Abstract
The ATP-binding cassette transporters p-glycoprotein and breast cancer resistance protein have been shown to be critical determinants limiting drug transport across the BBB into the brain. Several therapeutic agents have been shown to be substrates for these two transporters, and as a result they have limited distribution to the brain. Recently, it has been shown that these two drug transporters cooperate at the BBB and brain penetration of dual substrates increase significantly only when both are absent, e.g., in the Mdr1a/1b-/-Bcrp1-/- mice. The present study uses the brain penetration of sorafenib to investigate these findings and attempts to explain the mechanistic basis of this cooperation with a simple theory based on affinity and capacity dependent carrier-mediated transport. The brain efflux index method, combined with the organotypic brain slices, were used to determine the net contribution of P-gp and BCRP to the total clearance of sorafenib out of the brain and show that its efflux at the BBB is mediated primarily by BCRP. Sorafenib clearance out of the brain decreased 2-fold in the Bcrp1-/- mice and 2.5-fold in the Mdr1a/1b-/-Bcrp1-/- mice. Clearance out of brain when P-gp was absent did not change significantly compared to wild-type. We also investigated the expression of P-gp and BCRP in the genetic knockout animals and saw no differences in either P-gp or BCRP in the transporter deficient mice compared to the wild-type mice. In conclusion, this study explains the cooperation of P-gp and BCRP by analysis of the efflux clearance of sorafenib and correlating it to the ‘mechanisms’ that determine the clearance, i.e., affinity and capacity.
Keywords: Blood-brain barrier, P-glycoprotein, BCRP, Cooperation, Sorafenib, Efflux, Transporters
Introduction
The difficulty in delivering drugs to the central nervous system (CNS) is considered to be pivotal in the limited success of neurotherapeutics 1. The blood-brain barrier (BBB) is the most significant blood-CNS interface that restricts delivery of therapeutic agents to the brain 2. ATP binding cassette (ABC) transporters, such as P-glycoprotein (P-gp) and the breast cancer resistance protein (BCRP), that prevent the passage of drugs into the brain by pumping them back into the blood, form a vital component of this barrier 3. These two transporters have been extensively studied regarding their role in impeding drug penetration into the brain and it has been shown that both transporters are significant obstacles for delivering drugs to the brain 4.
Recent research has led to a new paradigm suggesting that P-gp and BCRP work as a “cooperative team of gatekeepers” at the BBB. In 2007, de Vries et al. showed that brain uptake of topotecan, a substrate for both P-gp and BCRP, was not increased in mice lacking BCRP (Bcrp1-/-) and increased only slightly in P-gp deficient mice (Mdr1a/1b-/-) 5. In contrast, topotecan brain uptake increased dramatically in mice lacking both P-gp and BCRP (Mdr1a/1b-/-Bcrp1-/-). Thus, absence of both P-gp and BCRP resulted in an effect that was significantly larger than the combined effects from the single transporter knockout mice. A similar phenomenon was reported by Polli et al. for lapatinib in P-gp/BCRP deficient mice 6. We have shown similar findings with other tyrosine kinase inhibitors, including dasatinib 7, gefitinib 8 and sorafenib 9. Even though these drugs are substrates for both P-gp and BCRP, absence of only one of the transporters did not significantly increase delivery of the drugs to the brain, and the greatest enhancement in brain penetration was seen when both transporters were absent at the BBB. Several studies now show that this is true for other dual P-gp and BCRP substrates as well 10-12.
These findings suggest that inhibition of either P-gp or BCRP can be compensated by the other transporter, and that both transporters “cooperate” with each other in preventing drugs from entering the brain13. Kodaira et al. explained this cooperation of P-gp and BCRP by determining the net contribution of each transporter to the overall efflux of various drugs at the BBB 11. The authors concluded that for many dual substrates, P-gp-mediated efflux out of the brain was greater than that by BCRP. However, we recently reported that BCRP is the dominant transporter in the efflux of the tyrosine kinase inhibitor sorafenib at the BBB 9. Therefore, several questions still remain to be answered. Why do some BCRP substrates show no enhancement in their brain penetration in the Bcrp1-/- mice? Is the compensatory mechanism a result of changes in expression of other transporters in the genetic knockout mice? If so, changes in transporter-mediated active clearance can explain some of the findings in the transporter deficient mice.
The objective of this study was to examine the cooperation of P-gp and BCRP in an experimental paradigm that would further explain the findings in the Mdr1a/b-/-, Bcrp1-/- and the combined Mdr1a/b-/-Bcrp1-/- mice. We use the brain efflux index method to determine the kinetics of sorafenib efflux out of the brain. We have previously demonstrated that P-gp and BCRP together limit the brain distribution of sorafenib with BCRP being the dominant transporter 9. In the current study, we determine the relative contributions of P-gp- and BCRP-mediated efflux to the total clearance of sorafenib from the brain. Moreover, since the expression of P-gp and BCRP at the BBB in the genetic knockout animals remains to be carefully characterized, the present study used immunoblotting to examine the expression of P-gp and BCRP at the BBB in the knockout mice. Finally, we present a simple explanation for the cooperation of P-gp and BCRP at the BBB. This hypothesis, based on differences in relative affinities and capacities of the two transporters, can reasonably explain the findings in the Mdr1a/b-/-Bcrp1-/- mice.
Experimental Section
Chemicals and Reagents
[3H] sorafenib (3.5 Ci/mmol, purity - 98.4) and [14C] inulin (7.5 mCi/mmol, purity – 98.5 %) were purchased from Moravek Biochemicals (La Brea, CA). All other chemicals were reagent grade and were purchased from Sigma Chemical Co (St. Louis, MO).
Brain Efflux Index (BEI) Study
FVB (wild-type), Mdr1a/b-/-, Bcrp1-/- and Mdr1a/b-/-Bcrp1-/- mice were from Taconic Farms, Inc. (Germantown, NY). All animals were 8 to 10 weeks old at the time of experiment. Animals were maintained under temperature-controlled conditions with a 12-h light/dark cycle and unlimited access to food and water. All studies were carried out in accordance with the guidelines set by the Principles of Laboratory Animal Care (National Institutes of Health) and were approved by The Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota.
The brain efflux index (BEI) technique was performed as described previously by Kakee and coworkers 14. Anesthetized mice were mounted on a stereotaxic device and a borehole was made 3.8 mm lateral to the bregma. The dosing solution was prepared by dissolving [3H]-sorafenib (10 μCi/ml) and [14C]-carboxyl-inulin (5 μCi/ml) in extracellular fluid (ECF) buffer (122 mM NaCl, 25 mM NaHCO3, 3mM KCl, 1.4 mM CaCl2, 1.2 mM MgSO4, 0.4 mM K2HPO4, 10 mM D-glucose, and 10 mM HEPES, pH 7.4). Using a 2.5-μL microsyringe fitted with a 32 gauge needle (Hamilton, Reno, NE), 0.2 μL of the dosing solution was injected over 2 minutes at a depth of 2.5 mm. The injection process was controlled by a Quintessential® stereotaxic injector (Stoelting Co., IL, USA). The needle was left in place for additional 4 minutes to minimize the backflow of injected solution after which the mice were euthanized at designated time points post dose. The right (ipsilateral), left (contralateral) cerebrum and cerebellum were harvested, weighed and homogenized in 3 volumes of 5% bovine serum albumin solution. A 100-μL sample of brain homogenate was mixed with 4 ml of scintillation fluid (Thermo Fisher Scientific, Waltham, MA) and the associated radioactivity was measured in a liquid scintillation counter (Beckman Coulter, Fullerton, CA).
100-BEI (%), defined as the percentage of the amount of test drug remaining in the ipsilateral cerebrum, was calculated according to the following equation,
| (1) |
Brain Slice Uptake Study
The volume of distribution of sorafenib in the brain was determined by using the brain slice uptake technique according to the procedure described by Friden et al.15 In brief, whole brain was sliced to obtain 400 μm coronal slices using a Vibratome™ (series 1000, The Vibratome Company, Bannockburn, IL). Slices were incubated in 3 ml of oxygenated ECF buffer containing [3H]-sorafenib (0.1 μCi/ml) and [14C]-inulin (0.05 μCi/ml) at 37 °C. The incubation solution was continuously aerated with an oxygen-CO2 mixture (95:5) and shaken on an orbital shaker at 60 r.p.m. At designated time points, the slices were removed from the drug solution, dried on filter paper and weighed. The radioactivity associated with the slice was determined by liquid scintillation counting. The brain volume of distribution (Vd,brain) was calculated as the amount of sorafenib in the brain slice divided by the concentration in the incubation solution, normalized by weight of the brain slice.
Isolation of Brain Capillaries and Membranes
Whole brain capillaries and crude membranes were prepared according to the method of Hartz et al. 16. Wild-type, Mdr1a/b-/-, Bcrp1-/- and Mdr1a/b-/-Bcrp1-/- mice were euthanized (n = 10 each) and whole brain was harvested. Brain tissue was carefully cleaned to remove all visible white matter and homogenized in ice-cold PBS buffer (2.7 mM KCl, 1.46 mM KH2PO4, 136.9 mM NaCl, 8.1 mM Na2HPO4, 0/9 mM CaCl2 and 0.5 mM MgCl2 supplemented with 5 mM D-glucose and 1 mM sodium pyruvate). The homogenate was mixed with Ficoll (final concentration 16 %) and centrifuged at 5800 g for 20 mins at 4 °C. The resulting pellet was resuspended in PBS buffer containing 1 % BSA solution and passed over a 0.4 mm glass bead column. Capillaries adhering to the glass beads were collected by gentle agitation using 1 % BSA. Capillaries were washed three times with PBS buffer followed by centrifugation at 7000 g for 10 mins at 4 °C to collect the capillary pellet. Pellets were then lysed using mammalian protein extraction reagent containing protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL). The lysate was centrifuged at 15,000 g for 15 mins and the denucleated supernatant was used as brain capillary lysate for Western blotting.
Western Blotting
Protein concentrations in the lysates from brain capillaries were determined by using the bicinchoninic acid protein assay (Thermo Fisher Scientific, Rockford, IL). Lysates were diluted in a reducing sample buffer and separated on a 10 % SDS-PAGE gel, run at 100 volts for 1 hour. Gels were then transferred to a PVDF membrane at 100 volts for 1 hour at 4 °C followed by blocking for 1 hour using 5 % milk. Membranes were incubated with primary antibody to BCRP (BXP-53, 1:50, 5 μg/ml), P-gp (2C19, 1:50, 2 μg/ml) or β-actin (1:1000, 1 μg/ml) overnight at 4 °C (Enzo Life Sciences, PA, USA). Membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibody (1:5000) for 1 hour. Blots were developed using the Pierce ECL Western blotting substrate (Thermo Fisher Scientific, Rockford, IL). Densitometry calculations were done on unmodified images using the Image J software (NIH, Bethesda, MD).
Pharmacokinetic and Statistical Analysis
The efflux rate constant in the BEI studies was estimated by linear regression of the log transformation of percent remaining in brain (100 – BEI) vs. time. The regression line was not forced through 100 % at time zero which was included as a data point. In the brain slice uptake studies, the volume of distribution was calculated as the average ratio of amount in brain slice to concentration in incubation solution, after equilibrium had been reached (last two time points). The apparent efflux clearance out of brain (Clapp,efflux) was calculated as the product of elimination rate constant (keff) and brain volume of distribution (Vd,brain).
| (2) |
The standard deviation of the efflux clearance was calculated according to the following formula17.
| (3) |
Sigma Stat was used to make statistical comparisons using the t-test for comparison of two groups with significance declared at a p value less than 0.05. Multiple groups were compared by one way ANOVA, using the Holm-Sidak post hoc test for multiple comparisons.
Results
Kinetics of Sorafenib Efflux from the Brain
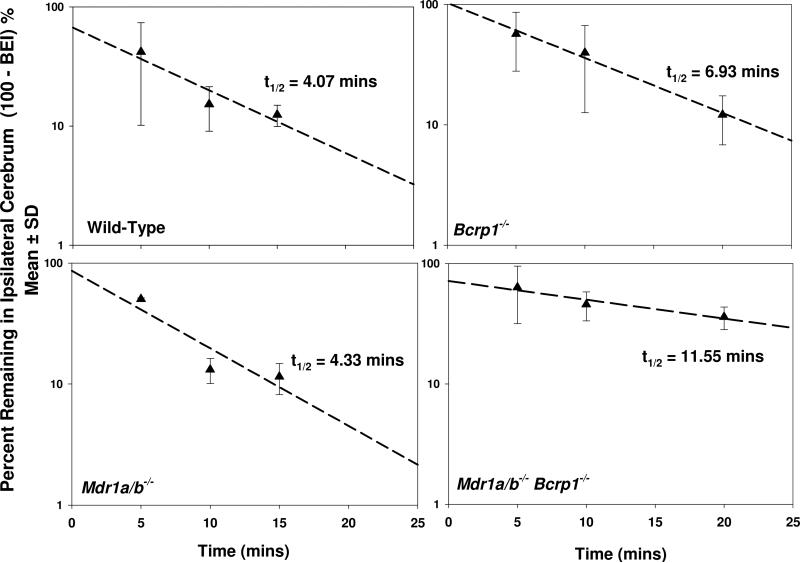
The brain efflux index method was used to study the kinetics of sorafenib elimination from the brain. Sorafenib was rapidly cleared out of the brain with an efflux rate constant of 0.17 ± 0.024 min-1 in the wild-type mice (Figure 1). The half-life in brain of wild-type mice was ~ 4 minutes. The efflux rate constant was unchanged when P-gp was absent in the Mdr1a/b-/- mice, the value being 0.16 ± 0.036 min-1 and the half life was 4.3 minutes. This suggested that P-gp did not have any significant contribution to the efflux of sorafenib from the brain. However, sorafenib efflux from brain was significantly reduced when BCRP was absent in the Bcrp1-/- mice, the rate constant decreasing to 0.10 ± 0.007 min-1 and the half-life increasing to 6.9 mins (Table 1, p < 0.05). This indicated that BCRP plays a significant role in the efflux of sorafenib from the brain. Similarly, the efflux rate constant was significantly smaller in the Mdr1a/b-/-Bcrp1-/- mice compared to wild-type (p < 0.05). The efflux rate constant and half-life in the Mdr1a/b-/-Bcrp1-/- mice was 0.062 ± 0.022 min-1 and 12 minutes, respectively (Figure 1, Table 1).
Figure 1. Brain efflux index of sorafenib in FVB mice.
Sorafenib was rapidly cleared out of the brain in wild-type and Mdr1a/b-/- mice with an efflux rate constant of 0.17 ± 0.02 and 0.16 ± 03 min-1, respectively. Sorafenib clearance out of brain decreased in the Bcrp1-/- and Mdr1a/b-/-Bcrp1-/- mice with efflux rate constants of 0.1 ± 0.01 and 0.06 ± 02 min-1, respectively. Data presented as mean ± S.D. (n = 3-5 per time point).
Table 1.
Efflux Clearance of Sorafenib from Brain in FVB wild-type, Mdr1a/b-/-,Bcrp1-/- and Mdr1a/b-/-Bcrp1-/- Mice.
| Genotype | keff (min-1) | Vd (ml/g) | Clapp,efflux (ml/min/g) | Half-life19 |
|---|---|---|---|---|
| Wild-type | 0.17 ± 0.024 | 63 ± 7.8c | 11 ± 2.0 | 4.0 |
| Mdr1a/b-/- | 0.16 ±0.036 | 91 ± 18 | 15 ± 4.4 | 4.3 |
| Bcrp1-/- | 0.10 ± 0.010a | 56 ± 8.7c | 5.6 ± 1.1a,d | 6.9 |
| Mdr1a/b-/-Bcrp1-/- | 0.062 ± 0.022a,b | 89 ± 12 | 4.3 ± 2.1a,d | 12 |
p < 0.05 compared to wild-type mice
p < 0.05 compared to Bcrp1-/- mice
p < 0.05 compared to Mdr1a/b-/- and Mdr1a/b-/-Bcrp1-/- mice
p < 0.05 compared to Mdr1a/b-/- mice
Sorafenib Volume of Distribution in the Brain
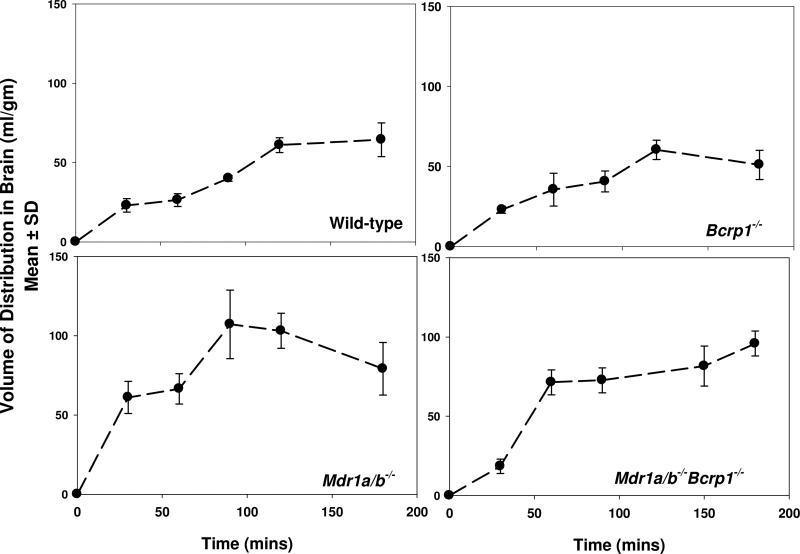
Volume of distribution of sorafenib in brain was determined by studying sorafenib uptake in brain slices from wild-type, Mdr1a/b-/-, Bcrp1-/- and Mdr1a/b-/-Bcrp1-/- mice. After incubation with [3H]-sorafenib equilibrium was attained within 2 hours in slices from all four mouse groups (Figure 2). The volume of distribution was 63 ± 7.8 ml/g in the wild-type, 91 ± 18 ml/g in the Mdr1a/b-/-, 56 ± 8.7 ml/g in the Bcrp1-/- and 89 ± 12 ml/g in the Mdr1a/b-/-Bcrp1-/- mice (Table 1).
Figure 2. Volume of distribution of sorafenib in brain.
Volume of distribution was determined by measuring uptake of [3H]-sorafenib in brain slices from wild-type, Mdr1a/b-/-, Bcrp1-/- and Mdr1a/b-/-Bcrp1-/- mice. Data presented as mean ± S.D. (n = 3-5 per time point).
Sorafenib Clearance out of Brain
Sorafenib clearance out of brain was calculated as the product of the efflux rate constant from the BEI study and the volume of distribution from the brain slice uptake study (Table 1). The clearance of sorafenib out of brain from the wild-type brain was 11 ± 2.0 ml/min/g. The clearance in the Mdr1a/b-/- mice was not significantly different (compared to wild-type) at 15 ± 4.4 ml/min/g. This suggested that P-gp did not contribute to the efflux of sorafenib at the BBB and its absence at the BBB does not influence sorafenib clearance from the brain. However, when BCRP was absent, sorafenib efflux clearance decreased significantly to 5.6 ± 1.1 ml/min/g in the Bcrp1-/- mice to 4.3 ± 2.1 ml/min/g in the Mdr1a/b-/-Bcrp1-/- mice. This confirms that a significant fraction of the total clearance of sorafenib out of the brain is mediated by BCRP at the BBB.
Detection of P-gp and BCRP in Brain Capillaries
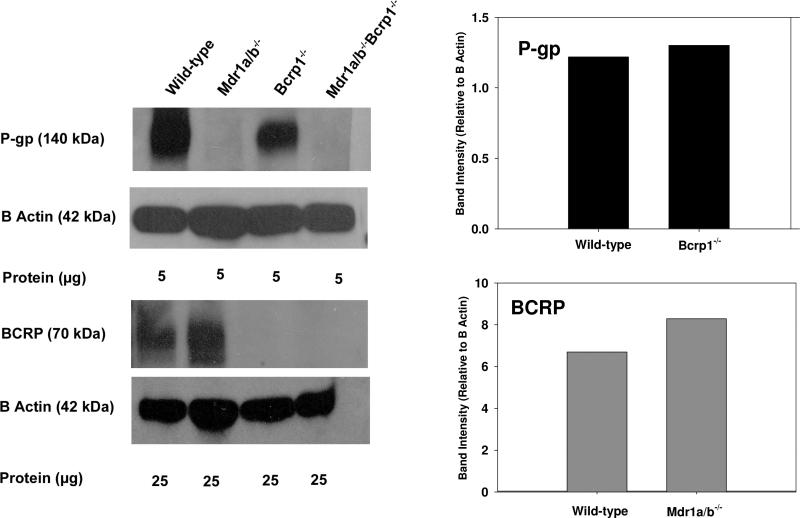
We used immunoblotting to examine expression of P-gp and BCRP in brain capillaries from FVB mice. Figure 3 illustrates Western blotting results in isolated brain capillaries showing no difference in expression of P-gp between the wild-type and Bcrp1-/- mice. Likewise, BCRP expression was not different between the wild-type and Mdr1a/b-/- mice. This suggests that there are no compensatory changes in expression of P-gp or BCRP at the BBB when one transporter is deficient in the genetic knockout mice.
Figure 3. Western blot analysis of P-gp and BCRP in isolated brain capillaries of wild-type, Mdr1a/b-/-, Bcrp1-/- and Mdr1a/b-/- Bcrp1-/- mice (pooled samples from 10 mouse brains from each genotype).
Quantification of Western blot shows no difference in expression of P-gp in the BCRP deficient mice and vice versa.
Discussion
Recent research on the role of ABC transporters, P-gp and BCRP, at the BBB has led to a new paradigm that suggests a cooperative role of the two transporters in the efflux of drugs out of the brain 6-8. There are several reports that show a greater than proportional increase in brain penetration of substrate drugs in Mdr1a/b-/-Bcrp1-/- mice compared to the single knockout mice 5, 8, 10-12. While there are some possibilities that explain this behavior of dual substrates in the combined P-gp/BCRP deficient mice, a clear understanding of the underlying mechanism is still absent. This study was undertaken with the objective to better understand the cooperation of P-gp and BCRP at the BBB and provide an explanation for the findings in the genetic knockout animals. We used the brain efflux index to analyze P-gp- and BCRP-mediated clearance of sorafenib from the brain. Sorafenib was chosen as the model substrate since we have previously shown that its brain penetration is restricted by BCRP and possibly by P-gp. This study shows that sorafenib clearance out of the brain decreases when BCRP is absent at the BBB. Interestingly, absence of P-gp alone does not alter the efflux clearance of sorafenib. These findings conclusively show that differences in brain distribution of sorafenib in the various genetic knockout mice are due to changes in transporter-mediated efflux clearance at the BBB. The data also qualitatively suggests that P-gp and BCRP expression at the BBB in the genetic knockout mice is not different from the wild-type mice. This finding is critical since it addresses an important question on the use of genetic knockout mice for brain distribution studies. This study also shows that there are no compensatory changes in expression of P-gp or BCRP at the BBB of the genetic knockout mice.
The brain efflux index studies show that sorafenib was rapidly cleared out of the brain with half-life of ~ 4 minutes in the wild-type mice (Figure 1). The half-life did not change when P-gp was absent in the Mdr1a/b-/- mice. However, sorafenib efflux clearance from brain was significantly reduced when BCRP was absent in the Bcrp1-/- mice (Table 1). These findings are consistent with our previous report that both P-gp and BCRP are involved in the efflux of sorafenib at the BBB. However, by determination of the efflux clearances, we now show that contribution of P-gp to the total efflux is small compared to BCRP. This is seen in the Mdr1a/b-/- mice where the clearance out of brain does not change compared to the wild-type mice. A 2-fold decrease in clearance in the Bcrp1-/- mice shows that BCRP contributes significantly to sorafenib efflux at the BBB. Thus, by using the BEI method, we were able to determine the contributions of P-gp and BCRP to the clearance of sorafenib out of the brain. To the best of our knowledge, this is the first study that attempts to explain the cooperation of P-gp and BCRP by experimentally determining the efflux clearance mediated by each of these transporters.
In 2004, Cisternino and colleagues reported that the BCRP mRNA was upregulated in the Mdr1a-/- mice, and suggested that genetic knockout animals are susceptible to compensatory upregulation of ABC transporters 18. This finding, if true for these genetic knockout mice, can explain why brain penetration does not increase when a single transporter is absent at the BBB. Compensatory increase in levels of other transporters (P-gp or BCRP) can be a reason behind the lack of effect in the single knockouts and the greater than additive increase in brain penetration in the combined P-gp/BCRP knockout mice. We examined this in the FVB mouse model by immunoblotting for P-gp and BCRP in the four mouse genotypes. Figure 3 illustrates Western blotting results in isolated brain capillaries showing similar expression of P-gp between the wild-type and Bcrp1-/- mice. Likewise, BCRP expression was not different between the wild-type and Mdr1a/b-/- mice. This finding is consistent with de Vries et al., who reported that there was no difference in the levels of BCRP protein in the wild-type and Mdr1a/b-/- mice 5. These results suggest that there might be no compensatory changes in expression of P-gp in the BCRP deficient mice, and vice versa. However, the above discussion is limited to P-gp and BCRP only, and it is possible there are compensatory changes in regulation of other influx and efflux transporters in these mice. A detailed and precise study examining the levels of all relevant transporters at the BBB is therefore necessary and can provide a more accurate depiction of compensatory changes (if any) in transporter function in the gene knockout mice.
Insights into the cooperation of P-gp and BCRP at the BBB can be gained by understanding the substrate-transporter interaction with respect to the transporter capacity (expression level) and substrate affinity. Recently, Kamiie et al. quantified the expression of various membrane transporters at the mouse BBB and reported approximately 5-fold higher P-gp protein levels compared to those of BCRP 19. The fact there is significantly more P-gp than BCRP at the mouse BBB can explain why P-gp appears to be the dominant transporter in the efflux of many dual substrates. Since the individual clearance by P-gp in itself is significantly greater than the remaining clearances out of brain, no enhancement is seen in brain penetration is seen in the Bcrp1-/- mice. This explains the findings that even though many dual substrates are transported by BCRP in vitro in highly expressing transfected cell systems, their brain penetration is not enhanced when BCRP alone is absent 5-8, 10, 11, 13. In comparison, due to lower protein levels, BCRP-mediated efflux appears to be minor and becomes apparent only when the greater clearance by P-gp is absent. Therefore, there is a slight increase in brain penetration. The true magnitude of the combined effect of P-gp and BCRP is seen only when both are absent in the Mdr1a/b-/-Bcrp1-/- mice, where there is a greater than additive increase in brain penetration of the dual substrates. For these compounds, the disproportionate increase in brain distribution is most likely because the true magnitude of any one transporter (especially BCRP) is masked in the single knockout mice. This phenomenon can be seen for most drugs, such as dasatinib, gefitinib, topotecan, lapatinib, erlotinib, which have all been shown to have at least some affinity for P-gp5, 7-9, 11.
However, in this case, P-gp has a weak affinity for sorafenib, which cannot be detected by in vitro assays, as compared to a higher BCRP affinity for sorafenib 9. Therefore, even though there is much more P-gp present in vivo, it cannot transport sorafenib as effeciently as BCRP. This is evident from the efflux clearance of sorafenib which is unchanged in absence of P-gp but decreases significantly when BCRP is absent. The scenario is thus opposite to that seen above with the other drugs and BCRP plays a more influential role in the efflux of sorafenib at the BBB. However, P-gp still effluxes sorafenib, which is evident from the lowest clearance in the Mdr1a/b-/-Bcrp1-/- mice and the highest brain distribution in these mice 9. The same is true for dantrolene, which is a weak P-gp substrate. Kodaira and coworkers showed that the brain distribution profile for dantrolene is similar to that of sorafenib with brain distribution increasing slightly in the BCRP knockout mice and increasing even more in the combined P-gp/BCRP knockout mice 11. Differences in relative affinities of P-gp and BCRP for substrate drugs as well as differential expression of the two transporters at the BBB can thus explain their cooperative role at the BBB. The above discussion is a simple theory to explain the interplay of P-gp and BCRP at the BBB. Of course, it does not take into consideration role of other transporters that may be involved in the efflux of these compounds at the BBB.
In conclusion, the present study explains the cooperation of P-gp and BCRP at the BBB by analyzing the clearance of sorafenib from the brain by using a simple affinity-capacity relationship that determines the role of the individual transporter in drug efflux. We show that sorafenib is effluxed at the BBB mainly by BCRP but P-gp also plays a significant role and its effect is seen only in the P-gp/BCRP knockout mice. We also show that there were no compensatory changes in expression of P-gp and BCRP in the genetic knockout mice. However, more detailed investigations are warranted so that the exact quantification of different influx and efflux transporters are determined and their influence on the transport of drugs to the brain can be ascertained.
Acknowledgements
The authors would like to thank Bjoern Bauer and Anika Hartz (University of Minnesota, Duluth) for their help and support in development of the brain capillary isolation method. This work was supported by National Institutes of Health - National Cancer Institute [CA138437] and a grant from the Children's Cancer Research Fund at the University of Minnesota (William F Elmquist). Financial support for Sagar Agarwal was provided by the Doctoral Dissertation Fellowship from the University of Minnesota.
List of Abbreviations
- CNS
central nervous system
- BBB
blood-brain-barrier
- P-gp
p-glycoprotein
- BCRP
breast cancer resistance protein
- TKI
tyrosine kinase inhibitors
- Mdr1
gene encoding the murine p-glycoprotein
- Bcrp1
gene encoding the murine breast cancer resistance protein
- ABC
ATP-binding cassette
- MDCKII
Madin Darby Canine Kidney II
- FVB
Friend Leukemia Virus Strain B
Footnotes
Supporting Information
This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardridge W, Rosenberg GA, Smith Q, Drewes LR. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7(1):84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 2.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O'Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12(3):169–82. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 4.Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13(21):6440–9. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- 6.Polli JW, Olson KL, Chism JP, John-Williams LS, Yeager RL, Woodard SM, Otto V, Castellino S, Demby VE. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethy l]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab Dispos. 2009;37(2):439–42. doi: 10.1124/dmd.108.024646. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther. 2009;330(3):956–63. doi: 10.1124/jpet.109.154781. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 2010;334(1):147–55. doi: 10.1124/jpet.110.167601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther. 2011;336(1):223–33. doi: 10.1124/jpet.110.175034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9569-1. [DOI] [PubMed] [Google Scholar]
- 11.Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther. 2010;333(3):788–96. doi: 10.1124/jpet.109.162321. [DOI] [PubMed] [Google Scholar]
- 12.Yang JJ, Milton MN, Yu S, Liao M, Liu N, Wu JT, Gan L, Balani SK, Lee FW, Prakash S, Xia CQ. P-glycoprotein and breast cancer resistance protein affect disposition of tandutinib, a tyrosine kinase inhibitor. Drug Metab Lett. 2010;4(4):201–12. doi: 10.2174/187231210792928279. [DOI] [PubMed] [Google Scholar]
- 13.Kusuhara H, Sugiyama Y. In vitro-in vivo extrapolation of transporter-mediated clearance in the liver and kidney. Drug Metab Pharmacokinet. 2009;24(1):37–52. doi: 10.2133/dmpk.24.37. [DOI] [PubMed] [Google Scholar]
- 14.Kakee A, Terasaki T, Sugiyama Y. Brain efflux index as a novel method of analyzing efflux transport at the blood-brain barrier. J Pharmacol Exp Ther. 1996;277(3):1550–9. [PubMed] [Google Scholar]
- 15.Friden M, Ducrozet F, Middleton B, Antonsson M, Bredberg U, Hammarlund-Udenaes M. Development of a high-throughput brain slice method for studying drug distribution in the central nervous system. Drug Metab Dispos. 2009;37(6):1226–33. doi: 10.1124/dmd.108.026377. [DOI] [PubMed] [Google Scholar]
- 16.Hartz AM, Mahringer A, Miller DS, Bauer B. 17-beta-Estradiol: a powerful modulator of blood-brain barrier BCRP activity. J Cereb Blood Flow Metab. 2010;30(10):1742–55. doi: 10.1038/jcbfm.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman LA. On the Exact Variance of Products. Journal of the American Statistical Association. 1960;55(292):708–713. [Google Scholar]
- 18.Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res. 2004;64(9):3296–301. doi: 10.1158/0008-5472.can-03-2033. [DOI] [PubMed] [Google Scholar]
- 19.Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, Sekine Y, Uchida Y, Ito S, Terasaki T. Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res. 2008;25(6):1469–83. doi: 10.1007/s11095-008-9532-4. [DOI] [PubMed] [Google Scholar]