Abstract
Purpose of the Report
To report our findings from a prospective pilot study evaluating the accuracy of molecular breast imaging (MBI) in assessing tumor response to neoadjuvant therapy (NT) for breast cancer.
Materials and Methods
Twenty patients with newly diagnosed invasive breast cancer who were scheduled to receive NT underwent MBI before beginning and after completing NT prior to surgery. MBI was performed using a dual-detector cadmium-zinc-telluride gamma camera system mounted on a modified mammography gantry after patients had received an intravenous injection of 20 mCi of technetium-99m (Tc-99m) sestamibi. Tumor extent was measured on MBI, and tumor-to-background (T/B) ratios of radiotracer uptake were determined through region-of-interest (ROI) analysis. Pathologic measurement of tumor size was used as a standard and compared to post-NT tumor size derived from MBI.
Results
Three patients in whom post-NT MBI could not be performed because of scheduling problems were excluded from analysis. Eighteen cancers were diagnosed in 17 patients. A correlation coefficient of r=0.681 (P=.002) was found between MBI and residual tumor size. The average T/B ratio on MBI decreased from a pretreatment value of 3.0 to a posttreatment value of 1.4. The relative decrease in T/B ratio did not appear to be predictive of response.
Conclusions
Measurements of tumor size by MBI and T/B ratios are limited in their predictive value regarding the pathologic extent of residual disease in women treated with NT for breast cancer. Alternate tumor-specific radiopharmaceuticals should be evaluated to provide information to improve planning and monitoring of breast cancer treatment.
Keywords: molecular breast imaging, neoadjuvant chemotherapy, neoadjuvant therapy
Introduction
Adjuvant therapy for breast cancer provides considerable benefit to patients at risk for relapse. Preoperative (neoadjuvant) systemic therapy is quite potent as an initial treatment for both inoperable and large operable breast cancers, and it is being used increasingly in patients with smaller resectable disease. Neoadjuvant therapy (NT) results in tumor regression and downsizing in most patients and may reduce the need for mastectomy. Furthermore, pathologic complete response has been associated with favorable outcomes (1–4). Data from recent clinical trials suggest that survival with preoperative systemic therapy is at least as good as that with postoperative systemic therapy (5,6). The use of NT as a new treatment for early-stage breast cancer can be assessed more quickly than can long-term adjuvant therapy after surgery for breast cancer (7). This approach is therefore being used more often in patients with earlier stage breast cancers. Accurate evaluation of the extent of residual disease after completion of NT can guide surgical options.
Clinical and radiologic measurements of breast tumors are often used to assess response to NT. Mammography (MMG) and ultrasound (US) are useful but have considerable limitations (8–10); magnetic resonance imaging (MRI) and positron emission tomography (PET) scans are generally considered more beneficial (11,12) but cost more. However, neither MRI nor PET scans appear to be sufficiently accurate to replace surgical excision of the tumor bed as the best method for determining a complete pathologic response. Since these imaging procedures are quite expensive, a less costly imaging technique would be of great value, especially in light of the increasing use of NT.
The nuclear medicine technique called molecular breast imaging (MBI) uses a small, dual-head, semiconductor-based gamma camera in a mammographic configuration to obtain high-resolution functional images of the uptake of technetium-99m (Tc-99m) sestamibi in the breast. We therefore sought to conduct a pilot study to evaluate whether MBI can accurately assess tumor response to NT in breast cancer patients.
Methods
Study Participants
In this prospective study, 20 patients with newly diagnosed invasive breast cancer were enrolled after discussing treatment options with the consulting breast surgeon and the medical oncologist and then deciding to undergo neoadjuvant chemotherapy or neoadjuvant endocrine therapy. Informed consent was obtained from all participants, and the protocol was approved by the Mayo Clinic Institutional Review Board and registered as NCT00566085 at http://www.ClinicalTrials.gov/.
Study Design
An MBI study was performed before initiation of NT, which consisted of various regimens of chemotherapy or hormonal therapy as selected by the treating oncologist. After completion of presurgical NT, another MBI was performed. Other imaging procedures (MMG, US, and MRI) were conducted as clinically indicated and hence were not performed on all patients. When results from these procedures were available, they were analyzed and compared with those from MBI.
MBI Study
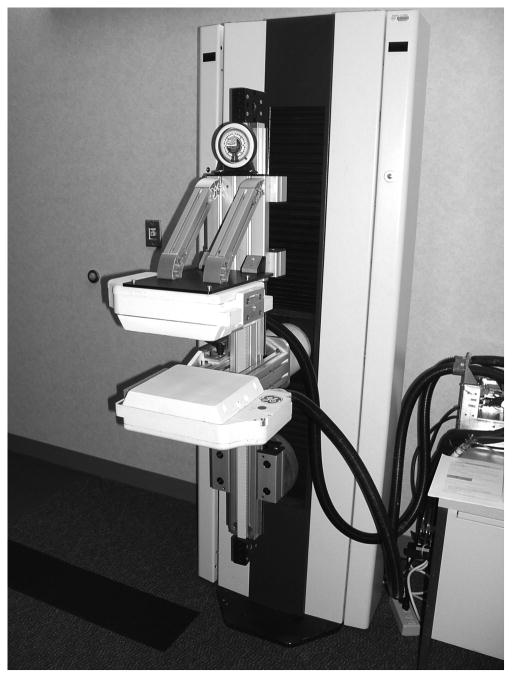
MBI was performed using 1 of 2 dual-detector cadmium-zinc-telluride (CZT) gamma camera systems mounted on a modified MMG gantry. One of the 2 dual-detector MBI systems that were used has 2 LumaGem detectors (Gamma Medica-Ideas, Inc, Northridge, California) (Figure 1). The second unit has 2 Alcyone detectors (GE Healthcare, Haifa, Israel) that are also mounted on a modified mammographic gantry. Both dual-head configurations allow the obtainment of standard craniocaudal and mediolateral oblique mammographic views and facilitate comparison of MBI and MMG images. These systems have been described in detail previously (13,14).
Figure 1.
Dual-Detector Molecular Breast Imaging System. This dual-detector cadmium-zinc-telluride gamma camera system uses 2 LumaGem detectors (Gamma Medica-Ideas, Inc, Northridge, California).
Patients received an intravenous injection of 20 mCi of Tc-99m sestamibi and underwent breast imaging about 5 minutes after injection. The breast was positioned between the 2 detectors and compressed with 15-lb force in a light, painless application that reduced breast thickness and limited movement artifact. Typically, 2 images of 10-minute duration were obtained of each breast in the craniocaudal and mediolateral oblique projections, requiring a total imaging time of 40 minutes.
MBI studies were interpreted by a dedicated breast radiologist with 1–2 years of experience in reading MBI examinations (C.L.T. or R.W.M.). These images were examined for the presence of abnormal radiotracer uptake.
Quantitative Assessment of Uptake on MBI
Tumor-to-background (T/B) ratios of radiotracer uptake were determined through region-of-interest (ROI) analysis of the pre-NT and post-NT MBI images performed with Mayo Clinic’s comprehensive imaging software suite Analyze 8.1 (AnalyzeDirect, Overland Park, Kansas). The MBI view in which the tumor appeared to be most discrete was selected for analysis. For each image, an ROI was drawn, with the aid of an automatic threshold-based segmentation method, to encompass the entire tumor region. A background ROI of the same size as the tumor ROI was positioned in a region of normal breast tissue; if the tumor was so large that an adequate area of normal tissue was not available, the background ROI was placed on an image of the contralateral breast. The T/B ratio was obtained by dividing the sum of counts in the tumor ROI by the sum of counts in the background ROI.
For the post-NT MBI studies, a T/B ratio of 1.1 or less was considered indicative of no residual disease and a T/B ratio greater than 1.1 was considered positive for the presence of residual disease. The T/B ratios of the pre-NT and post-NT MBI studies were compared to determine whether uptake in the tumor had increased, decreased, or remained stable.
Clinical Examination and Additional Imaging
Examination of the breasts for assessment of tumor size was performed both pre-NT and post-NT as deemed clinically necessary. Clinical breast examinations were performed by physicians working in a multidisciplinary breast clinic. Tumor size as determined by clinical examination (CE) was obtained retrospectively by abstracting data from the medical chart.
Additional imaging studies, including MMG, directed US, and bilateral contrast-enhanced breast MRI, were performed as deemed clinically necessary. Standard 4-view MMG was performed using either screen-film MMG (Lorad M-IV; Hologic, Inc, Bedford, Massachusetts) or digital (Selenia; Hologic) MMG. When necessary, lesions were examined by means of additional views (spot or direct magnification by microfocus). US examinations were performed (iU22 xMATRIX; Philips Healthcare, Andover, Massachusetts) with 12.5 and 17.5 megahertz transducers. MRI was performed on a 1.5 T MRI scanner (Signa, GE Medical Systems, Milwaukee, Wisconsin) with and without gadolinium contrast-enhancement in the axial and sagittal planes. CADstream (Confirma, Inc, Seattle, Washington) software was used to aid interpretation.
Measurement of Tumor Size
Tumor extent was measured from the MBI view in which the tumor appeared most conspicuous. When available, similar measurements were obtained by MMG, US, and MRI. At completion of the pilot study, all imaging interpretations were reviewed by a single dedicated breast radiologist (C.L.T.) for consistency.
Pathology Interpretation
All pathology slides were interpreted by an experienced pathologist specialized in diseases of the breast (B.C.). Residual tumor size was defined as the largest diameter of a viable tumor nest within the tumor bed when the tumor formed a more or less discrete mass. When viable tumor nests were present throughout the entire tumor bed, then the residual tumor size was reported as the size of the tumor bed. Pathologic complete response was defined as complete eradication of invasive cancer in the breast tissue.
Statistical Analysis
Correlation between the pre-NT tumor size on MBI and, when available, on CE, MMG, US, and MRI was performed using linear regression analysis. A 2-sided paired t test was used to determine whether there was a significant difference in tumor size estimates among methods. Pathologic measurement of tumor size was the standard for comparison with post-NT tumor size derived from MBI, and, when available, from CE, MMG, US, and MRI. A 2-sided paired t test was again used to check for difference in estimates of tumor size. A P value of≤.05 was considered statistically significant for paired t tests.
Results
Patient Recruitment
Between December 2007 and September 2009, we prospectively enrolled 20 patients with invasive breast cancer who were scheduled to undergo NT. Three patients in whom post-NT MBI could not be performed because of scheduling problems were excluded from analysis, leaving 17 patients whose data were analyzed.
Patient Demographics and Treatment
The median age of the 17 enrolled patients was 49 years (range, 38–76 years). Eighteen cancers were diagnosed in the 17 patients. One patient had a contralateral tumor that was not detected on CE or MMG, but was detected with MRI and MBI. Most (17/18) tumors were invasive ductal cancer; 1 was invasive lobular. Most (11/18 [61%]) tumors were estrogen-or progesterone-receptor positive, 2 (2/17 [12%]) were her-2 neu positive, and 5 (5/18 [28%]) were estrogen, progesterone, and her-2 neu negative (triple negative). Of 17 patients, 16 received neoadjuvant chemotherapy; 14 received doxorubicin/cyclophosphamide followed by paclitaxel; and 2 received paclitaxel/trastuzumab followed by fluorouracil, epirubicin, cyclophosphamide (FEC)/trastuzumab. One patient received neoadjuvant hormonal therapy (anastrozole).
Mean Tumor Size Before NT
Tumor size on CE ranged from 0 to 15 cm before NT and from 0 to 8 cm after NT. Mean tumor measurements both pre-NT and post-NT as determined by CE, MBI, various imaging procedures, and pathologic testing are depicted in Table 1. Not all patients underwent all imaging procedures, so these data reflect a subset of patients in whom the same imaging modality was performed before commencement of NT and after completion of NT.
Table 1.
Comparison of Tumor Size Before and After Neoadjuvant Therapy as Determined by Molecular Breast Imaging, Clinical Examination, Mammography, Ultrasound, and Magnetic Resonance Imaging, and at Pathology
| Type of Examination | No. of Breast Cancers | Tumor Size, cma |
||
|---|---|---|---|---|
| Pre-NT | Post-NT | At Pathology | ||
| MBI | 18 | 5.9 (2.6) | 1.8 (2.4) | 1.7 (3.0) |
| CE | 16 | 5.2 (3.7) | 1.7 (2.4) | 1.7 (3.2) |
| MMG | 8 | 5.0 (3.3) | 0.6 (1.4) | 0.4 (0.5) |
| US | 7 | 3.4 (1.2) | 1.2 (1.0) | 0.3 (0.4) |
| MRI | 5 | 6.5 (4.5) | 3.7 (3.5) | 3.7 (4.7) |
Abbreviations: CE, clinical examination; MBI, molecular breast imaging; MMG, mammography; MRI, magnetic resonance imaging; Post-NT, post-neoadjuvant therapy; Pre-NT, pre-neoadjuvant therapy; US, ultrasound.
Values are mean (SD).
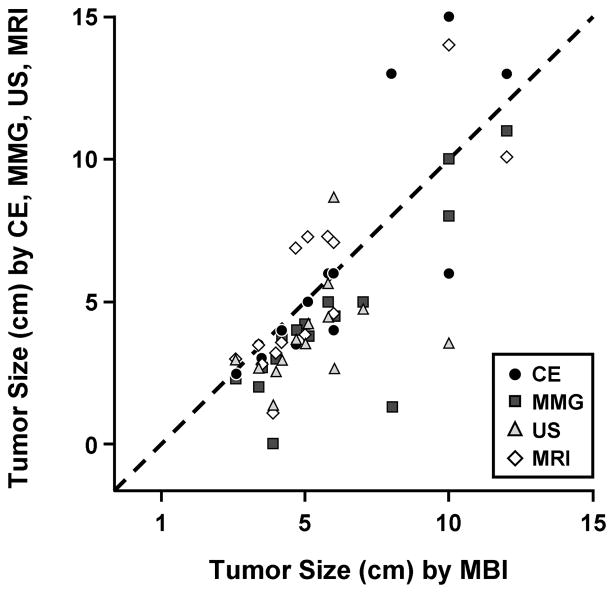
Figure 2 shows the relation between MBI and CE, MMG, US, and MRI in the pre-NT estimation of tumor size. The correlation coefficient between MBI and clinical and radiologic parameters was: r=0.831 for CE (P<.01), r=0.837 for MMG (P<.01), r=0.377 for US (P=.17), and r=0.844 for MRI (P<.01). Table 2 shows the P values from paired t tests comparing the pre-NT tumor sizes by the different modalities. Although we could not test for equivalence, these results show that estimates of tumor size from MMG and US differed significantly from all the other estimates of tumor size, particularly those made by MBI or MRI.
Figure 2.
Pre–Neoadjuvant Therapy Tumor Size. Correlation coefficients show the relation between molecular breast imaging (MBI) and clinical examination (CE) and the various imaging techniques in pre–neoadjuvant therapy estimation of tumor size: CE (r=0.831; P<.01), mammography (MMG [r=0.837; P<.01]), ultrasound (US [r=0.377; P=.17]), and magnetic resonance imaging (MRI [r=0.844; P<.01]).
Table 2.
Comparison of Molecular Breast Imaging, Clinical Examination, Mammography, Ultrasound, and Magnetic Resonance Imaging Before Neoadjuvant Therapy
| MBI | CE | MMG | US | MRI | |
|---|---|---|---|---|---|
| MBI | P=.59 | P<.01a | P=.02a | P=.86 | |
| CE | P=.59 | P=.14 | P=.34 | P=.31 | |
| MMG | P<.01a | P=.14 | P=.22 | P=.03a | |
| US | P=.02a | P=.34 | P=.22 | P=.04a | |
| MRI | P=.86 | P=.31 | P=.03a | P=.04a |
Abbreviations: CE, clinical examination; MBI, molecular breast imaging; MMG, mammography; MRI, magnetic resonance imaging; US, ultrasound.
P≤.05 indicates a significant difference in estimation of tumor size among modalities.
Mean Tumor Size After NT
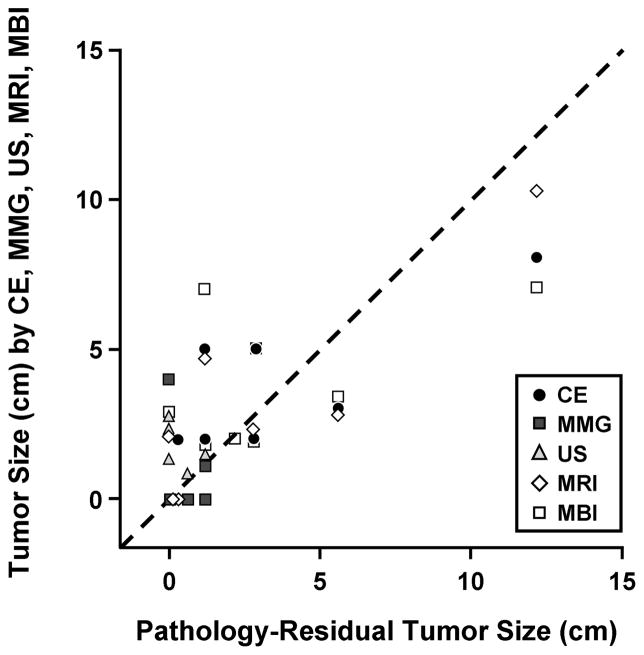
Correlation of all the different imaging modalities post-NT with residual tumor size was not possible because too few patients underwent all 4 imaging modalities, resulting in insufficient data sets for meaningful correlation. The correlation coefficient between MBI and residual tumor size was r=0.681 (P=.002). Figure 3 illustrates the relation between residual tumor size on pathologic examination and tumor size by CE, MMG, US, MRI, and MBI. There was considerable scatter in the data, with no clear correlation between the pathologic findings and any of the other estimates of tumor size.
Figure 3.
Post–Neoadjuvant Therapy Tumor Size. Relation of residual tumor size on pathologic examination to tumor size by clinical examination (CE), mammography (MMG), ultrasound (US), magnetic resonance imaging (MRI), and molecular breast imaging (MBI). No statistical analysis was possible for MMG, US, or MRI. Correlation coefficient between MBI and residual tumor size was r=0.681 (P=.002).
Assessing Pathologic Complete Response After NT
Six breast cancers showed a pathologic complete response within the involved breast. MBI evaluation was negative in 5 of these 6 cases, whereas it revealed a 2.9-cm area of low-intensity uptake in the 6th patient (no other post-NT imaging procedures were conducted in this patient). Tumor size estimates for these 6 patients as determined by CE, MMG, US, MRI, and MBI are summarized in Table 3.
Table 3.
Tumor Size Estimates (in cm) as Determined by Clinical Examination, Molecular Breast Imaging, Mammography, Ultrasound, and Magnetic Resonance Imaging After Neoadjuvant Therapy in 6 Patients Who Achieved a Pathologic Complete Response in the Breast
| Patient No. | Type of Examination
|
Residual Breast Tumor at Pathology | ||||
|---|---|---|---|---|---|---|
| CE | MBI | MMG | US | MRI | ||
| 1 | 0 | 0 | 4.0 | 2.7 | ND | 0 |
| 2 | 0 | 0 | ND | ND | 2.1 | 0 |
| 3 | 0 | 0 | 0 | 2.3 | ND | 0 |
| 4 | 0 | 0 | ND | 1.3 | ND | 0 |
| 5 | 0 | 2.9 | 0 | ND | ND | 0 |
| 6 | 0 | 0 | 0 | 0 | ND | 0 |
Abbreviations: CE, clinical examination; MBI, molecular breast imaging; MMG, mammography; MRI, magnetic resonance imaging; ND, not done; US, ultrasound.
Correlation of Negative MBI After NT With Findings at Pathology
MBI of 10 breasts involved by cancer was negative for uptake after NT. Pathologic testing did not identify any tumor in 5 of these cases; in the remaining 5 cases, the residual tumor size ranged from 0.1 cm to 1.2 cm.
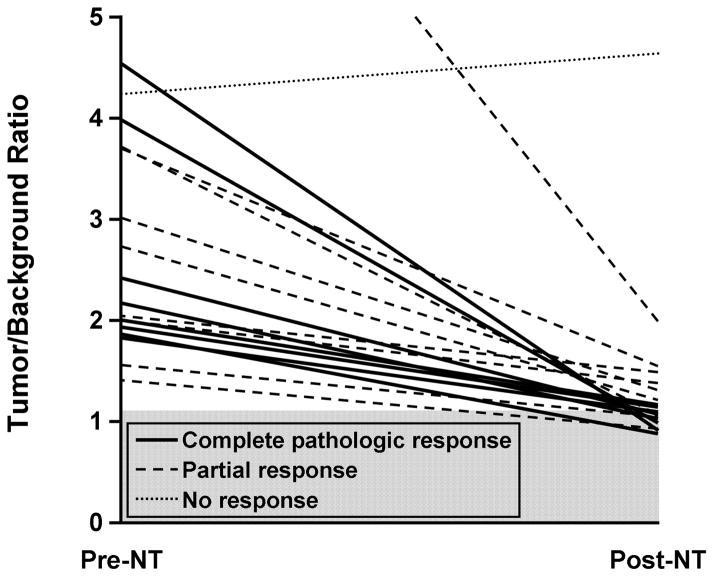
MBI T/B Ratio Before and After NT
Figure 4 plots the change in T/B ratio after NT. The average T/B ratio on MBI decreased from a pretreatment value of 3.0 to a posttreatment value of 1.4. The ratio decreased in all but 1 of 17 patients, a 76-year-old woman who received endocrine NT (anastrozole). Her T/B ratio increased from 4.2 to 4.6 (10%), whereas the tumor size as estimated by MBI measurements decreased from 5.0 cm pretreatment to 3.4 cm posttreatment.
Figure 4.
Tumor/Background Ratios Before and After Neoadjuvant Therapy for 18 Breast Cancers in 17 Patients. Tumor/background ratios of 1.1 or less were considered indicative of no residual tumor. Gray shading indicates no residual disease. Post-NT indicates post-neoadjuvant therapy; pre-NT, pre-neoadjuvant therapy.
In 11 breast cancers, the posttreatment T/B ratio ranged from 0.9 to 1.1, which was indicative of no residual disease. All 6 breasts with no residual breast tumor were included in this group. In the remaining 5 cancers, the residual tumor size ranged from 0.3 cm to 1.2 cm. In 6 tumors that demonstrated a partial response to NT, the T/B ratio ranged from 1.2 to 2.0 and the residual tumor size ranged from 0.6 cm to 2.9 cm. The relative decrease in T/B ratio (rather than the final T/B ratio) did not appear to be predictive of response and was −55% for those with a complete pathologic response in the breast and −50% for those with a partial response.
Imaging Examples
Figure 5 shows some examples of breast imaging. The pretreatment MMG, MRI, and MBI revealed multifocal and multicentric disease in the left breast (Figure 5A-5C). Enhancement on post-NT MRI remained in the lower outer left breast at the 4 o’clock position (Figure 5D), and post-NT MBI was negative (Figure 5E). Post-NT pathologic testing revealed a 0.3-cm residual tumor.
Figure 5.
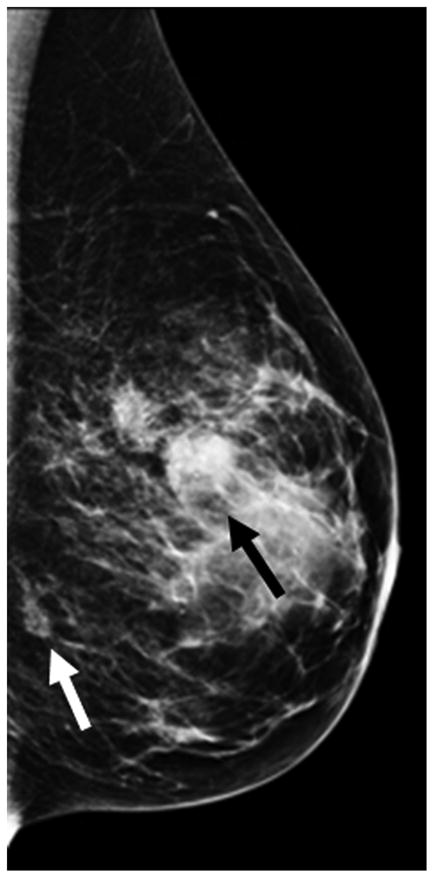
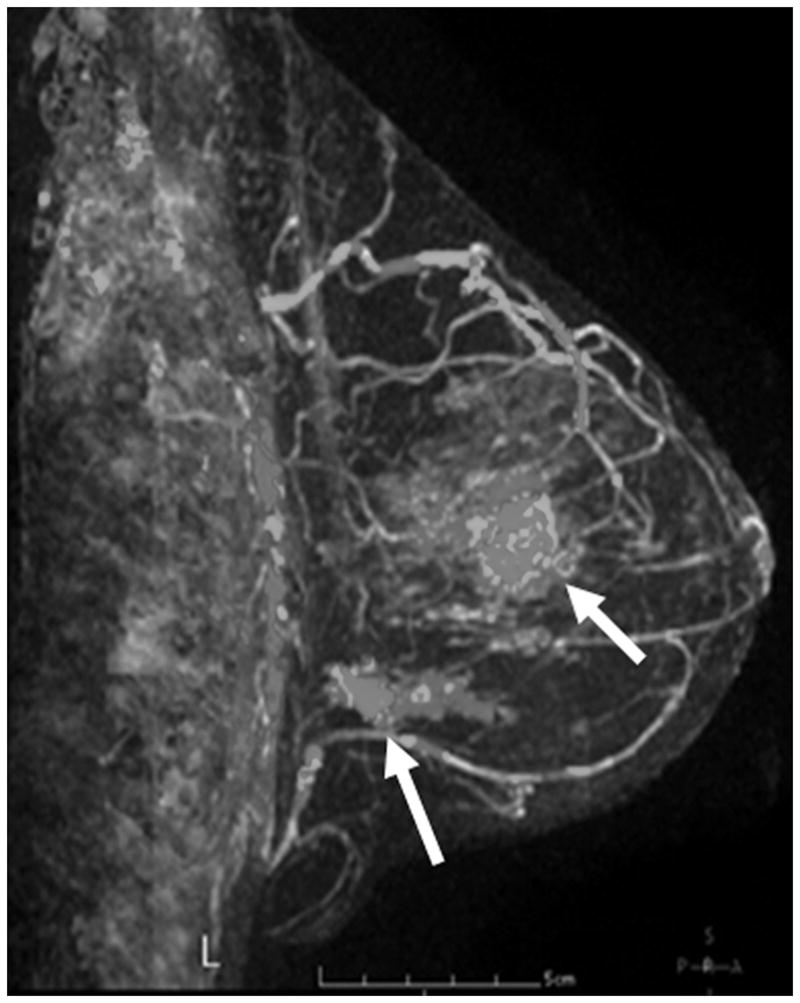
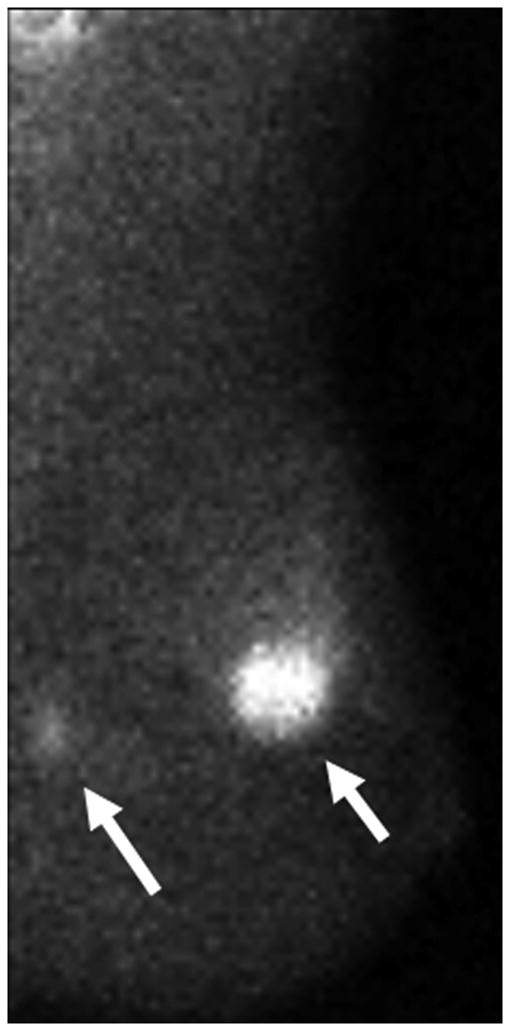
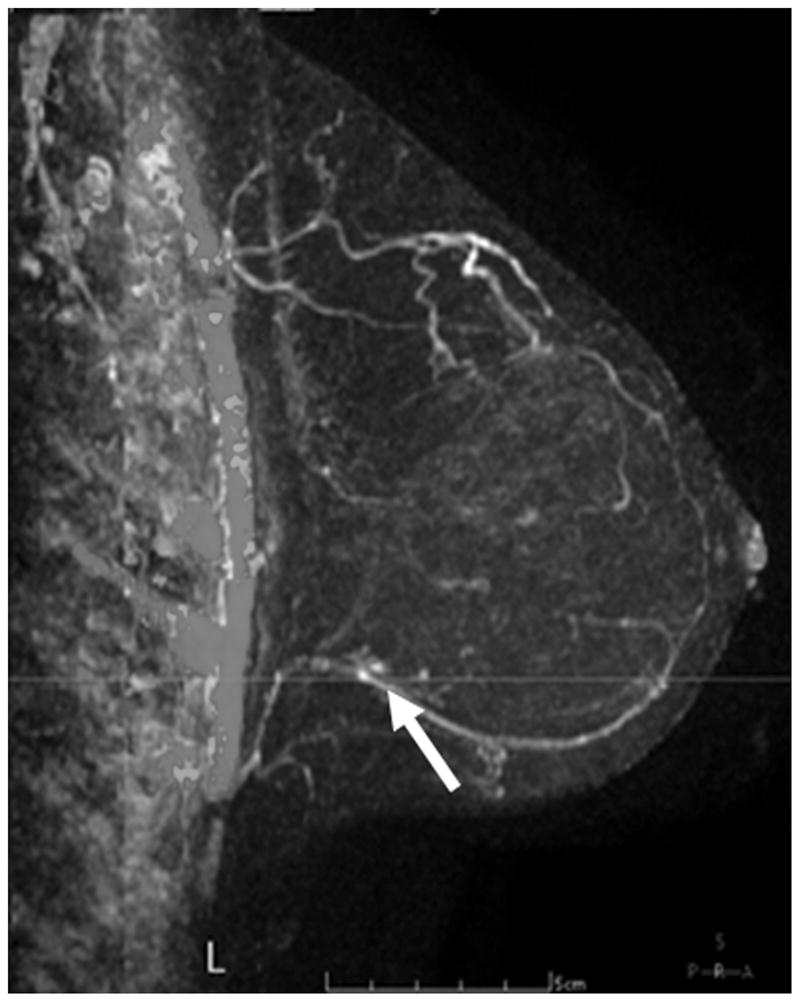

Pre–Neoadjuvant Therapy Imaging vs Post–Neoadjuvant Therapy Imaging. Pre–neoadjuvant therapy (pre-NT) imaging shows multifocal disease (A–D, arrows) by: (A) mammography (MMG), (B) magnetic resonance imaging (MRI), and (C) molecular breast imaging (MBI). Post–neoadjuvant therapy (post-NT) imaging (D) by MRI shows minimal enhancement remaining in lower outer breast and (E) by MBI is negative.
One patient had a large multicentric area of abnormal medium- to high-intensity tracer uptake on MBI in the right subareolar region of the upper outer and lower outer quadrants (Figure 6A and 6B). The lesion persisted after NT (Figure 6C and 6D), but the T/B ratio decreased from 3.7 to 1.5. Post-NT pathologic testing revealed a 2.9-cm residual tumor.
Figure 6.
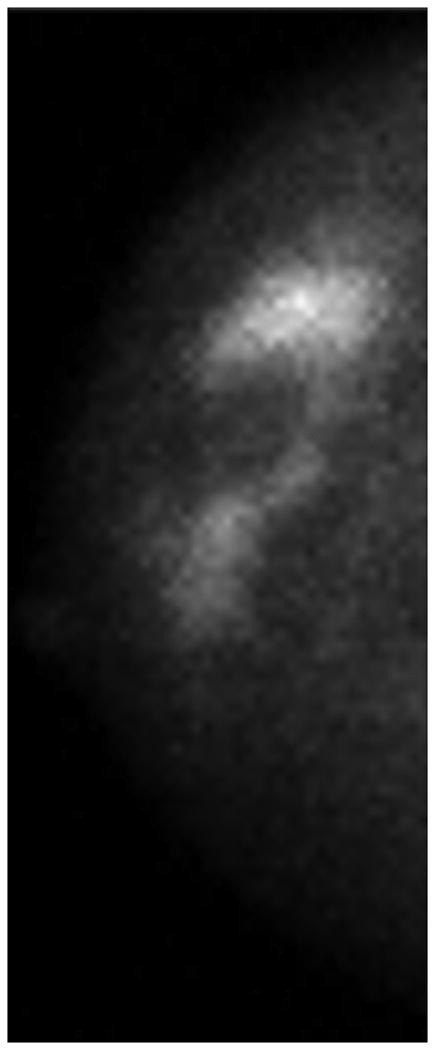
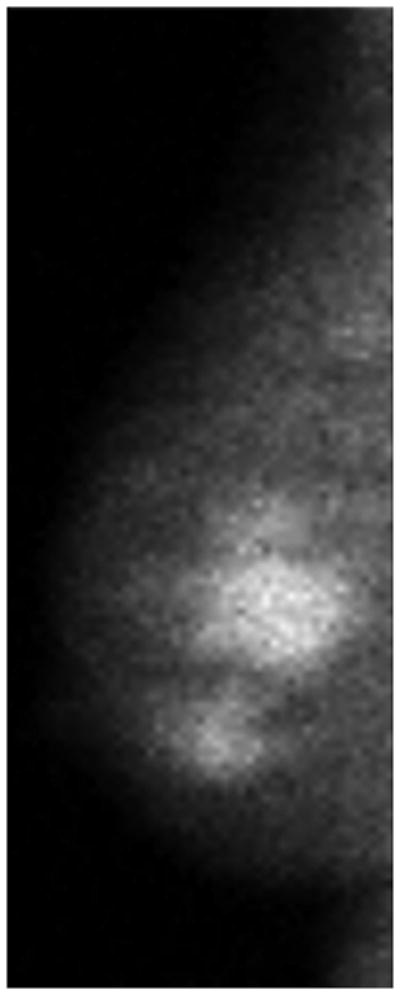
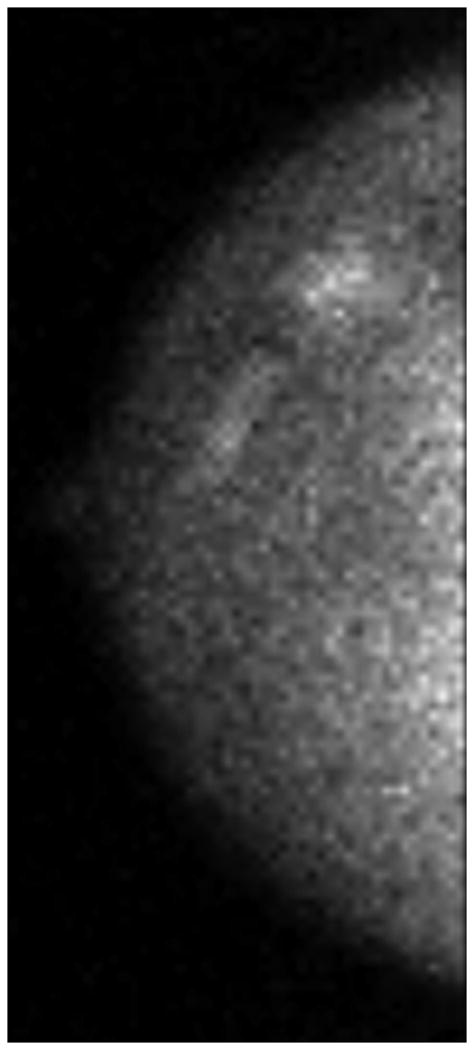
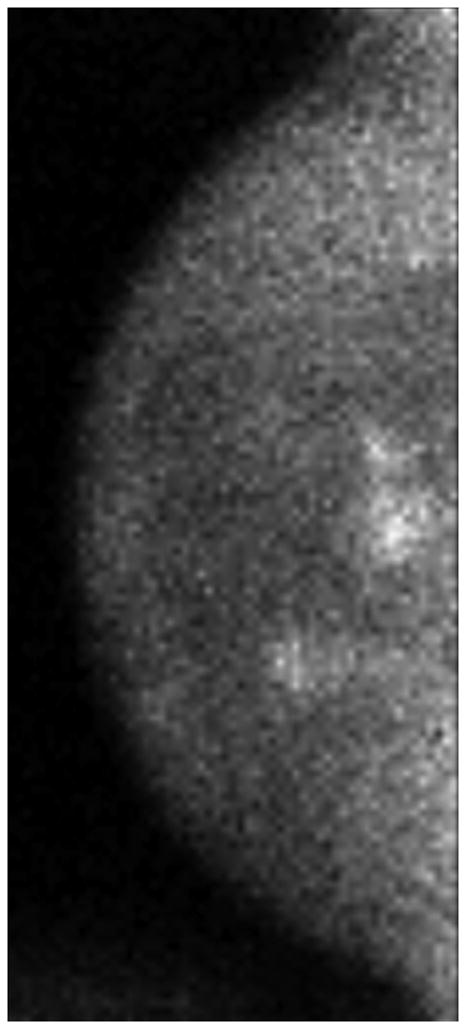
Molecular Breast Imaging Before and After Neoadjuvant Therapy. Pre-NT imaging by molecular breast imaging (MBI) shows a large multicentric area of abnormal medium- to high-intensity uptake in both (A) the craniocaudal view and (B) the mediolateral oblique view. On the post-NT MBI, the lesion persists in both (C) the craniocaudal view and (D) the mediolateral oblique view (tumor-to-background ratio, 1.5).
Figure 7 illustrates discordant findings on MBI vs MRI. In this patient, the pre-NT images from MBI and MRI showed a mass of 4–5 cm (Figure 7A and 7B). The MBI T/B ratio decreased from 1.9 to 0.9, the post-NT MRI showed a persistent 2.1-cm lesion with decreased enhancement (Figure 7C), and the post-NT MBI was negative (Figure 7D). Post-NT pathologic testing showed no residual disease.
Figure 7.
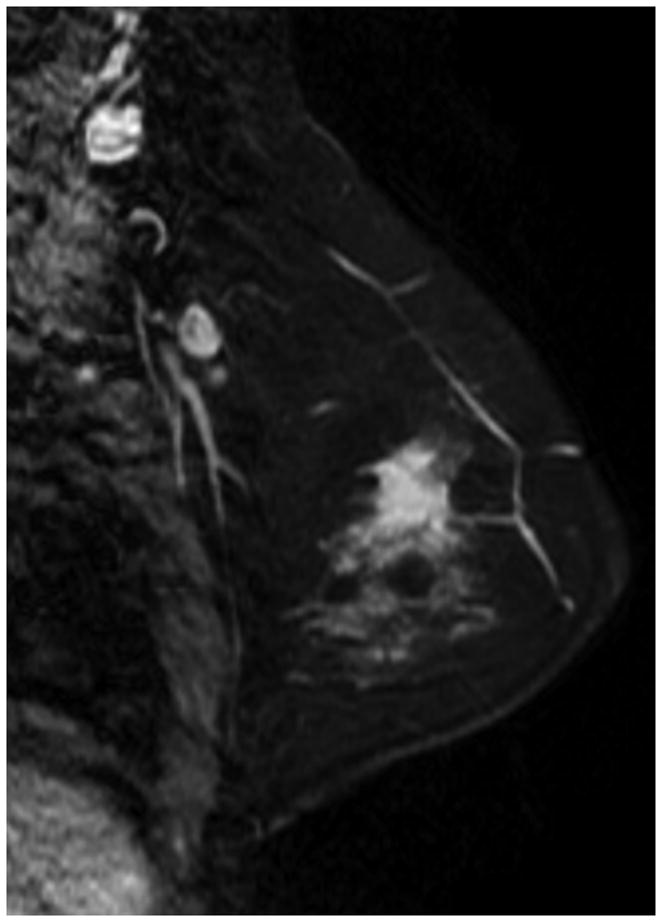
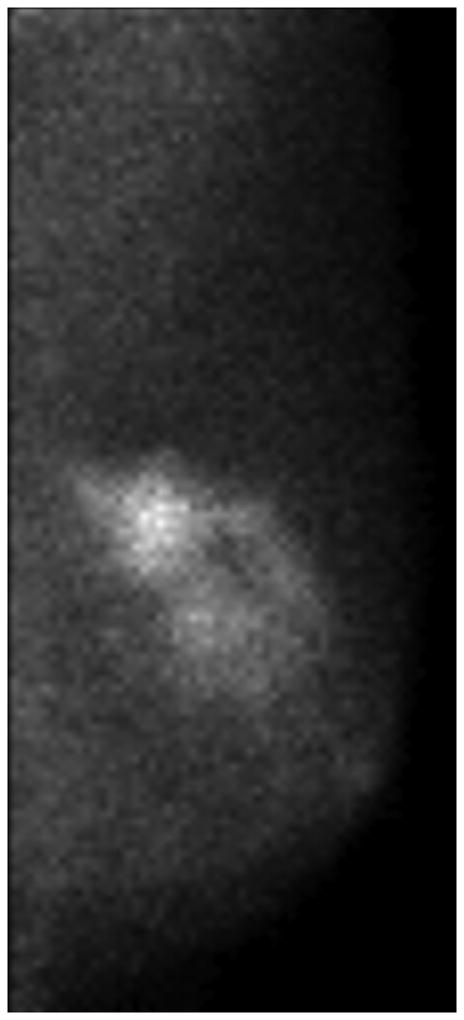
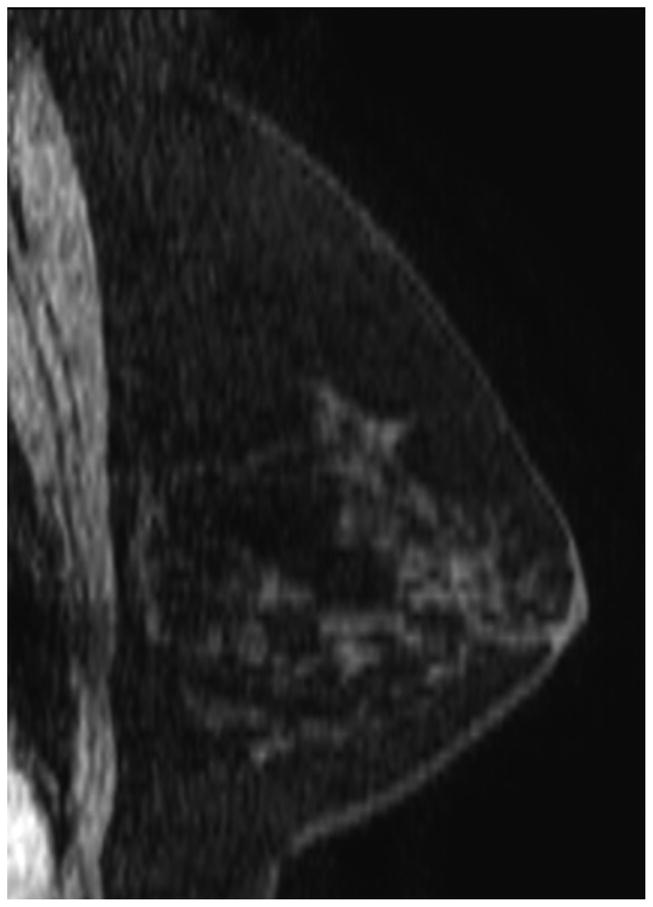

Comparison of Magnetic Resonance Imaging and Molecular Breast Imaging Before and After Neoadjuvant Therapy. The pre-NT imaging shows a 4.5-cm mass on (A) magnetic resonance imaging (MRI) and (B) molecular breast imaging (MBI), whereas the post-NT imaging (C) by MRI shows a persistent 2.1-cm lesion with decreased enhancement, and (D) by MBI is negative (tumor-to-background ratio, <1.1).
Discussion
Multiple studies that have evaluated the usefulness of CE, MMG, and US in monitoring response to neoadjuvant chemotherapy have found limited agreement between tumor size measured at final pathology, and the extent of residual disease as measured by CE, MMG, and US (8,11,15–17). The limited results that we obtained in this study confirm these findings and indicate that both MMG and US appear to be of limited value in monitoring response to NT. Results with MRI and F-18 FDG (fluorodeoxyglucose) PET have been more promising (11,12,18), and studies have shown that both modalities can differentiate responders from nonresponders early in the course of therapy and can predict ultimate tumor response. However, each of these modalities is expensive, and a less costly alternative would be of value, particularly with the growing use of NT in patients with smaller tumor masses.
The purpose of this pilot study was to revisit the potential use of Tc-99m sestamibi imaging in monitoring the response of breast tumors to NT. Thus, we elected to use small semiconductor-based gamma cameras that achieve about a 3-fold improvement in spatial resolution of breast imaging compared to that achieved with conventional gamma cameras. This approach facilitated the monitoring of changes both in tumor size and in function.
The use of Tc-99m sestamibi in monitoring response to NT has been studied in conventional scintimammography by Mankoff et al (19) and Schelling et al (20). Their findings indicated that Tc-99m sestamibi has a sensitivity of 65% to 100% for predicting complete pathologic response to NT. In our study, we found that all 5 of 6 patients who had a complete pathologic response in the breast showed no evidence of disease on MBI (tumor size of 0). In the 6th patient, the MBI showed a low-uptake 2.9-cm lesion that corresponded to an area of tumor bed fibrosis measuring 3.6 cm in greatest diameter. The extent of residual disease on MBI correlated well with pathologic findings from surgical resection with a correlation coefficient between MBI and residual tumor size of r=0.681.
Estimates of tumor size from MBI appeared to closely parallel the findings obtained with MRI and, not unexpectedly, both showed poor correlation with findings from MMG and US. Both MMG and US are known to be poor predictors of tumor response, whereas MRI appears to provide a better indication of tumor extent (8–12).
However, measurement of tumor size alone is a relatively simple indicator of response and does not take into account any change in tumor function. Schelling et al (20) have shown that a decrease in tumor function may precede a change in tumor size. A more promising index that can be derived from MBI is T/B ratio. Figure 4 shows the changes in T/B ratio from pre-NT to post-NT. This measurement appears to provide a better index of response to therapy than does a simple measurement of size.
However, the true value of a test such as MBI is not necessarily the documentation of a complete pathologic response, but rather the early prediction during NT of what might be expected to be the final pathologic complete response. As can be seen from Figure 4, considerable overlap exists in changes to the T/B ratio during therapy between tumors that demonstrate a complete pathologic response and those that demonstrate a partial response. The American Joint Committee on Cancer Staging has recommended separating the 3 degrees of response to NT (complete, partial, and no response). Only 1 of our 17 patients showed no response to NT. As shown in Figure 4, the MBI T/B ratio adequately separated this “no response” from those responses that were “complete” or “partial.” There was, however, considerable overlap between those patients with a complete response and those with a partial response. These findings are similar to results with MRI reported by Tan et al (21). Clearly, 2 time points provide inadequate information to determine how a tumor is responding to therapy. Most likely, any response will not be linear, and instead may be biphasic or multiphasic in nature as shown with F-18 FDG PET by Schelling et al (20) and with MRI by Tan et al (21). Hence, multiple assessments of the T/B ratio during the course of therapy are required to fully document how a tumor is responding to therapy. Thus, the relative low cost of MBI is an attractive alternative monitoring tool for tumor response (in our laboratory, MBI is currently less expensive than MRI by a factor of 6).
Despite the benefits of MBI, it does have several limitations. Its limited intrinsic resolution of 1.6 to 2.5 mm precludes the detection of disease smaller than about 3 mm. Hence, residual microscopic and/or diffuse disease may be difficult to detect. The current radiopharmaceutical agent used in MBI may also not be the most appropriate compound for monitoring tumor response. Sestamibi is a lipophilic cation whose uptake by breast tumors is thought to be partly due to increased blood flow in tumors (compared with that in normal breast tissue) and to increased retention due to altered cellular membrane potentials and a higher concentration of intracellular mitochondria.
The possible influence of P-glycoprotein-mediated multidrug resistance is a concern related to the use of sestamibi for evaluating the response to therapy. Mouse and cell culture studies have shown that high P-glycoprotein expression indicates cells with markedly reduced sestamibi uptake (19). Thus, the reduced uptake of sestamibi in tumors after NT may reflect the presence of multidrug-resistant P-glycoprotein in the tumors, rather than a true reduction in tumor size and function. Furthermore, the uptake of sestamibi does not appear to be strongly correlated with tumor grade. Hence, it may not be the ideal radiopharmaceutical agent for evaluation of response to NT.
Several alternative imaging agents that target other aspects of cell function may be better suited to the task of monitoring response to NT. Tc-99m αvβ3 is a radioligand with high affinity for the αvβ3 integrin localized on endothelial cells in the regions of angiogenesis (22). It is generally accepted that the growth of solid cancers to a diameter greater than 2 to 3 mm requires angiogenesis (23). Initial studies have shown similar uptake of Tc-99m sestamibi and Tc-99m αvβ3 in breast tumors, making Tc-99m αvβ3 a possible marker of tumor response.
Other promising compounds include radiolabeled analogs of glucose such as Tc-99m ethylenedicysteine-glucosamine and Tc-99m glucarate. These compounds are expected to behave in tumors in a way similar to the action of F-18 FDG in tumors. Studies have shown F-18 FDG uptake in tumors to be a good predictor of response early in the course of therapy (20). An alternate mechanism of studying tumor response to therapy is by assessing cell apoptosis using compounds such as Tc-99m annexin V. Symmans et al (24) demonstrated that the apoptotic index was an accurate way to detect apoptosis induced by paclitaxel in women receiving induction chemotherapy for primary breast cancer. Given the potential of these and other compounds such as Tc-99m DMSA (V) (25) and Tc-99m bombesin (26,27) to monitor tumor function, this field holds great promise as an inexpensive method for early monitoring of response to NT.
Conclusion
Our findings indicate that measurement of tumor size by MBI or T/B ratio has limited predictive value regarding the pathologic extent of residual disease in women with NT-treated breast cancer. Alternate tumor-specific radiopharmaceuticals should be developed and evaluated to provide functional and anatomical information to improve diagnosis, prognosis, planning, and monitoring of breast cancer treatment.
Acknowledgments
This research was funded by National Institutes of Health, and Mayo Foundation.
Abbreviations
- CE
clinical examination
- CZT
cadmium-zinc-telluride
- F-18 FDG
fluorodeoxyglucose
- FEC
fluorouracil, epirubicin, and cyclophosphamide
- MBI
molecular breast imaging
- MMG
mammography
- MRI
magnetic resonance imaging
- NT
neoadjuvant therapy
- PET
positron emission tomography
- ROI
region of interest
- T/B
tumor-to-background
- Tc-99m
technetium-99m
- US
ultrasound
Footnotes
Presented at the 23rd Annual Congress of the European Association of Nuclear Medicine, Vienna, Austria, October 9–13, 2010.
Conflict of interest: Mayo Clinic, Carrie B. Hruska, PhD, and Michael K. O’Connor, PhD, receive royalties from development of technologies licensed to Gamma Medica-Ideas, Inc, Northridge, California. There are no other conflicts of interest.
References
- 1.Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 2.van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 3.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. Epub 2007 Sep 4. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. Erratum in: J Clin Oncol. 2008;26:2793. [DOI] [PubMed] [Google Scholar]
- 5.Smith IE, Lipton L. Preoperative/neoadjuvant medical therapy for early breast cancer. Lancet Oncol. 2001;2:561–570. doi: 10.1016/S1470-2045(01)00490-9. [DOI] [PubMed] [Google Scholar]
- 6.Shenkier T, Weir L, Levine M, et al. Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: 15. Treatment for women with stage III or locally advanced breast cancer. CMAJ. 2004;170:983–994. doi: 10.1503/cmaj.1030944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow LW, Yiu CC, Yip AY, et al. The future perspectives of breast cancer therapy. Biomed Pharmacother. 2006;60:259–262. doi: 10.1016/j.biopha.2006.06.010. Epub 2006 Jun 23. [DOI] [PubMed] [Google Scholar]
- 8.Herrada J, Iyer RB, Atkinson EN, et al. Relative value of physical examination, mammography, and breast sonography in evaluating the size of the primary tumor and regional lymph node metastases in women receiving neoadjuvant chemotherapy for locally advanced breast carcinoma. Clin Cancer Res. 1997;3:1565–1569. [PubMed] [Google Scholar]
- 9.Helvie MA, Joynt LK, Cody RL, et al. Locally advanced breast carcinoma: accuracy of mammography versus clinical examination in the prediction of residual disease after chemotherapy. Radiology. 1996;198:327–332. doi: 10.1148/radiology.198.2.8596826. [DOI] [PubMed] [Google Scholar]
- 10.Chagpar AB, Middleton LP, Sahin AA, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg. 2006;243:257–264. doi: 10.1097/01.sla.0000197714.14318.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren RM, Bobrow LG, Earl HM, et al. Can breast MRI help in the management of women with breast cancer treated by neoadjuvant chemotherapy? Br J Cancer. 2004;90:1349–1360. doi: 10.1038/sj.bjc.6601710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosen EL, Blackwell KL, Baker JA, et al. Accuracy of MRI in the detection of residual breast cancer after neoadjuvant chemotherapy. AJR Am J Roentgenol. 2003;181:1275–1282. doi: 10.2214/ajr.181.5.1811275. [DOI] [PubMed] [Google Scholar]
- 13.Hruska CB, Phillips SW, Whaley DH, et al. Molecular breast imaging: use of a dual-head dedicated gamma camera to detect small breast tumors. AJR Am J Roentgenol. 2008;191:1805–1815. doi: 10.2214/AJR.07.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor M, Rhodes D, Hruska C. Molecular breast imaging. Expert Rev Anticancer Ther. 2009;9:1073–1080. doi: 10.1586/era.09.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schott AF, Roubidoux MA, Helvie MA, et al. Clinical and radiologic assessments to predict breast cancer pathologic complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2005;92:231–238. doi: 10.1007/s10549-005-2510-1. [DOI] [PubMed] [Google Scholar]
- 16.Londero V, Bazzocchi M, Del Frate C, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004;14:1371–1379. doi: 10.1007/s00330-004-2246-z. Epub 2004 Feb 18. [DOI] [PubMed] [Google Scholar]
- 17.Trecate G, Ceglia E, Stabile F, et al. Locally advanced breast cancer treated with primary chemotherapy: comparison between magnetic resonance imaging and pathologic evaluation of residual disease. Tumori. 1999;85:220–228. doi: 10.1177/030089169908500402. [DOI] [PubMed] [Google Scholar]
- 18.Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and breast cancer imaging. Radiographics. 2007;27 (Suppl 1):S215–229. doi: 10.1148/rg.27si075517. [DOI] [PubMed] [Google Scholar]
- 19.Mankoff DA, Dunnwald LK, Gralow JR, Livingston RB, et al. Monitoring the response of patients with locally advanced breast carcinoma to neoadjuvant chemotherapy using [technetium 99m]-sestamibi scintimammography. Cancer. 1999;85:2410–2423. [PubMed] [Google Scholar]
- 20.Schelling M, Avril N, Nahrig J, et al. Positron emission tomography using [(18)F] Fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689–1695. doi: 10.1200/JCO.2000.18.8.1689. [DOI] [PubMed] [Google Scholar]
- 21.Tan PC, Pickles MD, Lowry M, et al. Lesion T(2) relaxation times and volumes predict the response of malignant breast lesions to neoadjuvant chemotherapy. Magn Reson Imaging. 2008;26:26–34. doi: 10.1016/j.mri.2007.04.002. Epub 2007 Jun 15. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 23.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 24.Symmans WF, Volm MD, Shapiro RL, et al. Paclitaxel-induced apoptosis and mitotic arrest assessed by serial fine-needle aspiration: implications for early prediction of breast cancer response to neoadjuvant treatment. Clin Cancer Res. 2000;6:4610–4617. [PubMed] [Google Scholar]
- 25.van Leeuwen FW, Buckle T, Batteau L, et al. Potential value of color-coded dynamic breast-specific gamma-imaging; comparing (99m)Tc-(V)-DMSA, (99m)Tc-MIBI, and (99m)Tc-HDP in a mouse mammary tumor model. Appl Radiat Isot. 2010;68:2117–2124. doi: 10.1016/j.apradiso.2010.05.008. Epub 2010 May 16. [DOI] [PubMed] [Google Scholar]
- 26.Scopinaro F, Varvarigou A, Ussof W, et al. Breast cancer takes up 99mTc bombesin: a preliminary report. Tumori. 2002;88:S25–28. doi: 10.1177/030089160208800331. [DOI] [PubMed] [Google Scholar]
- 27.Scopinaro F, De Vincentis G, Varvarigou AD. Use of radiolabeled bombesin in humans. J Clin Oncol. 2005;23:3170–3171. doi: 10.1200/JCO.2005.05.363. [DOI] [PubMed] [Google Scholar]