Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back D. W., Wilson S. B., Morris S. M., Jr, Goodridge A. G. Hormonal regulation of lipogenic enzymes in chick embryo hepatocytes in culture. Thyroid hormone and glucagon regulate malic enzyme mRNA level at post-transcriptional steps. J Biol Chem. 1986 Sep 25;261(27):12555–12561. [PubMed] [Google Scholar]
- Bodner M., Karin M. A pituitary-specific trans-acting factor can stimulate transcription from the growth hormone promoter in extracts of nonexpressing cells. Cell. 1987 Jul 17;50(2):267–275. doi: 10.1016/0092-8674(87)90222-4. [DOI] [PubMed] [Google Scholar]
- Buchanan J., Tapley D. F. Stimulation by thyroxine of amino acid incorporation into mitochondria. Endocrinology. 1966 Jul;79(1):81–89. doi: 10.1210/endo-79-1-81. [DOI] [PubMed] [Google Scholar]
- Buetti E., Kühnel B. Distinct sequence elements involved in the glucocorticoid regulation of the mouse mammary tumor virus promoter identified by linker scanning mutagenesis. J Mol Biol. 1986 Aug 5;190(3):379–389. doi: 10.1016/0022-2836(86)90009-4. [DOI] [PubMed] [Google Scholar]
- Carr F. E., Jump D. B., Oppenheimer J. H. Distribution of thyroid hormone-responsive translated products in rat liver polysome and postribosomal ribonucleoprotein populations. Endocrinology. 1984 Nov;115(5):1737–1745. doi: 10.1210/endo-115-5-1737. [DOI] [PubMed] [Google Scholar]
- Carr F. E., Need L. R., Chin W. W. Isolation and characterization of the rat thyrotropin beta-subunit gene. Differential regulation of two transcriptional start sites by thyroid hormone. J Biol Chem. 1987 Jan 25;262(3):981–987. [PubMed] [Google Scholar]
- Casanova J., Copp R. P., Janocko L., Samuels H. H. 5'-Flanking DNA of the rat growth hormone gene mediates regulated expression by thyroid hormone. J Biol Chem. 1985 Sep 25;260(21):11744–11748. [PubMed] [Google Scholar]
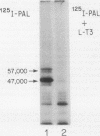
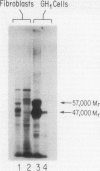
- Casanova J., Horowitz Z. D., Copp R. P., McIntyre W. R., Pascual A., Samuels H. H. Photoaffinity labeling of thyroid hormone nuclear receptors. Influence of n-butyrate and analysis of the half-lives of the 57,000 and 47,000 molecular weight receptor forms. J Biol Chem. 1984 Oct 10;259(19):12084–12091. [PubMed] [Google Scholar]
- Catanzaro D. F., West B. L., Baxter J. D., Reudelhuber T. L. A pituitary-specific factor interacts with an upstream promotor element in the rat growth hormone gene. Mol Endocrinol. 1987 Jan;1(1):90–96. doi: 10.1210/mend-1-1-90. [DOI] [PubMed] [Google Scholar]
- Conneely O. M., Sullivan W. P., Toft D. O., Birnbaumer M., Cook R. G., Maxwell B. L., Zarucki-Schulz T., Greene G. L., Schrader W. T., O'Malley B. W. Molecular cloning of the chicken progesterone receptor. Science. 1986 Aug 15;233(4765):767–770. doi: 10.1126/science.2426779. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Riegel A. T., Hager G. L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987 Jan 30;48(2):261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- Crew M. D., Spindler S. R. Thyroid hormone regulation of the transfected rat growth hormone promoter. J Biol Chem. 1986 Apr 15;261(11):5018–5022. [PubMed] [Google Scholar]
- Damm K., Beug H., Graf T., Vennström B. A single point mutation in erbA restores the erythroid transforming potential of a mutant avian erythroblastosis virus (AEV) defective in both erbA and erbB oncogenes. EMBO J. 1987 Feb;6(2):375–382. doi: 10.1002/j.1460-2075.1987.tb04765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton A. I., Selden J. R., Laws G., Dorney D. J., Finan J., Tripputi P., Emanuel B. S., Rovera G., Nowell P. C., Croce C. M. A human c-erbA oncogene homologue is closely proximal to the chromosome 17 breakpoint in acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4495–4499. doi: 10.1073/pnas.81.14.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillmann W. H., Barrieux A., Reese G. S. Effect of diabetes and hypothyroidism on the predominance of cardiac myosin heavy chains synthesized in vivo or in a cell-free system. J Biol Chem. 1984 Feb 25;259(4):2035–2038. [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Birnberg N. C., Rosenfeld M. G. Glucocorticoid and thyroid hormones transcriptionally regulate growth hormone gene expression. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7659–7663. doi: 10.1073/pnas.79.24.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett A. W., Clark W. A., Chizzonite R. A., Zak R. Change in synthesis rates of alpha- and beta-myosin heavy chains in rabbit heart after treatment with thyroid hormone. J Biol Chem. 1983 Feb 25;258(4):2421–2425. [PubMed] [Google Scholar]
- Flug F., Copp R. P., Casanova J., Horowitz Z. D., Janocko L., Plotnick M., Samuels H. H. cis-acting elements of the rat growth hormone gene which mediate basal and regulated expression by thyroid hormone. J Biol Chem. 1987 May 5;262(13):6373–6382. [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- GROSS J., PITT-RIVERS R. 3:5:3' -triiodothyronine. 1. Isolation from thyroid gland and synthesis. Biochem J. 1953 Mar;53(4):645–650. doi: 10.1042/bj0530645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., PITT-RIVERS R. 3:5:3'-triiodothyronine. 2. Physiological activity. Biochem J. 1953 Mar;53(4):652–657. doi: 10.1042/bj0530652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., PITT-RIVERS R. The identification of 3:5:3'-L-triiodothyronine in human plasma. Lancet. 1952 Mar 1;1(6705):439–441. doi: 10.1016/s0140-6736(52)91952-1. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Franco R., Weinberger C., Albert V. R., Evans R. M., Rosenfeld M. G. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature. 1987 Oct 22;329(6141):738–741. doi: 10.1038/329738a0. [DOI] [PubMed] [Google Scholar]
- Goodwin R. G., Rottman F. M., Callaghan T., Kung H. J., Maroney P. A., Nilsen T. W. c-erbB activation in avian leukosis virus-induced erythroblastosis: multiple epidermal growth factor receptor mRNAs are generated by alternative RNA processing. Mol Cell Biol. 1986 Sep;6(9):3128–3133. doi: 10.1128/mcb.6.9.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Beug H. Role of the v-erbA and v-erbB oncogenes of avian erythroblastosis virus in erythroid cell transformation. Cell. 1983 Aug;34(1):7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Gruol D. J. 3,5,3'-triiodothyronine receptor-containing chromatin fragments: production by nuclease digestion. Endocrinology. 1980 Oct;107(4):994–999. doi: 10.1210/endo-107-4-994. [DOI] [PubMed] [Google Scholar]
- Harington C. R., Barger G. Chemistry of Thyroxine: Constitution and Synthesis of Thyroxine. Biochem J. 1927;21(1):169–183. doi: 10.1042/bj0210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harington C. R. Chemistry of Thyroxine: Isolation of Thyroxine from the Thyroid Gland. Biochem J. 1926;20(2):293–299. doi: 10.1042/bj0200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Hervas F., Morreale de Escobar G., Escobar Del Rey F. Rapid effects of single small doses of L-thyroxine and triiodo-L-thyronine on growth hormone, as studied in the rat by radioimmunoassy. Endocrinology. 1975 Jul;97(1):91–101. doi: 10.1210/endo-97-1-91. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Giguere V., Segui P., Evans R. M. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell. 1987 Apr 10;49(1):39–46. doi: 10.1016/0092-8674(87)90753-7. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Jansson M., Philipson L., Vennström B. Isolation and characterization of multiple human genes homologous to the oncogenes of avian erythroblastosis virus. EMBO J. 1983;2(4):561–565. doi: 10.1002/j.1460-2075.1983.tb01463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch J. M., Krozowski Z., Quirin-Stricker C., Gronemeyer H., Simpson R. J., Garnier J. M., Krust A., Jacob F., Chambon P. Cloning of the chicken progesterone receptor. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5424–5428. doi: 10.1073/pnas.83.15.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump D. B., Seelig S., Schwartz H. L., Oppenheimer J. H. Association of the thyroid hormone receptor with rat liver chromatin. Biochemistry. 1981 Nov 24;20(24):6781–6789. doi: 10.1021/bi00527a007. [DOI] [PubMed] [Google Scholar]
- Karin M., Eberhardt N. L., Mellon S. H., Malich N., Richards R. I., Slater E. P., Barta A., Martial J. A., Baxter J. D., Cathala G. Expression and hormonal regulation of the rat growth hormone gene in transfected mouse L cells. DNA. 1984;3(2):147–155. doi: 10.1089/dna.1984.3.147. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Brent G. A., Warne R. L., Larsen P. R., Moore D. D. Thyroid hormone receptor binds to a site in the rat growth hormone promoter required for induction by thyroid hormone. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5670–5674. doi: 10.1073/pnas.84.16.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner D., Schwartz H. L., Surks M. I., Oppenheimer J. H. Binding of selected iodothyronine analogues to receptor sites of isolated rat hepatic nuclei. High correlation between structural requirements for nuclear binding and biological activity. J Biol Chem. 1975 Aug 25;250(16):6417–6423. [PubMed] [Google Scholar]
- Kourides I. A., Gurr J. A., Wolf O. The regulation and organization of thyroid stimulating hormone genes. Recent Prog Horm Res. 1984;40:79–120. doi: 10.1016/b978-0-12-571140-1.50007-4. [DOI] [PubMed] [Google Scholar]
- Krust A., Green S., Argos P., Kumar V., Walter P., Bornert J. M., Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986 May;5(5):891–897. doi: 10.1002/j.1460-2075.1986.tb04300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumara-Siri M. H., Surks M. I. Regulation of growth hormone mRNA synthesis by 3,5,3'-triiodo-L-thyronine in cultured growth hormone-producing rat pituitary tumor cells (GC cells). Dissociation between nuclear iodothyronine receptor concentration and growth hormone mRNA synthesis during the deoxyribonucleic acid synthesis phase of the cell cycle. J Biol Chem. 1985 Nov 25;260(27):14529–14537. [PubMed] [Google Scholar]
- Larsen P. R., Harney J. W., Moore D. D. Repression mediates cell-type-specific expression of the rat growth hormone gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8283–8287. doi: 10.1073/pnas.83.21.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Harney J. W., Moore D. D. Sequences required for cell-type specific thyroid hormone regulation of rat growth hormone promoter activity. J Biol Chem. 1986 Nov 5;261(31):14373–14376. [PubMed] [Google Scholar]
- Larsen P. R., Silva J. E., Kaplan M. M. Relationships between circulating and intracellular thyroid hormones: physiological and clinical implications. Endocr Rev. 1981 Winter;2(1):87–102. doi: 10.1210/edrv-2-1-87. [DOI] [PubMed] [Google Scholar]
- Latham K. R., Ring J. C., Baxter J. D. Solubilized nuclear "receptors" for thyroid hormones. Physical characteristics and binding properties, evidence for multiple forms. J Biol Chem. 1976 Dec 10;251(23):7388–7397. [PubMed] [Google Scholar]
- Lefevre C., Imagawa M., Dana S., Grindlay J., Bodner M., Karin M. Tissue-specific expression of the human growth hormone gene is conferred in part by the binding of a specific trans-acting factor. EMBO J. 1987 Apr;6(4):971–981. doi: 10.1002/j.1460-2075.1987.tb04847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw C. W., Towle H. C. Characterization of a thyroid hormone-responsive gene from rat. J Biol Chem. 1984 Jun 10;259(11):7253–7260. [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- MacLeod K. M., Baxter J. D. Chromatin receptors for thyroid hormones. Interactions of the solubilized proteins with DNA. J Biol Chem. 1976 Dec 10;251(23):7380–7387. [PubMed] [Google Scholar]
- Magnuson M. A., Dozin B., Nikodem V. M. Regulation of specific rat liver messenger ribonucleic acids by triiodothyronine. J Biol Chem. 1985 May 25;260(10):5906–5912. [PubMed] [Google Scholar]
- Magnuson M. A., Nikodem V. M. Molecular cloning of a cDNA sequence for rat malic enzyme. Direct evidence for induction in vivo of rat liver malic enzyme mRNA by thyroid hormone. J Biol Chem. 1983 Oct 25;258(20):12712–12717. [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Godowski P. J., Maler B. A., Yamamoto K. R. Glucocorticoid receptor mutants that define a small region sufficient for enhancer activation. Science. 1987 Apr 24;236(4800):423–427. doi: 10.1126/science.3563519. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Borgmeyer U., Nowock J. Interaction of the TGGCA-binding protein with upstream sequences is required for efficient transcription of mouse mammary tumor virus. EMBO J. 1987 May;6(5):1355–1360. doi: 10.1002/j.1460-2075.1987.tb02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Crenshaw E. B., 3rd, Franco R., Lira S. A., Albert V. R., Evans R. M., Rosenfeld M. G. Discrete cis-active genomic sequences dictate the pituitary cell type-specific expression of rat prolactin and growth hormone genes. Nature. 1986 Aug 7;322(6079):557–562. doi: 10.1038/322557a0. [DOI] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Koerner D., Schwartz H. L., Surks M. I. Specific nuclear triiodothyronine binding sites in rat liver and kidney. J Clin Endocrinol Metab. 1972 Aug;35(2):330–333. doi: 10.1210/jcem-35-2-330. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Mariash C. N., Kinlaw W. B., Wong N. C., Freake H. C. Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev. 1987 Aug;8(3):288–308. doi: 10.1210/edrv-8-3-288. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Pascual A., Casanova J., Samuels H. H. Photoaffinity labeling of thyroid hormone nuclear receptors in intact cells. J Biol Chem. 1982 Aug 25;257(16):9640–9647. [PubMed] [Google Scholar]
- Perlman A. J., Stanley F., Samuels H. H. Thyroid hormone nuclear receptor. Evidence for multimeric organization in chromatin. J Biol Chem. 1982 Jan 25;257(2):930–938. [PubMed] [Google Scholar]
- Raaka B. M., Samuels H. H. Regulation of thyroid hormone nuclear receptor levels in GH1 cells by 3,5,3'-triiodo-L-thyronine. Use of dense amino acid labeling to determine the influence of hormone on the receptor half-life and the rate of appearance of newly synthesized receptor. J Biol Chem. 1981 Jul 10;256(13):6883–6889. [PubMed] [Google Scholar]
- Raines M. A., Lewis W. G., Crittenden L. B., Kung H. J. c-erbB activation in avian leukosis virus-induced erythroblastosis: clustered integration sites and the arrangement of provirus in the c-erbB alleles. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2287–2291. doi: 10.1073/pnas.82.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Shapiro L. E. Thyroid hormone stimulates de novo growth hormone synthesis in cultured GH1 cells: evidence for the accumulation of a rate limiting RNA species in the induction process. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3369–3373. doi: 10.1073/pnas.73.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Relationship of receptor affinity to the modulation of thyroid hormone nuclear receptor levels and growth hormone synthesis by L-triiodothyronine and iodothyronine analogues in cultured GH1 cells. J Clin Invest. 1979 Jun;63(6):1229–1240. doi: 10.1172/JCI109418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Control of growth hormone synthesis in cultured GH1 cells by 3,5,3'-triiodo-L-thyronine and glucocorticoid agonists and antagonists: studies on the independent and synergistic regulation of the growth hormone response. Biochemistry. 1979 Feb 20;18(4):715–721. doi: 10.1021/bi00571a025. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Dose-dependent depletion of nuclear receptors by L-triiodothyronine: evidence for a role in induction of growth hormone synthesis in cultured GH1 cells. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3877–3881. doi: 10.1073/pnas.73.11.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Shapiro L. E. Modulation of thyroid hormone nuclear receptor levels by 3,5,3'-triiodo-L-thyronine in GH1 cells. Evidence for two functional components of nuclear-bound receptor and relationship to the induction of growth hormone synthesis. J Biol Chem. 1977 Sep 10;252(17):6052–6060. [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S., Casanova J., Stanley F. Thyroid hormone action: in vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. J Clin Invest. 1974 Oct;54(4):853–865. doi: 10.1172/JCI107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3488–3492. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action. Demonstration of similar receptors in isolated nuclei of rat liver and cultured GH1 cells. J Clin Invest. 1974 Feb;53(2):656–659. doi: 10.1172/JCI107601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Schadlow A. R., Surks M. I., Schwartz H. L., Oppenheimer J. H. Specific triiodothyronine binding sites in the anterior pituitary of the rat. Science. 1972 Jun 16;176(4040):1252–1254. doi: 10.1126/science.176.4040.1252. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Oppenheimer J. H. Nuclear triiodothyronine receptor sites in brain: probable identity with hepatic receptors and regional distribution. Endocrinology. 1978 Jul;103(1):267–273. doi: 10.1210/endo-103-1-267. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Trence D., Oppenheimer J. H., Jiang N. S., Jump D. B. Distribution and metabolism of L- and D-triiodothyronine (T3) in the rat: preferential accumulation of L-T3 by hepatic and cardiac nuclei as a probable explanation of the differential biological potency of T3 enantiomers. Endocrinology. 1983 Oct;113(4):1236–1243. doi: 10.1210/endo-113-4-1236. [DOI] [PubMed] [Google Scholar]
- Seo H., Vassart G., Brocas H., Refetoff S. Triiodothyronine stimulates specifically growth hormone mRNA in rat pituitary tumor cells. Proc Natl Acad Sci U S A. 1977 May;74(5):2054–2058. doi: 10.1073/pnas.74.5.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. E., Samuels H. H., Yaffe B. M. Thyroid and glucocorticoid hormones synergistically control growth hormone mRNA in cultured GH1 cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):45–49. doi: 10.1073/pnas.75.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupnik M. A., Chin W. W., Habener J. F., Ridgway E. C. Transcriptional regulation of the thyrotropin subunit genes by thyroid hormone. J Biol Chem. 1985 Mar 10;260(5):2900–2903. [PubMed] [Google Scholar]
- Sinha A. M., Umeda P. K., Kavinsky C. J., Rajamanickam C., Hsu H. J., Jakovcic S., Rabinowitz M. Molecular cloning of mRNA sequences for cardiac alpha- and beta-form myosin heavy chains: expression in ventricles of normal, hypothyroid, and thyrotoxic rabbits. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5847–5851. doi: 10.1073/pnas.79.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler B. J., MacLeod K. M., Ring J., Baxter J. D. Thyroid hormone receptors. Binding characteristics and lack of hormonal dependency for nuclear localization. J Biol Chem. 1975 Jun 10;250(11):4113–4119. [PubMed] [Google Scholar]
- Spindler S. R., Mellon S. H., Baxter J. D. Growth hormone gene transcription is regulated by thyroid and glucocorticoid hormones in cultured rat pituitary tumor cells. J Biol Chem. 1982 Oct 10;257(19):11627–11632. [PubMed] [Google Scholar]
- Spurr N. K., Solomon E., Jansson M., Sheer D., Goodfellow P. N., Bodmer W. F., Vennstrom B. Chromosomal localisation of the human homologues to the oncogenes erbA and B. EMBO J. 1984 Jan;3(1):159–163. doi: 10.1002/j.1460-2075.1984.tb01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T. Y., Towle H. C. Coordinate regulation of rat liver genes by thyroid hormone and dietary carbohydrate. Ann N Y Acad Sci. 1986;478:20–30. doi: 10.1111/j.1749-6632.1986.tb15518.x. [DOI] [PubMed] [Google Scholar]
- Tata J. R. The formation and distribution of ribosomes during hormone-induced growth and development. Biochem J. 1967 Jul;104(1):1–16. doi: 10.1042/bj1040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata J. R., Widnell C. C. Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J. 1966 Feb;98(2):604–620. doi: 10.1042/bj0980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. C., Weinberger C., Lebo R., Evans R. M. Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science. 1987 Sep 25;237(4822):1610–1614. doi: 10.1126/science.3629259. [DOI] [PubMed] [Google Scholar]
- Towle H. C., Mariash C. N., Schwartz H. L., Oppenheimer J. H. Quantitation of rat liver messenger ribonucleic acid for malic enzyme during induction by thyroid hormone. Biochemistry. 1981 Jun 9;20(12):3486–3492. doi: 10.1021/bi00515a028. [DOI] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Domain structure of human glucocorticoid receptor and its relationship to the v-erb-A oncogene product. Nature. 1985 Dec 19;318(6047):670–672. doi: 10.1038/318670a0. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- West B. L., Catanzaro D. F., Mellon S. H., Cattini P. A., Baxter J. D., Reudelhuber T. L. Interaction of a tissue-specific factor with an essential rat growth hormone gene promoter element. Mol Cell Biol. 1987 Mar;7(3):1193–1197. doi: 10.1128/mcb.7.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight P. A., Crew M. D., Spindler S. R. Discrete positive and negative thyroid hormone-responsive transcription regulatory elements of the rat growth hormone gene. J Biol Chem. 1987 Apr 25;262(12):5659–5663. [PubMed] [Google Scholar]
- Yaffe B. M., Samuels H. H. Hormonal regulation of the growth hormone gene. Relationship of the rate of transcription to the level of nuclear thyroid hormone-receptor complexes. J Biol Chem. 1984 May 25;259(10):6284–6291. [PubMed] [Google Scholar]
- Ye Z. S., Samuels H. H. Cell- and sequence-specific binding of nuclear proteins to 5'-flanking DNA of the rat growth hormone gene. J Biol Chem. 1987 May 5;262(13):6313–6317. [PubMed] [Google Scholar]