Abstract
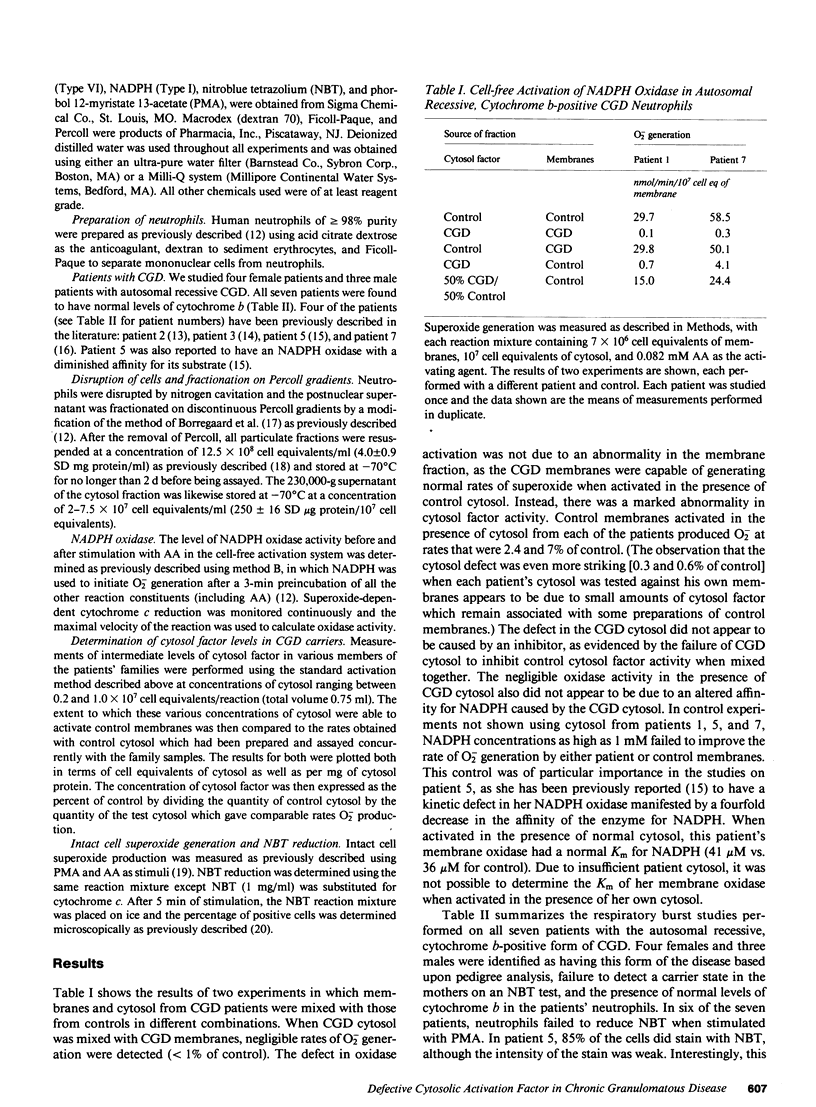
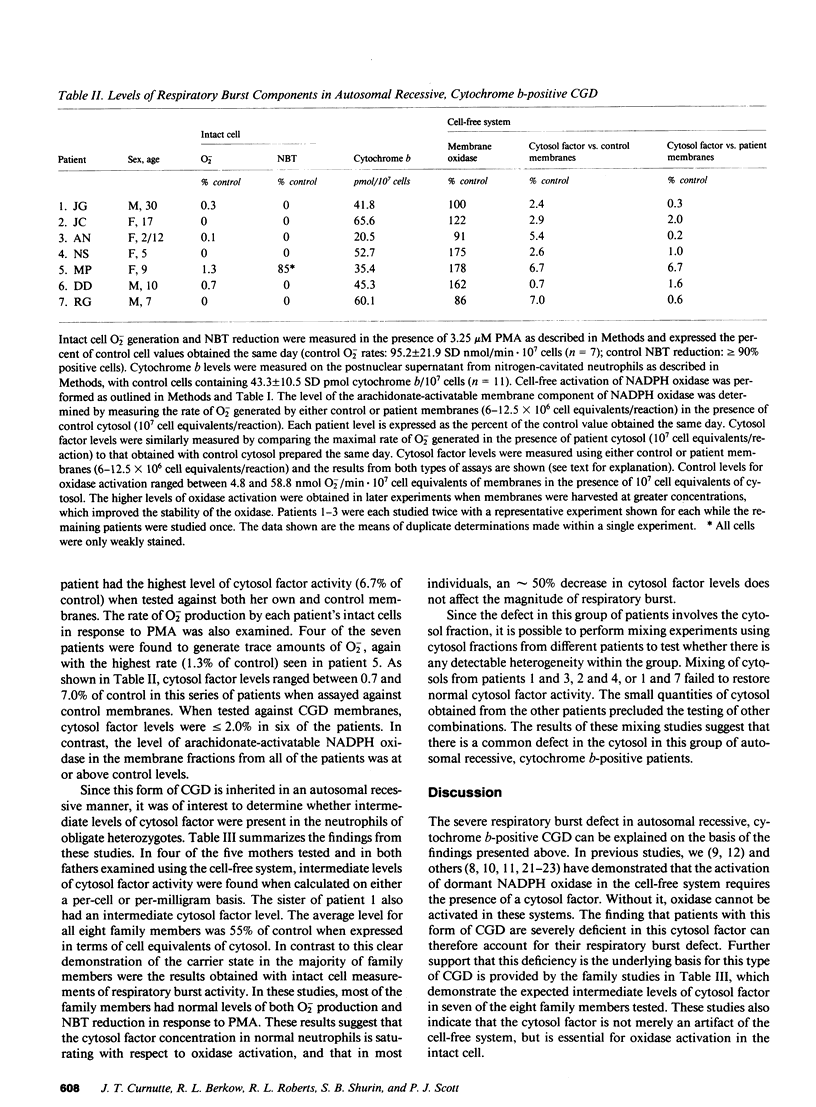
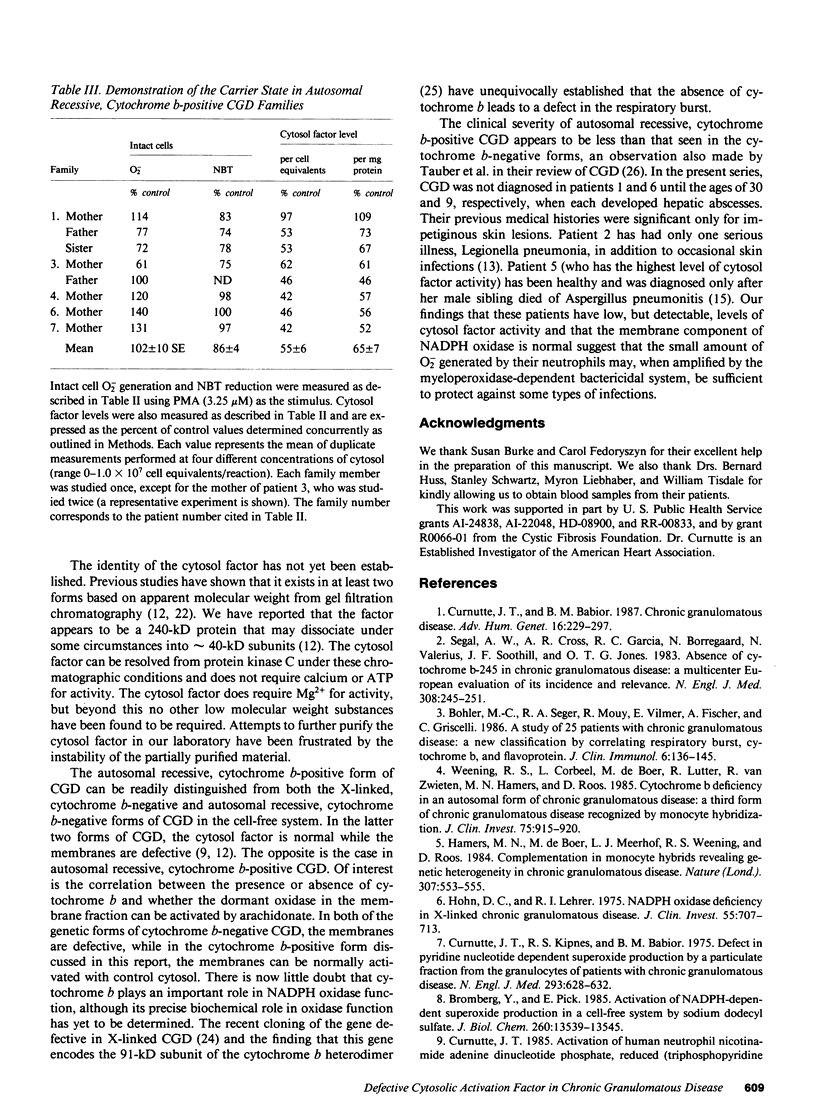
The superoxide-generating enzyme of human neutrophils, NADPH oxidase, is present in a dormant state in unstimulated neutrophils. It can be converted to an active form in a cell-free system if both the plasma membrane and cytosol fractions are incubated together in the presence of arachidonic acid. This system was used to determine the nature of the biochemical defect in seven patients with the autosomal recessive, cytochrome b-positive form of chronic granulomatous disease (CGD). A severe deficiency in the cytosol factor was identified in each patient. The defective activity was not caused by the presence of an inhibitor, nor could it be restored to normal by combining cytosol fractions from different patients. In contrast, the membrane fractions from all seven patients contained normal levels of NADPH oxidase when activated in the presence of control cytosol. Of family members tested (obligate heterozygotes for this disorder), seven of eight had intermediate levels of cytosol factor activity. The respiratory burst defect in this form of CGD is caused by an abnormality in the cytosolic factor required for NADPH oxidase activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badwey J. A., Curnutte J. T., Robinson J. M., Berde C. B., Karnovsky M. J., Karnovsky M. L. Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem. 1984 Jun 25;259(12):7870–7877. [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Bohler M. C., Seger R. A., Mouy R., Vilmer E., Fischer A., Griscelli C. A study of 25 patients with chronic granulomatous disease: a new classification by correlating respiratory burst, cytochrome b, and flavoprotein. J Clin Immunol. 1986 Mar;6(2):136–145. doi: 10.1007/BF00918746. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg Y., Pick E. Activation of NADPH-dependent superoxide production in a cell-free system by sodium dodecyl sulfate. J Biol Chem. 1985 Nov 5;260(25):13539–13545. [PubMed] [Google Scholar]
- Clark R. A., Leidal K. G., Pearson D. W., Nauseef W. M. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J Biol Chem. 1987 Mar 25;262(9):4065–4074. [PubMed] [Google Scholar]
- Cox J. A., Jeng A. Y., Sharkey N. A., Blumberg P. M., Tauber A. I. Activation of the human neutrophil nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase by protein kinase C. J Clin Invest. 1985 Nov;76(5):1932–1938. doi: 10.1172/JCI112190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J Clin Invest. 1985 May;75(5):1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Chronic granulomatous disease. Adv Hum Genet. 1987;16:229–297. doi: 10.1007/978-1-4757-0620-8_4. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Babior B. M. Activation of the respiratory burst oxidase in a fully soluble system from human neutrophils. J Biol Chem. 1987 May 15;262(14):6450–6452. [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Scott P. J. Activation of neutrophil NADPH oxidase in a cell-free system. Partial purification of components and characterization of the activation process. J Biol Chem. 1987 Apr 25;262(12):5563–5569. [PubMed] [Google Scholar]
- Dinauer M. C., Orkin S. H., Brown R., Jesaitis A. J., Parkos C. A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. 1987 Jun 25-Jul 1Nature. 327(6124):717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., English D., Akard L. P., Schell M. J. Regulation of neutrophil NADPH oxidase activation in a cell-free system by guanine nucleotides and fluoride. Evidence for participation of a pertussis and cholera toxin-insensitive G protein. J Biol Chem. 1987 Feb 5;262(4):1685–1690. [PubMed] [Google Scholar]
- Hamers M. N., de Boer M., Meerhof L. J., Weening R. S., Roos D. Complementation in monocyte hybrids revealing genetic heterogeneity in chronic granulomatous disease. Nature. 1984 Feb 9;307(5951):553–555. doi: 10.1038/307553a0. [DOI] [PubMed] [Google Scholar]
- Heyneman R. A., Vercauteren R. E. Activation of a NADPH oxidase from horse polymorphonuclear leukocytes in a cell-free system. J Leukoc Biol. 1984 Dec;36(6):751–759. doi: 10.1002/jlb.36.6.751. [DOI] [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., Shirley P. S., Clayton C. C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J Clin Invest. 1985 May;75(5):1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y., Buescher E. S., Roberts R., Metcalf J. A., Gallin J. I. Reevaluation of cytochrome b and flavin adenine dinucleotide in neutrophils from patients with chronic granulomatous disease and description of a family with probable autosomal recessive inheritance of cytochrome b deficiency. Blood. 1986 Apr;67(4):1132–1138. [PubMed] [Google Scholar]
- Peerless A. G., Liebhaber M., Anderson S., Lehrer R. I., Stiehm E. R. Legionella pneumonia in chronic granulomatous disease. J Pediatr. 1985 May;106(5):783–785. doi: 10.1016/s0022-3476(85)80355-3. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Kunkel L. M., Monaco A. P., Goff S. C., Newburger P. E., Baehner R. L., Cole F. S., Curnutte J. T., Orkin S. H. Cloning the gene for an inherited human disorder--chronic granulomatous disease--on the basis of its chromosomal location. Nature. 1986 Jul 3;322(6074):32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Tiller R. E., Berkow R. L. Invasive pulmonary aspergillosis in an infant: an unusual presentation of chronic granulomatous disease. Pediatr Infect Dis J. 1987 Feb;6(2):215–217. [PubMed] [Google Scholar]
- Segal A. W., Cross A. R., Garcia R. C., Borregaard N., Valerius N. H., Soothill J. F., Jones O. T. Absence of cytochrome b-245 in chronic granulomatous disease. A multicenter European evaluation of its incidence and relevance. N Engl J Med. 1983 Feb 3;308(5):245–251. doi: 10.1056/NEJM198302033080503. [DOI] [PubMed] [Google Scholar]
- Shurin S. B., Cohen H. J., Whitin J. C., Newburger P. E. Impaired granulocyte superoxide production and prolongation of the respiratory burst due to a low-affinity NADPH-dependent oxidase. Blood. 1983 Sep;62(3):564–571. [PubMed] [Google Scholar]
- Tauber A. I., Borregaard N., Simons E., Wright J. Chronic granulomatous disease: a syndrome of phagocyte oxidase deficiencies. Medicine (Baltimore) 1983 Sep;62(5):286–309. [PubMed] [Google Scholar]
- Weening R. S., Corbeel L., de Boer M., Lutter R., van Zwieten R., Hamers M. N., Roos D. Cytochrome b deficiency in an autosomal form of chronic granulomatous disease. A third form of chronic granulomatous disease recognized by monocyte hybridization. J Clin Invest. 1985 Mar;75(3):915–920. doi: 10.1172/JCI111792. [DOI] [PMC free article] [PubMed] [Google Scholar]



