Abstract
Background: Diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration (EUS FNA) varies relating to the equipment used and the site targeted. Multiple needle passes are usually required to obtain a diagnosis. A new needle incorporating a side port carries a theoretical advantage regarding acquisition of cytological material.
Methods: To demonstrate the safety and efficacy of the Olympus side-port needle across a spectrum of indications for EUS FNA, a prospective collection of 16 consecutive cases was undertaken at a tertiary gastroenterology referral centre in metropolitan Sydney, Australia. EUS FNA was performed with the novel Olympus side-port needle. EUS FNA was otherwise performed in the conventional fashion. The number of needle passes required for diagnosis, number of passes total, diagnosis on cytology and conclusive diagnosis were recorded.
Results: Diagnostic material was obtained at the first pass in 56.2% of patients. Mean number of passes required to reach a diagnosis was 2.1. Diagnosis was made on first pass in 62.5% of solid non-lymph-node lesions. The diagnosis was reached in 94%.
Conclusions: The novel side-port needle is safe and effective; further evaluation with a prospective randomized trial is warranted.
Keywords: endoscopic ultrasound, fine needle aspiration biopsy, needle, procore
Introduction
Detailed images of the upper gastrointestinal tract and adjacent structures can be obtained with endoscopic ultrasound (EUS). Fine-needle aspiration (FNA) of lesions visualized with EUS plays a crucial role in the diagnosis and staging of benign and malignant mass lesions of these structures [Giovannini et al. 1995; Shin et al. 2002; Vilmann et al. 1993; Wiersema et al. 1994], and complication rates are low [Adler et al. 2005; Affi et al. 2001; Eloubeidi et al. 2006; Giovannini et al. 1995; Varadarajulu and Eloubeidi, 2004] occurring in between 1% and 2.5% of cases.
The role of EUS guided FNA (EUS FNA) in the investigation of suspected pancreatic malignancy, abnormal lymph nodes and pancreatic cystic lesions is well established [De Witt, 2006; Giovannini et al. 1995; Gress et al. 2001; Rocca et al. 2007; Vazquez-Sequeiros, 2007; Vilmann et al. 1995]. Multiple passes at EUS FNA are often required to obtain sufficient material for diagnosis; five or six passes for solid pancreatic lesions and four passes for lymph nodes [Conway et al. 2009; Erickson et al. 2000; Yamao et al. 2009]. This prolongs the procedure and exposes the patient to an increased risk of biopsy related complications [Sakamoto et al. 2009; Wiersema et al. 1997].
The diagnostic yield of EUS FNA varies relating to the equipment used [Erickson et al. 2000; Yusuf et al. 2009] and site targeted [LeBlanc et al. 2004; O’Toole et al. 2001]. Recent advances may increase yield [Bruno et al. 2009; Erickson et al. 2000; Larghi et al. 2005; Nguyen et al. 2008; O’Toole et al. 2001; Palazzo et al. 1999; Puri et al. 2009; Siddiqui et al. 2009], however this rarely exceeds 90%.
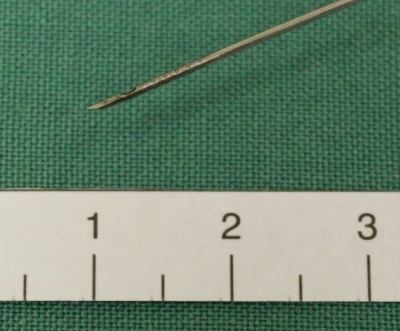
The Olympus Prototype Side-Port Needle (Olympus Corp, Japan) (Figure 1) was developed by the authors in conjunction with Olympus Tokyo, to increase tissue acquisition and reduce the required number of passes at EUS FNA. This device is a disposable 22-gage needle with a side port in addition to the standard end port. This report details the pilot experience with this needle. We evaluated the feasibility, safety and diagnostic yield of the side-port needle across a range of indications for EUS-FNA.
Figure 1.
Olympus side-port needle. Laser etching on the tip for increased echogenicity, side port 4 mm from the end port.
Patients and methods
Consecutive patients were recruited from a single tertiary referral centre between July and December 2009. Patients deemed suitable for EUS FNA were assessed by a physician prior to the procedure. Patients were given an information sheet and informed consent was obtained. Approval was gained from the ethics review board of the Sydney South West Area Health Service.
Exclusion criteria were standard contraindications to EUS FNA (profound thrombocytopenia or coagulopathy, severe comorbidities) and age less than 18. All lesions and indications for EUS FNA were included.
EUS FNA was performed in the left lateral position under conscious sedation with midazolam, fentanyl and propofol. Noninvasive blood pressure monitoring, pulse oximetry and cardiac monitoring were performed. Recovery was supervised for a minimum of 2 hours, and in follow up. An Olympus Linear Echoendoscope (UCT140-AL5) was used for each procedure. All procedures were performed by a single operator (AK), and were conducted on an outpatient basis.
A cytologist was present in the room for all cases except the fluid aspiration case (n = 1). FNA was performed according to standard protocol, with the Olympus side-port needle used at each pass. The Olympus side-port needle (Figure 1) is identical to the standard 22 gage EUS FNA needle, but has a second opening located 4 mm from the tip on the opposite side to the bevel. As with a standard EUS FNA needle, this needle moves freely within a protective sheath, permits passage of a stylet, and has an attachment for a suction device at the endoscopist end.
The results of each needle pass, and the number of passes taken to collect material sufficient for diagnosis were recorded. Prior to repeat aspiration, the cytologist examined the aspirated material. The decision to stop performing FNA was made when acquisition of adequate material was determined by the cytologist. When requested, further passes were taken for cell block and immunohistochemical analysis.
Results
Data were collected on our first 16 patients with a mean age of 57.6 years, 56% were female (Table 1). Seven cases were of masses in or around the pancreas (five head, two neck), six of enlarged thoracic lymph nodes and one each of a pancreatic tail mass, a pancreatic tail cyst and a gastric subepithelial lesion.
Table 1.
Results.
| Age | Sex | Indication | Site biopsied | Passes for diagnosis | Total number of passes | Results | Final diagnosis |
|---|---|---|---|---|---|---|---|
| 63 | M | Pancreas mass | HOP | 1 | 1 | Adenocarcinoma | Adenocarcinoma |
| 60 | M | Pancreas mass | Ascites | 1 | 1 | Adenocarcinoma | Adenocarcinoma |
| 45 | F | Mediastinal LN, query sarcoid | LN | 4 | 4 | Consistent with sarcoidosis | Consistent with sarcoidosis |
| 46 | F | Subcarinal LN, query sarcoid | LN | 4 | 4 | Consistent with sarcoidosis | Consistent with sarcoidosis |
| 68 | F | Pancreatic tail cyst | Pancreas tail cyst | 1 | 1 | Pus drained | Klebsiella infection in pancreatic cyst |
| 64 | M | Pancreas mass | HOP | 2 | 2 | Dysplastic mucinous tumor | Adenocarcinoma at CT guided core biopsy |
| 66 | M | Subepithelial mass at COJ | s/m lesion | 1 | 2 | GIST | GIST |
| 64 | F | Mediastinal lymph node, query sarcoid | LN | 3 | 3 | Consistent with sarcoidosis | Consistent with sarcoidosis |
| 65 | F | Mass near pancreatic head | Mass | 1 | 4 | Adenocarcinoma | Adenocarcinoma |
| 57 | M | Subcarinal LN mass | LN mass | 1 | 3 | Abscess | Abscess |
| 63 | F | Pancreas mass | HOP | 5 | 5 | Suspicious for adenocarcinoma | Adenocarcinoma |
| 53 | F | Pancreas mass | Neck | 1 | 1 | Adenocarcinoma | Adenocarcinoma |
| 63 | F | Pancreas mass | Neck | 5 | 5 | Suspicious for adenocarcinoma | Adenocarcinoma |
| 75 | F | Lymphadenopathy | Mediastinal LN | 2 | 2 | Reactive lymph node | Reactive lymph node |
| 28 | M | LN mass | Mediastinal LN | 1 | 1 | Granuloma | Tuberculosis |
| 41 | M | Pancreas mass | HOP | 1 | 1 | Inflammatory cells | Pancreatitis |
LN, lymph node; HOP, head of pancreas; s/m, submucosal; COJ, cardio-oesophageal junction; GIST, gastrointestinal stromal tumor; CT, computed tomography; M, male; F, female.
The mean number of needle passes to obtain material sufficient for a diagnosis was 2.1. Material sufficient for diagnosis was obtained at the first needle pass in nine patients (56.2%). In solid non-lymph-node lesions the diagnosis was made on the first pass in five patients (62.5%). No more than five passes were required to make the diagnosis in any patient. The diagnosis was obtained in 15 of 16 patients (94%), or 13 of 16 patients (81%) if findings suspicious for adenocarcinoma are considered nondiagnostic.
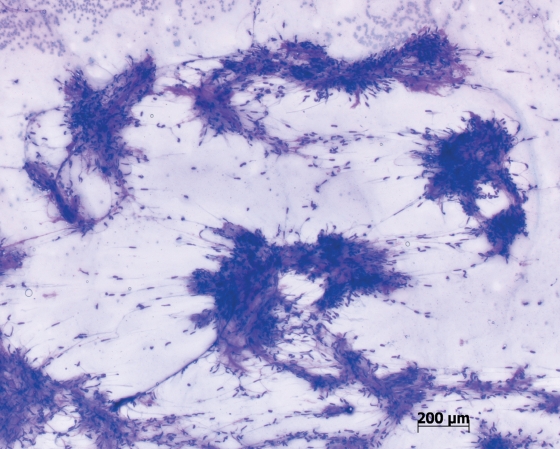
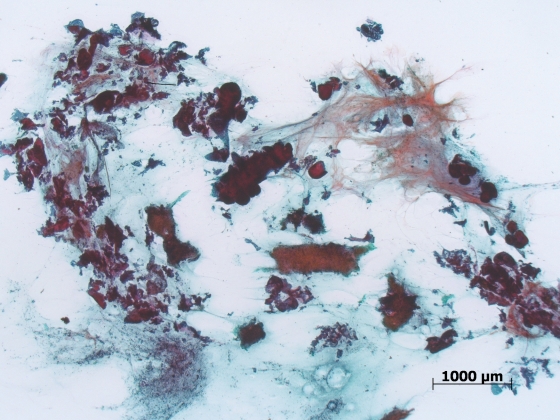
There were no complications recorded at the time of procedure or in follow up. There were no patients in whom the device was unable to be used, and no incidence of device failure, buckling or blockage. The material obtained was felt to be more cellular and contain more stroma by the pathologist (Figures 2 and 3).
Figure 2.
Cytology smear showing large volume cellular acquisition from a gastric gastrointestinal stromal tumor.
Figure 3.
Cytology smear from a pancreatic adenocarcinoma showing large volumes of malignant sheets and stromal tissue, a feature rarely seen with standard final needle aspiration.
Discussion
EUS FNA is important in the diagnosis and staging of benign and malignant mass lesions of the upper gastrointestinal tract and adjacent structures. The technique is safe, reported complications rates varying from less than 1–2%. Complications such as bleeding, infection, perforation and pancreatitis vary with the tissue sampled, relevant anatomy and technical aspects of the procedure.
Reported sensitivities of EUS FNA vary widely according to site targeted and equipment used. Recent attention has been focused on increasing the diagnostic yield of EUS FNA with emphasis on needle caliber [Conway et al. 2009; Wiersema et al. 1997; Siddiqui et al. 2009; Nguyen et al. 2008] and new devices, such as brushes [Bruno et al. 2009] and low [Puri et al. 2009] or high [Larghi et al. 2005] suction devices for EUS FNA. The diagnostic yield in these studies rarely exceeds 90% of cases.
Whilst it is not clear that an increasing number of needle passes increases the risks associated with EUS FNA, the prolonged procedure time associated with multiple passes is unpleasant, and may result in increased complications. During multiple passes, the risk of needle buckling or kinking increases. Despite high diagnostic rates with standard 22 and 25 gage needles, devices requiring fewer passes are needed.
The side port appears to have a different method of tissue acquisition to traditional needles. The exact mechanism for this is unknown. Cellular acquisition from the side port may be from a grating effect when cells are sucked into the port and the in–out movement is then applied shearing tissue that has been sucked into the needle. We used syringe suction for all cases and have not evaluated this needle in the setting of no suction. An alternative explanation is that twice as much cellular material is engaged with suction as there are twice the number of holes in the needle tip.
There are clear limitations to this pilot study. The sample size is small, and whilst successful and safe use was demonstrated across a spectrum of indications, this meant case selection was heterogeneous, making analysis of the success of this needle in particular situations difficult. Also, not all cases were of pancreatic masses, a group typically requiring more needle passes for a diagnosis.
In addition we only evaluated suction FNA when many experts suggest FNA without suction to be adequate. Further studies comparing this needle to traditional needles and techniques are required. We believe this needle may provide a benefit in the drainage of fluids such as cysts and ascites. Subjectively, fluid drainage was easier and the additional side port may explain this. Side ports in many drainage devices and catheters exist as a standard feature.
This report details a pilot experience of EUS FNA with the Olympus side-port needle. The technique of FNA using this needle shows promise and has a high diagnostic yield, often at first pass. This technique appears safe and effective in standard indications for EUS FNA without any incidents of complication or device failure in this series. Further evaluation of this device is warranted, and prospective trials are underway.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflicts of interest in preparing this article.
References
- Adler D.G., Jacobson B.C., Davila R.E., Hirota W.K., Leighton J.A., Qureshi W.A., et al. (2005) ASGE guideline: complications of EUS. Gastrointest Endosc 61: 8–12 [DOI] [PubMed] [Google Scholar]
- Affi A., Vazquez-Sequeiros E., Norton I.D., Clain J.E., Wiersema M.J. (2001) Acute extraluminal hemorrhage associated with EUS-guided fine needle aspiration: frequency and clinical significance. Gastrointest Endosc 53: 221–225 [DOI] [PubMed] [Google Scholar]
- Bruno M., Bosco M., Carucci P., Pacchioni D., Repici A., Mezzabotta L., et al. (2009) Preliminary experience with a new cytology brush in EUS-guided FNA. Gastrointest Endosc 70: 1220–1224 [DOI] [PubMed] [Google Scholar]
- Conway J., Kundu S., Mishra G., Stefanescu S. (2009) Is there a difference in diagnostic yield between 22g and 25g needles in endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) of solid lesions? Gastrointest Endosc 69: S248–S248 [Google Scholar]
- De Witt J. (2006) Pancreatic neoplasms. In: Hawes R., Fockens P. (eds), Interventional Endosonography. London: Elsevier, pp. 177–203 [Google Scholar]
- Eloubeidi M.A., Tamhane A., Varadarajulu S., Wilcox C.M. (2006) Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc 63: 622–629 [DOI] [PubMed] [Google Scholar]
- Erickson R.A., Sayage-Rabie L., Beissner R.S. (2000) Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc 51: 184–190 [DOI] [PubMed] [Google Scholar]
- Giovannini M., Seitz J.F., Monges G., Perrier H., Rabbia I. (1995) Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy 27: 171–177 [DOI] [PubMed] [Google Scholar]
- Gress F., Gottlieb K., Sherman S., Lehman G. (2001) Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med 134: 459–464 [DOI] [PubMed] [Google Scholar]
- Larghi A., Noffsinger A., Dye C.E., Hart J., Waxman I. (2005) EUS-guided fine needle tissue acquisition by using high negative pressure suction for the evaluation of solid masses: a pilot study. Gastrointest Endosc 62: 768–774 [DOI] [PubMed] [Google Scholar]
- LeBlanc J.K., Ciaccia D., Al-Assi M.T., McGrath K., Imperiale T., Tao L.-C., et al. (2004) Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc 59: 475–481 [DOI] [PubMed] [Google Scholar]
- Nguyen T.T.H., Lee C.E., Whang C.S., Ashida R., Lee J.G., Chang K., et al. (2008) A Comparison of the diagnostic yield and specimen adequacy between 22 and 25 gauge needles for endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) of solid pancreatic lesions (SPL): is bigger better? Gastrointest Endosc 67(5): AB100–AB100 [Google Scholar]
- O’Toole D., Palazzo L., Arotcarena R., Dancour A., Aubert A., Hammel P., et al. (2001) Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc 53: 470–474 [DOI] [PubMed] [Google Scholar]
- Palazzo L., Canard J., Carayon P., Dumas R., Escourrou J., Gay G., et al. (1999) L’cho-endoscopie en France en 1998: resultats d’une enquete prospective nationale de la Societe Francaise d’Endoscopie Digestive. Gastroenterol Clin Biol 23: 42–42 [Google Scholar]
- Puri R., Vilmann P., Saftoiu A., Skov B.G., Linnemann D., Hassan H., et al. (2009) Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol 44: 499–504 [DOI] [PubMed] [Google Scholar]
- Rocca R., De Angelis C., Daperno M., Carucci P., Ravarino N., Bruno M., et al. (2007) Endoscopic ultrasound-fine needle aspiration (EUS-FNA) for pancreatic lesions: effectiveness in clinical practice. Dig Liver Dis 39: 768–774 [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Kitano M., Komaki T., Noda K., Chikugo T., Dote K., et al. (2009) Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol 24: 384–390 [DOI] [PubMed] [Google Scholar]
- Shin H.J.C., Lahoti S., Sneige N. (2002) Endoscopic ultrasound-guided fine-needle aspiration in 179 cases: the M. D. Anderson Cancer Center experience. Cancer 96: 174–180 [DOI] [PubMed] [Google Scholar]
- Siddiqui U.D., Rossi F., Rosenthal L.S., Padda M.S., Murali-Dharan V., Aslanian H.R. (2009) EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc 70: 1093–1097 [DOI] [PubMed] [Google Scholar]
- Varadarajulu S., Eloubeidi M.A. (2004) Frequency and significance of acute intracystic hemorrhage during EUS-FNA of cystic lesions of the pancreas. Gastrointest Endosc 60: 631–635 [DOI] [PubMed] [Google Scholar]
- Vazquez-Sequeiros E. (2007) Endoscopic ultrasound and fine needle aspiration in inflammatory and cystic pancreatic pathology. Minerva Medica 98: 357–360 [PubMed] [Google Scholar]
- Vilmann P., Hancke S., Henriksen F.W., Jacobsen G.K. (1993) Endosonographically-guided fine needle aspiration biopsy of malignant lesions in the upper gastrointestinal tract. Endoscopy 25: 523–527 [DOI] [PubMed] [Google Scholar]
- Vilmann P., Hancke S., Henriksen F.W., Jacobsen G.K. (1995) Endoscopic ultrasonography-guided fine-needle aspiration biopsy of lesions in the upper gastrointestinal tract. Gastrointest Endosc 41: 230–235 [DOI] [PubMed] [Google Scholar]
- Wiersema M.J., Vilmann P., Giovannini M., Chang K.J., Wiersema L.M. (1997) Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 112: 1087–1095 [DOI] [PubMed] [Google Scholar]
- Wiersema M.J., Wiersema L.M., Khusro Q., Cramer H.M., Tao L.C. (1994) Combined endosonography and fine-needle aspiration cytology in the evaluation of gastrointestinal lesions. Gastrointest Endosc 40: 199-206 [DOI] [PubMed] [Google Scholar]
- Yamao K., Bhatia V., Mizuno N., Sawaki A., Shimizu Y., Irisawa A. (2009) Interventional endoscopic ultrasonography. J Gastroenterol Hepatol 24: 509–519 [DOI] [PubMed] [Google Scholar]
- Yusuf T.E., Ho S., Pavey D.A., Michael H., Gress F.G. (2009) Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy 41: 445–448 [DOI] [PubMed] [Google Scholar]