Abstract
Osteochondral allografting has been proved to be a useful method to treat diseased or damaged areas of joint surfaces. Operational long-term stocks of grafts which supply a buffer between procurement and utilization would contribute to the commercialization or industrialization of this technology. Vitrification has been thought to be a promising method for successful preservation of articular cartilage (AC), but high concentration cryoprotectants (CPAs) are used which may cause high cellular toxicity. An effective way to reduce CPA toxicity is to increase CPA concentration gradually while the temperature is lowered. Understanding the mechanism of CPA permeation at subzero temperatures is important for designing the cryopreservation protocol. In this research, the permeation of dimethyl sulfoxide (Me2SO) in ovine AC at subzero temperatures was studied experimentally. Pretreated AC discs were exposed in Me2SO solutions for different time (0, 5, 15, 30, 50, 80, and 120 min) at three temperature levels (−10, −20, and −30 °C). The Me2SO concentration within the tissue was determined by ultraviolet (UV) spectrophotometry. The diffusion coefficients were estimated to be 0.85×10−6, 0.48×10−6, and 0.27×10−6 cm2/s at −10, −20, and −30 °C, respectively, and the corresponding activation energy was 29.23 kJ/mol. Numerical simulation was performed to compare two Me2SO addition protocols, and the results demonstrated that the total loading duration could be effectively reduced with the knowledge of permeation kinetics.
Keywords: Articular cartilage, Vitrification, Dimethyl sulfoxide, Permeation, Diffusion coefficient, Subzero temperature
1. Introduction
Articular cartilage (AC) is vital for normal joint function. Unlike other tissues, traumatic or degenerative cartilages do not heal well. Cartilage is described as an immunologically privileged tissue, and fresh osteochondral allografts have been proved to be effective and functional for transplantation (Chu et al., 1999; Aubin et al., 2001; Williams et al., 2007; Raikin, 2009). Furthermore, a recent study showed that fresh osteochondral autograft and fresh allograft tissues are not statistically different with respect to bony incorporation, AC composition, and biomechanical properties up to six months after implantation (Glenn et al., 2006). However, this kind of treatment is limited by the scarcity of healthy grafts and the time difference between donation and clinical use, which necessitates the construction of an osteochondral allograft bank to preserve the AC that is from cadaver tissue donors or which might be manufactured by tissue engineering in the future.
Ice-free cryopreservation, or vitrification, has been considered as a promising method for the long-term storage of biological tissues. Vitrification refers to the amorphous solidification of a liquid without crystallization. This solidification retains the normal molecular and ionic distribution of the tissue. Generally, vitrification is accomplished through the addition of a single cryoprotectant (CPA) or CPA mixtures at high concentration plus rapid cooling and thawing rates to minimize nucleation and ice-crystal growth (Rall and Fahy, 1985; Song et al., 2000; Wusteman et al., 2008; Xu et al., 2009). This type of vitrification of AC has been studied by several research groups (Song et al., 2004a; 2004b; Brockbank et al., 2010). Recently, as an alternative, Pegg et al. (2006c) proposed a novel approach called “liquidus-tracking” (LT) method to vitrify cartilage samples. The ability of post-thawing cartilage specimens to incorporate sulfate (35S) into newly synthesized glycosaminoglycans (GAGs) was found to approach 70% of that of fresh control groups. In further work, this data reached 87% (Wang et al., 2007).
The knowledge of CPA permeation kinetics in the biological tissues is important for designing the CPA addition/removal protocols despite what kind of vitrification is used. The CPA permeation in AC at above zero temperatures has been reported in a few studies. Mukherjee et al. (2008) studied the diffusions of high concentration single CPA [6.9 mol/L, dimethyl sulfoxide (Me2SO)] and multi-component CPA mixture (VS55, 3.1 mol/L Me2SO+2.2 mol/L 1,2-propanediol+3.1 mol/L formamide) in immature bovine AC. Sharma et al. (2007) and Jomha et al. (2009) examined the time-dependent permeations of Me2SO, propylene glycol (PG), ethylene glycol (EG), and glycerol into intact porcine AC at concentrations suitable for vitrification. Carsi et al. (2004) determined the diffusion coefficients of Me2SO and glycerol in human AC at different temperatures and concentrations. The permeation of CPA in AC at subzero temperatures, however, has not been reported except when Pegg et al. (2006b) measured the concentrations of Me2SO in AC at the end of each processing step to ensure that the AC sample was not frozen. However, the diffusion coefficients were not determined, and the CPA addition/removal protocols were more or less empirical.
The successful prediction of CPA permeation into AC at subzero temperatures will aid in minimizing cell death due to CPA toxicity, osmotic damage, and ice-crystal formation when using the LT method. In this paper, the experiment was elaborately designed to obtain the apparent diffusion coefficients of Me2SO in isolated ovine AC at three subzero temperatures (−10, −20, and −30 °C), and the Arrhenius relation was used to express the manner in which the apparent diffusion coefficient changes with temperature. Finally, the use of these permeation kinetics in optimizing the Me2SO addition protocol of the LT method was discussed.
2. Materials and methods
2.1. Chemicals and solutions
Unless otherwise specified, all chemicals used were of analytical grade and all solutions were prepared with deionized water. The main solutions were prepared as follows. A standard Me2SO stock solution (100 mg/L) was prepared by dissolving 100 mg of Me2SO (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) in deionized water, which was then used to prepare working Me2SO standards (18 to 60 mg/L with an increment of 6 mg/L) through suitable dilutions. The Me2SO solutions of varied concentrations used in pre-equilibration and exposure (see Section 2.5) were prepared with a N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulfonic acid] (HEPES)-buffered carrier solution (145 mmol/L Na+, 3.3 mmol/L K+, and 20 mmol/L HEPES) instead of Dulbecco’s phosphate buffer solution (PBS) to avoid phosphate precipitation (Wusteman et al., 2002). The NaCl and KCl were supplied by Sinopharm Chemical Reagent Co. Ltd., Shanghai, China, and the HEPES was from Amresco, USA.
2.2. Calibration curve
The sulphoxide functional group present in Me2SO provides a potential avenue of detection of this substance by ultraviolet (UV) absorption. The absorbance of the Me2SO standard solutions (see Section 2.1) was measured using a TU-1810PC UV-vis spectrophotometer (Purkinje General Instrument Co. Ltd., Beijing, China) at 208 nm. Absorbance (A) was plotted as a function of the Me2SO concentration (C, mg/L), and a linear regression equation was obtained, expressed as A=0.010 89+0.010 52C with R 2=0.999.
2.3. Specimen preparation
AC samples were obtained from the back knee joints of approximately 12-month-old lambs that were killed for food in a local commercial abattoir. AC discs without bone (6 mm diameter, 0.6‒1.0 mm thickness) were taken from the weight-bearing portion using a corneal trephine and a scalpel, and weighed. They were then stored in ice-cold HEPES-buffered medium (see Section 2.1) for later use. In this research, only AC discs without bone attached were used, since studies (Xia et al., 1994; Sharma et al., 2007) have shown that isolated cartilage discs exhibit identical diffusion characteristics as those with bone attached.
2.4. Experimental setup
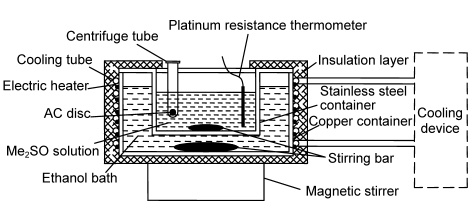
Fig. 1 shows the setup for the subzero permeation experiment. The fixed temperatures needed (−10, −20, and −30 °C) were provided by a custom-built cooling device. The temperature of the Me2SO solution was measured by a four-wire platinum resistance thermometer and recorded using an Agilent 34970A Data Acquisition Unit (Agilent Technologies, Santa Clara, CA). The AC samples were held in 2-ml centrifuge tubes where some holes (3 mm diameter) were drilled in the walls. Around the stirring bar, which ensured good mass transfer near the surfaces of the AC samples, seven centrifuge tubes could be placed simultaneously.
Fig. 1.
Schematic sketch of experimental setup
2.5. Experimental procedure
The AC discs were assigned randomly and pre-equilibrated in Me2SO solutions of lower concentrations at room temperature (22 °C, water bath) before they were exposed to Me2SO solutions of higher concentrations at subzero temperatures. Table 1 shows the details of the experiment. The exposure time at each subzero temperature was set as 0, 5, 15, 30, 50, 80, and 120 min. Four replications were undertaken for each exposure holding time. After exposure, the discs were removed and handled as follows: (1) The discs were lightly blotted with Kimwipes® tissues (Kimberly-Clark, Roswell, GA); (2) They were quickly rinsed with the deionized water; (3) They were lightly blotted again with Kimwipes® tissues; (4) Each was placed in a sealed centrifuge tube containing 2 ml deionized water, and held overnight at 4 °C in dark conditions to allow the Me2SO within the tissue to fully migrate out; (5) 0.3 ml of the resulting Me2SO/deionized water solution surrounding the cartilage was transferred into a flask of 50 ml for dilution; (6) the UV absorbance of the diluted solution was measured using the UV-vis spectrophotometer. As the control group, fresh AC discs without exposure to Me2SO solutions were directly immersed in 2 ml deionized water as in Step 4.
Table 1.
Details of the experiment
| Group | Tp (°C) | cp (%)a | tp (min)b | cpc (%)c | Te (°C) | ce (%) |
| 1 | 22 | 30.0 | 35 | 27.0 | −10 | 47.0 |
| 2 | 22 | 41.8 | 48 | 37.6 | −20 | 57.6 |
| 3 | 22 | 49.4 | 57 | 44.5 | −30 | 64.5 |
T p: pre-equilibration temperature; c p: pre-equilibration concentration (w/v); t p: pre-equilibration time; c pc: Pre-equilibrated concentration in cartilage (w/v); T e: exposure temperature; c e: exposure concentration (w/v)
Determined with an assumption that the concentration in tissue reached 90% of that of the surrounding solution at the end of the pre-equilibration
The pre-equilibration time needed for each pre-equilibration concentration at 22 °C was conservatively estimated from our previous studies (Yu et al., 2010)
To avoid freezing under the corresponding exposure temperature, the equilibrium concentration in cartilage needed was determined according to the equation in (Pegg, 1986). For computational purposes, the HEPES-buffered medium was assumed to be pure NaCl at mass fraction of 0.9%
The Me2SO concentration in the AC disc was expressed as weight of Me2SO/volume of AC interstitial fluid with the following two assumptions: (1) The volumes of the disc and fluid inside cartilage were constant; (2) The volume of the fresh AC interstitial fluid was approximated by water volume with a density of 1 g/cm3. The water content (mass fraction) of the fresh AC was about 77.7% (Yu et al., 2010).
2.6. Diffusion model
Cartilage is described as a network of pores and tortuosity. Hence, the apparent diffusion coefficient is used. The delivery of Me2SO in free-swelling cartilage discs is assumed to be spatially homogeneous and isotropic. The geometry of the AC disc is considered as a cylinder, and Me2SO can penetrate into the disc from the peripheral side as well as the top and bottom surfaces. Fick’s second law of diffusion in cylindrical coordinates can be expressed as
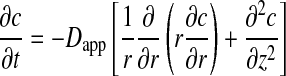 , ,
|
(1) |
where c is the local Me2SO concentration in cartilage, D app is the apparent diffusion coefficient of Me2SO in cartilage, r is the radial position, z is the axial position, and t is the time. D app is assumed to be constant although there is a very short time (less than 20 s according to numerical estimation) for the disc temperature to drop down.
The initial condition is
| c(t=0)=c0, | (2) |
where c 0 is the Me2SO concentration in cartilage after pre-equilibration. For boundary conditions, it is assumed that the mass transfer resistance at the surfaces of the disc is negligible. Therefore, the surface concentration is equal to Kc s, where c s is the concentration of the bathing solution, and K is the partition coefficient of Me2SO between the tissue and the solution. In this research, K is set to 0.9 (Mukherjee et al., 2008).
The partial differential equation was solved with MATLAB software. The value of D app at each temperature was evaluated based on the least-squares best fit of the experimental data to the model prediction.
3. Results and discussion
The absorbance values of the control group at 208 nm were less than 0.005 (n=4, data not shown), which were insignificant compared to those of the treatment groups (all large than 0.2, data not shown). Consequently, the effect of possible impurity from cartilage on the measurement accuracy was not considered here.
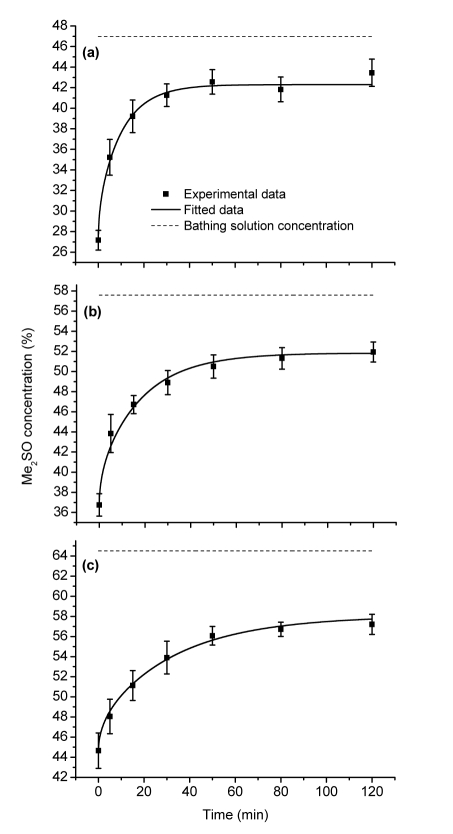
The experimental results, also the fitting curves, for the three subzero temperature levels are shown in Fig. 2. The best fits for the apparent diffusion coefficients are 0.85×10−6 cm2/s at −10 °C, 0.48×10−6 cm2/s at −20 °C, and 0.27×10−6 cm2/s at −30 °C. In no case did the final concentration in cartilage reach the concentration of the bathing solution, but stabilized at about 90% of that value (Table 2).
Fig. 2.
Uptake of Me2SO (%, w/v) by AC discs at three different subzero temperatures over time: −10 (a), −20 (b), and −30 °C (c)
Error bars represent the standard deviations (SDs)
Table 2.
Concentration of Me2SO in AC discs at each time point and temperature
| Immersion time (min) | Me2SO concentration (%)# |
||
| −10 °C | −20 °C | −30 °C | |
| 0 | 27.17±0.96 | 36.75±1.11 | 44.65±1.76 |
| 5 | 35.23±1.74 | 43.84±1.89 | 48.05±1.72 |
| 15 | 39.21±1.59 | 46.72±0.90 | 51.13±1.49 |
| 30 | 41.27±1.10 | 48.90±1.20 | 53.90±1.63 |
| 50 | 42.56±1.19 | 50.50±1.15 | 56.07±0.92 |
| 80 | 41.83±1.20 | 51.31±1.07 | 56.72±0.71 |
| 120 | 43.45±1.33 | 51.94±1.00 | 57.20±1.00 |
| 0.92* | 0.90* | 0.89* | |
Data (concentration, w/v) are expressed as mean±SD
Ratio of 120 min concentration to external concentration
The Arrhenius relation is used to express the manner in which the apparent diffusion coefficient changes with temperature:
| Dapp=Aexp(−Ea/RT), | (3) |
where E a is the activation energy, R is the universal gas constant, T is the temperature, and A is the pre-exponential factor. Fig. 3 shows the natural logarithm of D app plotted against the reciprocal Kelvin temperature, with E a determined to be 29.23 kJ/mol. In Fig. 3, the three data points at temperatures 1, 22, and 37 °C are obtained by fitting our previous experimental data (Yu et al., 2010) to the current model.
Fig. 3.
Natural logarithm of apparent diffusivity as a function of reciprocal Kelvin temperature
Due to the importance of the CPA permeation characteristics to the cryopreservation of tissues, many methods have been developed to investigate the penetration rates of CPA in tissue samples, such as using nuclear magnetic resonance (NMR) techniques (Muldrew et al., 1996; Carsi et al., 2004; Mukherjee et al., 2008), high performance liquid chromatography (HPLC) (Carpenter and Dawson, 1991; Hu and Wolfinbarger, 1994; Pegg, 2006), radiotracer (35S) (Elford, 1970), osmometer (Sharma et al., 2007; Jomha et al., 2009; Yu et al., 2010), conductivity meter (Chen et al., 2009), or electronic microbalance (Pegg, 2006). In this study, the quantity of Me2SO in ovine AC was measured using a spectrophotometer. The range of 0–60 mg/L was chosen for measurement calibration. In fact, the Beer’s law can also be used well in a larger range of Me2SO concentrations according to our trials (data not shown). The application of spectrophotometry is feasible in determining the quantity of Me2SO, which is a common CPA in the cryopreservation field.
Investigation of Me2SO permeation into AC has been studied with ovine (Pegg et al., 2006b), porcine (Muldrew et al., 1996; Sharma et al., 2007; Jomha et al., 2009), bovine (Mukherjee et al., 2008), and human AC (Carsi et al., 2004). In these studies, the temperature levels were between approximately 0 and 37 °C. The reported apparent diffusion coefficients ranged from 1.5×10−6 to 17.9×10−6 cm2/s depending on the temperature and tissue species. The current study obtained apparent diffusion coefficients of Me2SO in ovine AC at subzero temperatures −10, −20, and −30 °C. Arrhenius behavior was followed with R 2=0.998 (Fig. 3). The activation energy E a presented here was higher than those reported [17.99 kJ/mol (Jomha et al., 2009), 6.35 kJ/mol (Sharma et al., 2007)], but those two values were obtained with only three suprazero apparent diffusion coefficients for porcine AC.
From Table 2, it can be seen that the final concentration of Me2SO in cartilage samples after 120 min immersion did not reach the concentration value of the bathing solution. This phenomenon has been observed at above-zero temperatures (Pegg et al., 2006b; Sharma et al., 2007; Yu et al., 2010), which might be due to multiple factors. Briefly, (1) there exists an equilibrium pressure difference between the cartilage and the surrounding bathing solution (Elmoazzen et al., 2005); (2) “Bound” water exists in tissues, i.e., not all of the tissue water is available to dissolve Me2SO (Pegg et al., 1987; Zhang and Pegg, 2007).
With the Me2SO diffusion kinetics known, it is possible to do some work in the optimization of the loading protocol. Here, as an example, two protocols named as Protocol I (Table 3) and Protocol II (Table 4) are compared. The temperatures and concentrations of the bathing solution for both protocols follow Pegg et al. (2006c)’s values. In Protocol I, as did Pegg et al. (2006c), the duration for each step is set to be 30 min except for two steps at 22 °C, while in Protocol II, the duration for each subzero step is varied so that the T m,c [melting point of tissue calculated by the final center concentration of current step using the published equation (Pegg, 1986)] at the end of current step is 1 °C lower than the exposure temperature of the next step. The initial concentration in AC is zero and the objective is to increase the center concentration (mass fraction) to 47.6%, the minimal concentration for vitrification (Hua and Ren, 1994). It can be noted that for Protocol II, although there was one more step than Protocol I, the total loading time (104 min) is nearly one third less than that of Protocol I (140 min). The time cutoff is due to the relatively steep concentration change at the initial stage of each step as shown in Fig. 2. The exposure reduction would decrease the cellular toxicity of the CPA, but it should be noted that the final bathing solution concentration of Protocol II is higher than that of Protocol I despite a lower temperature. For further optimization of the Me2SO addition protocol, the CPA toxicity kinetics (cell viability loss from CPA exposure as a function of time, concentration, and temperature) are critical, which can be used to improve the coupling of the bathing solution temperature, concentration, and the duration for each step. The toxicity of Me2SO to chondrocytes [isolated (Tomford et al., 1984; Pegg et al., 2006a) or in situ (Elmoazzen et al., 2007; Jomha et al., 2008)] at above-zero temperatures has been investigated, but no systematic data for subzero temperatures are available.
Table 3.
Protocol I: fixed 30 min for each step except the two 22 °C steps with 10 min
| Step | BST (°C) | BSC (%) | Duration (min) | ICC (%) | IMC (%) | FCC (%) | FMC (%) | Tm,c (°C) |
| 1 | 22.0 | 10 | 10 | 0.0 | 0.0 | 8.8 | 8.9 | |
| 2 | 22.0 | 20 | 10 | 8.8 | 8.9 | 17.8 | 17.9 | |
| 3 | −5.0 | 29 | 30 | 17.8 | 17.9 | 25.8 | 25.9 | −5.8 |
| 4 | −8.5 | 38 | 30 | 25.8 | 25.9 | 33.6 | 33.9 | −10.2 |
| 5 | −16.0 | 47 | 30 | 33.6 | 33.9 | 40.9 | 41.5 | −17.6 |
| 6 | −23.0 | 56 | 30 | 40.9 | 41.5 | 47.6 | 48.8 | −28.5 |
BST: bathing solution temperature; BSC: bathing solution concentration (mass fraction); ICC: initial center concentration (mass fraction) in AC; IMC: initial mean concentration (mass fraction) in AC; FCC: final center concentration (mass fraction) in AC; FMC: final mean concentration (mass fraction) in AC
Table 4.
Protocol II: variable duration for each subzero step
| Step | BST (°C) | BSC (%) | Duration (min) | ICC (%) | IMC (%) | FCC (%) | FMC (%) | Tm,c (°C) |
| 1 | 22.0 | 10 | 10.0 | 0.0 | 0.0 | 8.8 | 8.9 | |
| 2 | 22.0 | 20 | 10.0 | 8.8 | 8.9 | 17.8 | 17.9 | |
| 3 | −5.0 | 29 | 17.4 | 17.8 | 17.9 | 24.8 | 25.4 | −5.8 |
| 4 | −8.5 | 38 | 23.1 | 24.8 | 25.4 | 33.1 | 33.6 | −9.5 |
| 5 | −16.0 | 47 | 14.8 | 33.1 | 33.6 | 38.2 | 39.9 | −17.0 |
| 6 | −23.0 | 56 | 18.3 | 38.2 | 39.9 | 44.7 | 47.1 | −24.0 |
| 7 | −35.0 | 63 | 10.7 | 44.7 | 47.1 | 47.6 | 50.8 | −36.0 |
The abbreviations are the same as those shown in Table 3
4. Conclusions
Vitrification has been proved to be promising for the long-term preservation of AC and other tissues. This paper presented the measurements of Me2SO permeation into ovine AC at three subzero temperatures using the method of UV spectrophotometry. The equilibrium concentration of Me2SO in AC was approximately 90% of that of the bathing solution. The fitted apparent diffusion coefficients followed the Arrhenius relation well. Using the obtained diffusivities, two addition protocols of Me2SO were simulated and compared, which demonstrated that the total loading duration could be effectively reduced with the knowledge of permeation kinetics. Otherwise, the LT method could be further optimized when coupling permeation kinetics with toxicity kinetics. Investigations of Me2SO toxicity kinetics to AC at subzero temperatures are now under way in our laboratory.
Acknowledgments
We would like to thank Mrs. Debra KWITEROVICH-HOOVER (Villanova University, USA) for revising the grammar of the paper.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 50606032) and the Graduate Innovation Research Program of Zhejiang Province (No. YK2008020), China
References
- 1.Aubin PP, Cheah HK, Davis AM, Gross AE. Long-term followup of fresh femoral osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat R. 2001;391(Suppl.):318–327. doi: 10.1097/00003086-200110001-00029. [DOI] [PubMed] [Google Scholar]
- 2.Brockbank KGM, Chen ZZ, Song YC. Vitrification of porcine articular cartilage. Cryobiology. 2010;60(2):217–221. doi: 10.1016/j.cryobiol.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter JF, Dawson PE. Quantitation of dimethyl sulfoxide in solutions and tissues by high performance liquid chromatography. Cryobiology. 1991;28(3):210–215. doi: 10.1016/0011-2240(91)90025-J. [DOI] [PubMed] [Google Scholar]
- 4.Carsi B, Lopez-Lacomba JL, Sanz J, Marco F, Lopez-Duran L. Cryoprotectant permeation through human articular cartilage. Osteoarthr Cartilage. 2004;12(10):787–792. doi: 10.1016/j.joca.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Chen HH, Zhou XM, Shu ZQ, Woods EJ, Gao DY. Electrical conductivity measurements for the ternary systems of glycerol/sodium chloride/water and ethylene glycol/sodium chloride/water and their applications in cryopreservation. Biopreserv Biobank. 2009;7(1):13–17. doi: 10.1089/bio.2009.0001. [DOI] [PubMed] [Google Scholar]
- 6.Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation—clinical results in the knee. Clin Orthop Relat R. 1999;360:159–168. doi: 10.1097/00003086-199903000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Elford BC. Diffusion and distribution of dimethyl sulphoxide in the isolated guinea-pig taenia coli. J Physiol. 1970;209(1):187–208. doi: 10.1113/jphysiol.1970.sp009162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmoazzen HY, Elliott JAW, McGann LE. Cryoprotectant equilibration in tissues. Cryobiology. 2005;51(1):85–91. doi: 10.1016/j.cryobiol.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Elmoazzen HY, Poovadan A, Law GK, Elliott JAW, McGann LE, Jomha NM. Dimethyl sulfoxide toxicity kinetics in intact articular cartilage. Cell Tissue Bank. 2007;8(2):125–133. doi: 10.1007/s10561-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 10.Glenn RE, McCarty EC, Jr, Potter HG, Juliao SF, Gordon JF, Spindler KP. Comparison of fresh osteochondral autografts and allografts: a canine model. Am J Sports Med. 2006;34(7):1084–1093. doi: 10.1177/0363546505284846. [DOI] [PubMed] [Google Scholar]
- 11.Hu JF, Wolfinbarger L., Jr Dimethyl sulfoxide concentration in fresh and cryopreserved porcine valved conduit tissues. Cryobiology. 1994;31(5):461–467. doi: 10.1006/cryo.1994.1056. [DOI] [PubMed] [Google Scholar]
- 12.Hua TC, Ren HS. Cryobiomedical Technologies. Beijing, China: Science Press; 1994. p. 120. (in Chinese) [Google Scholar]
- 13.Jomha NM, McGann LE, Elmoazzen HY. Modeling cryoprotectant toxicity in articular cartilage. J Bone Joint Surg Br. 2008;90B(Suppl.):105. [Google Scholar]
- 14.Jomha NM, Law GK, Abazari A, Rekieh K, Elliott JA W, McGann LE. Permeation of several cryoprotectant agents into porcine articular cartilage. Cryobiology. 2009;58(1):110–114. doi: 10.1016/j.cryobiol.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee IN, Li Y, Song YC, Long RC, Jr, Sambanis A. Cryoprotectant transport through articular cartilage for long-term storage: experimental and modeling studies. Osteoarthr Cartilage. 2008;16(11):1379–1386. doi: 10.1016/j.joca.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muldrew K, Sykes B, Schachar N, McGann LE. Permeation kinetics of dimethyl sulfoxide in articular cartilage. Cryoletters. 1996;17:331–340. [Google Scholar]
- 17.Pegg DE. Equations for obtaining melting points and eutectic temperatures for the ternary system dimethyl sulphoxide/sodium chloride/water. Cryoletters. 1986;7:387–394. [Google Scholar]
- 18.Pegg DE. Immersion weighing as a method for monitoring the permeation of tissues by cryoprotectants. Cryobiology. 2006;53(3):383. doi: 10.1016/j.cryobiol.2006.10.038. [DOI] [Google Scholar]
- 19.Pegg DE, Hunt CJ, Fong LP. Osmotic properties of the rabbit corneal endothelium and their relevance to cryopreservation. Cell Biochem Biophys. 1987;10(2):169–189. doi: 10.1007/BF02797398. [DOI] [PubMed] [Google Scholar]
- 20.Pegg DE, Wusteman MC, Wang LH. Cryopreservation of articular cartilage. Part 1: conventional cryopreservation methods. Cryobiology. 2006;52(3):335–346. doi: 10.1016/j.cryobiol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Pegg DE, Wang LH, Vaughan D, Hunt CJ. Cryopreservation of articular cartilage. Part 2: mechanisms of cryoinjury. Cryobiology. 2006;52(3):347–359. doi: 10.1016/j.cryobiol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Pegg DE, Wang LH, Vaughan D. Cryopreservation of articular cartilage. Part 3: the liquidus-tracking method. Cryobiology. 2006;52(3):360–368. doi: 10.1016/j.cryobiol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Raikin SM. Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am. 2009;91(12):2818–2826. doi: 10.2106/JBJS.I.00398. [DOI] [PubMed] [Google Scholar]
- 24.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at −196 °C by vitrification. Nature. 1985;313(6003):573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 25.Sharma R, Law GK, Rehieh K, Abazari A, Elliott JA W, McGann LE, Jomha NM. A novel method to measure cryoprotectant permeation into intact articular cartilage. Cryobiology. 2007;54(2):196–203. doi: 10.1016/j.cryobiol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Song YC, Khirabadi BS, Lightfoot F, Brockbank KG M, Taylor MJ. Vitreous cryopreservation maintains the function of vascular grafts. Nat Biotechnol. 2000;18(3):296–299. doi: 10.1038/73737. [DOI] [PubMed] [Google Scholar]
- 27.Song YC, An YH, Kang QK, Li CY, Boggs JM, Chen ZZ, Taylor MJ, Brockbank KGM. Vitreous preservation of articular cartilage grafts. J Invest Surg. 2004;17(2):65–70. doi: 10.1080/08941930490422438. [DOI] [PubMed] [Google Scholar]
- 28.Song YC, Lightfoot FG, Chen ZZ, Taylor MJ, Brockbank KGM. Vitreous preservation of rabbit articular cartilage. Cell Preserv Technol. 2004;2(1):67–74. doi: 10.1089/153834404322708772. [DOI] [Google Scholar]
- 29.Tomford WW, Fredericks GR, Mankin HJ. Studies on cryopreservation of articular cartilage chondrocytes. J Bone Joint Surg Am. 1984;66(2):253–259. [PubMed] [Google Scholar]
- 30.Wang LH, Pegg DE, Lorrison J, Vaughan D, Rooney P. Further work on the cryopreservation of articular cartilage with particular reference to the liquidus tracking (LT) method. Cryobiology. 2007;55(2):138–147. doi: 10.1016/j.cryobiol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Williams SK, Amiel D, Ball ST, Allen RT, Tontz WL, Emmerson BC, Jr, Badlani NM, Emery SC, Haghighi P, Bugbee WD. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35(12):2022–2032. doi: 10.1177/0363546507305017. [DOI] [PubMed] [Google Scholar]
- 32.Wusteman MC, Pegg DE, Robinson MP, Wang LH, Fitch P. Vitrification media: toxicity, permeability, and dielectric properties. Cryobiology. 2002;44(1):24–37. doi: 10.1016/S0011-2240(02)00002-0. [DOI] [PubMed] [Google Scholar]
- 33.Wusteman MC, Simmonds J, Vaughan D, Pegg DE. Vitrification of rabbit tissues with propylene glycol and trehalose. Cryobiology. 2008;56(1):62–71. doi: 10.1016/j.cryobiol.2007.10.177. [DOI] [PubMed] [Google Scholar]
- 34.Xia Y, Farquhar T, Burton-Wurster N, Ray E, Jelinski LW. Diffusion and relaxation mapping of cartilage-bone plugs and excised disks using microscopic magnetic resonance imaging. Magn Reson Med. 1994;31(3):273–282. doi: 10.1002/mrm.1910310306. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Shi HC, Zang WF, Lu D. An experimental research on cryopreserving rabbit trachea by vitrification. Cryobiology. 2009;58(2):225–231. doi: 10.1016/j.cryobiol.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Yu XY, Zhang SZ, Xu MJ, Chen GM. Study on the permeation of dimethyl sulfoxide into articular cartilage. J Eng Thermophys. 2010;31(8):1363–1366. (in Chinese) [Google Scholar]
- 37.Zhang SZ, Pegg DE. Analysis of the permeation of cryoprotectants in cartilage. Cryobiology. 2007;54(2):146–153. doi: 10.1016/j.cryobiol.2006.12.001. [DOI] [PubMed] [Google Scholar]