Abstract
Purpose
In our ongoing investigation into the consequences of a radiological terrorism event, we assessed whether a dose range that is believed to be subthreshold for the development of lung endpoints results in late pathological changes and, secondarily, whether those late changes affect the lung’s ability to respond to subsequent challenge.
Materials and methods
C57BL/6J mice received total body irradiation (0.5-10 Gy) and were followed for 6-18 months after irradiation. At 12 and 15 months, a subset of mice was exposed to a second challenge (aerosolized lipopolysaccharide [LPS]).
Results
Cytokines shown to be upregulated early (hours) following irradiation (interleukin [IL]6,keratinocyte chemoattractant [KC], IL1B, and IL1R2) demonstrated increases in messenger ribose nucleic acid (mRNA) expression at late time points, beginning at 9 months. Although persistent, dose-dependent increases in T cell counts were seen, no other overt changes in pathophysiology were observed. Nonetheless, animals that were exposed to a secondary challenge at late time points demonstrated an increased inflammatory cell recruitment and persistence in response relative to controls.
Conclusions
We propose that, following doses that elicit little change in pathophysiology, sub-clinical radiation-induced injury increases the lungs’ susceptibility to a secondary challenge, possibly through a radiation-induced alteration in the immune defense system.
Keywords: lung late effects, secondary challenge, late cytokine expression
Introduction
Recent history has shown that, following radiological and/or nuclear dispersion events, early medical intervention can result in many patients surviving the immediate consequences of exposures that result in an acute radiation syndrome (ARS). However, despite the temporally early successes, of those who have been exposed to the higher dose levels, many subsequently die as a result of multiple late complications, frequently related to the lung (Vlasov & Kvacheva 1996, Hirama & Akashi 2005). This observation has led some investigators to suggest that, in the context of lethal or near-lethal total body irradiation (TBI), the combination of radiation-induced toxicities in critical normal tissues can result in a form of Multi-Organ Dysfunction Syndrome(MODS) (Mizock 2009, Meineke & Fliedner 2005).
This syndrome can result from a range of tissue insults (for example, injury or infection) and in its initial stages, MODS often involves an uncontrolled inflammation as part of a systemic response, even when the injury is relatively localized. Subsequently, there is progressive breakdown in critical organs, with respiratory failure being a common component. Although MODS has been subjected to a significant degree of study (Aikawa, 1996, Pinsky, 1996, Akashi 2005, Mizock 2009), only a few investigators have considered radiation-induced MODS (RI-MODS) as contributing to late morbidity following TBI (Gale 1987, Konchalovsky et al. 2005, Monti et al. 2005, Meineke & Fliedner 2005), and fewer still have investigated the possible underlying mechanisms. Interestingly, despite the fact that the lung appears to play a significant role in the development of both MODS and RI-MODS (Hirama & Akashi 2005, Mizock 2009), it is not clear what role, if any, pulmonary complications may play in the development of late outcomes that would be seen in the survivors of a mass casualty radiation event, particularly in those whose exposure dose is sufficiently low that they may not receive medical assistance during the acute (ARS) period. This level of uncertainty is increased further when consideration is given to the fact that, at the upper limits of the dose range where survival of ARS may be possible with sufficient medical care (~6-8 Gy in the human [Anno et al. 2003]), these doses still are below the established dose threshold levels for the clinical radiation-induced endpoints seen in the lung (pneumonitis and fibrosis) (Hopewell et al. 2000).
In earlier work, we and others have demonstrated that, following clinically-relevant high doses of thoracic radiation, there are persistent waves of increased cytokine expression in the lung tissue (Johnston et al. 1996, Johnston et al. 2002, Chiang et al. 2005); this observation led to the proposal that these cyclical periods of upregulation in expression are a critical component of normal tissue late effect progression (Rubin et al. 1995, McBride et al. 2004). More recently, our group has shown that, even following doses that are considered subthreshold for pulmonary late effects (≤10 Gy), increases in cytokine expression are still seen in the lung and peripheral circulation in the immediate period following irradiation (Johnston et al. 2010). The principal goal of our study was, therefore, to test the hypothesis that such subthreshold doses of radiation, if delivered as part of a total body exposure, would not significantly alter the mechanism of cytokine activation seen in the lung after higher doses, but would, instead, lead to a delay in the onset of the physiological and pathological symptoms of tissue damage, as well as the lung’s participation in any resultant systemically-based adverse outcome.
Finally, the likelihood of total body exposure as part of a terrorist event leads to the possibility of a systemic response. This is not normally assessed as part of late normal tissue toxicity since, in general, such research focuses on therapy-related, high dose, localized radiation fields. However, as discussed in recent publications (McBride et al. 2004, Schaue & McBride 2010), the immune system may play a greater role in normal tissue late effects than was previously realized and, if true, this would be particularly relevant to the lung due to its portal role; since the lungs’ primary function is gas exchange with an external environment, there is a considerable risk of contamination with various pathogens and inert particles. Consequently, the lung’s immune microenvironment must be highly responsive to challenge, although it also must maintain a balance between controlling potential pathogens while avoiding prolonged inflammation.
In terms of responsiveness, potent proinflammatory cytokines and chemokines, such as tumor necrosis factor (TNF)-α, interleukins (IL)-1α and IL1ß and monocyte chemoattractant protein (MCP)-1, have been shown to be rapidly expressed following a range of pulmonary injuries, e.g., infection, irradiation, and inhaled toxicants (Johnston et al. 1996, Finkelstein et al. 1997, Olman et al. 2002, Johnston et al. 2002, Porter et al. 2002, Sedgwick et al. 2002, Calveley et al. 2005). This upregulation leads to the subsequent triggering of inflammatory cell recruitment and is believed to be part of the important initial step in a host’s defense response. However, since cytokines and chemokines also are important regulators of innate and adaptive immunity, any persistent alterations in the cytokine milieu could, potentially, impact downstream immune responses to subsequent pulmonary injury (McBride et al, 2004, Schaue & McBride 2010).
Therefore, the goals in this study were to establish a time course for the changes in cytokine expression after irradiation with doses of 10 Gy or lower and to provide analysis of the progression of late lung toxicity. In addition, we included a late, secondary injury in our studies, using an inhaled endotoxin challenge (LPS), in the belief that this might reveal sub-clinical lung damage not otherwise apparent from histopathological and physiological examination. Such findings would have implications on the follow-up schedule and treatment of an exposed population following a nuclear or radiological accident or event. Finally, the use of cytokine assays as biochemical markers holds out the promise of relatively easy quantification and, if reproducible, their use as predictors and monitors of radiation injury; therefore, we anticipated identifying changes in cytokine expression that may be used as biomarkers or surrogates of radiation exposure and late effect progression, which, in turn, could be utilized in mitigation assessment.
Materials and methods
Experimental animals and irradiation
Adult C57BL/6J mice (7-8 week-old females) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Animals were allowed to acclimate from shipping for 1 week prior to experimentation. Mice were maintained five per cage under filter caps in pathogen-free rooms and were supplied with standard laboratory diet and water ad libitum.
In each of two independent, but identical, studies, groups of 10 mice received TBI at doses of 0-10 Gy from a 137Cs γ-ray source (J.L. Shepherd & Associates, San Fernando, CA, USA) operating at a dose rate of approximately 1.6 Gy/min; those animals receiving the highest dose (10 Gy) had a single hind-limb excluded from the radiation field in order to provide some sparing to the bone marrow. Mice were confined to plastic jigs and were not anesthetized during the procedure; age-matched control animals were sham-irradiated and maintained under identical conditions for the course of the experiment. Mice were sacrificed between 6 and 18 months after radiation. All treatment protocols were approved by the University of Rochester institutional animal care and use committee.
Secondary (LPS inhalation) challenge
At 12 and 15 months following irradiation, surviving mice were randomly assigned to one of two groups. One group was sacrificed immediately, whilst the second group was exposed for eight minutes to LPS (Pseudomonas aeruginosa Lipopolysaccharide, Sigma Chemical Co., St. Louis, MO, USA). This form of LPS was chosen since Pseudomonas aeruginosa is an opportunistic pathogen that is ubiquitous in our environment and is the cause of a broad spectrum of lung infections (Williams et al. 2010). However, it rarely infects the healthy human lung and, therefore, is used by many as a means of investigating the integrity of the host defense response. Aerosols of LPS were generated by using an AERO-Mist compressed air nebulizer (CIS-US, Inc., Bedford, MA, USA); 100 μl of LPS (100 μg) in 6 ml saline was added to the nebulizer, which had an output airflow of 20 liter/minute. Mice were exposed in a 30 liter whole-body exposure chamber in individual compartments. The LPS aerosol had a mass median aerodynamic diameter of 0.6 μm, with a geometric standard deviation of 1.6, and the estimated total broncheolar and alveolar dose was approximately 26 endotoxin units (EU). The respiratory tract deposition fraction was estimated using a multiple path particle dosimetry model (MPPD) (Applied Research Associates, Albuquerque, NM, USA), which does not account for any additional dose from ingestion (i.e. following grooming). However, the delivered dose is relatively low compared to the doses of LPS that are, in general, administered in studies using either the intraperitoneal or intratracheal routes. However, we have previously shown that equivalent low doses, when delivered by inhalation, reach far deeper into the lung, hence generating an induction of cytokines equivalent to significantly higher doses seen by either of the other two routes of administration (Johnston et al. 1998a). The chosen dose and route of administration, therefore, limits the host response to the lung alone and minimizes any systemic effect. Mice were allowed to recover in room air for 1, 3, or 7 days post-inhalation and then were sacrificed. Control mice were exposed to filtered room air in an adjacent chamber.
Peripheral blood analysis
Peripheral blood was collected by intracardial puncture from a minimum of 5 animals from each group and pooled for analysis. Samples were centrifuged at 6000g for 10 minutes, filtered through a 1.2-μm polyvinylidene fluoride filter plate (Millipore, Billerica, MA, USA), and then incubated overnight using a Beadlyte® Mouse Multi-Cytokine BeadmasterTM Kit (Millipore) and accompanying Beadmates (Millipore). The cytokines analyzed included IL1α, IL1ß, IL2, IL3, IL4, IL5, IL6, IL9, IL10, IL12 (p40), IL12 (p70), IL13, IL17, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, KC/chemokine (C-X-C motif) ligand 1 (CXCL1), MCP1, macrophage inflammatory protein (MIP)-1ß, Regulated on Activation Normal T-Cell Expressed and Secreted (RANTES), TNFα, and vascular endothelial growth factor (VEGF). Cytokines were quantified using Luminex xMAP technology (Luminex Corporation, Austin, TX, USA) on a BioPlex 200 System (Bio-Rad Laboratories, Hercules, CA, USA).
Methodology for serial blood capture
An additional subgroup of animals (n = 20/dose) were subjected to serial blood collection in order to determine if there was a direct correlation between the level of acute changes in cytokine expression detected in the plasma to the late response in each individual animal. Blood collection from the submandibular vein was used as an alternative to retro-orbital blood collection, which allowed for the maximum allowable sample volume (≤15% circulating blood volume; approximately 150 μl from a 20 g mouse) with minimal trauma to the animal. Samples were taken at a frequency of no less than 30 days, as permitted under the University of Rochester IACUC’s policy on blood collection. Blood samples were taken at 1-24 hours, 1, 3, 6, 9, 12, and 15 months following radiation. At the time of sampling, each mouse was secured by grasping the loose skin over the shoulders and behind the ears, keeping the skin taut over the mandible. Using an 18-gauge short bevel needle that had been first dipped in 100 μ/ml Heparin Lock Flush (Abraxis Pharmaceutical Products, Schaumburg, IL, USA), the submandibular vein was punctured slightly behind the mandible, but in front of the ear canal. Drops of blood were collected directly into labeled ethylenediaminetetraacetic acid (EDTA)-coated microtainer tubes (Becton Dickinson and Company, Franklin Lakes, NJ, USA), which were immediately inverted several times to mix. The blood samples were then centrifuged at 2500 rpm for 10 minutes and the plasma was collected. The plasma samples were stored frozen at −85°C until analyzed, as described for the peripheral blood analyses.
Tissue analysis
Histological preparation
After sacrifice, the animals’ lungs were inflated using zinc-buffered formalin (Z-Fix) (Anatech Ltd., Battle Creek, MI, USA) using a gravity perfusion apparatus. Additional target organs (spleen, gut, and thymus) were dissected from the mice and all tissues were post-fixed for 16-24 hours in Z-Fix. Tissues were dehydrated through graded alcohols, cleared through several changes of xylene, and infiltrated with paraffin. All tissues were embedded in paraffin, sectioned at 5 μm, and mounted on microscope slides (Harvey Instruments, Buffalo, NY, USA). Collagen staining was achieved using Gomori’s trichrome (Manual of the Special Stains Laboratory, University of Rochester Medical Center, Rochester, NY, USA).
Immunohistochemical techniques
Paraffin sections were deparaffinized in xylene (BDH/VWR International, West Chester, PA, USA) and rehydrated through graded ethanol (Ultrapure, North Darien, CT, USA) up to distilled water. Tissues for immunohistochemistry were pretreated in a citrate buffer (pH 6.0) (Dako, Carpinteria, CA, USA) for 38 minutes at a temperature of 95-97°C in a water bath. Following pretreatment, sections were incubated for five minutes with 3% hydrogen peroxide to inactivate endogenous peroxidases, followed by three rinses in Tris buffered saline-Tween (TBS-T) (Dako). Tissues then were blocked with Rodent Block M (Biocare Medical, Concord, CA, USA) for 20 minutes at room temperature followed by application of the primary antibody, diluted in Da Vinci Green Diluent (Biocare Medical), which was applied to the sections and incubated at 4°C overnight; neutrophils were stained using Rat Anti-Mouse Neutrophil (Cat. No. MCA771G; Serotec, Raleigh, NC, USA), macrophages using a Rat Anti-Mouse F4/80 (Cat. No. MCAP497; Dako), and T-lymphocytes were identified using Rat Anti-Human cluster of differentiation (CD)3 (Cat. No. MCA1477; Serotec) antibodies, respectively. The latter (anti-human) antibody is cross-reactive with mouse and a section of human tonsil is included in each staining run as a control to ensure specificity. Slides then were rinsed three times in TBS-T and the Rat Probe (Rat-on-Mouse HRP (horseradish peroxidase)-Polymer kit [Cat. No. RT517H; Biocare Medical]) was applied for 15 minutes at room temperature. Sections then were rinsed three times in TBS-T and incubated with Rat Polymer HRP for 15 minutes. Sections again were rinsed three times in TBS-T and then developed with DAB (3,3′-diaminobenzidine tetrahydrochloride) 1000 Chromagen System (Biocare Medical) for two to five minutes at room temperature. Slides were then rinsed in five changes of distilled water and counterstained with CAT Hematoxylin (Biocare Medical). Finally, nuclei were blued with two to three dips in 0.08% ammonia water, rinsed in three changes of distilled water, dehydrated in graded ethanols and coverslipped from xylene.
Image analysis
Tissue sections of immunohistochemically-stained tissues were imaged (Spot Pursuit camera, Diagnostic Instruments, Inc., Sterling Heights, MI, USA) into 3×6 montages that were taken at a 10x magnification in order to provide the greatest coverage of the entire lung sections. Computer imaging of stained lung sections was performed using Image Pro Plus 6.3 software (Media Cybernetics, Silver Spring, MD, USA). Total tissue area was measured using manually generated areas of interest (AOI) followed by programmatic deduction of the air (white) space. For inflammatory cell analysis, serial sections for each mouse lung were used to stain for macrophages, neutrophils and T cells, thus matching AOI from previous analyses could be used to calculate total tissue area. Finally, total cell counts were made and the population density for each lung was calculated.
Ribonuclease (RNase) protection assay
Total RNA was isolated from lung tissue using TRIzol Reagent (Life Technologies, Grand Island, NY, USA) according to the manufacturer’s instructions. Each frozen lung lobe (50-100 mg) was homogenized in 1 ml of TRIzol Reagent and the final RNA pellets were then resuspended in 50 μl of diethylpyrocarbonate-treated water. The RNA concentration and purity was quantified using the Gene Quant RNA/deoxyribonucleic acid (DNA) Calculator (Pharmacia Biotech, Piscataway, NJ, USA). Steady-state cytokine mRNA levels were quantified using a multi-cytokine ribonuclease protection assay (RPA), with custom riboprobe templates for IL1RI, IL1R2, IL10, IL1α, IL1ß, IL1Ra, IL18/IGIF, IL6, L32, andglyceraldehyde-3-phosphate dehydrogenase (GAPDH). The protected, radiolabeled RNA fragments were electrophoresed on a 5% acrylamide/8 M urea sequencing gel, and the dried gel was used to expose X-AR film (Eastman Kodak, Rochester, NY, USA) at −80°C with intensifying screens (Quanta III; Dupont, Wilmington, DE, USA).
Statistics
The relationships between cytokine expression levels and dose were examined using linear regression after logarithmic transformation. Expression levels across groups were compared using Student’s t tests. Results of RNase protection for the analysis of mRNA abundance were quantified by PhosphorImager analysis. Values for each mRNA species were normalized to the internal standards GAPDH or L32. Results of replicate experiments from five separate animals, analyzed in triplicate, were evaluated by analysis of variance (ANOVA) for independent measures, followed by Tukey or Dunnett tests of significance in multiple group comparisons. Similarly, the primary outcomes of inflammation and fibrosis, as measured by image analysis, included inflammatory cell infiltration and collagen deposition; these outcomes were treated as continuous variables and evaluated using ANOVA. The two-tailed level of significance for all analyses was set at p < 0.05.
Results
Analysis of body weights
Mice were weighed at 8 weeks of age, prior to TBI, and at each subsequent time point. No significant difference in body weight was observed between the sham and irradiated groups through 12 months after radiation. However, by18 months, mice that had received 10 Gy TBI had a significantly lower body weight (p < 0.01), weighing approximately 7 grams less than age matched mice in all other groups (Figure 1).
Figure 1.
Line plot showing the change in body weight of female C57BL/6J mice following a range of total body irradiation (TBI) doses from 0-10 Gy. No difference in body weights was observed between sham (0 Gy) and irradiated groups until 18 months after irradiation, when there was a significant decrease in the mean weight of the 10 Gy TBI group compared to all other groups (*p < 0.01; 10 Gy vs. controls). Error bars indicate the standard error of the mean (SEM) for 2 independent experiments; n=20/group.
Analysis of cytokine expression in peripheral blood
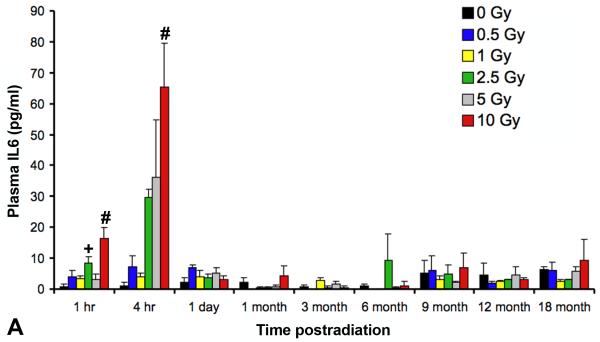
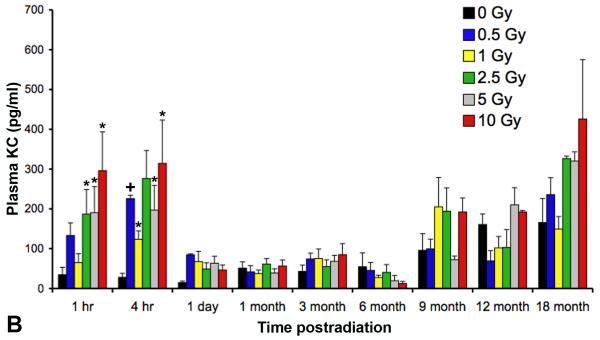
As previously reported (Johnston et al. 2010), two cytokines, IL6 and KC/CXCL1, had demonstrated significant time- and dose-related increases in the plasma in the first 2 days following irradiation. The current study continued to assess these cytokines until 18 months after radiation at all doses ranging from 0 Gy to 10 Gy (Figures 2A and B). We demonstrated at the later time points that further changes in cytokine plasma levels were not detected until 9 months, and by 18 months, doses greater than 1 Gy showed an apparent age-related and dose-dependent increase in KC plasma levels, although neither increases reached statistical significance due to inter- and intra-group variability.
Figure 2.
Analysis using a multiplex bioassay of expression of (A) interleukin (IL)6 and (B)keratinocyte chemoattractant (KC)/CXCL1 in the plasma of female C57BL/6J following TBI at time points ranging from 1 hour to 18 months after radiation. A significant, dose-dependent increase in expression was seen at early (one and four hours following irradiation) time points in the expression levels of both IL6 (+p < 0.01; #p < 0.001) and KC/CXCL1 (*p < 0.05; +p < 0.01) followed by a return to baseline levels by 1 day. At ≥9 months after irradiation, there appeared to be a dose-dependent increase in KC expression, however this failed to reach statistical significance. Error bars indicate the SEM for 2 independent experiments; n=10/group.
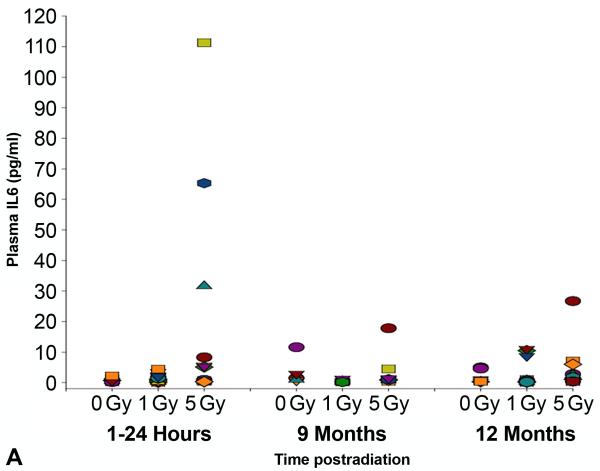
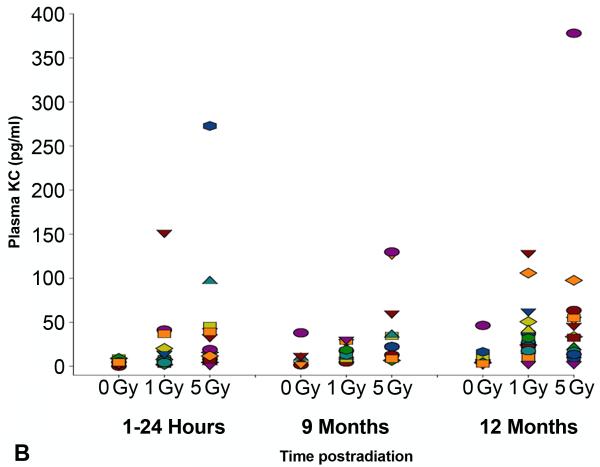
In an independent study, we verified the observed changes in cytokine expression levels by analyzing blood samples taken serially from individual mice exposed to 0, 1 or 5 Gy doses of TBI (Figure 3A and B). Similar to the pooled results obtained from the groups of animals sacrificed at each time point, both IL6 and KC/CXCL1 showed dose-dependent increases at the earliest sampling times following 5 Gy TBI in the individual animals. At 12 months after radiation, expression levels of KC/CXCL1 in some of the individual mice again indicated a small increase following both 1 and 5 Gy TBI, although the levels did not reach statistical significance. Importantly, no correlation was observed between early and late cytokine expression levels.
Figure 3.
Scatterplot showing levels of expression of (A) interleukin (IL)6 and (B) keratinocyte chemoattractant (KC) in the plasma of individual, serially bled female C57BL/6J mice following0, 1 or 5 Gy TBI, as measured by multiplex bioassay (n=10-20 animals/dose/time point). Each symbol represents the serial measurements made in the same individual mouse. No correlation was found between the levels of expression assessed at acute (≤24 hours) and late (≥9 months) time points.
Image analysis of inflammatory cells postirradiation
Image analysis of the inflammatory cells, neutrophils, macrophages, and CD3+ T-lymphocytes, at the late time points indicated that, at 9 months after radiation, there was a small accumulation of CD3+ T-lymphocytes at the highest dose level (10 Gy). This trend continued at later time points, with increasing numbers of lymphocytes being seen. The outcomes of interest were measured at 12 months on the T-lymphocytes, with the sum of cell counts, area of interest, and mean count per unit area being of potential interest. The correlation between each measurement pair was greater than 0.99, so only the mean count per unit area was considered. On the untransformed scale, linearity between dose and outcome was evident. However, the variance of the outcome increased as the dose increased, violating one of the regression assumptions. Therefore, a weighted least squares regression was used to estimate the slope, and the weights were taken to be proportional to the inverse empirical variance. The estimated slope was 38.6 (P = 0.00001), confirming a significant dose response effect. Figure 4 shows the relationship between the outcome and dose.
Figure 4.
Scatterplot of square-root (mean cell count) of CD3-positive (T cell) versus dose at 12 months following TBI, with a superimposed line from locally-weighted polynomial regressions (n=3/group) indicating a dose-related increase in T cell accumulation.
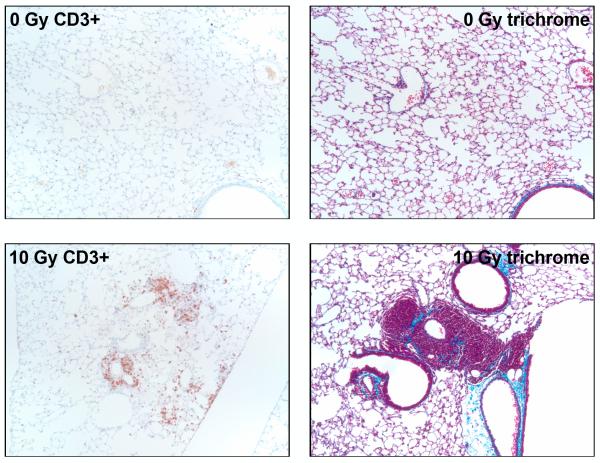
Histological examination indicated that the infiltrating cells were found in focal clusters, as well as cuffing airways and blood vessels (Figure 5). In addition, trichrome stained sections suggested that the focal clusters of lymphocytes were closely associated with increasing deposition of collagen (Figure 5). However, time points later than 12 months did not demonstrate any further changes in pathophysiology and the inflammation was not associated with changes in respiration, as determined by breathing rates measured by plethysmograph (data not shown). In those animals that had been serially bled, no correlation was seen between early increases in cytokine expression and any late pathological changes.
Figure 5.
Representative light micrographs of lung tissue from C57BL/6J mice at 12 months following TBI showing sections that have been stained for either CD3 (T cells) or trichrome (blue color = collagen). The micrographs showing CD3+ cells (brown stain) illustrate the focal accumulation of T-lymphocytes, frequently adjacent to airways or blood vessels. The trichrome-stained sections suggest the onset of fibrosis, with increased levels of collagen in areas of tissue remodeling and inflammation. Original magnification is 10x.
Analysis of tissue mRNA postirradiation
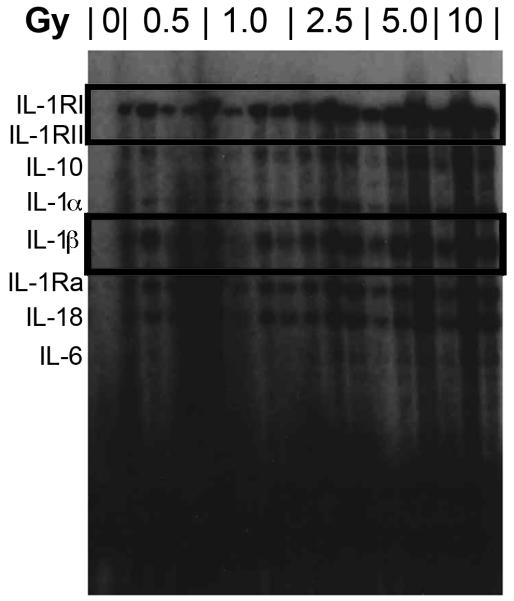
Using the ribonuclease protection assay (RPA), we had previously demonstrated increases in the mRNA abundance of IL1B and IL1R2 during the acute period after irradiation (Johnston et al. 2010); message induction peaked at six hours before returning to near baseline levels by 1 day. In the present study, we continued to analyze message levels of the interleukin family members out to 18 months. Levels for the interleukin family were unaltered from those of controls at 1, 3, and 6 months following irradiation; however, at 9 months, IL1B and IL1R2 message abundance appeared to be induced in a dose-dependent manner (Figure 6). However, although increased levels continued to persist through 18 months, this was a qualitative observation only since message levels in the controls were at background level, precluding normalization of the data.
Figure 6.
RNase protection assay illustrating alterations in interleukin mRNA expression, notably increases in IL1RI and IL1ß message at 9 months following TBI.
Analysis of Pulmonary Inflammatory Cell Recruitment and Cytokine Expression in Peripheral Blood Following Secondary Challenge (LPS)
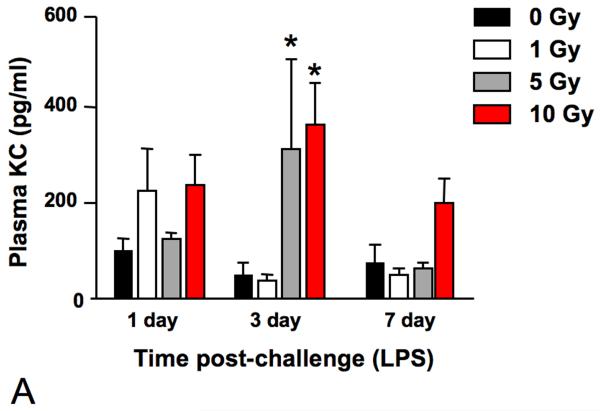
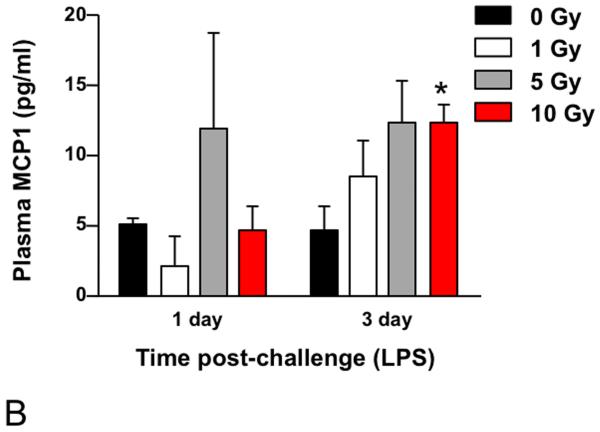
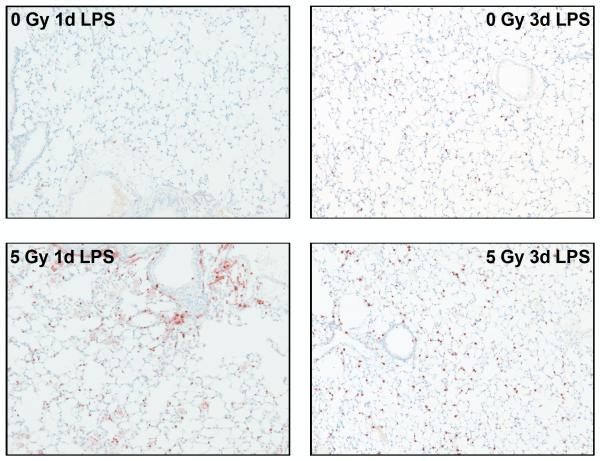
In order to assess the competence of the irradiated lungs at late time points, at both 12 and 15 months following irradiation, some of the surviving animals were exposed to LPS inhalation and sacrificed 1, 3, or 7 days after exposure as a test of the innate immune system. Tissues were analyzed for inflammatory cell infiltration and, in addition, analysis of chemokine levels in serum was performed using multiplex bead technology. Elevated serum levels were detected for KC/CXCL1 (Figure 7A) and MCP1 (Figure 7B). KC/CXCL1 is a chemoattractant for neutrophils and this finding correlated with the observation of greater numbers of polymorphonuclear leukocytes (PMN) cells in the alveolar tissue compared to the 0 Gy age-matched controls (Figure 8), as well as prolonged resolution in the 10 Gy irradiated group. Similarly, MCP1 is a chemoattractant for macrophages and an increased number of macrophages were indeed seen in the lungs of the irradiated groups relative to the 0 Gy age-matched controls (Figure 8).
Figure 7.
Analysis using multiplex bioassay of the expression of (A) KC/CXCL1 and (B) MCP1 in the plasma of female C57BL/6J following LPS inhalation, administered at 12 months or 15 months, respectively, after 0, 1, 5, or 10 Gy TBI. A significantly more robust response was seen with respect to both chemokines relative to age-matched controls at all dose levels. The 10 Gy animals demonstrated a delayed peak in response (3 days versus 1 day after LPS challenge) for both cytokines and expression levels of KC remained elevated in the 10 Gy group until >7 days after challenge. Data are expressed as mean ± SEM and were analyzed using a two-factor analysis of variance and Bonferroni post tests (*p < 0.05 vs. same day 0 Gy controls; n=3/group).
Figure 8.
Representative micrographs of macrophage and polymorphonuclear leukocyte (PMN) recruitment into mouse lungs at 1 and 3 days, respectively, after LPS inhalation given 15 months following 5 Gy TBI. Macrophages (left) were targeted using an antibody toward the F4/80 antigen and PMNs (right) were stained using a rat anti-mouse probe; both were detected with DAB chromagen. Micrographs show an increased number of macrophages present in the 5 Gy group relative to age-matched controls at 1 day after LPS challenge and a persistent PMN response at 3 days. Positively stained cells are brown. Original images were taken at 10x.
Discussion
Pulmonary pneumonitis and fibrosis represent the classically recognized endpoints of acute and late lung response, respectively, to clinically-relevant radiation doses and the risk of their development imposes significant dose limitations on thoracic radiotherapy regimens as part of cancer treatment. As a result, researchers have tried for decades to dissect the mechanisms that underlie the initiation and progression of these events in the hope of identifying potential targets for prevention and mitigation, with the majority of this work making use of rodent (mainly murine) models that have been subjected to localized high dose, single exposures followed by tissue analysis of the subsequent molecular, biochemical, and pathologic changes (Rubin et al. 1992, Hong et al. 2003, Dagle & Sanders 1984, Calveley et al. 2010). A consistent finding in such studies has been a chronically dysregulated lung environment, characterized by cyclical “waves” of cytokine expression in the irradiated tissue, principally proinflammatory in nature; these “waves” have been shown to appear at the time of injury, persist through the initiation of pneumonitis and continuing until the appearance of tissue remodeling and fibrosis. This so-called “perpetual cascade” of cytokines has been hypothesized by some to be a critical factor in the progression to normal tissue late effects (Rubin et al. 1995).
Interestingly, such studies would predict that, following radiation injury delivered in a dose range of ≤10 Gy, a pulmonary late effect is unlikely to occur in a murine model since it is widely accepted that a dose threshold of ~12 Gy needs to be exceeded for pneumonitis to occur (Sharplin & Franko 1989, Travis et al. 1980, Hong et al. 2003, Down & Yanch 2010). Thus, when recent work from our group indicated that TBI doses that are “sub-threshold” for pneumonitis induced significant increases in cytokine expression during the immediate period following irradiation (Johnston et al. 2010), we were led to propose two possible hypotheses for the development of lung injury as a component of TBI: firstly, that earlier preclinical studies had not made use of a sufficiently long follow-up period and that exposing the lungs to a “sub-threshold” dose range would, indeed, result in the same classic pulmonary endpoints, but with a previously unrecognized temporal delay; the alternative hypothesis was that low dose irradiation does not, in fact, induce an overt pathophysiologic endpoint, but may, instead, result in a sub-clinically injured environment, damage that could be uncovered if the lung is subjected to a subsequent injury or challenge.
Of the two hypotheses, the first would imply that TBI, per se, would have little influence over any observed pulmonary response since, whether the irradiation injury is localized or systemic (total body), the lung tissue itself would experience the same dose. Further, if temporal delay is the only consequence of a lower initial injury, then one would anticipate continuing to see the cyclical periods of dysregulated cytokine expression, albeit at a likely less robust level. Such a result was not evident from our studies since, beyond 24 hours after irradiation, no further changes in expression were seen in any of the cytokines analyzed at any of the time points until ≥9 months later (Figures 2a and 2b), and when increased expression did occur, upregulation did not attain equivalent levels to those previously measured following higher doses (Johnston et al. 1996, Johnston et al. 1998b).
Intuitively, the early presence of such cytokines as IL6 and KC/CXCL1 in the plasma may simply have reflected a normal canonical response to the TBI-induced systemic injury. For example, in the lung following doses of >12 Gy, IL6, an early response proinflammatory cytokine which influences antigen-specific immune responses through the activation of T cells, has been shown to be rapidly upregulated in the bronchiolar epithelium in the hours following radiation damage and then, subsequently, during the onset of pneumonitis (Rube et al. 2005). The failure to demonstrate any increases in IL6 expression beyond the immediate first few hours (Figure 2A), when it was seen in the circulation only, supports its role in a systemic, rather than lung-specific, response. In contrast, KC/CXCL1, an indirect homologue to interleukin (IL)-8, is involved in neutrophil recruitment to sites of inflammation (Hol et al. 2009) and we recently demonstrated the association between KC expression in plasma and neutrophilic infiltration into the lung during the immediate recovery period following irradiation (Johnston et al. 2010). Although the late (≥9 months) increases in KC expression seen in the previously irradiated mice failed to reach statistical significance (Figure 2B) and were not associated with pulmonary PMN infiltration, nonetheless these data, taken together with the elevated levels of IL1B in the pulmonary tissue (Figure 6) and the airway-associated clusters of T cells (Figure 5), suggests that they may indeed be signaling the presence of ongoing damage.
However, if one makes the assumption that inflammation plays a role in pulmonary late effect progression, then, with respect to the second hypothesis, i.e. a cryptic injury that is revealed by a subsequent challenge, systemic injury involving the bone marrow could indeed affect the inherent inflammatory response and thereby exacerbate pulmonary outcome. Certainly, some investigators previously have suggested that the observed recurring fluctuations in cytokine expression are due to disruptions in communication between responding infiltrating cells and the damaged cells in the irradiated microenvironment, resulting in compromise of the immune response (Schaue & McBride 2010). In addition, the clinical outcomes from events such as Chernobyl, Tokai-mura, and others would suggest that, when the lung is injured as part of a whole body exposure, the level of pulmonary toxicity expressed are exacerbated by the systemic injury (Goans & Wald 2005, Vlasov & Kvacheva 1996, Svendsen et al. 2010, Uozaki et al. 2005). This observation is supported by data from the bone marrow transplant literature, where acute respiratory distress syndrome/late pneumonitis are relatively common, and frequently lethal, outcomes (Cooke 2005, Ortonne et al. 2001).
Nonetheless, since significant changes were not seen in cytokine expression other than in the immediate phase, does this mean that a chronically dysregulated environment was not present? Although earlier results from our own group have suggested that it is the “perpetual cascade” of cytokines and chemokines that is the critical element in the development of radiation pneumonitis and, ultimately, late radiation fibrosis (Rubin et al. 1995, Johnston et al. 1996), other investigators have suggested that chronic dysregulation may be due to other mechanisms, such as the maintenance of damage within the mitochondria, leading to an activated inflammatory site as a result of chronic free radical production (Epperly et al. 2003, Kanai et al. 2007, Zhao & Robbins 2009). Therefore, observation of persistent periods of increased cytokine expression may not be a requirement for confirmation of the presence of ongoing pulmonary damage.
If the possibility of cryptic damage in the lung is accepted, then this provides support for the concept of pulmonary injury being manifested as a result of combined or multiple injury. Furthermore, if unrealized or hidden damage in the lung is a component of TBI, then systemic damage to the immune and inflammatory systems also is likely to play a role. Indeed, a limited number of studies have demonstrated that ionizing radiation can impact the pulmonary immune response. For example, Ishizaka et al. has shown E.Coli-induced acute lung injury in guinea pigs was attenuated by 12 Gy whole body X-irradiation three days prior to infection (Ishizaka et al. 2000), whereas 3 Gy whole body gamma irradiation, 10 days prior to LPS exposure, exacerbated LPS-induced weight loss, but attenuated LPS-induced increases in spleen mass (Gridley et al. 2007). Radiation-induced decreases in alveolar macrophages and white blood cell counts were observed in both studies and the attenuated responses were attributed to this observation (Ishizaka et al. 2000, Gridley et al. 2007). Although we are not aware of other groups looking at greater periods after irradiation, earlier studies from our own group have demonstrated the longevity of immune dysregulation, describing a several fold increase in lavageable protein in mice that had received LPS via inhalation 24 weeks after a 15 Gy dose of thoracic irradiation (Johnston et al. 2004), a result that was accompanied by prolonged chemokine expression. Our current data further suggests that radiation injury prolongs resolution to secondary challenges following doses that induce little evidence of overt alterations in the inflammatory or cytokine/chemokine expression status. Therefore, despite older studies that suggested a dose-related delay in recovery following single dose irradiation of pulmonary normal tissue (Terry et al. 1988), these findings underscore our overall lack of a clear understanding of the mechanisms underlying the lung’s long-term response to radiation damage and support the caution that should be taken when prescribing re-irradiation as part of cancer treatment (Bourgier et al. 2010).
It is worth noting here that, although the doses investigated were below the single dose threshold for pneumonitis induction in rodent models, a recent review has indicated a great deal of concurrence between species, including humans (Hopewell et al. 2000), with respect to this threshold. Therefore, the finding that irradiated animals as late as 12 months after injury had an increased and prolonged response to LPS challenge (Figures 7 and 8) raises concerns should a radiological or nuclear event occur involving mass casualties. The data suggest that the vast majority of survivors, who will have received relatively low doses of radiation (≤1 Gy) and will exhibit little in the way of readily-apparent acute or late consequences, nonetheless may be at risk of being unable to raise a fully effective defense against later challenge, e.g. against an influenza outbreak, requiring greater follow-up than may currently be considered. Unfortunately, the failure to show any correlation between the acute cytokine response to radiation injury and the late elevations in cytokine expression and/or pathophysiologic changes, such as the T cell accumulations seen at ≥9 months (Figures 3A and 3B), implies that early cytokine indicators may not be used as surrogate biomarkers of late effects and do not currently allow us to identify those at risk. Nonetheless, we are continuing in our studies to assess the competency of the lung to respond to challenges against both the innate and adaptive immune systems and hope to identify countermeasures that may be used to mitigate such effects.
Acknowledgements
We would like to thank Dr. Bruce Fenton and Mr. Scott Paoni for their assistance with the image analysis and Ms. Amy Huser for her editorial and research assistance with this manuscript.
This work was supported by both the Centers for Medical Countermeasures against Radiation Program, National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) U19-AI067733 and U19-AI091036, and National Institute of Environmental Health Sciences (NIEHS)/Environmental Health Sciences (EHS) P30 ES01247.
References
- Aikawa N. Cytokine storm in the pathogenesis of multiple organ dysfunction syndrome associated with surgical insults. Journal of the Japanese Surgical Society. 1996;97:771–777. [PubMed] [Google Scholar]
- Akashi M. Role of infection and bleeding in multiple organ involvement and failure. British Journal of Radiology (Suppl) 2005;27:69–74. [Google Scholar]
- Anno GH, Young RW, Bloom RM, Mercier JR. Dose response relationships after acute ionizing-radiation lethality. Health Physics. 2003;84:565–575. doi: 10.1097/00004032-200305000-00001. [DOI] [PubMed] [Google Scholar]
- Bourgier C, Vozenin M-C, Deutsch E. Reirradiation of normal tissues: preclinical radiobiology data. Cancer Radiotherapie. 2010;14:412–415. doi: 10.1016/j.canrad.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. International Journal of Radiation Biology. 2005;81:887–899. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- Calveley VL, Jelveh S, Langan A, Mahmood J, Yeung IW, Van Dyk J, Hill RP. Genistein can mitigate the effect of radiation on rat lung tissue. Radiation Research. 2010;173:602–611. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CS, Liu WC, Jung SM, Chen FH, Wu CR, McBride WH, Lee CC, Hong JH. Compartmental responses after thoracic irradiation of mice: strain differences. International Journal of Radiation Oncology, Biology, Physics. 2005;62:862–871. doi: 10.1016/j.ijrobp.2005.02.037. [DOI] [PubMed] [Google Scholar]
- Cooke KR. Acute lung injury after allogeneic stem cell transplantation: from the clinic, to the bench and back again. Pediatric Transplant. 2005;9((Suppl)7):25–36. doi: 10.1111/j.1399-3046.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- Dagle GE, Sanders CL. Radionuclide injury to the lung. Environmental Health Perspectives. 1984;55:129–137. doi: 10.1289/ehp.8455129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Down JD, Yanch JC. Identifying the high radiosensitivity of the lungs of C57L mice in a model of total-body irradiation and bone marrow transplantation. Radiation Research. 2010;174:258–263. doi: 10.1667/RR2149.1. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Gretton JE, Sikora CA, Jefferson M, Bernarding M, Nie S, Greenberger JS. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiation Research. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- Finkelstein JN, Johnston C, Barrett T, Oberdorster G. Particulate-cell interactions and pulmonary cytokine expression. Environmental Health Perspectives. 1997;105((Suppl) 5):1179–1182. doi: 10.1289/ehp.97105s51179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale RP. Immediate medical consequences of nuclear accidents. Lessons from Chernobyl. Journal of the American Medical Association. 1987;258:625–628. [PubMed] [Google Scholar]
- Goans RE, Wald N. Radiation accidents with multi-organ failure in the United States. British Journal of Radiology. 2005;27(Suppl):41–46. [Google Scholar]
- Gridley DS, Miller GM, Pecaut MJ. Radiation and primary response to lipopolysaccharide: bone marrow-derived cells and susceptible organs. In Vivo. 2007;21:453–461. [PubMed] [Google Scholar]
- Hirama T, Akashi M. Multi-organ involvement in the patient who survived the Tokai-mura criticality accident. British Journal of Radiology. 2005;27(Suppl):17–20. [Google Scholar]
- Hong JH, Jung SM, Tsao TC, Wu CJ, Lee CY, Chen FH, Hsu CH, McBride WH, Chiang CS. Bronchoalveolar lavage and interstitial cells have different roles in radiation-induced lung injury. International Journal of Radiation Biology. 2003;79:159–167. doi: 10.1080/0955300031000076894. [DOI] [PubMed] [Google Scholar]
- Hol J, Wilhelmsen L, Haraldsen G. The murine IL-8 homologues KC, MIP-2, and LIX are found in endothelial cytoplasmic granules but not in Weibel-Palade bodies. Journal of Leukocyte Biology. 2010;87:501–508. doi: 10.1189/jlb.0809532. [DOI] [PubMed] [Google Scholar]
- Hopewell JW, Rezvani M, Moustafa HF. The pig as a model for the study of radiation effects on the lung. International Journal of Radiation Biology. 2000;76:447–452. doi: 10.1080/095530000138439. [DOI] [PubMed] [Google Scholar]
- Ishizaka A, Sayama K, Hasegawa N, Fujita H, Asano K, Kanazawa M, Kubo A. Attenuation of live E. coli-induced acute lung injury by X-ray irradiation in guinea pigs. Lung. 2000;178:331–340. doi: 10.1007/s004080000036. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Piedboeuf B, Rubin P, Williams JP, Baggs R, Finkelstein JN. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiation Research. 1996;145:762–767. [PubMed] [Google Scholar]
- Johnston CJ, Finkelstein JN, Gelein R, Oberdörster G. Pulmonary cytokine and chemokine mRNA levels after inhalation of lipopolysaccharide in C57BL/6 mice. Toxicological Sciences. 1998a;46:300–307. doi: 10.1006/toxs.1998.2557. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Wright TW, Rubin P, Finkelstein JN. Alterations in the expression of chemokine mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Experimental Lung Research. 1998b;24:321–337. doi: 10.3109/01902149809041538. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Williams JP, Okunieff P, Finkelstein JN. Radiation-induced pulmonary fibrosis: examination of chemokine and chemokine receptor families. Radiation Research. 2002;157:256–265. doi: 10.1667/0033-7587(2002)157[0256:ripfeo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Williams JP, Elder ACP, Hernady E, Finkelstein JN. Inflammatory cell recruitment following thoracic irradiation. Experimental Lung Research. 2004;30:369–382. doi: 10.1080/01902140490438915. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Hernady E, Reed C, Thurston SW, Finkelstein JN, Williams JP. Early alterations in cytokine expression in adult compared to developing lung in mice after radiation exposure. Radiation Research. 2010;173:522–535. doi: 10.1667/RR1882.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai A, Zabbarova I, Amoscato A, Epperly M, Xiao J, Wipf P. Mitochondrial targeting of radioprotectants using peptidyl conjugates. Organic Biomolelcular Chemistry. 2007;5:307–309. doi: 10.1039/b613334g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konchalovsky MV, Baranov AE, Kolganov AV. Multiple organ involvement and failure: selected Russian radiation accident cases re-visited. British Journal of Radiology. 2005;27(Suppl):26–29. [Google Scholar]
- McBride WH, Chiang C-S, Olson JL, Wang CC, Hong JH, Pajonk F, Dougherty GJ, Iwamoto KS, Pervan M, Liao YP. A sense of danger from radiation. Radiation Research. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- Meineke V, Fliedner TM. Radiation-induced multi-organ invovlement and failure: challenges for radiation accident medical management and future resarch. British Journal of Radiology. 2005;27(Suppl):196–200. [Google Scholar]
- Mizock BA. The multiple organ dysfunction syndrome. Disease-A-Month. 55:476–526. doi: 10.1016/j.disamonth.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Monti P, Wysocki J, Van der Meeren A, Griffiths NM. The contribution of radiation-induced injury to the gastrointestinal tract in the development of multi-organ dysfunction syndrome or failure. British Journal of Radiology. 2005;27(Suppl):89–94. [Google Scholar]
- Olman MA, White KE, Ware LB, Cross MT, Zhu S, Matthay MA. Microarray analysis indicates that pulmonary edema fluid from patients with acute lung injury mediates inflammation, mitogen gene expression, and fibroblast proliferation through bioactive interleukin-1. Chest. 2002;121(Suppl 3):69S–70S. doi: 10.1378/chest.121.3_suppl.69s. [DOI] [PubMed] [Google Scholar]
- Ortonne N, Ribaud P, Meignin V, Sarfati C, Esperou H, Devergie A, Gluckman E, Socie G, Janin A. Toxoplasmic pneumonitis leading to fatal acute respiratory distress syndrome after engraftment in three bone marrow transplant recipients. Transplantation. 2001;72:1838–1840. doi: 10.1097/00007890-200112150-00022. [DOI] [PubMed] [Google Scholar]
- Pinsky MR. Organ-specific therapy in critical illness: interfacing molecular mechanisms with physiological interventions. Journal of Critical Care. 1996;11:95–107. doi: 10.1016/s0883-9441(96)90024-6. [DOI] [PubMed] [Google Scholar]
- Porter DW, Ye J, Ma J, Barger M, Robinson VA, Ramsey D, McLaurin J, Khan A, Landsittel D, Teass A, Castranova V. Time course of pulmonary response of rats to inhalation of crystalline silica: NF-kappa B activation, inflammation, cytokine production, and damage. Inhalation Toxicology. 2002;14:349–367. doi: 10.1080/08958370252870998. [DOI] [PubMed] [Google Scholar]
- Rübe CE, Uthe D, Wilfert W, Ludwig D, Yang K, König J, Palm J, Schuck A, Willich N, Remberger K, Rübe C. The bronchiolar epithelium as a prominent source of proinflammatory cytokines after lung irradiation. Internaional Journal of Radiation Oncology, Biology, Physics. 2005;61:1482–1492. doi: 10.1016/j.ijrobp.2004.12.072. [DOI] [PubMed] [Google Scholar]
- Rubin P, Finkelstein J, Shapiro D. Molecular biology mechanisms in the radiation induction of pulmonary injury syndromes: interrelationship between the alveolar macrophage and the septal fibroblast. International Journal of Radiation Oncology, Biology, Physics. 1992;24:93–101. doi: 10.1016/0360-3016(92)91027-k. [DOI] [PubMed] [Google Scholar]
- Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. International Journal of Radiation Oncology, Biology, Physics. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- Schaue D, McBride WH. Linkes between innate immunity and normal tissue radiobiology. Radiation Research. 2010;173:406–417. doi: 10.1667/RR1931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick JB, Menon I, Gern JE, Busse WW. Effects of inflammatory cytokines on the permeability of human lung microvascular endothelial cell monolayers and differential eosinophil transmigration. Journal of Allergy and Clinical Immunology. 2002;110:752–756. doi: 10.1067/mai.2002.128581. [DOI] [PubMed] [Google Scholar]
- Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the intermediate and late phases. Radiation Research. 1989;119:15–31. [PubMed] [Google Scholar]
- Svendsen ER, Kolpakov IE, Stepanova YI, Vdovenko VY, Naboka MV, Mousseau TA, Mohr LC, Hoel DG, Karmaus WJ. 137Cesium exposure and spirometry measures in Ukrainian children affected by the Chernobyl nuclear incident. Environmental Health Perspectives. 2010;118:720–725. doi: 10.1289/ehp.0901412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry NH, Tucker SL, Travis EL. Residual radiation damage in murine lung assessed by pneumonitis. International Journal of Radiation Oncology, Biology, Physics. 1988;14:929–938. doi: 10.1016/0360-3016(88)90015-6. [DOI] [PubMed] [Google Scholar]
- Travis EL, Down JD, Holmes SJ, Hobson B. Radiation pneumonitis and fibrosis in mouse lung assayed by respiratory frequency and histology. Radiation Research. 1980;84:133–143. [PubMed] [Google Scholar]
- Uozaki H, Fukayama M, Nakagawa K, Ishikawa T, Misawa S, Doi M, Maekawa K. The pathology of multi-organ involvement: two autopsy cases from the Tokai-mura criticality accident. British Journal of Radiology. 2005;27(Suppl):13–16. [Google Scholar]
- Vlasov PA, Kvacheva IE. The pathomorphology of the pulmonary infectious complications in acute radiation sickness (based on the autopsy data from persons who died as a result of the accident at the Chernobyl Atomic Electric Power Station. Terapevticheskii Arkhiv. 1996;68:23–26. [PubMed] [Google Scholar]
- Williams BJ, Dehnbostel J, Blackwell TS. Pseudomonas aeruginosa: host defence in lung diseases. Respirology. 2010;15:1037–1056. doi: 10.1111/j.1440-1843.2010.01819.x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Current Medicinal Chemistry. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]