Abstract
Ipilimumab is a fully human monoclonal antibody that enhances antitumor immunity by way of cytotoxic T-lymphocyte antigen 4 blockade. It has already been approved by the US Food and Drug Administration for the treatment of metastatic melanoma and is being investigated for treating other solid tumors such as renal cell, prostate and lung cancers. This review details the potential of ipilimumab in the management of non-small cell lung cancer (NSCLC). In particular, ipilimumab showed promising results in a first-line NSCLC phase II study combining carboplatin/paclitaxel chemotherapy with concurrent or phased ipilimumab. The median immune-related progression-free survival was 5.68 months for the phased ipilimumab arm versus 4.63 months for chemotherapy alone (hazard ratio [HR] = 0.68, p = 0.026) and 5.52 months for the concurrent ipilimumab arm versus 4.63 months for chemotherapy alone (HR = 0.77, p = 0.094). The main adverse events were immune related, such as hypophysitis, enterocolitis, and hyperthyroidism. These adverse events may be improved with high-dose glucocorticoids and may be correlated with tumor response. Phase III studies are ongoing. Future studies may investigate ipilimumab in the management of early stage lung cancer. Strategies for potential translational research studies are also discussed to identify prognostic and predictive biomarkers for the use of ipilimumab in the treatment of patients with NSCLC.
Keywords: biomarkers, immunotherapy, ipilimumab, lung cancer, targeted therapy
Introduction
Ipilimumab is a fully human immunoglobulin G and anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) monoclonal antibody. It has already been studied in several solid malignancies and has been approved by the US Food and Drug Association (FDA) for the treatment of advanced melanoma. Studies of ipilimumab for the treatment of lung cancer are described in this review, and the clinical potential of ipilimumab is discussed.
Biological background: mechanisms of action
The adaptive immune response requires two signals between the antigen-presenting cells (APCs) and the effector T cells. The first signal is mediated by the T-cell receptor and the major histocompatibility complex classes I or II antigenic peptide. The second signal is a costimulatory signal mediated by the engagement of CD28 on the T-cell surface by members of the B7 family on APCs. Both signals result in the activation and interleukin-2 (IL-2)-dependant clonal proliferation of T cells. This proliferation needs to be regulated to avoid autoimmunity. After activation, T cells express CTLA-4, a member of the immunoglobulin super family and homologue to CD28 first cloned in 1987 [Brunet et al. 1987]. CTLA-4 binds members of the B7 family with a much higher affinity than CD28 and downregulates the T-cell response. It has been proven that one reason for the poor immunogenicity of many tumors such as lung cancer is CTLA-4 activity and that in vivo administration of antibodies to CTLA-4 can enhance antitumor immunity [Leach et al. 1996].
CTLA-4 also regulates tumor immunity via Tregs. Tregs are a suppressive CD4+ T-cell population that expresses high levels of surface CTLA-4. Tregs have nonspecific immunosuppressive functions through several mechanisms. Among these mechanisms is upregulation of CTLA-4 on the surface of Tregs, which can suppress the activation and expansion of effector cells [Gabriel and Lattime, 2007]. Therefore, whereas CTLA-4-expressing Tregs may play a critical role in maintaining self-tolerance, they may also facilitate nonresponsiveness to tumor antigens. Tregs have been shown to be present in tumors and coexist with primed effector T cells. Thus, blockade of Tregs function via anti-CTLA-4 antibodies has the potential to remove Tregs suppression and enhance antitumor immunity.
O’Mahony and colleagues studied the biological consequences of the administration of ipilimumab 3 mg/kg and then 1.5 mg/kg monthly in patients with advanced malignancies after cancer vaccine failure. The first hypothesis for the biological mechanism of action of ipilimumab was that blockade of CTLA-4 on CD8+ T cells would result in an expansion of vaccine-specific T-cell responses. However, no increase in peripheral blood CD3+, CD8+ T cells or vaccine-specific CD8+ T cells was detected. These data, consistent with previous studies, suggest that the increase in CD8+ T-cell response through blockade of CTLA-4 signaling is not the major mechanism of tumor response [O’Mahony et al. 2007]. However, Yang and colleagues published a retrospective analysis of four phase II studies of ipilimumab in stage IV melanoma. Patients with clinical benefit had a statistically significant increase in CD8+ T cells in comparison with patients without clinical benefit (p = 0.0294) [Yang et al. 2010]. The second hypothesis was the potential for ipilimumab to deplete Tregs. Flow cytometry was used to quantitate Tregs number. The authors showed a significant decrease of Tregs at 1–4 days after each administration of ipilimumab. But the number of Tregs had increased above baseline at the time of next administration. This suggests that modulation of Tregs plays a role in tumor regression and that the transient decrease in Tregs allows for immune activation [Yang et al. 2010].
Proof of concept of ipilimumab activity in solid malignancies
Ipilimumab has been studied in several solid malignancies, and the main results are summarized here to allow comparison with the NSCLC development of ipilimumab.
Melanoma
Hodi and colleagues conducted a phase III study of ipilimumab in the treatment of metastatic melanoma. Overall, 676 patients with HLA-A0201-positive unresectable stage III or IV melanoma whose disease had progressed after a first-line therapy were randomized to receive ipilimumab 3mg/kg every 3 weeks plus gp100 or ipilimumab alone or gp100 alone. Ipilimumab alone improved overall survival (OS) in comparison with gp100 alone (HR for survival = 0.66, p = 0.003). Similarly, ipilimumab plus gp100 improved OS in comparison with gp100 alone (HR = 0.68, p < 0.001). There was no difference in OS between the ipilimumab groups (p = 0.76) [Hodi et al. 2010] or across subgroups [Lebbé et al. 2010; Haanen et al. 2010; Lawrence et al. 2010].
Renal cell carcinoma
Yang and colleagues conducted a phase II study of ipilimumab monotherapy 3 mg/kg followed by 1 mg/kg or 3 mg/kg every 3 weeks in patients with metastatic renal cell carcinoma (RCC). Overall, 15% of patients with the higher dose had partial response according to the Response Evaluation Criteria for Solid Tumors (RECIST) criteria [95% confidence interval (CI) for cohort response rate 4–27%) [Yang et al. 2007].
Prostate cancer
Fong and colleagues published a phase I study of 24 patients with metastatic castration-resistant prostate cancer treated with granulocyte macrophage colony-stimulating factor (GM-CSF) and increasing doses of ipilimumab. Three of the six patients with the highest dose had prostate-specific antigen declines of more than 50%. Expansion of activated circulating CD25+ CD69+ CD8+ T cells was greater than in patients from two separate clinical trials who received ipilimumab or GM-CSF as monotherapy [Fong et al. 2009].
Ipilimumab in lung cancer
Despite recent advances in lung cancer treatment with third-generation chemotherapies and targeted therapies, the prognosis of patients with lung cancer remains poor and there is a need for new therapeutic strategies to improve lung cancer patients’ survival. Lung cancer in not known to be an immunogenic-mediated malignancy. Ding and colleagues performed DNA sequencing of a large number of genes in 188 lung cancer samples and identified 26 genes mutated at significantly high frequencies and thus potentially involved in lung cancer carcinogenesis [Ding et al. 2008]. None of the genes were involved in immunity. However, immunotherapy remains an attractive therapeutic approach because of its theoretical specificity and potential long-term disease control. Consequently, several immunotherapies are under investigation in NSCLC and some of them already showed promising results. Some immunotherapies have a specific immune target; for example, MAGE-A3, Lucanix™ (belagenpumatucel-L, a transforming growth factor β2-based vaccine, NovaRx, San Diego, CA, USA), Stimuvax® (BLP25 liposome vaccine, a vaccine targeting mucin 1, Oncothyreon, Seattle, WA, USA) and Saccharomyces-CEA vaccine. Some immunotherapies do not have identified target, such as GVAX (GM-CSF vaccine), talactoferrin (a genetically engineered form of the human protein lactoferrin), and ipilimumab [Morgensztern et al. 2010]. As an example, a randomized phase II study of talactoferrin versus placebo in patients with advanced NSCLC that progressed after chemotherapy showed an improvement in OS (HR for survival = 0.68; p = 0.04) [Parikh et al. 2011]. In the same way, a phase II study of MAGE-A3 immunotherapy as adjuvant therapy in stage IB/II NSCLC showed a positive trend for activity of MAGE-A3 in NSCLC with a relative improvement of disease-free survival of 27% [Vansteenkiste et al. 2007]. However, there has been no vaccine or other immune-related therapy, FDA or European Medicines Agency (EMEA) approved, for NSCLC. Furthermore, Stimuvax, talactoferrin, MAGE-A3, and ipilimumab are still actively being investigated for the treatment of patients with NSCLC through ongoing phase III studies.
The rationale for the use of ipilimumab in association with chemotherapy is based on the assumption that tumor-specific antigen released during chemotherapy-induced tumor necrosis may increase tumor-specific immunity and therefore enhance ipilimumab or other immunotherapeutic efficacy. This hypothesis may explain the trend in favor of the sequential association of ipilimumab and chemotherapy in comparison with the concurrent association as highlighted in several phase II studies, including lung cancer studies as reported below. In addition, a randomized phase I study assessed the pharmacokinetic interaction between ipilimumab and paclitaxel/carboplatin in patients with untreated advanced melanoma and there was no clinically relevant pharmacokinetic interaction [Weber et al. 2010]. Complete-dose chemotherapy may increase antigen exposure to immunotherapy but may also decrease the population of immune cells mediating the response to immunotherapy. This is the reason why phasing with chemotherapy is also of importance. Actually, low-dose intravenous infusions of cyclophosphamide may overcome the immune suppression seen in carcinomas, and cyclophosphamide has shown, in various animal models, its ability to augment delayed-type hypersensitivity responses, increase antibody production, abrogate tolerance, and potentiate antitumor immunity [Bass and Mastrangelo, 1998]. To date, use of ipilimumab with pretreatment cyclophosphamide remains to be studied.
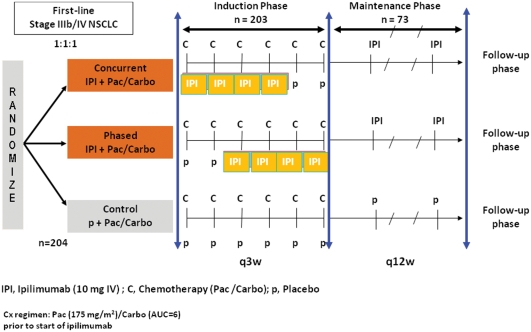
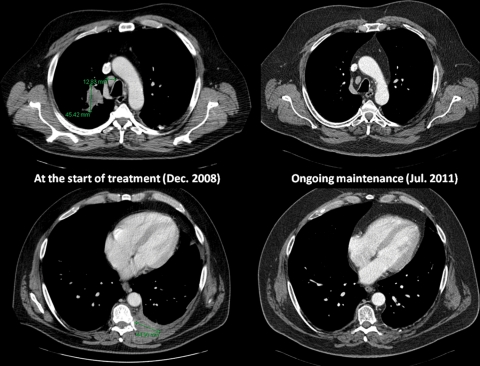
Ipilimumab showed promising results in the treatment of NSCLC in a phase II study. The CA184-041 phase II study randomized previously untreated patients with advanced NSCLC to receive chemotherapy with carboplatin (area under the curve 6) and paclitaxel (175 mg/m2) alone or in association with concurrent ipilimumab (10 mg/kg from cycle 1 to cycle 4) or with phased ipilimumab (10 mg/kg from cycle 3 to cycle 6) (see Figure 1 for design) [Lynch et al. 2010]. Overall, 204 patients were included in the study with common characteristics for patients with advanced NSCLC (74% men, 79% stage IV disease, 51% adenocarcinoma, and 71% European Cooperative Oncology Group performance status 1). The primary endpoint was immune-related progression-free survival (irPFS), according to the guidelines for the evaluation of immune therapy activity in solid tumors [Wolchok et al. 2009]. Clinical response to immunotherapeutic agents is sometimes represented by an initial increase in tumor size or even appearance of new lesions, therefore considered as a disease progression according to RECIST or World Health Organization (WHO) criteria. This novel pattern of clinical response limits the use of the RECIST or WHO criteria when using immunotherapy. Therefore, immune-relative response criteria were defined in an attempt to capture additional response patterns observed in immunotherapy. In the CA184-041 study, irPFS was improved in the ipilimumab arms in comparison with chemotherapy alone. Indeed, the median irPFS was 5.68 months for the phased ipilimumab arm versus 4.63 months for chemotherapy alone (HR = 0.68, p = 0.026) and was 5.52 months for the concurrent ipilimumab arm versus 4.63 months for chemotherapy alone (HR = 0.77, p = 0.094). However, there was no significant difference between the arms in terms of OS (p = 0.104) but a trend seemed to favor the sequential combination of ipilimumab plus chemotherapy. Indeed, OS was 8.3 months in the placebo arm versus 9.7 months in the concurrent arm (HR = 0.98, p = 0.47) and 12.2 months in the phased arm (HR = 0.86, p = 0.23). The disease control rate (DCR) seemed also to favor the phased combination of ipilimumab with chemotherapy with a DCR of 57.1 versus 77.9 and 72.7 for chemotherapy alone, phased and concurrent ipilimumab, respectively. Although the absolute difference in irPFS seems small (1 month), it is statistically significant and we have to focus on HR (0, 68). In this disease with such a poor prognosis, there are very few immune therapies showing such promising results. Furthermore, some patients experienced almost complete and longlasting responses (Figure 2), highlighting the need for phase III studies and assessment for potential predictive biomarkers. The association of ipilimumab and chemotherapy was generally well tolerated and ipilimumab did not potentiate chemotherapy-related toxicity. However, safety results suggest a moderate added toxicity in the arms containing ipilimumab (grade 3–4 adverse events were observed in 58% of patients in the concurrent arm and 52% of patients in the phased arm versus 42% of patients in the placebo arm). The more specific immune-related toxicities are detailed below.
Figure 1.
Study design of the CA184-041 phase II study investigating the potential of concurrent and phased ipilimumab (IPI) versus placebo (p) with concomitant carboplatin (C, Carbo) plus paclitaxel (P, Pac) as first-line treatment in advanced stages of non-small cell lung cancer (NSCLC).
q3w, every 3 weeks; q12w, every 12 weeks.
Figure 2.
Thoracic computed tomography scans showing baseline (left panels) and current (right panels) scans of a patient with stage IV non-small cell lung cancer participating in the CA184-041 phase II study, and showing an almost complete and long-lasting response to the combination of ipilimumab plus concurrent carboplatin plus paclitaxel chemotherapy.
In 2011, Lynch and colleagues presented the results of a subgroup analysis of the CA184-041 phase II study looking at efficacy by histological subtypes [Lynch et al. 2011]. This subgroup hypothesis-generating analysis suggested that patients with squamous NSCLC might derive a greater benefit of the ipilimumab plus chemotherapy combination when compared with patients with nonsquamous NSCLC, especially for the phased combination. Indeed, the median irPFS was 6.2 and 5.7 months for the phased ipilimumab arms versus 4.2 and 5.3 months for chemotherapy alone in squamous and nonsquamous NSCLC, respectively (HR = 0.55 and 0.82, respectively). The retrospective nature of this analysis and the small sample size warrants caution in interpretation.
Safety profile in solid malignancies
The major toxicities due to ipilimumab are immune-related adverse events (irAEs). The most common major toxicity was enterocolitis (21% of patients) [Beck et al. 2006], defined by diarrhea or biopsy documentation (neutrophilic and lymphocytic inflammation). The major risk is represented by bowel perforation. Most patients with enterocolitis respond to high-dose systemic corticosteroids, without affecting tumor response, but a significant association between irAEs and tumor regression (response rate 30% with irAEs versus 0% without irAEs) was also shown [Yang et al. 2007]. Weber and colleagues conducted a double-blind placebo-controlled phase II study of prophylactic budesonide in the management of grade ≥2 diarrhea in patients with unresectable advanced melanoma receiving ipilimumab. Budesonide did not affect the rate of grade ≥2 diarrhea (32.7% versus 35%) [Weber et al. 2009]. Immune-related endocrinopathies have also been described. Autoimmune hypophysitis has been reported in 17% (all grades) of patients with melanoma and RCC treated with ipilimumab. Furthermore, cases of hypopituitarism have been described in patients with prostate cancer undergoing experimental therapy with ipilimumab. In most cases, high-dose glucocorticoid treatment improved symptoms [Dillard et al. 2010]. In the same way, cases of hyperthyroidism after ipilimumab therapy for patients with advanced melanoma have been described [Min et al. 2011]. Thus, the early screening and treatment of autoimmune endocrinopathies is recommended in patients undergoing clinical trials with ipilimumab. Ipilimumab can also induce uveitis and hepatitis that may be severe and corticosteroid resistant [Chmiel et al. 2011].
In the CA184-041 phase II study dedicated to patients with NSCLC, irAEs were more frequent in the ipilimumab arms (19% and 15% grade 3–4 irAEs for concurrent and phased arms, respectively, versus 6% in the placebo arm) [Lynch et al. 2010]. There were mostly grade 3 irAEs, with dermatologic and gastrointestinal irAEs the most frequent. A fatal grade 5 toxic epidermal necrolysis was observed in a patient treated in the concurrent arm. No hypopituitarism or adrenal insufficiency was observed, but two patients experienced a grade 1–2 hypothyroidism.
Perspectives
The results of the CA184-041 study actually led to the design of a phase III study of the association of ipilimumab with paclitaxel/carboplatin in previously untreated advanced NSCLC with squamous histology (clinicaltrials.gov, NCT 01285609 and NCT00527735).
Table 1.
Immune-related response criteria (irRC) [Wolchok, 2009].
| Complete response | All lesions gone |
|---|---|
| Partial response | ≥ 50% decrease in SPD of index + new* lesions |
| Stable disease | SPD of index + new* lesions not irCR, irPR or irPD |
| Progressive disease | SPD of index + new* lesions increases ≥25% |
SPD, sum of products of perpendicular diameters; irCR, immune related Complete Response; irPR, immune related Partial Response; irPD, immune related Progressive Disease;*, differences between irRC and modified World Health Organization response criteria.
However, the potential efficacy of ipilimumab in NSCLC together with its particular mechanism of action might also lead to investigation in earlier stages of the disease, such as the early stages of NSCLC in the adjuvant or neoadjuvant setting, but also in all the situations when a maintenance treatment might be useful (response after induction chemotherapy in NSCLC, management of unresectable stage III, etc.). The investigation of ipilimumab in early stage lung cancer may expose patients to long-term irAEs. Long-term results of a phase III trial of ipilimumab in metastatic melanoma describe only 1% of irAEs in the ‘ipilimumab alone’ arm, mostly grade 1 or 2, with no grade 4 or 5 toxicities. The long-term benefit of ipilimumab on OS has been demonstrated [Haanen et al. 2010]. Furthermore, in comparison with adjuvant chemotherapy for resected lung cancer, the long-term results of the International Adjuvant Lung Cancer Trial on OS favor adjuvant chemotherapy, but not significantly, after 5 years even if non-lung cancer mortality is increased in the chemotherapy arm [Arriagada et al. 2010].
In addition, predictive and prognostic biomarkers to drive the use of ipilimumab should be investigated in tumor or blood. We already know that increased tumor-infiltrating lymphocytes in patients with squamous disease were correlated with improved survival in NSCLC [Hiraoka et al. 2006]. Several other biomarkers are under investigation, such as the increase in CD8+ T cells or the decrease of CTLA-4-expressing Tregs, but these studies frequently face the complexity of interactions between tumor and host that impact on antitumor immune responses. Although assessing predictors of response to immunotherapies is difficult, recommendations are available in the cancer immunotherapy biomarkers resource document [Bedognietti et al. 2011]. It provides key references relevant to the discovery, evaluation and clinical application of immune biomarkers [Bedognietti et al. 2011]. In addition, Breunis and colleagues provide data suggesting that genetic variations in CTLA-4 could influence response to CTLA-4 blockade [Breunis et al. 2008], representing a first step in biomarker development in the field of ipilimumab studies.
Conclusions
Ipilimumab is an anti-CTLA-4 antibody. A phase II study of ipilimumab in the first-line treatment of metastatic NSCLC in association with chemotherapy showed an improvement of irPFS in comparison with chemotherapy alone, without major added toxicity. Phase III studies, especially in the squamous-NSCLC population, are ongoing. In the future, ipilimumab should be investigated in early stage or locally advanced lung cancer, such as other immunotherapies. There is also a need for the research of biomarkers to individualize and optimize the use of ipilimumab in NSCLC.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors have participated in clinical trials based on ipilimumab in lung cancer.
References
- Arriagada R., Dunant A., Pignon J.P., Bergman B., Chabowski M., Grunenwald D., et al. (2010) Long-term results of the International Adjuvant Lung Cancer Trial evaluating cisplatine-based chemotherapy in resected lung cancer. J Clin Oncol 28: 35–42 [DOI] [PubMed] [Google Scholar]
- Bass K.K., Mastrangelo M.J. (1998) Immunopotentiation with low-dose cyclophosphamide in the active specific immumnotherapy of cancer. Cancer Immunol Immunother 47: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K.E., Blansfield J.A., Tran K.Q., Feldman A.L., Hughes M.S., Royal R.E., et al. (2006) Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 24: 2283–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedognetti D., Balwit J.M., Wang E., Disis M.L., Britten C.M., Delogu L.G., et al. (2011) SITC/iSBTc cancer immunotherapy biomarkers resource document: online resources and useful tools – a compass in the land of biomarker discovery. J Transl Med 9: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunis W.B., Tarazona-Santos E., Chen R., Kiley M., Rosenberg S.A., Chanock S.J. (2008) Influence of cytotoxic T lymphocyte- associated antigen 4 (CTLA4) common polymorphisms on outcome in treatment of melanoma patients with CTLA-4 blockade. J Immunother 31: 586–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet J.F., Denizot F., Luciani M.F., Roux-Dosseto M., Suzan M., Mattei M.G., Golstein P. (1987) A new member of the immunoglobulin superfamily – CTLA-4. Nature 328: 267–270 [DOI] [PubMed] [Google Scholar]
- Chmiel K.D., Suan D., Liddle C., Nankivell B., Ibrahim R., Bautista C., et al. (2011) Resolution of severe ipilimumab-induced hepatitis after antithymocyteglobulin therapy. J Clin Oncol 29: e237–e240 [DOI] [PubMed] [Google Scholar]
- Dillard T., Yedinak C.G., Alumkal J., Fleseriu M. (2010) Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary 13: 29–38 [DOI] [PubMed] [Google Scholar]
- Ding L., Getz G., Wheeler D.A., Mardis E.R., McLellan M.D., Cibulskis K., et al. (2008) Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455: 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong L., Kwek S.S., O’Brien S., Kavanagh B., McNeel D.G., Weinberg V., et al. (2009) Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res 69: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel E.M., Lattime E.C. (2007) Anti-CTL associated antigen 4: are regulatory T cells a target? Clin Cancer Res 13: 785–788 [DOI] [PubMed] [Google Scholar]
- Haanen J.B., Hodi F.S., O’Day S.J., McDermott D.F., Robert C., Schadendorf D., et al. (2010) Ipilimumab improves survival in patients with previously treated advanced melanoma: long-term survival results from a phase III trial. Ann Oncol 21(Suppl. 8): 402, abstract no. 1327P [Google Scholar]
- Hiraoka K., Miyamoto M., Cho Y., Suzuoki M., Oshikiri T., Nakakubo Y., et al. (2006) Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer 94: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D.P., Hamid O., McDermott D.F., Puzanov I., Sznol M., Clark J., et al. (2010) Phase II trial of ipilimumab monotherapy in melanoma patients with brain metastases. J Clin Oncol 28(Suppl.): abstract no. 8523 [Google Scholar]
- Leach D.R., Krummel M.F., Allison J.P. (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271: 1734–1736 [DOI] [PubMed] [Google Scholar]
- Lebbé C., McDermott D.F., Robert C., Lorigan P., Ottensmeier C.H., Wolchok J., et al. (2010) Ipilimumab improves survival in previously treated advanced melanoma patients with poor prognosis factors: subgroup analyses from a phase III trial. Ann Oncol 21(Suppl. 8): 401, abstract no. 13240 [Google Scholar]
- Lynch T.J., Bondarenko I.N., Luft A., Serwatowski P., Barlesi F., Chacko R.T., et al. (2010) Phase II trial of ipilimumab and paclitaxel/carboplatin in first-line stage IIIb/IV non-small cell lung cancer. J Clin Oncol 28(Suppl.): 208, abstract no. 7531 [Google Scholar]
- Lynch T.J., Bondarenko I.N., Luft A., Serwatowski P., Barlesi F., Chacko R.T., et al. (2011) Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in non-small cell lung cancer: analysis by baseline histology in a phase 2 trial. WCLC abstract no. 701 [DOI] [PubMed] [Google Scholar]
- Min L., Vaidya A., Becker C. (2011) Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol 164(2): 303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgensztern D., Goodgame B., Govindan R. (2010) Vaccines and immunotherapy for non-small cell lung cancer. J Thorac Oncol 5(12 Suppl. 6): S463–S465 [DOI] [PubMed] [Google Scholar]
- O’Mahony D., Morris J.C., Quinn C., Gao W., Wilson W.H., Gause B., et al. (2007) A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancies. Clin Cancer Res 13: 958–964 [DOI] [PubMed] [Google Scholar]
- Parikh P.M., Vaid A., Advani S.H., Digumarti R., Madhavan J., Nag S., et al. (2011) Randomized, double-blind, placebo-controlled phase II study of single-agent oral talactoferrin in patients with locally advanced or metastatic non-small-cell lung cancer that progressed after chemotherapy. J Clin Oncol 29: 4129–4136 [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J., Zielinski M., Linder A., Dahabre J., Esteban E., Malinowski W., et al. (2007) Final results of a multi-center, double-blind, randomized, placebo-controlled phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC). J Clin Oncol 25(Suppl.): 398s, abstract no. 7554 [Google Scholar]
- Weber J., Thompson J.A., Hamid O., Minor D., Amin A., Ron I., et al. (2009) A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 15: 5591–5598 [DOI] [PubMed] [Google Scholar]
- Weber J.S., Hamid O., Wolchok J., Amin A., Masson E., Goldberg S., et al. (2010) Assessment of pharmacokinetic interaction between ipilimumab and chemotherapy in a randomized study. Ann Oncol 21(Suppl. 8): 403, abstract no. 1329P19628568 [Google Scholar]
- Wolchok J.D., Hoos A., O’Day S., Weber J.S., Hamid O., Lebbe C., et al. (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15: 7412–7420 [DOI] [PubMed] [Google Scholar]
- Yang A., Kendle R.F., Ginsberg B.A., Roman R., Heine A.I., Pogoriler E., et al. (2010) CTLA-4 blockade with ipilimumab increases peripheral CD8+ T cells: correlation with clinical outcomes. J Clin Oncol 28(Suppl.): abstract no. 2555 [Google Scholar]
- Yang J.C., Hughes M., Kammula U., Royal R., Sherry R.M., Topalian S.L., et al. (2007) Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 30: 825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]