Abstract
Duodenal polyps are a rare finding in patients presenting for gastroscopy, being found in 0.3–4.6% of cases. The majority of patients are asymptomatic. The most common lesions necessitating removal are duodenal adenomas which should be differentiated from other mucosal lesions such as ectopic gastric mucosa, and submucosal lesions such as carcinoids and gastrointestinal stromal tumours (GISTs). Adenomas can occur sporadically or as part of a polyposis syndrome. Both groups carry malignant potential but this is higher in patients with a polyposis syndrome. The majority of sporadic duodenal adenomas are flat or sessile and occur in the second part of the duodenum. Historically duodenal adenomas have been managed by radical surgery, which carried significant mortality and morbidity, or more conservative local surgical excision which resulted in high local recurrence rates. There is growing evidence for the use of endoscopic mucosal resection (EMR) techniques for treatment of sporadic nonampullary duodenal adenomas, with good outcomes and low complication rates. Endoscopic submucosal dissection (ESD) carries greater risk of complications and should be reserved for experts in this technique. Patients with sporadic duodenal adenomas carry an increased risk of colonic neoplasia and should be offered colonoscopy. The impact of endoscopic resection on the course of polyposis syndromes such as familial adenomatous polyposis (FAP) needs further study.
Keywords: adenoma, carcinoid, duodenum, endoscopic mucosal resection, endoscopic submucosal dissection, familial adenomatous polyposis, gastrointestinal stromal tumour
Introduction
Duodenal polyps are reported in 0.3–4.6% of patients attending for upper gastrointestinal endoscopy [Ghazi et al. 1984; Hochter et al. 1984; Jepsen et al. 1994; Reddy et al. 1981]. Management of these polyps is dependent on symptoms, histopathology and endoscopic features. This review focuses on the endoscopic management of nonampullary duodenal polyps and in particular duodenal adenomas.
Historically duodenal adenomas have been managed by radical surgery or more conservative local surgical excision. However, these approaches have carried significant morbidity and mortality risks, plus a high recurrence rate following local excision. For these reasons, endoscopic management of duodenal adenomas has become increasingly popular. However there is a lack of published evidence regarding the optimal endoscopic management of duodenal polyps, both sporadic and in those associated with a polyposis syndrome. Small case series show promising but variable results of endoscopic management. A recent UK National Institute for Health and Clinical Excellence (NICE) identified the lack of data in this field [NICE, 2010]
Endoscopic treatment of duodenal polyps provides a challenge both in terms of accurate diagnosis, staging and endoscopic resection in the presence of the thin duodenal wall and rich vascularity. However, an endoscopic approach offers considerable advantages in terms of organ preservation, risks, recovery and length of hospital stay.
The aim of this article is to review the available literature on endoscopic resection of duodenal polyps and highlight the advantages and disadvantages of an endoscopic approach.
Types of duodenal polyps
A variety of lesions can be found in the duodenum on endoscopic examination. These lesions can be distinguished based on endoscopic appearance, endoscopic ultrasound (EUS) and histology. Biopsy is recommended to determine histology and thus guide management.
Submucosal lesions
Inflammatory fibroid polyps are a rare finding in the duodenum, being much more common in the stomach [Wysocki et al. 2007]. They are usually submucosal which makes accurate endoscopic diagnosis difficult as histopathology is rarely diagnostic. Use of EUS may aid diagnosis prior to resection. If small and asymptomatic these lesions can be left alone. However polyps up to 12.5 cm in size have been reported and large symptomatic lesions warrant removal [Ott et al. 1980].
Carcinoid tumours of the duodenum are rare, accounting for less than 5% of all carcinoids. They arise from the submucosa and hence biopsies can be nondiagnostic. In a case series of 27 lesions, 59% were found in the first part of the duodenum [Zyromski et al. 2001]. Data on endoscopic resection of carcinoid tumours is limited to very small case series. Endoscopic resection is feasible for carcinoids <1 cm and possibly up to 2 cm in diameter. EUS assessment is required prior to resection to ensure that the lesion does not involve the muscularis propria [Ahmad et al. 2002; Dalenback and Havel, 2004; Karagiannis et al. 2009; Pungpapong et al. 2006; Shim and Jung, 2005; Urso et al. 2007; Zyromski et al. 2001]. The risk of perforation and bleeding can be high due to the submucosal origin of carcinoids, plus the rich vascular supply and thin wall of the duodenum. Therefore, resection of carcinoids in the duodenum should be reserved for experts working in high-volume centres with experience of performing endoscopic mucosal resection (EMR) in the duodenum.
Solitary Peutz–Jeghers-type polyps can be found in the duodenum in the absence of Peutz–Jeghers syndrome although there are only a handful of case reports. These lesions have been described as having a lobular or nodular surface, whitish colour and whitish spots on the surface. Endoscopic removal is indicated as there is a small risk of malignant transformation [Suzuki et al. 2008].
Gastrointestinal stromal tumours (GISTs) are submucosal tumours and cannot be diagnosed by standard endoscopy or biopsy. EUS is key in confirming the diagnosis and determining the layer of origin. Those arising from the muscularis mucosae can potentially be removed by EMR but the majority will require surgery. GISTs of the duodenum are less common than gastric GISTs but more likely to be symptomatic [Miki et al. 2010]. Prognosis of duodenal GISTs compared with gastric GISTs is debatable with some authors suggesting recurrence after resection may be more frequent, whilst others report a more favourable prognosis [Miki et al. 2010; Yang et al. 2009].
Mucosal lesions
Gastric heterotopia is most commonly found as multiple small polyps in the duodenal bulb as reported by Jepsen and colleagues [Jepsen et al. 1994]. However, other retrospective studies of duodenal polyps report no cases of gastric heterotopias, raising the possibility that as a relatively common finding, these lesions may not be reported by many endoscopists. They have no malignant potential or clinical significance and do not require any endoscopic treatment.
Brunner’s gland tumours are extremely rare with a prevalence of less than 1 in 10,000 in an autopsy series [Osborne et al. 1973]. These lesions are predominately found in the duodenal bulb and small polyps are usually asymptomatic. They usually follow a benign course but dysplasia and malignancy have occasionally been reported. Larger polyps tend to be pedunculated and can present with upper gastrointestinal haemorrhage requiring endoscopic intervention [Mukherjee et al. 1999; Walden and Marcon, 1998].
Duodenal adenomas
Rarer duodenal tumours such as those described so far only constitute a small minority of the lesions for which duodenal endoscopic resection is indicated. The bulk of experience in this area is in the treatment of duodenal adenomas. Duodenal adenomas have malignant potential in a similar fashion to colonic adenomas [Sellner, 1990; Spigelman et al. 1994]. This is the case both in patients with polyposis syndromes such as familial adenomatous polyposis (FAP) and also in those with sporadic duodenal adenomas (SDAs). The risk of carcinoma is greater in ampullary adenomas compared with nonampullary, and increases with the size of adenoma [Eswaran et al. 2006; Perzin and Bridge, 1981].
Historical series of surgical resection specimens show the majority of patients to be symptomatic, usually presenting with advanced lesions [Perzin and Bridge, 1981; Sellner, 1990]. With the increasingly widespread use of endoscopy over the past few decades, duodenal adenomas are being detected at an earlier and often asymptomatic stage. Recent series of endoscopically managed duodenal polyps report 66–80% of patients to be asymptomatic at the time of diagnosis [Abbass et al. 2010; Alexander et al. 2009; Perez et al. 2003; Takahashi et al. 2009]. However, all adenomas carry a risk of malignant potential, so depending on the patient’s fitness and life expectancy, resection of adenomas should be considered.
Duodenal adenomas in familial adenomatous polyposis
Duodenal adenomas occur in up to 90% of patients with FAP, most commonly at the ampulla, peri-ampullary region or distal duodenum, which is thought to be in part due to the exposure of the duodenal mucosa to bile in a predisposed patient [Bulow et al. 2004; Gallagher et al. 2006; Spigelman et al. 1989]. The lifetime risk of duodenal cancer in patients with FAP is estimated to be 3– 5% [Bulow et al. 2004; Groves et al. 2002; Lepisto et al. 2009; Vasen et al. 1997]. Duodenal cancer and desmoids tumours are now the main cause of death in patients with FAP, rather than colorectal cancer [Arvanitis et al. 1990; Spigelman et al. 1989]. A staging system for assessing the severity of the duodenal polyp burden was developed by Spigelman and colleagues (see Table 1). This has been used to assess the duodenal polyp burden in patients with FAP and predict the risk of developing duodenal cancer.
Table 1.
Spigelman grading of duodenal adenomas in familial adenomatous polyposis.
| Points | 1 | 2 | 3 |
|---|---|---|---|
| Number of polyps | 1–4 | 5–20 | >20 |
| Polyp size | 1–4 | 5–10 | >10 |
| Histology | Tubular | Tubulovillous | Villous |
| Dysplasia | Mild | Moderate | Severe |
Stage 0 = 0 points; stage I = 1–4 points; stage II = 5–6 points; stage III = 7–8 points; stage IV = 9–12 points.
The risk of developing duodenal cancer is greatest for patients with stage IV disease, with rates of 7–36% described over follow-up periods of 7.6–10 years [Bulow et al. 2004; Groves et al. 2002]. This risk is confirmed in studies of duodenal resection specimens of patients with stage IV disease which revealed unsuspected cancer in 28–31% of cases [Gallagher et al. 2004; Penna et al. 1998]. In comparison the risk of developing duodenal cancer in stage 0–III disease over a similar period is low at 0.7% [Bulow et al. 2004]. Progression to higher stages occurs over time with one study showing 15% of patients progressing from stages 0–III to stage IV disease over a period of 8 years [Bulow et al. 2004]. The risk of developing stage IV disease by age 70 is estimated to be between 20% and 50% [Bjork et al. 2001; Bulow et al. 2004; Heiskanen et al. 1999].
There is evidence that use of nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce progression and even cause regression of small adenoma [Phillips et al. 2002]. These potential advantages need to be balanced against potential cardiovascular and renal side effects and the risk of gastrointestinal bleeding.
Surgical management involves duodenectomy with or without preservation of the pancreas and pylorus. This carries significant morbidity and mortality risks, greater than those for non-FAP patients undergoing similar surgery [Gallagher et al. 2004; Ruo et al. 2002]. This may be related to increased complexity of surgery due to previous prophylactic colectomy and the presence of desmoids tumours in patients with Gardener’s syndrome. Surgical duodenotomy and polypectomy has proven ineffective with high rates of recurrence [Penna et al. 1998].
Surveillance of duodenal familial adenomatous polyposis
In view of this high risk of duodenal cancer in patients with advanced duodenal polyposis, upper gastrointestinal endoscopic surveillance has been proposed, with examinations beginning from age 25–30 [Cairns et al. 2010; Gallagher et al. 2006]. Forward and side viewing endoscopes should be used to provide adequate visualization of all the duodenal mucosa [Bulow et al. 2004; Groves et al. 2002]. Recent studies have shown that the use of indigocarmine chromoendoscopy leads to greater numbers of duodenal adenomas being detected in patients with FAP and an upgrading of the Spigelman stage in 12% of patients [Dekker et al. 2009; Picasso et al. 2007]. Patients with no visible polyps detected have been shown to have adenomatous tissue on random biopsies in up to 7.6% of cases. However, this approach may no longer be necessary with the more widespread use of chromoendoscopy and electronic-imaging techniques such as NBI (Narrow Band Imaging), FICE (Flexible Spectral Imaging Colour Enhancement) and iScan enhancing pickup of small adenomas. The frequency of endoscopic examinations is determined by the Spigelman stage, with a shorter duration between examinations for more advanced stages, the aim being to detect stage IV disease before duodenal or ampullary cancer has developed. Patients with stage 0 or I disease receive repeat surveillance endoscopy after 5 years, those with stage II disease after 3 years and those with stage III disease every 1–2 years. Patients found to have stage IV disease should be referred to a pancreato-biliary surgeon for consideration of prophylactic duodenectomy [Groves et al. 2002]. There is a fair degree of agreement in published recommendations regarding the frequency of endoscopic surveillance in patients of a specific Spigelman stage. Recommendations differ slightly in the use of imaging, chemoprophylaxis and endoscopic intervention; see Table 2
Table 2.
Recommendations for duodenal familial adenomatous polyposis surveillance.
| Spigelman stage | Bulow et al. | Gallagher et al. | Groves et al. |
|---|---|---|---|
| 0 | 5 yearly endoscopy | 5 yearly endoscopy | 5 yearly endoscopy |
| 1 | 5 yearly endoscopy | 5 yearly endoscopy | 5 yearly endoscopy |
| 2 | 3 yearly endoscopy | 2–3 yearly endoscopy | 3 yearly endoscopy + endoscopic therapy |
| 3 | 1–2 yearly endoscopy | 1–2 yearly endoscopy +/− endoscopic therapy Celecoxib | 1–2 yearly endoscopy (consider GA (General Anaesthetic)) + endoscopic therapy Celecoxib |
| 4 | EUS Consider Surgery | EUS/CT Consider surgery | Consider surgery |
EUS, endoscopic ultrasound; CT, computed tomography; GA (General Anaesthetic).
Endoscopic treatment
Duodenal surveillance in this group of patients has not been proven to improve survival. A decision analysis study suggested only a modest benefit in terms of life expectancy [Vasen et al. 1997]. Optimum management of patients with lesser stages of disease has yet to be determined. The advent of EMR has led to a new potential therapeutic option for large flat duodenal polyps in FAP. This has been proposed for patients with stage II and III disease [Groves et al. 2002] and in a small series has been shown to downstage disease in a proportion of patients, but with a significant complication rate of 24% [Gallagher et al. 2006]. At present it is not clear whether endoscopic intervention significantly alters the course of the disease, or improves survival or quality of life in patients with a significant duodenal polyp burden. However, it is our current practice to endoscopically resect all large duodenal adenomas in patients with FAP unless there is suspicion of advanced histology and submucosal invasion. Other endoscopic options include argon plasma coagulation (APC), photodynamic therapy (PDT) and thermal ablation. In our view APC is the only other modality which has a role, when used for eradication of tiny adenomas less than 5 mm in size, which are often very numerous. Until more data is available, endoscopic treatment should be individualized based on the patient’s overall polyp burden, size and location of polyps, comorbidities and patient preferences. Further studies are needed in this area to determine the impact of endoscopic intervention on the course of duodenal disease in FAP.
Sporadic duodenal adenomas
Background
Nonampullary SDAs are those which arise in patients without a known polyposis syndrome. They are usually solitary and the majority are sessile or flat rather than pedunculated [Ahmad et al. 2002; Apel et al. 2005; Honda et al. 2009; Kedia et al. 2010; Kim et al. 2010]. The mean age at diagnosis is usually in the seventh decade and incidence is approximately equal amongst men and women. The majority of patients are asymptomatic at the time of diagnosis, although bleeding, anaemia and abdominal pain are the commonest reported symptoms.
Around 80–94% of SDAs are found in the second part of the duodenum with mean size in various series ranging from 13 to 29 mm [Alexander et al. 2009; Apel et al. 2005; Honda et al. 2009; Kim et al. 2010; Lepilliez et al. 2008]. Contrast radiography is unreliable in detecting duodenal adenomas, because of the flat or sessile nature of the majority of lesions, and the vast majority are detected during upper gastrointestinal endoscopy. These morphological features plus their tendency to grow along folds means that duodenal adenomas can be more difficult to detect than colonic adenomas and may be missed by the untrained eye (Figures 1 and 3). Whilst the risk of malignant transformation of SDAs is lower than that of duodenal adenomas in patients in FAP, these lesions do carry malignant potential and removal is recommended. Historical case series of surgically resected adenomas in symptomatic patients show cancer prevalence rates in the resected polyps of between 33% and 47%. The risk of carcinomatous change in an asymptomatic group with duodenal adenomas detected at endoscopy is harder to quantify, but is likely to be far lower [Galandiuk et al. 1988; Jepsen et al. 1994; Miller et al. 1980; Sellner, 1990; Witteman et al. 1993].
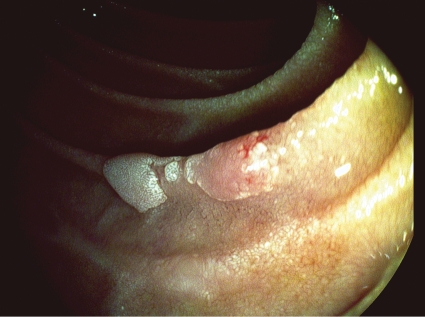
Figure 1.
Small duodenal adenoma.
Figure 3.
Large sessile duodenal adenoma.
Treatment strategies involve radical surgical resection of the duodenum such as Whipple’s pancreatectomy, pylorus preserving pancreatectomy (PPP) or pylorus and pancreas preserving duodenectomy (PPPD). These procedures carry a mortality risk of 1–6.4% and morbidity risk of 37–41% [Cameron et al. 2006; Mukherjee et al. 2009].
Local surgery involving duodenotomy and local excision or wedge excision have shown high rates of recurrence and still carry significant operative risk and is not currently practised by most surgeons [Galandiuk et al. 1988; Haglund et al. 1985]. This has led to a surge in endoscopic assessment and resection of duodenal adenomas.
Endoscopic assessment
The principles of EMR at other sites apply equally to duodenal adenomas. The lesion is first carefully assessed to determine size, involvement of mucosal folds and proportion of the circumference of the duodenal lumen involved. The relationship of the adenoma to the Ampulla of Vater should be determined, using a side-viewing endoscope. Delineating the borders of the adenoma may be aided by the use of chromoendoscopy. Intravenous glucagon or hyoscine should be given during assessment or resection to limit duodenal peristalsis and improve the view and access to the lesion.
Careful evaluation is required before planning any endoscopic intervention. Endoscopic assessment should focus on endoscopic resectability of the lesion and detection of any features which may predict submucosal invasion and hence make endoscopic treatment unsuitable. Features which suggest higher risk of submucosal invasion include a depressed element (IIc component of the Paris classification), type 5 Kudo’s pit pattern, surface bleeding, ulceration, induration or the nonlifting sign when submucosal injection is attempted. Hirasawa and colleagues reviewed the Japanese literature regarding the size of duodenal adenomas, Paris Classification (Paris Workshop 2003) and risk of lymph node metastases [Hirasawa et al. 1997]. They concluded that endoscopic resection could be performed on lesions with a depressed element (Paris IIc, IIc + IIa or IIa + IIc) up to 10 mm in diameter and on nondepressed adenomas up to 50 mm diameter as the risk of invasive malignancy in these groups was very low. It should be noted that these studies, performed by Japanese experts, relied on very accurate assessment of surface patterns (Kudo’s) and lesion morphology which may not translate to a Western setting. There are case reports of lesions up to 80 mm without evidence of invasive disease and in our centre we have treated similar sized lesions which have proven benign. However, the risks of EMR increase with the size of lesion [Hirasawa et al. 1997].
The role of EUS in the assessment of SDAs has not been determined. However, it is our practice to perform EUS/CT in lesions >20 mm diameter, excluding any invasive element prior to endoscopic resection. This is not evidence based and there is a need for further studies to evaluate the role of EUS in assessment of duodenal adenomas.
Endoscopic resection
Endoscopic resection is an established technique for treatment of large adenomas in the colon and precancerous lesion and early cancers in the oesophagus and stomach. To date only a few small series reporting endoscopic resection of SDAs have been published.
As most duodenal adenomas have a flat or sessile morphology, a submucosal injection of lifting solution is performed, raising the lesion on a cushion of submucosal fluid which separates the mucosal and muscle layers. Several different fluid bases for lifting solution have been studied but those in widest use are 0.9% saline, gelofusin, glycerol and hyaluronic acid. There is evidence that hypertonic fluids provide better and longer-lasting lift than 0.9% saline [Uraoka et al. 2009]. The addition of adrenaline (epinephrine) to the solution can prevent early bleeding, giving a clearer field of view, and has been used in several series. The use of indigocarmine in the mixture stains the submucosa blue helping delineate the submucosal layer from the underlying muscle layer [Alexander et al. 2009; Honda et al. 2009; Kedia et al. 2010; Takahashi et al. 2009]. Endoscopic resection without pre-injection of submucosal lifting solution may lead to incomplete resection of adenomatous material resulting in higher rates of recurrence [Apel et al. 2005]. The thin duodenal wall makes the risk of perforation high and prior submucosal injection should reduce this risk.
Once sufficient lift has been achieved the majority of published series used a snare technique to perform EMR. This enables lesions up to approximately 20 mm in diameter to be resected in a single piece. For lesions larger than 20 mm piecemeal resection is likely to be required [Alexander et al. 2009; Kedia et al. 2010]. When contemplating a piecemeal resection the endoscopist must have a clear plan of action for a systematic resection, working from one side of the lesion to the other, without leaving any islands of adenomatous tissue in the EMR base. Modified cutting current such as Endocut Effect 2 (blended current) should be used, rather than coagulation current, to reduce the risk of perforation. APC can be used as an adjunct to ablate any tiny islands of residual adenomatous tissue at the edges of the EMR base. In our practice we also use clips to close the EMR base after resection (see Figure 2). The combination of these techniques seems to reduce the risk of late bleeding following duodenal EMR, although evidence for this is limited [Lepilliez et al. 2008].
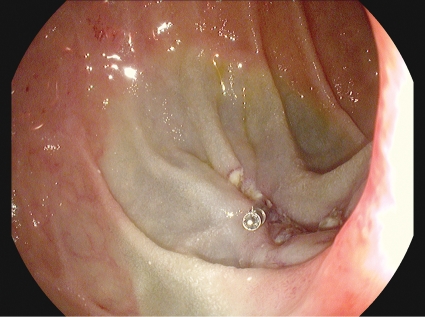
Figure 2.
Duodenal EMR base closed with clips.
Published success rates of EMR for complete removal of duodenal adenomas range from 79% to 100%. Of 6 series with specific data for EMR of nonampullary duodenal adenomas a total of 126 of 139 adenomas were successfully treated endoscopically, giving an overall success rate of 92% (see Table 3). In the studies that reported the number of EMR sessions required to achieve complete resection, 80% of cases were completed in a single EMR session, 17% in two sessions and the remaining 3% in three sessions. The intention of the endoscopist should always be to resect the whole lesion in one attempt as the risk of complications rises with each further attempt at EMR.
Table 3.
Published case series of Endoscopic Resection of Duodenal Adenomas : Outcomes and Complications.
| Author & Journal | Number of Cases | Lesion Size | Staging | Technique | EMR/ESD Sessions | Complete resection achieved | Perforations | Bleeding | Follow-up | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| Hirasawa et al, GIE 1997 | 13 pts, 14 lesions | range 5-20mm | EMR | 14 | 100% | 0% | 0% | Mean 19 months | 0% | |
| Ahmad et al, GIE 2002 | 23 | 1-4cm | EUS in 19 | EMR | Not reported seperately for duodenal cases | 79% (non-ampullary) | 0% (number of sessions not reported) | Not reported seperately for duodenal cases | Not reported | Not reported |
| Apel et al, Endoscopy 2005 | 18 pts, 20 lesions | median 27.5mm (range 8-50mm) | Not reported | EMR + APC | 26 | 85% | 0% | 7.1% procedural bleed | Mean 71 months | 29.40% |
| Lepilliez et al, Endoscopy 2008 | 36 pts, 37 lesions | mean 19mm (range 4-50mm) | EUS not used routinely | EMR + APC | 43 | 100% | 2.30% | 11.6% delayed bleeds | Mean 15 months | 0% |
| Alexander et al, GIE 2009 | 21 | mean 27.6mm | Not reported | EMR | 25 | 100% | 0% (1 possible serositis admitted overnight, normal CT Abdo) | 4% | Median 13 months | 25% |
| Abbass et al, GIE 2010 | N/A | mean 17.2mm (range 3-50mm) | 5% had EUS | EMR + APC | N/A | Separate data for adenomas not available | Separate data for adenomas not available | Mean 26 months | 37% | |
| Kedia et al, GIE 2010 | 33 | 9 <20mm, 27 >20mm | EUS in some | EMR + APC | 28 | 67% | 0% | 17.9% procedural bleeds | Not reported | Not reported |
| Kim et al, Gut and Liver 2010 | 17 | mean 13mm (range 8-27) | Rare use of EUS | EMR | 17 | 100% | 0% | 5.8% procedural bleeds | Mean 29 months | 0% |
| Honda et al, Digestive Endoscopy 2009 | 14pts, 15 lesions | ESD - mean 23.8mm, EMR mean 7.5mm | Not reported | ESD & EMR | 9 ESD 6 Hybrid EMR | 100% | 2 perfs in ESD group (1 immediate - clipped, 1 delayed - surgery) | ESD 22.2% delayed bleeds EMR Hybrid 16.7% delayed bleed | Not reported | Not reported |
| Takehashi et al, Scandinavian J Gast 2009 | 4 | mean 20.5mm (range 10-31mm) | Not reported | ESD | 4 | 100% | 2 - managed conservatively. | 0% | Mean 18 months | 0% |
| Means | 92% | EMR 0.6% ESD 30.7% | Procedural bleeds 9.0% Delayed bleeds 3.9% | 19.90% |
Kedia and colleagues studied the relationship between the size of lesion, percentage of luminal circumference involved and success rate of EMR [Kedia et al. 2010]. A success rate of 94.7% was achieved for lesions involving <25% of luminal circumference compared to 45.5% in those involving 25–50% of the circumference and 0% success in those involving >50%. Maximum lesion diameter was not significantly associated with failure to complete resection but there was a trend towards failure in larger lesions which may have reached statistical significance in a larger series.
In those series reporting complication rates specifically for EMR of adenomas, perforation occurred once in 155 endoscopic resection procedures (0.6%); this case required surgical intervention. In addition there was one case of serositis with a normal CT scan which was managed conservatively. Fourteen procedural bleeds occurred during 155 EMR sessions (9.0%), all of which were successfully managed endoscopically with either clipping, APC or adrenaline injection. None of these procedural bleeds required transfusion. Because there is no standard definition of procedural bleeding it is difficult to assess whether these reported bleeds were all of similar severity, or clinically significant. Six cases of delayed bleeding occurred (3.9% of procedures), all successfully managed endoscopically. Two of these six cases required blood transfusion [Alexander et al. 2009; Apel et al. 2005; Hirasawa et al. 1997; Kedia et al. 2010; Lepilliez et al. 2008]. Minor bleeding during EMR is common and can easily be controlled. Owing to the thin duodenal wall, special care should be taken to minimize current delivery to reduce the subsequent risk of perforation. A bipolar device is preferable to monopolar. The overall complication rate was 13.9% in 155 EMR procedures performed for duodenal adenomas.
Following successful eradication of nonampullary SDAs most authors recommend a follow-up endoscopy at 3–6 months to check for recurrence, followed by further endoscopies at intervals of 6–12 months. This may be influenced by the degree of dysplasia in the original lesion [Ahmad et al. 2002]. Our standard practice is to perform a repeat endoscopy at 3 months and if no evidence of residual adenoma is found, arrange annual endoscopies for 3–5 years.
Reported recurrence rates vary greatly from 0% to 36%. Leppiliez and colleagues reported no cases of recurrence although mean follow up in this series was only 15 months and 42% of patients had only had one follow-up endoscopy [Leppiliez et al. 2008]. Kim and colleagues also had 0% recurrence after mean 29 months follow up [Kim et al. 2010]. Conversely Abbass and colleagues reported 36% recurrence of adenomatous lesions after a mean follow up of 26 months, and noted a greater recurrence rate of 63% in lesions >2 cm in diameter [Abbass et al. 2010]. Alexander and colleagues reported recurrence in 25% of cases with up-to-date follow up [Alexander et al. 2009]. All of these were successfully retreated with either snare resection or APC ablation. In a study by Apel and colleagues recurrence occurred in 5 of 17 cases (29%) with 2 of 5 being successfully retreated [Apel et al. 2005]. Overall mean recurrence for those series that provided clear data was 19.9%. These high recurrence rates highlight the need for meticulous removal or ablation of all adenomatous tissue during the initial treatment phase and careful follow up to detect and treat early recurrence.
Argon plasma coagulation
APC as a sole treatment for sessile or flat duodenal adenomas has been studied in one series of 15 patients. Initial treatment was successful in 13 of 15 cases (87%). Of those successfully treated, the recurrence rate was 39% over a mean follow up of 40 months, with 4 of 5 recurrences being successfully retreated. No patients developed duodenal adenocarcinoma, however these success rates do not appear comparable to endoscopic resection [Lienert and Bagshaw, 2007]. Use of the Nd:YAG laser is the duodenum is not recommended as the potential for deep thermal injury risks duodenal perforation [van Stolk et al. 1987]. APC is now primarily used as an adjunct to EMR to reduce the risk of recurrence and we would support its use in this context, rather than as a primary treatment modality.
Endoscopic submucosal dissection
ESD involves a submucosal injection to lift the lesion in a similar fashion to EMR. A mucosal incision is then made using an electrosurgical knife and the submucosal plane meticulously dissected to remove the lesion en bloc. The advantages of ESD over conventional EMR are the higher rates of en bloc resection but at the cost of higher risk of perforation and increased procedure duration. Two series have described the use of ESD for the treatment of duodenal adenomas and early duodenal cancers, showing excellent success rates. High rates of en bloc resection were achieved, aiding histopathological staging [Honda et al. 2009; Takahashi et al. 2009]. However the complication rate of ESD is greater than EMR with perforation occurring in 4 of 13 ESD cases (31%) and late bleeding in 2 of 13 (15%). Owing to these risks this approach is only suitable for those with advanced experience in ESD. The endoscopist should have sufficient experience of performing ESD in other locations such as stomach and colon before attempting duodenal ESD as the risks and consequences of complications are very serious. We would recommend that this is only performed in a research setting.
Histology of resected specimen
Precise histological examination of the resection specimen is required to determine the degree of dysplasia, and assess for any evidence of invasion. In comparison to early cancers of the stomach and colon, little data is available regarding the risk of lymph node metastases in early duodenal cancer. It appears that intramucosal cancer carries a very low risk of lymph node involvement provided that there are no other adverse histological features, namely poorly differentiated carcinoma, signet ring type or lymphovascular invasion. In lesions with submucosal invasion (sm1 to sm3) the risk appears to be at least 5% [Friedrich-Rust and Ell, 2005; Hirasawa et al. 1997; Nagatani et al. 1993; Witteman et al. 1993]. Therefore, those patients found to have submucosal invasion should be considered for radical surgery. This emphasizes the importance of specimen retrieval. It is quite easy for the resected specimen to be lost down the distal small bowel during endoscopic resection of duodenal adenomas. It is our standard practice to move resected tissue fragments to the stomach before resecting further portions of adenoma, thus reducing the risk of lost specimens.
Risk of colorectal adenomas
Nonampullary SDAs have been linked with an increased risk of colorectal neoplasia. Apel and colleagues found that 16 of 22 patients (72.7%) with a sporadic duodenal adenoma had colorectal adenomas detected at colonoscopy [Apel et al. 2004]. A retrospective case–control study by Murray and colleagues found colorectal neoplasia in 56% of patients with a duodenal adenoma versus 33% in controls without a duodenal adenoma (p = 0.03) [Murray et al. 2004]. There was a nonsignificant increase in advanced colorectal neoplasia (38% versus 19%) and colorectal cancer (21% versus 8%). In a similar study by Pequin and colleagues, overall rates of neoplasia were not significantly different between patients with duodenal adenomas and controls without duodenal adenomas (37.1% and 25.7%, respectively) [Pequin et al. 2007]. However, the risk of advanced neoplasia or advanced adenoma were significantly increased (28.6% versus 4.3% and 22.9% versus 2.9%, respectively). In view of these results, all patients found to have a sporadic duodenal adenoma should be offered colonoscopy to screen for colorectal neoplasia.
Conclusions
Differentiating duodenal adenomas from other types of duodenal polyp, both mucosal and submucosal is crucial to allow optimum management. The evidence base for endoscopic resection of duodenal adenomas is limited, but promising results and good outcomes have been achieved by experts in these techniques. Further data is required to guide future management of duodenal adenomas.
Key Points
Duodenal polyps are rare, but detection has increased with the more widespread use of upper gastrointestinal endoscopy. The majority of patients are asymptomatic.
Chromoendoscopy and electronic imaging techniques should be used to assess lesions prior to initial resection and to check for recurrence during follow up.
Endoscopic therapy should be considered for Spigelman stage II/III duodenal FAP on an individual patient basis. Further study of the impact of endoscopic intervention in this group of patients is needed.
FAP patients with Spigelman stage IV disease should have the option of radical surgery discussed.
Nonampullary SDAs should be considered for endoscopic resection provided there are no adverse features.
EMR technique should involve submucosal injection of lifting solution and blended current.
Footnotes
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no conflicts of interest in preparing this article.
References
- Abbass R., Rigaux J., Al-Kawas F.H. (2010) Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc 71: 754–759 [DOI] [PubMed] [Google Scholar]
- Ahmad N.A., Kochman M.L., Long W.B., Furth E.E., Ginsberg G.G. (2002) Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc 55: 390–396 [DOI] [PubMed] [Google Scholar]
- Alexander S., Bourke M.J., Williams S.J., Bailey A., Co J. (2009) EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc 69: 66–73 [DOI] [PubMed] [Google Scholar]
- Apel D., Jakobs R., Spiethoff A., Riemann J.F. (2005) Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy 37: 444–448 [DOI] [PubMed] [Google Scholar]
- Apel D., Jakobs R., Weickert U., Riemann J.F. (2004) High frequency of colorectal adenoma in patients with duodenal adenoma but without familial adenomatous polyposis. Gastrointest Endosc 60: 397–399 [DOI] [PubMed] [Google Scholar]
- Arvanitis M.L., Jagelman D.G., Fazio V.W., Lavery I.C., McGannon E. (1990) Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum 33: 639–642 [DOI] [PubMed] [Google Scholar]
- Bjork J., Akerbrant H., Iselius L., Bergman A., Engwall Y., Wahlstrom J., et al. (2001) Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology 121: 1127–1135 [DOI] [PubMed] [Google Scholar]
- Bulow S., Bjork J., Christensen I.J., Fausa O., Jarvinen H., Moesgaard F., et al. (2004) Duodenal adenomatosis in familial adenomatous polyposis. Gut 53: 381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns S.R., Scholefield J.H., Steele R.J., Dunlop M.G., Thomas H.J., Evans G.D., et al. (2010) Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 59: 666–689 [DOI] [PubMed] [Google Scholar]
- Cameron J.L., Riall T.S., Coleman J., Belcher K.A. (2006) One thousand consecutive pancreaticoduodenectomies. Ann Surg 244: 10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalenback J., Havel G. (2004) Local endoscopic removal of duodenal carcinoid tumors. Endoscopy 36: 651–655 [DOI] [PubMed] [Google Scholar]
- Dekker E., Boparai K.S., Poley J.W., Mathus-Vliegen E.M., Offerhaus G.J., Kuipers E.J., et al. (2009) High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy 41: 666–669 [DOI] [PubMed] [Google Scholar]
- Eswaran S.L., Sanders M., Bernadino K.P., Ansari A., Lawrence C., Stefan A., et al. (2006) Success and complications of endoscopic removal of giant duodenal and ampullary polyps: a comparative series. Gastrointest Endosc 64: 925–932 [DOI] [PubMed] [Google Scholar]
- Friedrich-Rust M., Ell C. (2005) Early-stage small-bowel adenocarcinoma: a review of local endoscopic therapy. Endoscopy 37: 755–759 [DOI] [PubMed] [Google Scholar]
- Galandiuk S., Hermann R.E., Jagelman D.G., Fazio V.W., Sivak M.V. (1988) Villous tumors of the duodenum. Ann Surg 207: 234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M.C., Phillips R.K., Bulow S. (2006) Surveillance and management of upper gastrointestinal disease in Familial Adenomatous Polyposis. Fam Cancer 5: 263–273 [DOI] [PubMed] [Google Scholar]
- Ghazi A., Ferstenberg H., Shinya H. (1984) Endoscopic gastroduodenal polypectomy. Ann Surg 200: 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves C.J., Saunders B.P., Spigelman A.D., Phillips R.K. (2002) Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut 50: 636–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund U., Fork F.T., Genell S., Rehnberg O. (1985) Villous adenomas in the duodenum. Br J Surg 72: 26–27 [DOI] [PubMed] [Google Scholar]
- Heiskanen I., Kellokumpu I., Jarvinen H. (1999) Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy 31: 412–416 [DOI] [PubMed] [Google Scholar]
- Hirasawa R., Iishi H., Tatsuta M., Ishiguro S. (1997) Clinicopathologic features and endoscopic resection of duodenal adenocarcinomas and adenomas with the submucosal saline injection technique. Gastrointest Endosc 46: 507–513 [DOI] [PubMed] [Google Scholar]
- Hochter W., Weingart J., Seib H.J., Ottenjann R. (1984) [Duodenal polyps. Incidence, histologic substrate and significance]. Dtsch Med Wochenschr 109: 1183–1186 [DOI] [PubMed] [Google Scholar]
- Honda T., Yamamoto H., Osawa H., Yoshizawa M., Nakano H., Sunada K., et al. (2009) Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc 21: 270–274 [DOI] [PubMed] [Google Scholar]
- Jepsen J.M., Persson M., Jakobsen N.O., Christiansen T., Skoubo-Kristensen E., Funch-Jensen P., et al. (1994) Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol 29: 483–487 [DOI] [PubMed] [Google Scholar]
- Karagiannis S., Eshagzaiy K., Duecker C., Feyerabend B., Mozdzanowski E., Faiss S. (2009) Endoscopic resection with the cap technique of a carcinoid tumor in the duodenal bulb. Endoscopy 41(Suppl. 2): E288-E289 [DOI] [PubMed] [Google Scholar]
- Kedia P., Brensinger C., Ginsberg G. (2010) Endoscopic predictors of successful endoluminal eradication in sporadic duodenal adenomas and its acute complications. Gastrointest Endosc 72: 1297–1301 [DOI] [PubMed] [Google Scholar]
- Kim H.K., Chung W.C., Lee B.I., Cho Y.S. (2010) Efficacy and long-term outcome of endoscopic treatment of sporadic nonampullary duodenal adenoma. Gut Liver 4: 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepilliez V., Chemaly M., Ponchon T., Napoleon B., Saurin J.C. (2008) Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy 40: 806–810 [DOI] [PubMed] [Google Scholar]
- Lepisto A., Kiviluoto T., Halttunen J., Jarvinen H.J. (2009) Surveillance and treatment of duodenal adenomatosis in familial adenomatous polyposis. Endoscopy 41: 504–509 [DOI] [PubMed] [Google Scholar]
- Lienert A., Bagshaw P.F. (2007) Treatment of duodenal adenomas with endoscopic argon plasma coagulation. ANZ J Surg 77: 371–373 [DOI] [PubMed] [Google Scholar]
- Miki Y., Kurokawa Y., Hirao M., Fujitani K., Iwasa Y., Mano M., et al. (2010) Survival analysis of patients with duodenal gastrointestinal stromal tumors. J Clin Gastroenterol 44: 97–101 [DOI] [PubMed] [Google Scholar]
- Miller J.H., Gisvold J.J., Weiland L.H., McIlrath D.C. (1980) Upper gastrointestinal tract: villous tumors. AJR Am J Roentgenol 134: 933–936 [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Kocher H.M., Hutchins R.R., Bhattacharya S., Abraham A.T. (2009) Impact of hospital volume on outcomes for pancreaticoduodenectomy: a single UK HPB centre experience. Eur J Surg Oncol 35: 734–738 [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Mainzer T.A., Murthy U.K. (1999) Endoscopic injection and polypectomy for bleeding Brunner’s gland hamartoma. Gastrointest Endosc 50: 597–598 [DOI] [PubMed] [Google Scholar]
- Murray M.A., Zimmerman M.J., Ee H.C. (2004) Sporadic duodenal adenoma is associated with colorectal neoplasia. Gut 53: 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani K., Takekoshi T., Baba Y., Kaku S., Fujii A., Ogata E., et al. (1993) Indications for endoscopic treatment of early duodenal cancer: based on cases reported in the literature. Endosc Digest 7: 969–976 [Google Scholar]
- NICE (2010) Endoscopic mucosal resection and endoscopic submucosal dissection of non-ampullary duodenal lesions. Available at: http://www.nice.org.uk/guidance/IPG359
- Nagatani K., Takekoshi T., Baba Y., Kaku S., Fujii A., Ogata E., et al. (1973) Brunner’s gland adenoma of the duodenum. Am J Dig Dis 18: 689–694 [DOI] [PubMed] [Google Scholar]
- Ott D.J., Wu W.C., Shiflett D.W., Pennell T.C. (1980) Inflammatory fibroid polyp of the duodenum. Am J Gastroenterol 73: 62–64 [PubMed] [Google Scholar]
- Penna C., Bataille N., Balladur P., Tiret E., Parc R. (1998) Surgical treatment of severe duodenal polyposis in familial adenomatous polyposis. Br J Surg 85: 665–668 [DOI] [PubMed] [Google Scholar]
- Pequin P., Manfredi S., Quentin V., Heresbach D., Boyer J., Siproudhis L., et al. (2007) Patients with sporadic duodenal adenoma are a high-risk group for advanced colorectal neoplasia: Results of a case-control study. Aliment Pharmacol Ther 26: 277–282 [DOI] [PubMed] [Google Scholar]
- Perez A., Saltzman J.R., Carr-Locke D.L., Brooks D.C., Osteen R.T., Zinner M.J., et al. (2003) Benign nonampullary duodenal neoplasms. J Gastrointest Surg 7: 536–541 [DOI] [PubMed] [Google Scholar]
- Perzin K.H., Bridge M.F. (1981) Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer 48: 799–819 [DOI] [PubMed] [Google Scholar]
- Phillips R.K., Wallace M.H., Lynch P.M., Hawk E., Gordon G.B., Saunders B.P., et al. (2002) A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 50: 857–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picasso M., Filiberti R., Blanchi S., Conio M. (2007) The role of chromoendoscopy in the surveillance of the duodenum of patients with familial adenomatous polyposis. Dig Dis Sci 52: 1906–1909 [DOI] [PubMed] [Google Scholar]
- Pungpapong S., Woodward T.A., Wallace M.B., Krishna M., Raimondo M. (2006) EUS-assisted EMR of a large duodenal carcinoid tumor. Gastrointest Endosc 63: 703-704 discussion 704 [DOI] [PubMed] [Google Scholar]
- Reddy R.R., Schuman B.M., Priest R.J. (1981) Duodenal polyps: diagnosis and management. J Clin Gastroenterol 3: 139–147 [DOI] [PubMed] [Google Scholar]
- Ruo L., Coit D.G., Brennan M.F., Guillem J.G. (2002) Long-term follow-up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J Gastrointest Surg 6: 671–675 [DOI] [PubMed] [Google Scholar]
- Sellner F. (1990) Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer 66: 702–715 [DOI] [PubMed] [Google Scholar]
- Shim C.S., Jung I.S. (2005) Endoscopic removal of submucosal tumors: preprocedure diagnosis, technical options, and results. Endoscopy 37: 646–654 [DOI] [PubMed] [Google Scholar]
- Spigelman A.D., Talbot I.C., Penna C., Nugent K.P., Phillips R.K., Costello C., et al. (1994) Evidence for adenoma-carcinoma sequence in the duodenum of patients with familial adenomatous polyposis. The Leeds Castle Polyposis Group (Upper Gastrointestinal Committee). J Clin Pathol 47: 709–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spigelman A.D., Williams C.B., Talbot I.C., Domizio P., Phillips R.K. (1989) Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 2: 783–785 [DOI] [PubMed] [Google Scholar]
- Suzuki S., Hirasaki S., Ikeda F., Yumoto E., Yamane H., Matsubara M. (2008) Three cases of Solitary Peutz–Jeghers-type hamartomatous polyp in the duodenum. World J Gastroenterol 14: 944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Ando T., Kabeshima Y., Kawakubo H., Shito M., Sugiura H., et al. (2009) Borderline cases between benignancy and malignancy of the duodenum diagnosed successfully by endoscopic submucosal dissection. Scand J Gastroenterol 44: 1377–1383 [DOI] [PubMed] [Google Scholar]
- Uraoka T., Saito Y., Yamamoto K., Fujii T. (2009) Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des Devel Ther 2: 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso E., Pucciarelli S., Cassaro M., Agostini M., Nitti D. (2007) Long-term follow-up after endoscopic forceps biopsies for early stage duodenal carcinoid: case report and review of endoscopic treatments. Endoscopy 39(Suppl. 1): E128. [DOI] [PubMed] [Google Scholar]
- van Stolk R., Sivak M.V., Jr., Petrini J.L., Petras R., Ferguson D.R., Jagelman D. (1987) Endoscopic management of upper gastrointestinal polyps and periampullary lesions in familial adenomatous polyposis and Gardner’s syndrome. Endoscopy 19(Suppl. 1): 19–22 [DOI] [PubMed] [Google Scholar]
- Vasen H.F., Bulow S., Myrhoj T., Mathus-Vliegen L., Griffioen G., Buskens E., et al. (1997) Decision analysis in the management of duodenal adenomatosis in familial adenomatous polyposis. Gut 40: 716–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden D.T., Marcon N.E. (1998) Endoscopic injection and polypectomy for bleeding Brunnery’s gland hamartoma: case report and expanded literature review. Gastrointest Endosc 47: 403–407 [DOI] [PubMed] [Google Scholar]
- Witteman B.J., Janssens A.R., Griffioen G., Lamers C.B. (1993) Villous tumours of the duodenum. An analysis of the literature with emphasis on malignant transformation. Neth J Med 42: 5–11 [PubMed] [Google Scholar]
- Wysocki A.P., Taylor G., Windsor J.A. (2007) Inflammatory fibroid polyps of the duodenum: a review of the literature. Dig Surg 24: 162–168 [DOI] [PubMed] [Google Scholar]
- Yang W.L., Yu J.R., Wu Y.J., Zhu K.K., Ding W., Gao Y., et al. (2009) Duodenal gastrointestinal stromal tumor: clinical, pathologic, immunohistochemical characteristics, and surgical prognosis. J Surg Oncol 100: 606–610 [DOI] [PubMed] [Google Scholar]
- Zyromski N.J., Kendrick M.L., Nagorney D.M., Grant C.S., Donohue J.H., Farnell M.B., et al. (2001) Duodenal carcinoid tumors: how aggressive should we be? J Gastrointest Surg 5: 588–593 [DOI] [PubMed] [Google Scholar]