Abstract
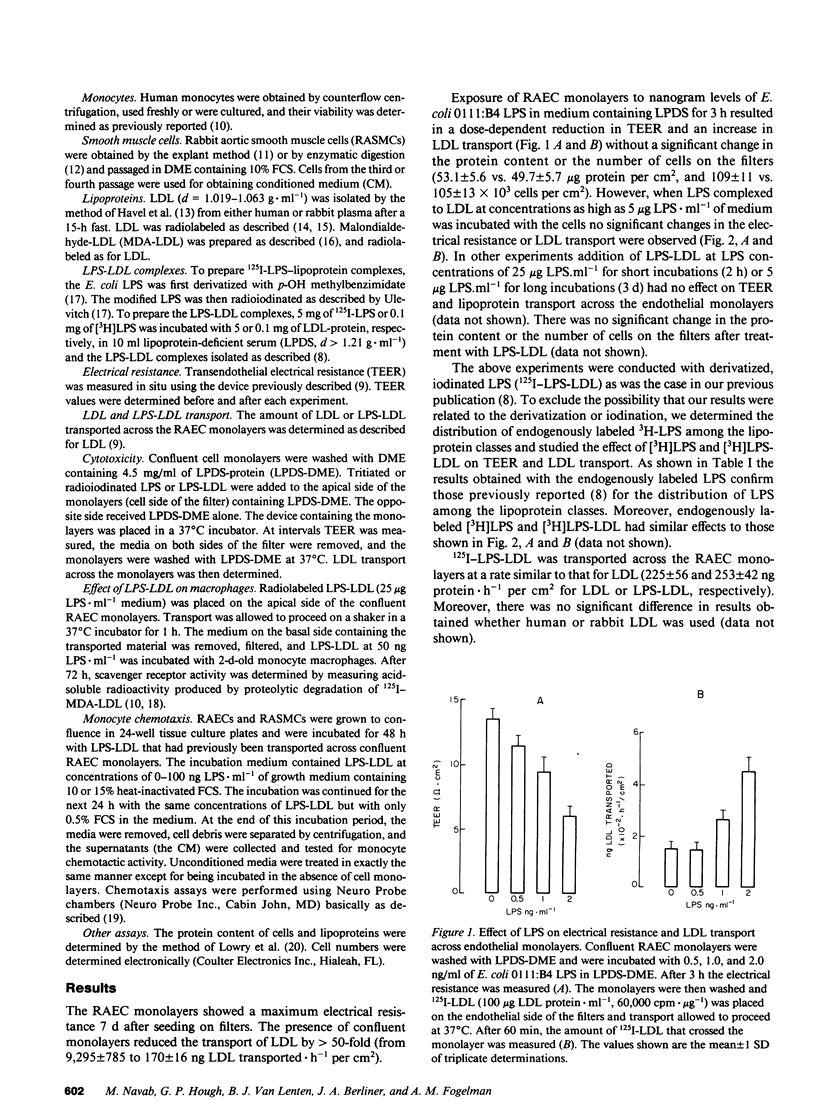
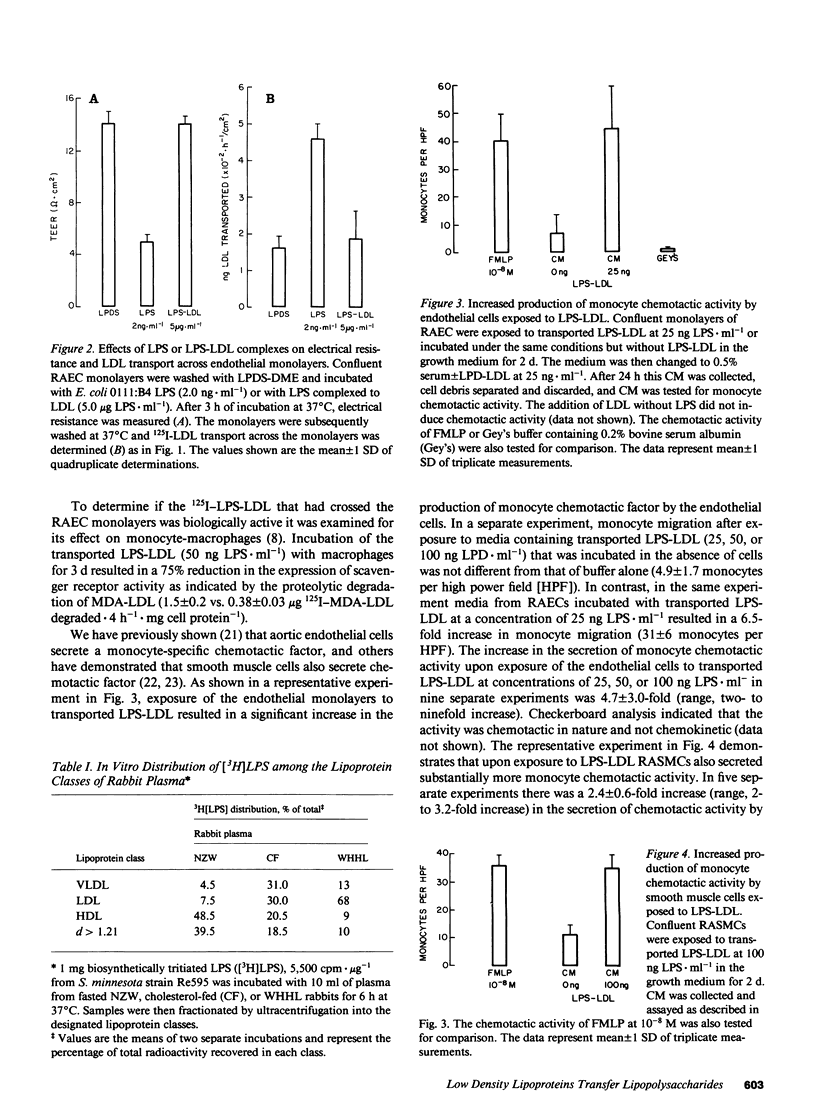
Rabbit aortic endothelial cells (RAECs) were grown on micropore filters in a device that allowed in situ determination of transendothelial electrical resistance (TEER). Incubation of confluent RAEC monolayers with 2 ng.ml-1 of bacterial LPS for 3 h did not change the protein content or the number of cells on the filters, but resulted in a marked decline in TEER (from 14.1 +/- 0.9 to 5.1 +/- 0.6 omega.cm2) and a significant increase in LDL transport across the monolayers (from 154 +/- 13 to 456 +/- 41 ng. h-1 per cm2). In contrast, exposure of RAEC monolayers for 3 d to as much as 5 micrograms.ml-1 of LPS complexed to LDL (LPS-LDL) did not alter the TEER or LDL transport. LPS-LDL was transported across the monolayers at the same rate as LDL. While microgram quantities of LPS complexed to LDL did not disrupt the integrity of the endothelial monolayer, incubation of RAECs with transported LPS-LDL at concentrations of 25-100 ng LPS.ml-1 resulted in a two- to ninefold increase in the secretion of monocyte chemotactic activity by these cells. Incubation of rabbit aortic smooth muscle cells with transported LPS-LDL at concentrations of 25-100 ng LPS.ml-1 resulted in a two- to threefold increase in the secretion of monocyte chemotactic activity. We propose that LDL protects endothelial cells from the acute toxicity of LPS but the resulting complexes are transported across the endothelium in a biologically active form that can initiate an inflammatory response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berliner J. A., Territo M., Almada L., Carter A., Shafonsky E., Fogelman A. M. Monocyte chemotactic factor produced by large vessel endothelial cells in vitro. Arteriosclerosis. 1986 May-Jun;6(3):254–258. doi: 10.1161/01.atv.6.3.254. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Colucci W. S., Brock T. A., Gimbrone M. A., Jr, Alexander R. W. Nonlinear relationship between alpha 1-adrenergic receptor occupancy and norepinephrine-stimulated calcium flux in cultured vascular smooth muscle cells. Mol Pharmacol. 1985 May;27(5):517–524. [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Goss J. A., Soby L. Control of monocyte recruitment by chemotactic factor(s) in lesion-prone areas of swine aorta. Arteriosclerosis. 1985 Jan-Feb;5(1):55–66. doi: 10.1161/01.atv.5.1.55. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Richardson M., Caplan B. A., Cade J. F., Hirsh J., Schwartz C. J. Endotoxin-induced vascular endothelial injury and repair. II. Focal injury, en face morphology, (3H)thymidine uptake and circulating endothelial cells in the dog. Exp Mol Pathol. 1976 Feb;24(1):59–69. doi: 10.1016/0014-4800(76)90057-5. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Olch C. L., Folgelman A. M. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984 Sep 25;259(18):11305–11311. [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Reidy M. A., Gajdusek C. M., Schwartz S. M., Striker G. E. Lipopolysaccharide-mediated bovine endothelial cell injury in vitro. Lab Invest. 1983 Mar;48(3):269–274. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mazzone T., Jensen M., Chait A. Human arterial wall cells secrete factors that are chemotactic for monocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5094–5097. doi: 10.1073/pnas.80.16.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Modulation of endotoxin-induced endothelial cell toxicity by low density lipoprotein. Lab Invest. 1986 Oct;55(4):419–426. [PubMed] [Google Scholar]
- Navab M., Hough G. P., Berliner J. A., Frank J. A., Fogelman A. M., Haberland M. E., Edwards P. A. Rabbit beta-migrating very low density lipoprotein increases endothelial macromolecular transport without altering electrical resistance. J Clin Invest. 1986 Aug;78(2):389–397. doi: 10.1172/JCI112589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy M. A., Bowyer D. E. Scanning electron microscopy: morphology of aortic endothelium following injury by endotoxin and during subsequent repair. Atherosclerosis. 1977 Mar;26(3):319–328. doi: 10.1016/0021-9150(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Tsukada T., Gown A. M., Ross R. Fatty streak initiation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987 Jan-Feb;7(1):9–23. doi: 10.1161/01.atv.7.1.9. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter I., Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J Lipid Res. 1981 Jan;22(1):63–71. [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J. The preparation and characterization of a radioiodinated bacterial lipopolysaccharide. Immunochemistry. 1978 Mar;15(3):157–164. doi: 10.1016/0161-5890(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Valente A. J., Fowler S. R., Sprague E. A., Kelley J. L., Suenram C. A., Schwartz C. J. Initial characterization of a peripheral blood mononuclear cell chemoattractant derived from cultured arterial smooth muscle cells. Am J Pathol. 1984 Dec;117(3):409–417. [PMC free article] [PubMed] [Google Scholar]
- Van Lenten B. J., Fogelman A. M., Haberland M. E., Edwards P. A. The role of lipoproteins and receptor-mediated endocytosis in the transport of bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2704–2708. doi: 10.1073/pnas.83.8.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]



