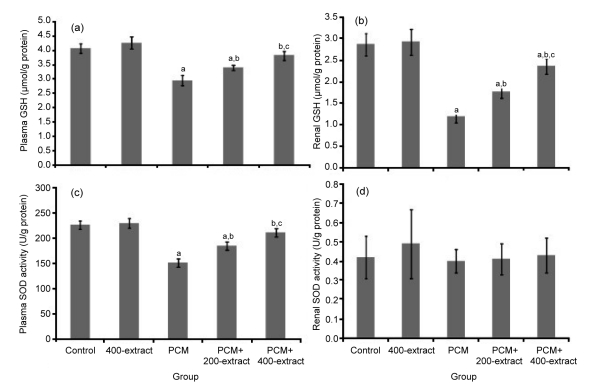
Administration of PCM for seven consecutive days caused a significant reduction (P<0.05) of GSH level in both plasma and renal homogenate (Figs. 3a and 3b). In contrast, administrations with either 200 or 400 mg/kg of Z. zerumbet extract significantly prevented (P<0.05) PCM-induced GSH reductions, with greater effects demonstrated in the PCM+400 mg/kg extract group, which was able to maintain the level of GSH despite the presence of PCM. Furthermore, supplementation of 400 mg/kg of Z. zerumbet extract alone showed no significant difference in plasma and renal levels of GSH as compared to the control group.
Fig. 3.
Effects of ethyl acetate extract of Z. zerumbet on the levels of plasma GSH (a), renal GSH (b), plasma SOD activity (c), and renal SOD activity (d) in rats after seven days of treatments
Each column represents mean±SD (n=10). a P<0.05 versus control group; b P<0.05 versus PCM group; c P<0.05 versus PCM+200-extract group
In addition to the GSH level, the activity of SOD enzymes in plasma and renal tissue was also determined. The group treated with PCM alone showed significantly lower (P<0.05) plasma SOD activity as compared to the control (Fig. 3c). Administration of extracts at both doses significantly increased (P<0.05) plasma SOD activity than the PCM group. In addition, supplementation with 400 mg/kg extract together with PCM further increased plasma SOD activity than the PCM+200 mg/kg extract groups, and its plasma SOD activity was almost similar as that in the control group. In contrast to the activity of plasma SOD, the renal homogenate SOD activity did not show any significant difference among all experimental groups (Fig. 3d).
3.4. Effect of Z. zerumbet extract on oxidative stress
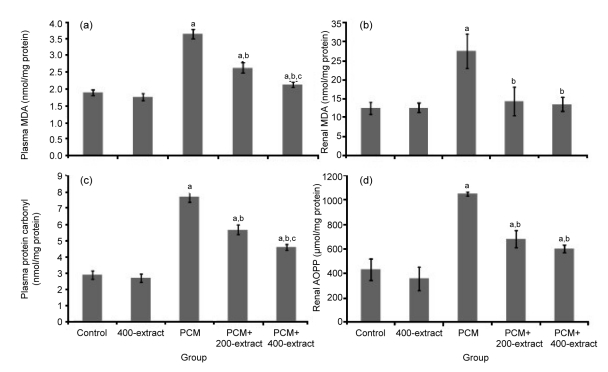
Administration of PCM for seven consecutive days induced oxidative stress with a significant increase (P<0.05) in the levels of renal homogenate and plasma MDA, plasma protein carbonyl, and renal AOPP (Fig. 4). Concurrent administration of PCM and Z. zerumbet extract at 200 and 400 mg/kg, however, reduced the oxidative stress, as evidenced by significantly reduced (P<0.05) level of renal homogenate and plasma MDA, plasma protein carbonyl, and renal AOPP than those in the PCM group. Interestingly, administration of both doses brought the MDA level of renal homogenate nearly to the value of the control group. In addition, no significant induction of oxidative stress was observed in rats administered with 400 mg/kg of the extract alone, as evidenced by no significant difference in the value of renal homogenate and plasma MDA, plasma protein carbonyl, and renal AOPP than in control.
Fig. 4.
Effects of ethyl acetate extract of Z. zerumbet on the levels of plasma MDA (a), renal MDA (b), plasma protein carbonyl (c), and renal AOPP (d) in rats after seven days of treatments
Each column represents mean±SD (n=10). a P<0.05 versus control group; b P<0.05 versus PCM group, c P<0.05 versus PCM+200-extract group
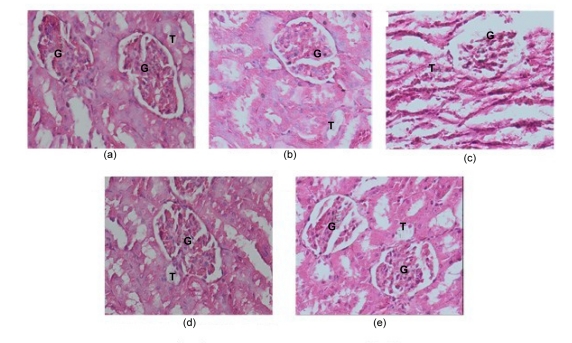
3.5. Effect of Z. zerumbet extract on histological features of the kidney
The histological features of the kidneys from various treatments groups are as presented in Fig. 5. Renal sections from the control and 400 mg/kg Z. zerumbet group showed no histological changes, as evidenced by the normal appearance of glomeruli and tubules (Figs. 5a and 5b). In contrast, kidney sections from the PCM group exhibited altered architecture with extensive destruction of glomeruli and tubular structures, as demonstrated by marked necrotic areas (Fig. 5c). On the other hand, administration of Z. zerumbet extract at 200 (Fig. 5d) or 400 mg/kg (Fig. 5e) concurrent with PCM administration, showed protective effects on the kidney, as evidenced by the less severe tubular and glomerular damages, with better protection being observed in the PCM+400 mg/kg extract group. These histological observations were in line with biochemical findings and all these results support each other.
Fig. 5.
Kidney sections of each group (hematoxylin and eosin stained)
(a) Control group showing normal appearance of glomeruli (G) and tubules (T); (b) 400 mg/kg extract group exhibiting normal features of glomeruli (G) and tubules (T); (c) PCM group showing severe glomerular (G) and tubular damages (T); (d) PCM+200 mg/kg extract group showing moderate glomerular (G) and tubular destruction (T); (e) PCM+400 mg/kg extract group showing almost normal appearance of glomeruli (G) and tubules (T)
4. Discussion
PCM-induced nephrotoxicity has been previously documented by a number of studies (Mitchell et al., 1977; McMurtry et al., 1978; Newton et al., 1983). In this study, we demonstrated that the administration of 750 mg/kg PCM for seven consecutive days was able to induce nephrotoxicity in rats, as demonstrated by the significantly increased level of plasma creatinine. The nephrotoxic effect of PCM was further confirmed by the severely destructed renal tissue, as shown in the histological analysis. This finding is in accordance with that of Abdel-Zaher et al. (2007) who showed that the administration of PCM at 750 mg/kg induced nephrotoxicity in rats. Administration of ethyl acetate extract of Z. zerumbet concurrent with PCM exposure prevented PCM-induced nephrotoxicity. Furthermore, the protective effect was found to be more remarkable in the PCM+400 mg/kg extract group than in the PCM+200 mg/kg extract group.
To date, a number of studies have suggested the involvement of oxidative stress as the mediator of PCM-induced nephrotoxicity (Das et al., 2010; Kheradpezhouh et al., 2010; Yousef et al., 2010). Hart et al. (1994) pointed out that PCM toxicity was shown to enhance NAPQI production. The accumulated NAPQI induces greater formation of free radicals, which are responsible in mediating cellular damages and renal toxicity.
Elevated levels of MDA in tissue have been regarded as an indicator for cellular damage due to excess lipid peroxidation processes that occur during malfunction of the antioxidant defense system (Kaplowitz, 2000). In our study, administration of 750 mg/kg PCM caused significant elevations of plasma and renal MDA. In contrast, treatment with ethyl acetate extract of Z. zerumbet abrogated this elevation with greater protection being shown with the 400 mg/kg extract. The ability of Z. zerumbet to protect lipid peroxidation is in agreement with Ruslay et al. (2007), who demonstrated that ethyl acetate fractions of Z. zerumbet possess remarkable lipid peroxidation inhibition and radical scavenging activities. Pietta (2000) also showed that the ethyl acetate fraction of Z. zerumbet contains flavonoid glycosides known as afzelin and diacetylafzelin, and derivatives of kaempferol, a 3-flavonol that was known to possess antioxidant property. In addition, Ibrahim et al. (2010) reported that pretreatment with zerumbone, a natural compound isolated from fresh rhizomes of Z. zerumbet protected against cisplatin-induced nephrotoxicity by attenuating the production of MDA and simultaneously promoting GSH production. Therefore, we believe that the effects manifested by the crude ethyl acetate extract of Z. zerumbet to overcome PCM-induced nephrotoxicity in our study were mediated by the presence of these bioactive compounds.
Excess reactive oxygen species (ROS) can promote protein oxidation, forming protein carbonyls (Chevion et al., 2000; Margetis et al., 2009) and AOPP, which have been described as reliable markers to estimate the degree of oxidant-mediated protein damage (Alderman et al., 2002). The significant elevation of plasma protein carbonyls and renal homogenate AOPP levels in the PCM group indicates the presence of oxidative stress and upregulation of NAPQI production, which stimulates protein oxidations. Concurrent delivery of Z. zerumbet extract at both doses, however, reduced significantly the production of plasma protein carbonyls and renal homogenate AOPP, and this demonstrates the capacity of the Z. zerumbet extract to overcome ROS production, presumably through its antioxidants property.
GSH is a well-known non-enzymatic antioxidant that is responsible as the frontier defense mechanism during oxidative stress and the depletion of intracellular GSH in a prolonged oxidative stress (Kaplowitz, 2000). As demonstrated in our findings, a significant decline in both plasma and renal homogenate GSH levels in PCM-treated rats is in line with the previous finding that oral administration of 750 mg/kg PCM to rats for seven days reduced renal cellular contents of GSH (Abdel-Zaher et al., 2007). The administration of Z. zerumbet ethyl acetate extract at 200 and 400 mg/kg preserved GSH levels and the effects were remarkably effective especially in rats received 400 mg/kg Z. zerumbet extract. Ibrahim et al. (2010) reported that zerumbone compound in Z. zerumbet extract indirectly induces the biosynthesis of GSH and provides a protective intracellular mechanism, presumably as a free radical scavenger for toxic agents. Hence, we can speculate that the Z. zerumbet extract in our study may induce the synthesis of endogenous GSH, which protects against PCM-induced oxidative damage and greater effects at 400 mg/kg treated rats are presumably conferred through higher amounts of zerumbone present in the extracts.
Administration of the Z. zerumbet extract at 200 and 400 mg/kg was again able to maintain the SOD activity during PCM overdose. Improvement in SOD activities does provide evidence to an overall improvement in endogenous antioxidant defense system (Hemabarathy et al., 2009). This relates to the fact that Z. zerumbet has been shown to have high antioxidant activities through induction of the endogenous antioxidants that reduce free radical activity (Nakamura et al., 2004). In contrast, SOD activities measured from renal homogenate were not significantly different among all groups. This finding is supported by Hemabarathy et al. (2009) who demonstrated that no significant alteration of liver SOD levels was observed during PCM-induced hepatotoxicity. This finding suggests that GSH is responsible as the foremost antioxidant defense mechanism against PCM-induced oxidative damage, and the significant alteration of GSH levels will appear before the depletion of SOD activities.
As demonstrated in this study, rats exposed to PCM in the absence of the Z. zerumbet extract showed severe cellular damage. In contrast, concurrent treatment with the Z. zerumbet extract managed to protect against severe cellular lesions, with only mild or moderate degeneration of glomerular and tubular kidney cells observed. In agreement with biochemical parameters, the best protection was manifested in rats treated with 400 mg/kg of the extract. Finally, it is also demonstrated that administration of ethyl acetate extract of Z. zerumbet at 400 mg/kg in rats possessed no nephrotoxicity effects, as evidenced by biochemical and histological analyses. However, despite no nephrotoxic evidence, rats administered with 400 mg/kg extract of Z. zerumbet alone showed significantly reduced kidney weight as compared to control rats. Therefore, at this stage, we cannot ascertain the definitive reason for the above condition; however, we suggest that the Z. zerumbet extract, when administered at 400 mg/kg, may affect the kidney without remarkable alteration of renal histology. Thus, the administration of the Z. zerumbet extract at 400 mg/kg or higher in rats may possess a risk of toxicity, and further study is required to elucidate this issue.
5. Conclusions
The administration of Z. zerumbet ethyl acetate extract was found to have protective effects and antioxidant activities against PCM-induced nephrotoxicity, as evidenced by the biochemical status and histological findings. The most remarkable effects were observed when the Z. zerumbet extract was delivered at 400 mg/kg as compared to a lower dose of 200 mg/kg of the extract. This indicates that the amount of antioxidant compounds present in Z. zerumbet contributes significantly to its antioxidant property. Together, the absence of renal damage and supportive evidence of its antioxidant properties may suggest the potential applications of Z. zerumbet as an alternative antidote against PCM-induced nephrotoxicity, as well as a novel antioxidant.
Acknowledgments
The authors are grateful to the Faculty of Health Sciences (FSK), Universiti Kebangsaan Malaysia for financial assistance. The authors would like to acknowledge everyone in the faculty who contributed directly or indirectly to this research.
References
- 1.Abdel-Zaher AO, Abdel-Rahman MM, Hafez MM, Omran FM. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2007;234(1-2):124–134. doi: 10.1016/j.tox.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Abdul AB, Abdelwahab SI, Al-Zubairi AS, Elhassan MM, Murali SM. Anticancer and antimicrobial activities of zerumbone from the rhizomes of Zingiber zerumbet . Int J Pharmacol. 2008;4(4):301–304. doi: 10.3923/ijp.2008.301.304. [DOI] [Google Scholar]
- 3.Alderman CJ, Shah S, Foreman JC, Chain BM, Katz DR. The role of advanced oxidation protein products in regulation of dendritic cell function. Free Radic Biol Med. 2002;32(5):377–385. doi: 10.1016/S0891-5849(01)00735-3. [DOI] [PubMed] [Google Scholar]
- 4.Beyer W, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161(2):559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 5.Blakely P, McDonald BR. Acute renal failure due to acetaminophen ingestion: a case report and review of the literature. J Am Soc Nephrol. 1995;6(1):48–53. doi: 10.1681/ASN.V6148. [DOI] [PubMed] [Google Scholar]
- 6.Borne RF. Nonsteroidal Anti-Inflammatory Drugs. In: Foye WO, Lemke TL, Williams DA, editors. Principles of Medicinal Chemistry. Williams & Wilkins; 1995. pp. 535–580. [Google Scholar]
- 7.Boutis K, Shannon M. Nephrotoxicity after acute severe acetaminophen poisoning in adolescents. Clin Toxicol. 2001;39(5):441–445. doi: 10.1081/CLT-100105413. [DOI] [PubMed] [Google Scholar]
- 8.Brown RA. Hepatic and renal damage with paracetamol overdosage. J Clin Pathol. 1968;21(6):793. doi: 10.1136/jcp.21.6.793-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter HM, Mudge GH. Acetaminophen nephrotoxicity: studies on renal acetylation and deacetylation. J Pharmacol Exp Ther. 1981;218(1):161–167. [PubMed] [Google Scholar]
- 10.Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic Res. 2000;11(33):S99–S108. [PubMed] [Google Scholar]
- 11.Das J, Ghosh J, Manna P, Sil PC. Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology. 2010;269(1):24–34. doi: 10.1016/j.tox.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Dilger RN, Baker DH. Oral N-acetyl-L-cysteine is a safe and effective precursor of cysteine. J Anim Sci. 2007;85(7):1712–1718. doi: 10.2527/jas.2006-835. [DOI] [PubMed] [Google Scholar]
- 13.Ellman GL. Tissue sulphydryl groups. Arch Biochem. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 14.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Meth Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-H. [DOI] [PubMed] [Google Scholar]
- 15.Gunnell D, Murray V, Hawton K. Use of paracetamol (acetaminophen) for suicide and nonfatal poisoning: worldwide patterns of use and misuse. Suicide Life Threat Behav. 2000;30(4):313–326. [PubMed] [Google Scholar]
- 16.Hart SG, Beierschmitt WP, Wyand DS, Khairallah EA, Cohen SD. Acetaminophen nephrotoxicity in CD-1 mice. I. Evidence of a role for in situ activation in selective covalent binding and toxicity. Toxicol Appl Pharmacol. 1994;126(2):267–275. doi: 10.1006/taap.1994.1116. [DOI] [PubMed] [Google Scholar]
- 17.Hemabarathy B, Budin SB, Feizal V. Paracetamol hepatoxicity in rats treated with crude extract of Alpinia galanga . J Biol Sci. 2009;9(1):57–62. doi: 10.3923/jbs.2009.57.62. [DOI] [Google Scholar]
- 18.Huang GC, Chien TY, Chen LG, Wang CC. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005;71(3):219–224. doi: 10.1055/s-2005-837820. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim MY, Abdul AB, Ibrahim TA, AbdelWahab SI, Elhassan MM, Mohan S. Attenuation of cisplatin-induced nephrotoxicity in rats using zerumbone. Afr J Biotechnol. 2010;9(28):4434–4441. [Google Scholar]
- 20.Jaganath IB, Ng LT. Herbs: The Green Pharmacy of Malaysia. , Malaysia: Vinpress Sdn. Bhd; 2000. pp. 95–99. [Google Scholar]
- 21.Kaderi MG, Habib MR, Nikkon F, Yeasmin T, Rashid MA, Rahman MM, Gibbons S. Zederone from the rhizomes of Zingiber zerumbet and its antistaphylococcal activity. Latin Am Caribbean Bull Med Arom Plants. 2010;9(1):63–68. (in Spanish) [Google Scholar]
- 22.Kaplowitz N. Mechanism of liver cell injury. J Hepatol. 2000;32(S1):39–47. doi: 10.1016/S0168-8278(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 23.Kheradpezhouh E, Panjehshahin MR, Miri R, Javidnia K, Noorafshan A, Monabati A, Dehpour AR. Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N-acetyl cysteine. Eur J Pharmacol. 2010;628(1-3):274–281. doi: 10.1016/j.ejphar.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Levine RL, Williams J, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Meth Enzymol. 1990;233:346–357. doi: 10.1016/S0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 25.Margetis PI, Antonelou MH, Petropoulos IK, Margaritis LH, Papassideri IS. Increased protein carbonylation of red blood cell membrane in diabetic retinopathy. Exp Mol Pathol. 2009;87(1):76–82. doi: 10.1016/j.yexmp.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 26.McMurtry RJ, Snodgrass WR, Mitchell JR. Renal necrosis, glutathione depletion, and covalent binding after acetaminophen. Toxicol Appl Pharmacol. 1978;46(1):87–100. doi: 10.1016/0041-008X(78)90139-4. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell JR, McMurtry RJ, Statham CN, Nelson SD. Molecular basis for several drug-induced nephropathies. Am J Med. 1977;62(4):518–526. doi: 10.1016/0002-9343(77)90407-7. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Yoshida C, Murakami A, Ohigashi H, Osawa T, Uchida K. Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes. FEBS Lett. 2004;572(1-3):245–250. doi: 10.1016/j.febslet.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 29.Nelson SD. Mechanisms of the formation and disposition of reactive metabolites that can cause acute liver injury. Drug Metab Rev. 1995;27(1-2):147–177. doi: 10.3109/03602539509029821. [DOI] [PubMed] [Google Scholar]
- 30.Newton JF, Yoshimoto M, Bernstein J, Rush GF, Hook JB. Acetaminophen nephrotoxicity in the rat. 1. Strain differences in nephrotoxicity and metabolism. Toxicol Appl Pharmacol. 1983;69(2):291–306. doi: 10.1016/0041-008X(83)90311-3. [DOI] [PubMed] [Google Scholar]
- 31.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 32.Placke ME, Wyand DS, Cohen SD. Extrahepatic lesions induced by acetaminophen in the mouse. Toxicol Pathol. 1987;15(4):381–387. doi: 10.1177/019262338701500401. [DOI] [PubMed] [Google Scholar]
- 33.Rashid RA, Hawariah A, Pihie L. The antiproliferative effects of Zingiber zerumbet extracts and fractions on the growth of human breast carcinoma cell lines. Malays J Pharma Sci. 2005;3(1):45–52. [Google Scholar]
- 34.Ruslay S, Abas F, Shaari K, Zainal Z, Maulidiani , Sirat H, Israf DA, Lajis NH. Characterization of the components present in the active fractions of health gingers (Curcuma xanthorrhiza and Zingiber zerumbet) by HPLC-DAD-ESIMS. Food Chem. 2007;104(3):1183–1191. doi: 10.1016/j.foodchem.2007.01.067. [DOI] [Google Scholar]
- 35.Saadiah MS, Halijah I. Zingiberaceae; Proceedings of the National Convention on Herbal Medicine; Kuala Lumpur: Forest Research Institute Malaysia; 1995. pp. 205–207. [Google Scholar]
- 36.Sharifah Sakinah SA, Handayani ST, Azimahtol LP. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Inter. 2007;7(1):4. doi: 10.1186/1475-2867-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slot C. Plasma creatinine determination. A new & specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17(4):381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 38.Somchit MN, Shukriyah MH. Anti-inflammatory property of ethanol and water extracts of Zingiber zerumbet . Ind J Pharmacol. 2003;35:181–182. [Google Scholar]
- 39.Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers AT, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49(5):1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 40.Yousef MI, Omar SA, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol. 2010;48(11):3246–3261. doi: 10.1016/j.fct.2010.08.034. [DOI] [PubMed] [Google Scholar]