Abstract
We describe an original activity assay for membrane transport that uses the proton motive force-dependent efflux pump MexAB from Pseudomonas aeruginosa. This pump is co-reconstituted into proteoliposomes together with bacteriorhodopsin (BR), a light-activated proton pump. In this system, upon illumination with visible light, the photo-induced proton gradient created by the BR is shown to be coupled to the active transport of substrates through the pump.
Multidrug efflux pumps from gram-negative bacteria are the focus of increasing interest because of their prominent role in the active efflux of xenobiotics out of the bacteria and, by extension, their prominent role in the worrisome problem of bacterial resistance. Efflux transporters are organised as multicomponent systems in which the RND pump (of the so-called Resistance, Nodulation, cell Division family) located in the inner membrane works in conjunction with a periplasmic protein (MFP, Membrane Fusion Protein) and an outer membrane protein (OMP)1. The cytoplasmic inner membrane protein, which has broad substrate specificity, acts as an energy-dependent pump that is driven by the proton-motive force (pmf). We focus on the OprM/MexA/MexB system from the gram-negative bacterium Pseudomonas aeruginosa (Figure 1a).
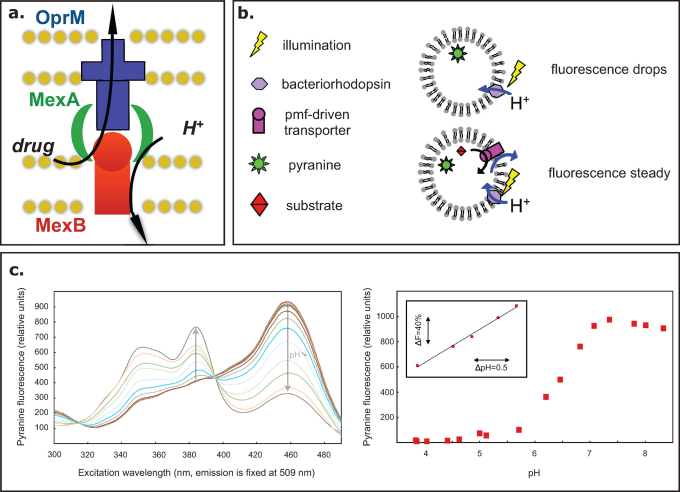
Figure 1. Rationale for the assay.
A. Schematic representation of the MexA-MexB-OprM pump from Pseudomonas aeruginosa. B. Cartoon representation of liposomes reconstituted with BR (upper scheme) or with both BR and a functional and active membrane transporter (lower scheme). C. Pyranine fluorescence as a function of pH. left, excitation spectrum (emission wavelength set to 509 nm) of pyranine measured at pHs ranging from 6.6 to 8.5; right, pyranine fluorescence (λex 455 nm, λem 509 nm) as a function of pH. inset: linear correlation between fluorescence and pH between pH 6 and pH 7. The following linear regression was calculated: Δ fluorescence (%) = 65.9 x ΔpH - 368 (R2 = 0.9946).
Although it has recently benefited from significant structural breakthroughs2,3, the efflux pump field still suffers from a critical lack of tools that allow the in vitro investigation of transport. This lack of tools arises from the difficulty of generating robust, quantitatively measurable and reproducible proton gradients across the membrane, a pre-requisite for the transporter to function. Initial attempts have been described that involve the use of valinomycin, a potassium-selective ionophore, which makes it possible to generate proton movement within liposomes that are first filled with KCl and subsequently diluted in a KCl-free buffer4,5. We have made recommendations that will allow this procedure to become unambiguous and reproducible6, but we have to acknowledge that even with these caveats in mind, the valinomycin assay remains tedious. Hence, we have decided to improve this protocol by adapting a classics of the membrane bioenergetics field, namely the work of Ephraïm Racker and Walther Stoeckenius in which they used the bacteriorhodopsin from the archaeal organism Halobacter halobium to generate the photo-induced proton gradients needed to allow for ATP synthesis through the pH-dependent ATP synthase7. We have decided to adapt this pioneering work to convert light into the energy needed to activate the RND pump MexB.
Results
Rationale for the assay
In our system, the membrane proteins are reconstituted into liposomes containing 8-hydroxypyrene-1, 3, 6-trisulphonic acid (pyranine), which provides an optical read-out of the pH changes occurring within the vesicles8,9. In Figure 1c, we show the fluorescence spectra of pyranine at different pHs. The pH dependence of the fluorescence is plotted and is shown to be linear over 1 pH unit. The proton gradient photo-induced by the BR is used by MexB to transport Hoechst 33342, a known substrate of the pump. Given that BR incorporation into liposomes favours one direction10 (proton pumping into the liposome lumen), only those MexB membrane proteins oriented inside-out will be potentially energised by the pH gradient (see Figure 1b).
The rationale for our assay is the following: In a control experiment in which only BR is present in the liposome membrane, the pH is supposed to decrease upon illumination, and hence, the pyranine fluorescence drops. In contrast, when BR is present together with an active and functional pmf-dependent transporter, the protons pumped inside the vesicle by BR will be counter-transported, thereby leading to compensation for the liposomal acidification and hence a steady fluorescence signal.
Mandatory controls
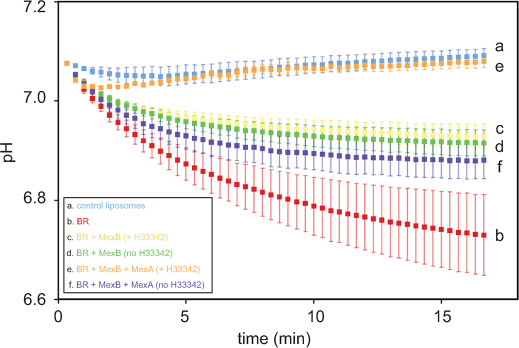
Of foremost importance regarding the rationale of our assay is that the proteoliposomes must be as tight as possible. Indeed, one should make sure that the above-mentioned steady signal is due to counter-transport by the pump and not to the passive leakage of protons out of the liposome. We have taken extreme care in the optimisation of the preparation of the liposomes6,10, and we systematically checked that the liposomes reconstituted with both membrane proteins were not significantly leakier than the control liposomes (see Supplementary information, Figure S1). Note that the proton gradients generated by the BR dissipate, leading to the eventual restoration of the initial fluorescence level after the liposomes are incubated in the dark; acidification can then be triggered from that point simply by illuminating again the suspension. Provided that the above prerequisites are fulfilled, the activity of the RND pump MexB can be monitored in vitro. In Figure 2, we present the averages of the results for six independent measurements.
Figure 2. Monitoring of the liposomal acidification upon illumination.
Pyranine fluorescence variations, measured as a function of time, were normalised to the corresponding pH variations based on the titration presented Figure 1c.
Investigation of transport
One can but appreciate the sensitivity of the BR-mediated proton pumping procedure. We have defined the reconstitution and measurement conditions such that a pH decrease of approximately 0.3 units occurs upon light-induced proton pumping11, thereby matching the ΔpH postulated to be required for activating the pump5. Surprisingly, when the measurement is performed with proteoliposomes containing BR and MexB, the pump seems to provide suboptimal compensation for the BR-mediated acidification: the fluorescence signal ends between that obtained with control liposomes devoid of any protein and that obtained with liposomes reconstituted with BR alone (see Figure 2, trace c versus trace a and trace b). Note that this behaviour is observed irrespective of the presence of substrate, as if MexB were able to pump protons even in the absence of any substrate (a so-called basal, substrate-independent, proton pumping activity), though at a rate seemingly too low to efficiently compensate for the gradient created by the BR. By contrast, when the measurement is performed with liposomes containing BR, the substrate, and both MexB and MexA, one now clearly observe the fluorescence signal expected for coupled proton counter transport: for one proton entering the liposome, one is transported out by the pump (see Figure 2, trace e). This observation is in line with the results of work of Pr Nikaido, who has shown that the MFP was mandatory for transport4,5. Interestingly, when proteoliposomes containing BR, MexA and MexB are prepared beforehand without substrate and subjected to our assay, no coupled activity is observed (see Figure 2, trace f). However, when the same proteoliposomes are subsequently incubated with the substrate in the dark for a sufficient period of time, proton counter transport is detected. It is likely that the incubation makes it possible for the substrate to partition into the membranes of the liposome and then diffuse laterally to eventually reach the pump via its vestibule; hence, transport is made possible.
Discussion
We have designed an activity assay that allows the monitoring of the activity of RND efflux pumps. Our results might well shed some light on the controversy regarding the possible implication of the MFP in transport. In agreement with the results of previous work by Nikaido and Aires, we show here that MexA is a key element of the actual coupled transport through the efflux pump. In contrast, the Venter's group showed that MexB is able to transport Hoechst 33342 alone, in the absence of MexA12. The difference between our respective results might very well arise from the different experimental procedures used in our respective laboratories. Indeed, in our protocol, inside-out reconstitution entraps the whole functional complex, and the resulting confinement increases the probability of interaction between MexB and its MFP partner. In contrast, Venter and colleagues performed a so-called "direct incorporation" experiment in which MexB is inserted unidirectionally, right-side out. Under such conditions, functional assembly will be more difficult to achieve because the MFP will have to explore a much larger volume in that case than in our inside-out liposomes.
We show that MexB behaves as if it were able to sustain a basal proton pumping activity that is potentiated by the presence of substrate. This behaviour is reminiscent of that of proteins in the the functionally related ABC protein family, which exhibit a basal ATPase activity that is enhanced by the presence of substrates13. Interestingly, it has been shown recently that MacB, an ABC transporter from E. coli that works in conjunction with a MFP and an OMP, exhibits basal ATPase activity that is enhanced in the presence of its cognate MFP protein MacA, which is closely related to MexA14,15. Note that we cannot exclude the possibility that what we believe to be substrate-free conditions are in fact not; it is possible that the system is contaminated by some endogenous compound (e.g., lipid, left-over from the purification) that can be transported by the pump.
Our protocol opens the way towards a better understanding of the activity of the RND efflux pump MexB and, more generally, of all pmf-activated membrane proteins. However, we also envisage using this protocol for screening Efflux Pump Inhibitors (EPIs) because our protocol can be easily scaled down, automatised (e.g., in a 96-well plate reader equipped with relevant optical filters) and parallelised: in this context, the use of BR as a switch for triggering the measurement is certainly an asset because it eliminates any second-hand manipulation of the suspension.
Methods
Materials
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) was purchased from Avanti Polar Lipids. Cholesterol, 8-hydroxypyrene-1,3,6-trisulphonic acid (pyranine), Triton X-100, and Hoechst 33342 were obtained from Sigma Aldrich. SM2 Bio-beads were obtained from Bio-Rad. n-Dodecyl-β-D-maltopyranoside (DDM) was purchased from Affymetrix (reference D310LA).
Preparation of the proteins
Expression and purification of the MexA, MexB and BR proteins were performed as previously described16,17,18. Briefly, MexA and MexB were heterologously expressed from pBAD-mexA or pET-mexB (pBAD33-GFPuv, Invitrogen; pET22b vector, Novagen) in E. coli cells (strain C43 DE3) and purified as His-tagged proteins from the membranes solubilised with DDM. The yields of homogeneously purified MexA and MexB were approximately 6–7 mg and 0.5 mg per litre of culture, respectively. The purified proteins were concentrated using Vivaspin (VivaScience) concentrators with cutoffs of 30 kDa and 100 kDa for MexA and MexB, respectively.
Halobacter halobium cells were grown under illumination at 37°C in a liquid growth medium containing NaCl 4 M, MgSO4 150 mM, trisodium citrate 10 mM, KCl 30 mM, yeast extract 5 g/L, and peptone 5 g/L. Purple membrane was isolated using a sucrose gradient and solubilised as follows: a membrane suspension containing 15–20 mg BR at 7 g/L was sonicated for 5 min and incubated overnight at room temperature with Triton X-100 at a 1:5 protein-to-detergent ratio (w/w). The concentration of solubilised BR was estimated using ε (570 nm) = 54.000 M−1.cm−1 and ε (280 nm) = 1.080.000 M−1.cm−1.
Preparation of unilamellar vesicules
DOPC and cholesterol were dissolved in HEPES 25 mM pH 7, K2SO4 100 mM and MgCl2 15 mM to final concentrations of 10 mg/mL and 1.5 mg/mL, respectively. The suspension was then heated for 10 min at 37°C. Then, pyranine and Hoechst 33342 were added to final concentrations of 2 mM and 140 μM, respectively. This solution was then sonicated for 10 minutes with 30′′ pulse/30′′ pause cycles. The liposomes were subsequently extruded through 200-nm membranes and then through 100-nm membranes (20 passes for each type of membrane). Triton X-100 was then added to reach a 2.8 detergent/lipid ratio (w/w). This suspension was then incubated overnight at 4°C with gentle stirring. Proteins were added to the solubilised liposome suspension at the following ratios (w/w): lipids/MexB = 20, MexB/MexA = 2.5, and lipids/BR = 30. Detergent removal was achieved upon addition of Bio-beads (previously washed with methanol, ethanol and water) at a Bio-bead/detergent ratio of 60 (w/w) for at least 3 h at 4°C in the dark. The proteoliposomes were then purified using a PD-10 desalting column (GE Healthcare) equilibrated with HEPES 25 mM pH 7, K2SO4 100 mM and MgCl2 15 mM and kept in the dark at 18°C. The proteoliposomes were stored for up to 2–3 weeks under these conditions.
Fluorescence assay
Fluorescence measurements were conducted at 25.0°C using a JASCO FP-6200 spectrofluorimeter. The measurements were performed using the dual-wavelength mode, taking measurements with excitation/emission wavelengths of 550 nm/550 nm for 18′′ (to activate BR) and then taking measurements with excitation/emission wavelengths of 455 nm/509 nm for 2′′ (to measure the pyranine fluorescence). The excitation and emission bandwidths were 5 nm. Note that the measurements were performed in the presence of 50 nM valinomycin to prevent the formation of a reverse ΔΨ.
Author Contributions
MP conceived the experiments. AV, IB and MP wrote the manuscript. AV performed the experiments.
Supplementary Material
Supplementary informationn
Acknowledgments
We thank Manuela Dezi and Daniel Lévy for helpful discussions, and Clara Jaquet for the initial fluorescence measurements. This work was supported by a grant from Région Ile-de-France (DIM-Malinf) and by the Agence Nationale de la Recherche (project ANR-07-BLAN-0263).
References
- Nikaido H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 78, 119–146 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M. A. et al. Structural Asymmetry of AcrB Trimer Suggests a Peristaltic Pump Mechanism. Science. 313, 1295–1298 (2006). [DOI] [PubMed] [Google Scholar]
- Murakami S., Nakashima R., Yamashita E., Matsumoto T. & Yamaguchi A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature. 443, 173–179 (2006). [DOI] [PubMed] [Google Scholar]
- Zgurskaya H. I. & Nikaido H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 96, 7190–7195 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aires J. R. & H. Nikaido. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli. Journal of Bacteriology. 187, 1923–1929 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M., Verchère A. & Broutin I. Monitoring the active transport of efflux pumps after their reconstitution into proteoliposomes: caveats and keys. Analytical Biochemistry 420, 194–196 (2012). [DOI] [PubMed] [Google Scholar]
- Racker E. & Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J. Biol. Chem. 249, 662–663 (1974). [PubMed] [Google Scholar]
- Clement N. R. & J. M. Gould. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry 20, 1534–1538 (1981). [DOI] [PubMed] [Google Scholar]
- Sedgwick E. G. & P. D. Bragg. Differential movement of ions in artificial phospholipid vesicles. FEBS Letters. 272, 81–84 (1990). [DOI] [PubMed] [Google Scholar]
- Rigaud J.-L. & D. Lévy. Reconstitution of membrane proteins into liposomes. Meth. Enzymol. 372, 65–86 (2003). [DOI] [PubMed] [Google Scholar]
- Seigneuret M. & Rigaud J-L. Analysis of Passive and Light-Driven Ion Movements in Large Bacteriorhodopsin Liposomes Reconstituted by Reverse-Phase Evaporation. 1. Factors Governing the passive proton permeability of the membrane. Biochemistry 25, 6716–6722 (1986). [Google Scholar]
- Welch A., Awah C. U., Jing S., Van Veen H. & Venter H. Promiscuous partnering and independent activity of MexB, the multidrug transporter protein from Pseudomonas aeruginosa. Biochem. J. 430, 355–364 (2010). [DOI] [PubMed] [Google Scholar]
- Al-Shawi MK. Catalytic and transport cycles of ABC exporters. Essays Biochem. 50, 63–83 (2011). [DOI] [PubMed] [Google Scholar]
- Tikhonova E. B., Devroy V. K., Lau S. Y. & Zgurskaya H. I. Reconstitution of the Escherichia coli macrolide transporter: the periplasmic membrane fusion protein MacA stimulates the ATPase activity of MacB. Mol Microbiol. 63, 895–910 (2007). [DOI] [PubMed] [Google Scholar]
- Modali S. D. & Zgurskaya H. I. The periplasmic membrane proximal domain of MacA acts as a switch in stimulation of ATP hydrolysis by MacB transporter. Mol Microbiol. 81, 937–951 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama H. Crystal Structure of the Membrane Fusion Protein, MexA, of the Multidrug Transporter in Pseudomonas aeruginosa. J. Biol. Chem. 279, 25939–25942 (2004). [DOI] [PubMed] [Google Scholar]
- Mokhonov V. et al. Multidrug transporter MexB of : overexpression, purification, and initial structural characterization. Protein Expression and Purification. 40, 91–100 (2005). [DOI] [PubMed] [Google Scholar]
- Oesterhelt D. & Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Meth. Enzymol. 31, 667–678 (1974). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary informationn