Abstract
Ever since the beginning of biochemical analysis, yeast has been a pioneering model for studying the regulation of eukaryotic metabolism. During the last three decades, the combination of powerful yeast genetics and genome-wide approaches has led to a more integrated view of metabolic regulation. Multiple layers of regulation, from suprapathway control to individual gene responses, have been discovered. Constitutive and dedicated systems that are critical in sensing of the intra- and extracellular environment have been identified, and there is a growing awareness of their involvement in the highly regulated intracellular compartmentalization of proteins and metabolites. This review focuses on recent developments in the field of amino acid, nucleotide, and phosphate metabolism and provides illustrative examples of how yeast cells combine a variety of mechanisms to achieve coordinated regulation of multiple metabolic pathways. Importantly, common schemes have emerged, which reveal mechanisms conserved among various pathways, such as those involved in metabolite sensing and transcriptional regulation by noncoding RNAs or by metabolic intermediates. Thanks to the remarkable sophistication offered by the yeast experimental system, a picture of the intimate connections between the metabolomic and the transcriptome is becoming clear.
IN addition to being the building blocks of proteins, amino acids have a central role in general metabolism. A major achievement of yeast research has been the determination of the complete metabolic pathways for amino acid utilization as carbon and nitrogen sources, amino acid biosynthesis, and the conversion of amino acids to other metabolites including nucleotides. Key reviews on these processes, of almost biblical stature, by Cooper (1982a)1 and Jones and Fink (1982) are notable since they summarized and integrated results from both biochemical and genetic analyses and thereby provided a solid framework to incorporate findings that have been highlighted in subsequent major reviews (Hinnebusch 1992; Johnston and Carlson 1992; Magasanik 1992). Extensive, albeit not fully complete, information regarding the metabolic networks involving amino acids and nucleotides is available in well-established databases with excellent user interfaces, e.g., the Saccharomyces Genome Database (SGD) (Hong et al. 2008) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) (Aoki-Kinoshita and Kanehisa 2007).
In cells, catabolic nitrogen source utilization and anabolic amino acid and nucleotide biosynthetic pathways function in parallel. These competing processes must be coordinated to enable cells to manifest a proper response to nutrient availability. A requisite for coordination of metabolism is the ability to monitor concentrations of nutrients in the extracellular environment and within cells (for review see Zaman et al. 2008). Plasma membrane-localized sensors that respond to the availability of diverse sets of nutrients, including many nitrogen sources, have recently been identified. These environmental sensors operate together with networks of intracellular sensing systems that are spread and function in the cytosol, vacuole/endosome, mitochondria, peroxisome, and nucleus. Furthermore, catabolic and anabolic pathways generate multiple metabolic intermediates that significantly contribute to the complexity of the chemical composition of cells. These metabolic intermediates are not necessarily inert, and there are examples of intermediates providing information (signals) regarding the metabolic status of cells and exerting regulatory effects. Yeast cells can clearly integrate multiple nutrient-based signals derived from spatially separated sensing systems.
Here we focus on regulatory mechanisms and highlight newly attained information regarding aspects of both catabolic and anabolic processes affecting amino acid and nucleotide metabolism. In addition, because nucleotide synthesis is phosphate consuming, regulation of phosphate uptake and utilization is included. Specific examples have been chosen to illustrate how multiple layers of metabolic control are coordinated. Briefly, yeast cells possess suprapathway mechanisms that, in response to metabolic changes, can reprogram large-scale patterns of gene expression. Suprapathway control is exerted at both the transcriptional and the translational levels. In contrast to these general modes of control, cells can also respond very precisely by regulating the activity of specialized transcription factors that bind a particular metabolite and in response activate or repress the expression of specific sets of genes. These mechanisms are complemented by post-translational modes of regulation, which provide cells with the means to rapidly adjust the catalytic properties of enzymes, modulating the degradation rates of enzymes and permeases and regulating the flow of metabolites in and out of intracellular organelles.
Amino Acids
Nitrogen source utilization: the flow of nitrogen to amino acids, purines, and pyrimidines
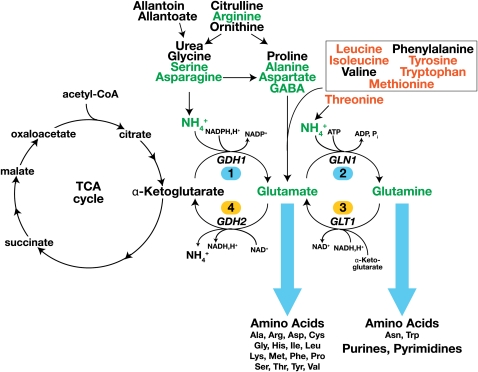
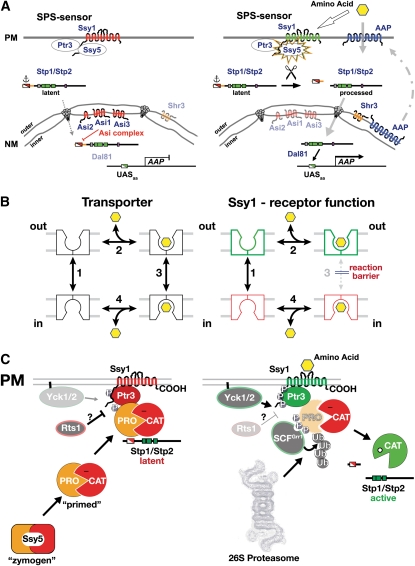
Yeast cells react to the nitrogen content of the growth environment by controlling nitrogen source uptake and by regulating catabolic and anabolic processes. As reviewed by Cooper (1982a) and schematically depicted in Figure 1, yeast can use a variety of nitrogenous compounds as sole sources of nitrogen for growth. Although some strain variability exists, all L-amino acids, with the exception of lysine, histidine, and cysteine, can support growth as the sole nitrogen source (Table 1). However, each amino acid supports a distinct rate of growth; in media with glucose as the main carbon source, generation times vary from ∼2 h (e.g., asparagine, glutamine, and arginine) to >4 h (e.g., methionine and tryptophan). The ability to use amino acids and other nitrogenous compounds requires their internalization, and accordingly, yeast cells possess multiple permeases to facilitate their transport across the plasma membrane (Table 4). Notably, the presence of external amino acids induces the expression of several broad-specificity permeases; hence, amino acids induce their own uptake. This transcriptional response is mediated by the plasma membrane localized Ssy1-Ptr3-Ssy5 (SPS) sensor (reviewed in Ljungdahl 2009). Once internalized, nitrogenous compounds can be used directly in biosynthetic processes, be deaminated to generate ammonium, or serve as substrates of transaminases that transfer amino groups to α-ketoglutarate to form glutamate (reviewed in Cooper 1982a; Magasanik 1992; Magasanik and Kaiser 2002). In cells grown on glucose, ammonium can be assimilated by two anabolic reactions, i.e., the synthesis of glutamate from ammonium and α-ketoglutarate catalyzed by the NADPH-dependent glutamate dehydrogenase (GDH1) (reaction 1) (Figure 1), and the synthesis of glutamine from ammonium and glutamate by glutamine synthetase (GLN1) (reaction 2). In cells grown on ethanol as a carbon source, a Gdh1 isozyme encoded by GDH3 is expressed and contributes to the assimilation of ammonium (Avendano et al. 1997; DeLuna et al. 2001). When glutamine is the sole nitrogen source, the NADH-dependent glutamate synthase (GLT1) is required to catalyze the synthesis of glutamate (reaction 3). The catabolic release of ammonia from glutamate (reaction 4) is catalyzed by the NAD+-linked glutamate dehydrogenase (GDH2). This latter reaction is also required to provide ammonium for the synthesis of glutamine when glutamate is the sole nitrogen source. The central importance of glutamate and glutamine in biosynthesis of nitrogenous compounds is illustrated in Figure 1 (blue arrows); ∼85% of the total cellular nitrogen is incorporated via the amino nitrogen of glutamate, and the remaining 15% is derived from the amide nitrogen of glutamine (Cooper 1982a).
Figure 1 .
Schematic diagram of the main pathways of nitrogen metabolism. The entry routes of several nitrogen sources into the central core reactions are shown. The class A preferred and class B nonpreferred nitrogen sources are in green and red text, respectively. The nitrogen of preferred nitrogen sources is incorporated into glutamate, and the resulting carbon skeletons are shunted into pyruvate and α-ketoglutarate. Nitrogen from branched-chain amino acids, aromatic amino acids, and methionine (within box) is transferred to α-ketoglutarate by transaminases forming glutamate; the resulting deaminated carbon skeletons are converted to noncatabolizable and growth-inhibitory fusel oils (Hazelwood et al. 2008). Nitrogenous compounds are synthesized with nitrogen derived from glutamate or glutamine as indicated (blue arrows). Central anabolic reactions 1 and 2 are catalyzed by NADPH-dependent glutamate dehydrogenase (GDH1) and glutamine synthetase (GLN1). Central catabolic reactions 3 and 4 are catalyzed by NADH-dependent glutamate synthase (GLT1) and NAD+-linked glutamate dehydrogenase (GDH2). For detailed descriptions of the pathways, the reader is referred to the SGD (http://pathway.yeastgenome.org/) or KEGG (http://www.genome.jp/kegg/pathway.html) databases.
Table 1. Compilation of literature values: generation times and glutamate and glutamine pool sizes in cells grown on various sole nitrogen sources.
| Generation time (hours.minutes) strain background | μmol 100 mg−1 dry weightd | ||||||
|---|---|---|---|---|---|---|---|
| Nitrogen source | Σ1278ba | Σ1278bb | S288cb | S288cc | Y48d | Glu | Gln |
| Preferred class Aa: high-moderate active NCR/high-moderate active UPR/inactive GAAC | |||||||
| NH4+ | 2.00 | 2.28 | 2.24 | 1.52 | 2.08 | 7.4 | 2.8 |
| Asn | 2.00 | 2.53 | 2.14 | 1.49 | 2.42 | 5.2 | 3.4 |
| Gln | 2.05 | 2.24 | 2.12 | 2.14 | 2.16 | 22.7 | 43.1 |
| Ser | 2.15 | 2.40 | 2.33 | 2.23 | 5.53 | 7.0 | 1.5 |
| Asp | 2.10 | 2.51 | 2.55 | 2.19 | 2.57 | 5.1 | 2.9 |
| Ala | 2.30 | 2.43 | 3.00 | 3.28 | 4.33 | 2.9 | 0.6 |
| Arg | 2.25 | 3.22 | 2.49 | 2.06 | 2.11 | 8.2 | 1.5 |
| Glu | 2.15 | 2.16 | 2.29 | 2.29 | 2.38 | 37.9 | 15.6 |
| Intermediatea: slight active NCR/moderate active UPR/inactive GAAC | |||||||
| Orn | 4.30 | 3.26 | 3.13 | 6.56 | 3.42 | 12.2 | 1.8 |
| Pro | 3.15 | 4.28 | 4.28 | 4.57 | 4.33 | 27.4 | 3.1 |
| Val | 3.00 | 3.32 | 3.24 | 4.05 | 8.20 | 4.5 | 1.0 |
| Phe | 3.20 | 2.33 | 3.44 | 3.39 | 2.51 | 5.3 | 0.9 |
| Intermediatea: inactive NCR/inactive-slight active UPR/inactive GAAC | |||||||
| Urea | 3.35 | 2.38 | 2.44 | — | — | — | — |
| Cit | 4.30 | 3.06 | 3.28 | — | 3.42 | 2.6 | 22.1 |
| Non-preferred class Ba: inactive NCR/slight active UPR/active GAAC | |||||||
| Leu | 3.25 | 4.47 | 4.31 | 6.18 | 3.14 | 10.1 | 1.5 |
| Ile | 3.55 | 4.48 | 3.42 | 6.18 | 9.06 | 5.4 | 0.5 |
| Met | 4.05 | — | 5.29 | 5.47 | 5.33 | 10.7 | 6.9 |
| Tyr | 4.10 | 2.44 | 5.26 | 5.20 | 4.21 | 9.1 | 1.1 |
| Thr | 4.20 | 2.34 | 5.12 | 5.47 | 10.00 | 2.3 | 0.6 |
| Trp | 4.45 | 4.01 | 4.20 | 6.18 | 8.20 | 6.4 | 1.0 |
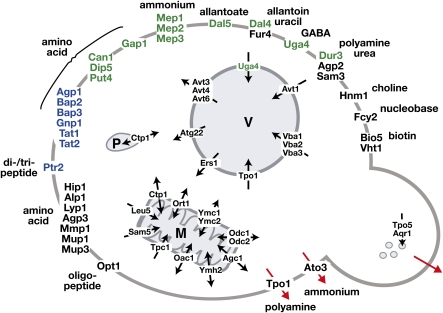
Table 4. Plasma membrane-localized transporters of nitrogenous substrates relevant to amino acid metabolism.
| Amino acid permease family | ||||
|---|---|---|---|---|
| Systematic name | Gene name | Functional description (substrate specificity) | Regulation | Reference |
| Core - Cluster Ia – SPS sensor regulated | ||||
| YCL025c | AGP1 | Broad substrate range, medium capacity permease (Val, Ile, Phe, Met, Ser, Leu, Thr, Cys, Asn, Tyr, Ala, Gly, Gln)b (Pro) | SPS-sensor, NCR, GAACc | Andréasson et al. (2004); Iraqui et al. (1999); Schreve et al. (1998) |
| YBR068c | BAP2 | Branched-chain amino acid permease (Val, Ile, Phe, Tyr, Leu, Trp, Met, Cys, Ala)b | SPS-sensor, GAACc | Grauslund et al. (1995) |
| YDR046c | BAP3 | Branched-chain amino acid permease (Val, Ile, Phe, Tyr, Trp, Leu, Met, Cys, Thr, Ala)b | SPS-sensor | Didion et al. (1998) |
| YDR508c | GNP1 | High-affinity glutamine permease (Thr, Gln, Ser, Cys, Leu, Met, Asn)b (Pro) | SPS-sensor | Andréasson et al. (2004); Zhu et al. (1996) |
| YBR069c | TAT1 | Tyrosine and tryptophan permease (Val, Thr)b (low-affinity His), (Leu) | SPS-sensor | Bajmoczi et al. (1998); Schmidt et al. (1994) |
| YOL020w | TAT2 | High-affinity tryptophan permease (Phe, Tyr, Trp, Gly, Ala)b | SPS-sensor | Schmidt et al. (1994) |
| Core - Cluster II | ||||
| YKR039w | GAP1 | General, high-capacity, amino acid permease (all L-amino acids, D-amino acids, GABA, peptides, polyamines) | NCR, GAACc | André et al. (1993); Jauniaux and Grenson (1990); van Zeebroeck et al. (2009) |
| YGR191w | HIP1 | Histidine permease | Tanaka and Fink (1985) | |
| YLL061w | MMP1 | High-affinity S-methyl methionine permease | Rouillon et al. (1999) | |
| YPL274w | SAM3 | High-affinity S-adenosyl methionine permease; High-affinity putrescine, spermidine, spermine (polyamine) | Rouillon et al. (1999); Uemura et al. (2007) | |
| Core - Cluster III | ||||
| YEL063c | CAN1 | Arginine permease (Arg) | NCR, GAACc | Hoffmann (1985) |
| YNL270c | ALP1 | Arginine permease (Arg)b | Sychrova and Chevallier (1994) | |
| YNL268w | LYP1 | Lysine permease (Lys, Met)b | GAACc | Sychrova and Chevallier (1993) |
| Core - Unclustered | ||||
| YOR348c | PUT4 | High-affinity proline permease (Val, Ala, Pro)b (GABA) | NCR | André et al. (1993); Jauniaux et al. (1987) |
| YPL265w | DIP5 | Dicarboxylic amino acid permease (Ser, Ala, Asn. Asp, Gln, Gly, Gln)b | NCR | Regenberg et al. (1998) |
| YDR160w | SSY1 | Receptor component of the SPS sensor | Didion et al. (1998); Iraqui et al. (1999); Klasson et al. (1999) | |
| YBR132c | AGP2 | Carnitine, spermidine, putrescine (polyamine) | Aouida et al. (2005); van Roermund et al. (1999) | |
| YFL055w | AGP3 | Broad-substrate specificity amino acid permease (Asp, Glu, Ser)b (Met) | Menant et al. (2006); Regenberg et al. (1999) | |
| Non-core - MUP Cluster | ||||
| YGR055w | MUP1 | High-affinity methionine permease (Cys) | Isnard et al. (1996); Kosugi et al. (2001) | |
| YHL036w | MUP3 | Low-affinity methionine permease; inhibited by broad-substrate spectrum (Met) | GAACc | Isnard et al. (1996) |
| Non-core - UGA Cluster | ||||
| YDL210w | UGA4 | GABA permease (δ-aminolevulinic acid, putrescine) vacuole localization | NCR | André et al. (1993); Uemura et al. (2004) |
| YKL174c | TPO5 | Polyamine secretion, Golgi localization | Tachihara et al. (2005) | |
| YGL077c | HNM1 | Choline permease (ethanolamine) | INO regulon | Nikawa et al. (1986) |
| YNR056c | BIO5 | Biotin permease (7-keto 8-aminopelargonic acid) | Phalip et al. (1999) | |
| Ammonium permease family | ||||
| YGR121C | MEP1 | Medium-affinity, high-capacity ammonium permease | NCR | Marini et al. (1994) |
| YNL142W | MEP2 | High-affinity, low-capacity ammonium permease, ammonium sensor | NCR | Lorenz and Heitman (1998); Marini et al. (1997) |
| YPR138C | MEP3 | Low-affinity, high-capacity ammonium permease | NCR | Marini et al. (1997) |
| Other transporters/permeases | ||||
| DAL5 Cluster | ||||
| YJR152w | DAL5 | Allantonate permease (ureidosuccinate/dipeptide) | NCR | Cai et al. (2007); Rai et al. (1987) |
| YLL055w | YCT1 | Cysteine transporter | Kaur and Bachhawat (2007) | |
| YGR260w | TNA1 | Nicotinic acid transporter | Llorente and Dujon (2000) | |
| YGR065c | VHT1 | Biotin transporter | GAACc | Stolz et al. (1999) |
| YCR028c | FEN2 | Panthothenate transporter | Stolz and Sauer (1999) | |
| YAL067c | SEO1 | Not determined – sulfur compound? | Isnard et al. (1996) | |
| FUR4 Cluster | ||||
| YBR021w | FUR4 | Uracil permease | Jund et al. (1988) | |
| YIR028w | DAL4 | Allantoin permease (uracil) | NCR | Yoo et al. (1992) |
| YBL042c | FUI1 | Uridine permease | Wagner et al. (1998) | |
| YLR237w | THI7 | Thiamine permease | Thi3 | Enjo et al. (1997) |
| YOR192c | THI72 | Thiamine permease | Enjo et al. (1997) | |
| YOR071c | NRT1 | Nicotinamide riboside (high-affinity), thiamine (low-affinity) | Belenky et al. (2008) | |
| FCY2 Cluster | ||||
| YER056c | FCY2 | Nucleobase permease (cytidine, cytosine, purine, adenine, guanine, hypoxanthine) | Weber et al. (1990) | |
| YGL186c | TPN1 | Pyridoxine transporter (vitamin B6) | GAACc | Stolz and Vielreicher 2003) |
| Unclustered | ||||
| YHL016c | DUR3 | High-affinity polyamine (urea) | NCR | El Berry et al. (1993); Uemura et al. (2007) |
| YKR093w | PTR2 | Peptide transporter (di-/tripeptides) | SPS-sensor, Cup9, NCR | Hauser et al. (2001); Island et al. (1991) |
| YJL212c | OPT1 | Oligopeptide transporter (glutathione, phytochelatin) | NCR | Hauser et al. (2001) |
| YPR194c | OPT2 | Homologous to OPT1 (role as transporter uncertain; inactivation leads to fragmented vacuoles) | NCR | Aouida et al. (2009) |
| Transporters involved with excretion of nitrogenous compounds | ||||
| YDR384c | ACO3 | Ammonium transport outward | SPS-sensor, GAAC | Guaragnella and Butow (2003); Palkova et al. (2002) |
| YNL065w | AQR1 | H+-antiporter, localized to multiple intracellular membranes/vesicles (secretion of homoserine, Thr, Ala, Asp, Glu) | Velasco et al. (2004) | |
| YLL028w | TPO1 | Polyamine transporter | Tomitori et al. (1999); Uemura et al. (2005) | |
Clustered based onon the basis of sequence homology according to Nelissen et al. (1997).
Substrate specificity as reported in Regenberg et al. (1999).
GAAC-regulated expression according to Natarajan et al. (2001).
Nitrogen source: quality of amino acids
The various nitrogen sources used by yeast are often qualitatively referred to as being preferred (good) or nonpreferred (poor). This less-than-precise classification has been empirically based on two criteria. The first criterion is how well the individual compounds support growth when present as sole source of nitrogen. The second criterion reflects the finding that preferred nitrogen sources generally repress processes required for the utilization of nonpreferred nitrogen sources (reviewed in Cooper 1982a; Magasanik 1992; Magasanik and Kaiser 2002). Nitrogen regulation of transcription is a general suprapathway response that is commonly referred to as nitrogen catabolite repression (NCR). NCR primarily functions to ensure that cells selectively use preferred nitrogen sources when they are available, and in the absence of a preferred nitrogen source, the general derepression of NCR-regulated genes enables cells to indiscriminately scavenge alternative, nonpreferred nitrogen sources. The classification of nitrogen sources is not absolute, and their repressive effects can vary significantly between different yeast strain backgrounds. For example, ammonium and, to a lesser extent glutamate are repressing nitrogen sources for Σ1278b-derived strains, whereas, for many S288c-derived strains, they are not, even though these nitrogen sources promote high rates of growth (Magasanik and Kaiser 2002). Genetic analyses have shown that the phenotypic differences between these genetic backgrounds are multifactorial and not fully understood (Magasanik and Kaiser 2002; Georis et al. 2009a).
Godard et al. (2007) carefully analyzed the patterns of gene expression in prototrophic wild-type cells (Σ1278b) growing in media containing glucose as the carbon source and different sources of sole nitrogen, including 16 individual amino acids. Importantly, the patterns of gene expression were monitored in cells from logarithmically expanding cultures fully adapted to growth with each individual nitrogen source. This analysis revealed several significant findings. First, the yeast cultures grew at variable rates characteristic for the nitrogen source (Table 1); however, in the comparisons with gene expression patterns, no significant variations in the levels of general stress response genes were detected. Consequently, cells clearly adapt to the quality of the nitrogen source to achieve a balanced state of growth. Second, the pattern of gene expression in urea-grown cells could be used as the reference for comparisons; urea supports intermediate growth and, notably, the major transcriptional regulatory systems, i.e., NCR, general amino acid control (GAAC), and the unfolded protein response (UPR), as well as the SPS-sensing system, are not active. Third, the ability of cells to sense the presence of extracellular amino acids via the SPS-sensing pathway and to prioritize their uptake is relatively independent of nitrogen source. Fourth, several of the nitrogen sources could unambiguously be classified as follows: class A, preferred nitrogen sources—nitrogen-sensitive gene expression is repressed (NCR is active), the UPR is moderately active, and GAAC is inactive; conversely, class B, nonpreferred nitrogen sources—nitrogen-sensitive gene expression is derepressed (NCR inactive), UPR is less active, and GAAC is highly active (Table 1). Finally, as pointed out by Godard et al. (2007), the utilization of the preferred amino acids as nitrogen sources yields carbon skeletons that are readily integrated in metabolism (Figure 1). Six of the seven preferred amino acids are substrates of transaminases or deaminases that yield pyruvate (alanine and serine), tricarboxylic acid (TCA) cycle intermediates oxaloacetate (asparagine and aspartate) or α-ketoglutarate (glutamate and glutamine). The nonpreferred class B amino acids are subject to transamination resulting in carbon skeletons that are converted via the Ehrlich pathway to noncatabolizable and growth-inhibitory fusel oils (Hazelwood et al. 2008).
Biosynthesis of amino acids
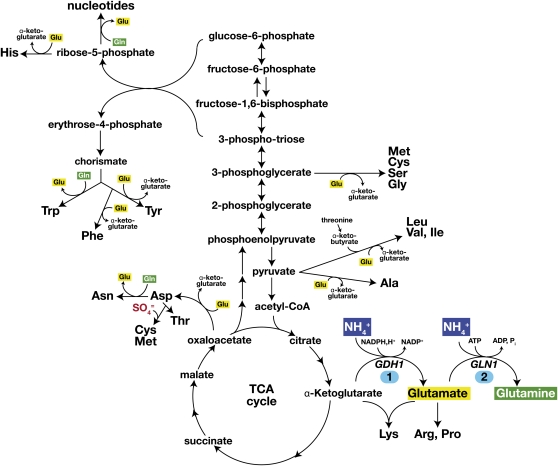
As schematically depicted in Figure 2, yeast cells provided with an appropriate source of carbon and ammonium can synthesize all L-amino acids used in protein synthesis (Jones and Fink 1982). Ammonia is incorporated during the formation of glutamate from α-ketoglutarate (reaction 1) by NADPH-dependent glutamate dehydrogenase (GDH1), and glutamine from glutamate (reaction 2) by glutamine synthetase (GLN1) (reviewed in Magasanik 2003). The families of amino acids derived from a common molecule are readily identifiable and include the glutamate family (glutamate, glutamine, arginine, proline, and lysine); the aromatic family (phenylalanine, tyrosine, and tryptophan); the serine family (serine, glycine, cysteine, and methionine); the aspartate family (aspartate, asparagine, threonine, and the sulfur-containing amino acids cysteine and methionine); and the pyruvate family (alanine and the branched amino acids valine, leucine, and isoleucine). The histidine and nucleotide biosynthetic pathways are connected. The importance of glutamate and glutamine, and consequently the central core reactions in nitrogen metabolism, becomes apparent by highlighting their involvement in transamination reactions required in the synthesis of each amino acid (Cooper 1982a; Magasanik 1992; Magasanik and Kaiser 2002).
Figure 2 .
General scheme for the biosynthesis of amino acids from glucose and ammonia. Ammonia is incorporated during the formation of glutamate from α-ketoglutarate (reaction 1) by NADPH-dependent glutamate dehydrogenase (GDH1) and of glutamine from glutamate (reaction 2) by glutamine synthetase (GLN1). The transamination reactions transferring nitrogen from glutamate (yellow) or glutamine (green) are shown. For detailed descriptions of the pathways, the reader is referred to the SGD (http://pathway.yeastgenome.org/) or KEGG (http://www.genome.jp/kegg/pathway.html) databases.
Nitrogen-regulated gene expression
NCR was first recognized as a physiological response in the early 1960s, and the literature regarding NCR is extensive; however, the primary mechanism underlying how cells sense the overall nitrogen status remains unknown (Cooper 2002; Magasanik and Kaiser 2002). This represents a major hole in understanding and a challenge for the future. The aim of the following discussion of NCR is to provide the basis for understanding the rapidly evolving concepts of how nitrogen source utilization pathways are regulated. The gene names defined as standard in the SGD will be used.
Although the nitrogen-sensing mechanism(s) operating upstream of NCR remain elusive, a rather comprehensive understanding of the downstream events of NCR can be outlined as follows. NCR-sensitive genes are controlled by a core set of regulatory components, including Ure2 and the four GATA transcription factors Gln3, Gat1, Dal80, and Gzf3. Gln3 and Gat1 function as activators of gene expression that are efficiently targeted to the nucleus under conditions that derepress the expression of NCR-sensitive genes. In contrast, Dal80 and Gzf3 act as repressors that constitutively localize to the nucleus. All four transcription factors possess zinc-finger DNA-binding motifs that bind core GATAAG consensus sequences present in the promoters of NCR-sensitive genes. The ability of the GATA factors to compete for cis-acting GATAAG sequence elements is influenced by nitrogen source availability and is even modulated by events within the nucleus (Georis et al. 2009b, 2011).
The expression of NCR-sensitive genes is constitutively depressed by mutations that inactivate Ure2 (Drillien and Lacroute 1972), indicating that Ure2 participates in repressing gene expression in cells grown in the presence of preferred nitrogen sources (Wiame et al. 1985; Courchesne and Magasanik 1988; Coschigano and Magasanik 1991). The derepression of NCR genes in the absence of Ure2 is largely dependent on Gln3; cells lacking GLN3 are unable to derepress NCR-sensitive gene expression (Mitchell and Magasanik 1984; Minehart and Magasanik 1991). Cells carrying mutations that inactivate URE2 are able to utilize nonpreferred nitrogen sources even in the presence of preferred ones, a finding that has been exploited to optimize industrial fermentations (Salmon and Barre 1998). The inactivation of Ure2 results in constitutive nuclear localization of Gln3. Microscopic analysis and subcellular fractionation studies suggest that a significant portion of Gln3 is membrane associated in cells grown in the presence of a preferred nitrogen source, which may have important consequences for the regulation of the Ure2–Gln3 interaction (Cox et al. 2002; Puria et al. 2008). Gat1 also targets the nucleus in cells grown in nonpreferred nitrogen sources (Kulkarni et al. 2006). However, in contrast to Gln3, Gat1 is not specifically excluded from the nucleus, and the loss of Ure2 does not greatly affect Gat1 localization. Consequently, Gat1 localization appears largely independent of Ure2; other factors thus must be important in determining Gat1 function (Georis et al. 2008, 2009a,b). This notion is consistent with the finding that Gzf3 interacts directly with Gat1 in the nucleus, an interaction that regulates Gat1 promoter binding (Georis et al. 2009b).
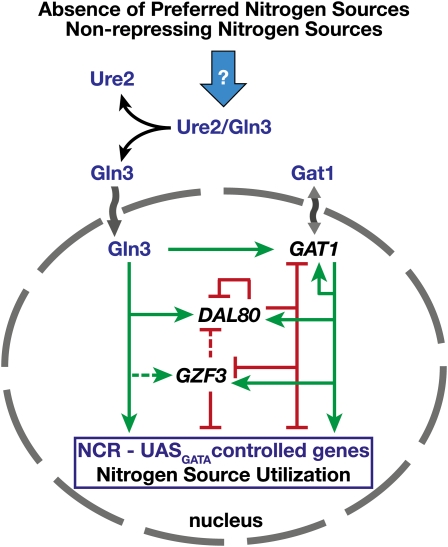
With the notable exception of GLN3, the genes for the other three GATA factors (GAT1, GZF3, and DAL80) are expressed under the control of promoters containing multiple GATAAG sequences, and their expression is sensitive to NCR (Cunningham and Cooper 1991; Coffman et al. 1996; Rowen et al. 1997; Soussi-Boudekou et al. 1997). These factors participate in regulating each other’s expression (cross-regulation), exhibiting either positive or negative regulation dependent on their corresponding roles. In certain instances, the factors regulate their own expression (Figure 3) (Coffman et al. 1997; Rowen et al. 1997; Soussi-Boudekou et al. 1997; Georis et al. 2009b).
Figure 3 .
Model of GATA factor and NCR-controlled gene expression. The promoters of GAT1, GZF3, and DAL80 contain multiple GATAAG sequences, and their expression is sensitive to NCR. These factors regulate each other’s expression (cross-regulation) and in certain instances exhibit partial autogenous regulation. GAT1 and DAL80 expression is primarily dependent on Gln3 and Dal80; the expression of these factors is the highest in cells grown under nonrepressive conditions. Inactivation of GZF3 results in the derepressed expression of several NCR-sensitive genes including GAT1, indicating that, in contrast to Dal80, Gzf3 is expressed at functionally significant levels and active in the presence of repressing nitrogen sources. Consistent with this latter finding, GZF3 expression is induced by Gat1 under conditions when Gln3 is apparently inactive (Rowen et al. 1997). Gzf3 maintains low levels of GAT1 expression by competing with Gat1 at GATAAG-binding sites; in essence, these two factors participate in an autoregulatory loop. Green lines and arrows indicate positive regulation; red lines and bars indicate negative regulation; and dashed lines reflect relatively weaker regulation. The model is modified from Coffman et al. (1997) and Georis et al. (2009a).
In growing cells, URE2 and GLN3 expression are not tightly regulated in response to nitrogen (Coschigano and Magasanik 1991; Georis et al. 2009b). Consequently, the Ure2–Gln3 interaction provides cells with a stably expressed regulatory complex, or switch, that can be rapidly controlled to directly activate gene expression. The Ure2–Gln3 switch appears to function as the master controller, which, together with the overlapping regulatory activities of the GATA factors, enables cells to adjust GATA factor levels in a manner appropriate for prevailing nitrogen source availability (Zaman et al. 2008). Activation of the switch in cells grown in the presence of nonrepressing (nonpreferred) nitrogen sources results in the suprapathway induction of ∼90 genes (Table 2). Although several models have been proposed for the regulation of the Ure2–Gln3 switch, the current literature does not support a consensus view, and clearly, deciphering the mechanism(s) controlling the Ure2–Gln3 switch remains the Holy Grail of the NCR field.
Table 2. NCR-controlled genes.
| Category | Experimentally verifieda | Predictedb |
|---|---|---|
| Amino acid – nitrogen metabolism | ASP3c, BAT2, CAR1, DAL1, DAL2, DAL3, DAL7, DCG1, DUR1,-2, GDH2, GDH3, GLN1, PUT1, PUT2, UGA1 | ARG4, CHA1, GDH1, GLT1, NIT1, SDL1 |
| Plasma membrane nitrogen uptake | AGP1, CAN1, DAL4, DAL5, DUR3, GAP1, MEP1, MEP2, MEP3, PUT4, UGA4 | DIP5, OPT1, OPT2. , PTR2 |
| Transcription factors | DAL80, GAT1, GZF3 | GCN4, MIG2, UGA3 |
| Vacuole function | ATG14, CPS1, LAP4, PEP4, PRB1 | AVT1, AVT4, AVT7, MOH1, VBA1, YHR138c |
| Mitochondrial carrier proteins | GGC1 | |
| Regulatory proteins | NPR2, PMP1, RTS3, YGK3 | |
| Nucleotide salvage pathways | AAH1, GUD1, NRK1, URK1 | |
| Carbon metabolism | ALD4, HXK1 | |
| Other | ECM38, VID30, YHI9, YGR125w | ECM37, LEE1, RNY1, RPS0B, RSM10, SLX9, UGX2, YDL237w, YDR090c, YGL196w, YJR011c, YLR149c, YLR257w, YOR052c |
Forty-one known NCR-regulated genes as compiled by (Godard et al. (2007).
Forty-four genes identified by (Godard et al. (2007).
Four copies are present in genome reference strain S288c.
Target of rapamycin (TOR) signaling and NCR are functionally distinct
It has also been proposed that TOR signaling directly regulates NCR by controlling the Ure2-mediated cytoplasmic retention of Gln3 (Beck and Hall 1999). Consistent with this notion, cells treated with rapamycin, a specific inhibitor of the TORC1 complex (Loewith et al. 2002), exhibit derepressed expression of NCR-sensitive genes. Rapamycin treatment reduces levels of Gln3 phosphorylation, which correlates with its nuclear targeting. In apparent support of this model, the TORC1-regulated phosphatase Tap42-Sit4, negatively controlled by TORC1, has been shown to influence the extent of Gln3 phosphorylation (Beck and Hall 1999; Cardenas et al. 1999; Hardwick et al. 1999; Bertram et al. 2000; Carvalho et al. 2001).
Although very important insights regarding NCR have been gained by examining rapamycin inhibition of TORC1 signaling, and without doubt TORC1 activity can influence NCR, this major signaling hub appears to operate independently, perhaps in parallel of the nitrogen sensor that “naturally” regulates NCR. Consistent with this notion, there is accumulating evidence that rapamycin exerts its effects in a manner that does not faithfully mimic nitrogen starvation (Cox et al. 2004; Crespo et al. 2004; Kulkarni et al. 2006; Georis et al. 2008, 2009a; Puria and Cardenas 2008; Puria et al. 2008; Tate et al. 2009, 2010). For example, in direct opposition to rapamycin treatment, a functional myc-tagged Gln3 construct becomes hyperphosphorylated during nitrogen and carbon starvation (Cox et al. 2004; Kulkarni et al. 2006), and the phosphorylation status of Gln3 does not affect its ability to bind Ure2 (Bertram et al. 2000). Also, Gln3 phosphorylation levels do not correlate with the presence of preferred or nonpreferred nitrogen sources, the intracellular localization of Gln3, or the capacity to support NCR-sensitive transcription (Cox et al. 2004; Tate et al. 2005; Kulkarni et al. 2006). Consequently, the mechanisms controlling Gln3 localization remain to be clarified.
Since the inactivation of TORC1 induces signals that impinge on the NCR-mediated transcriptional control pathway, it is imperative to distinguish between direct and indirect effects. There are several examples where this has been problematic. For example, in ammonium-grown cells, the mutational inactivation of NPR1 results in Gln3-dependent derepression of NCR-sensitive genes (Crespo et al. 2004). The kinase activity of Npr1 is required for proper post-transcriptional control of several ammonium-sensitive permeases (Boeckstaens et al. 2007). On the basis of experiments indicating that Npr1 is rapidly dephosphorylated upon rapamycin treatment (Schmidt et al. 1998), the derepression of nitrogen-regulated genes was interpreted to support the placement of TORC1 in a pathway negatively controlling NCR (Crespo et al. 2004). However, further analysis has shown that the derepression of NCR-regulated genes is linked to the known requirement of Npr1 in facilitating efficient ammonium uptake, i.e., the nitrogen source used in the initial studies. Indeed, NCR is active in npr1 mutants when ammonium uptake is restored using buffered media (see Wiame et al. 1985) or in heterologous expression of a non-Npr1-regulated ammonium transporter from the fungus Hebeloma cylindrosporum (Feller et al. 2006). Consistently, the presence of preferred amino acids glutamine, serine, or asparagine also represses NCR-sensitive genes in cells lacking Npr1 function (Tate et al. 2006). Also, Crespo et al. (2004) reported that inactivating mutations affecting the function of the E3-ubiquitin ligase Rsp5, or its associated proteins Bul1/Bul2, restores repression of NCR-regulated genes in cells lacking NPR1. In accordance with the current understanding of these components, and their role in governing the stability of plasma membrane permeases (for review see Lauwers et al. 2010), loss of Rsp5-mediated ubiquitylation prevents the degradation of nitrogen-sensitive permeases. Consequently, suppression of the npr1mutant phenotype is accounted for by the increased capacity of the npr1rsp5 double mutants to take up ammonium (Feller et al. 2006). These data demonstrate that Npr1 and TORC1 have indirect roles in regulating NCR, presumably by controlling the functional expression of ammonium permeases.
General amino acid control
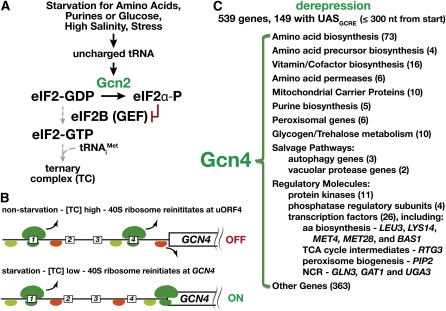
As noted by Jones and Fink (1982), many enzymes in multiple amino acid biosynthetic pathways are induced in response to starvation for any amino acid. This supra-, cross-pathway regulation is termed general amino acid control, or GAAC (reviewed in Hinnebusch and Natarajan 2002; Hinnebusch 2005). Amino acid starvation can be rapidly induced by the addition of antimetabolites [e.g., 3-amino-1,2,4-triazole, a competitive inhibitor of imidazoleglycerol-phosphate dehydratase (HIS3) that catalyzes the sixth step of histidine biosynthesis, and metsulfuron methyl, an inhibitor of acetohydroxyacid synthase (Ilv2) that catalyzes the first step of branched-chain amino acid biosynthesis] or by the removal of an amino acid required for growth of auxotrophic strains. GAAC is required for survival of cells grown on media prepared with amino acid compositions that elicit starvation through feedback inhibition of enzymes in shared pathways. For example, when cells are grown in the presence of both tyrosine and phenylalanine, mutants lacking GAAC cannot grow on media lacking tryptophan (Niederberger et al. 1981). In either of the starvation conditions just described, cells activate the expression of a large set of genes (>500) (Figure 4C), including representatives in every amino acid biosynthetic pathway, with the exception of cysteine (Table 3) (Natarajan et al. 2001; Hinnebusch and Natarajan 2002).
Figure 4 .
Schematic depiction of the GAAC pathway and the global affect of Gcn4-dependent transcription. (A) GAAC is activated when the levels of any amino acid become limiting, leading to alterations in the pools of charged tRNAs (Zaborske et al. 2009, 2010). Uncharged tRNAs bind and activate the Gcn2 kinase, which phosphorylates Ser-51 of the α-subunit of the translation initiation factor eIF2 (Wek et al. 1989; Dong et al. 2000; Qiu et al. 2001). The phosphorylated eIF2α exhibits an enhanced affinity for the GTP-GDP exchange factor eIF2Β (GEF), competitively inhibiting the rate of nucleotide exchange, resulting in a reduction in the rate of TC eIF2-GTP-Met-tRNAi formation (gray dashed arrows). (B) The gene encoding the transcription factor Gcn4, the effector of GAAC, is transcribed as an mRNA with four small open reading frames in the 5′-untranslated region (uORF; boxes 1–4) (Mueller and Hinnebusch 1986). As a scanning 40S ribosome with a TC (light green) encounters the first initiator codon of uORF1, the GTP bound to the TC is hydrolyzed to GDP, releasing the eIF2-GDP, and the 60S ribosome is recruited and translation initiates (80S ribosome, dark green). Translation terminates at the uORF1 stop codon, and the 60S ribosome dislocates; the 40S ribosome continues to scan but is unable (red) to initiate translation until it reacquires a TC. Under non-inducing conditions with a high level of TC, the 40S ribosome regains competence (light green) to initiate translation at uORF4. The translation of uORF4 interferes with initiation at GCN4. Under GAAC-inducing conditions, due to a low level of ternary complex, the 40S ribosome regains competence after passing uORF4 and initiates translation at GCN4. (C) Gcn4 binds to promoters of genes possessing the consensus UASGCRE sequence motif GAGTCA. Activation of GAAC leads to major reprograming of transcription (>500 genes are induced and >1000 are repressed) (Natarajan et al. 2001). The number of induced genes (parentheses) in the categories of proteins relevant to amino acid and nucleotide metabolism is indicated. As indicated in A, a variety of stimuli have been shown to result in increased levels of Gcn4 (Hinnebusch and Natarajan 2002). Some of these responses impinge directly on Gcn2 (Cherkasova et al. 2010; Zaborske et al. 2009, 2010) and some function independently, apparently in parallel. Notably, Gcn4 stability is increased under amino acid starvation (Kornitzer et al. 1994; Shemer et al. 2002; Bomeke et al. 2006; Aviram et al. 2008; Streckfuss-Bomeke et al. 2009).
Table 3. General Amino Acid Control (GAAC) - Gcn4-controlled genes linked to amino acid biosynthesis.
| Pathway | Genes |
|---|---|
| Histidine | HIS1, HIS2, HIS3, HIS4, HIS5, HIS7 |
| Glutamate/Glutamine | GLT1, GDH2 |
| Proline | PRO2 |
| Arginine | ARG1, ARG2, ARG3, ARG4, ARG5, 6, ARG7, ARG8, ARG80, CPA1, CPA2 |
| Lysine | LYS1, LYS2, LYS4, LYS5, LYS9, LYS12, LYS14, LYS20, LYS21 |
| Aromatic (Phe, Trp, Tyr) | ARO1, ARO2, ARO3, ARO4, ARO8, ARO9, ARO10, TRP2, TRP3, TRP4, TRP5 |
| Serine/Glycine | SER1, SER3, SER33, ICL1, AGX1, GCV1, GCV2, GCV3, LPD1, SHM2 |
| Aspartate | AAT2 |
| Asparagine | ASN1, ASN2 |
| Threonine | HOM2, HOM3, THR1, THR4 |
| Methionine | SUL1, SUL2, MET1, MET2, MET3, MET4, MET10, MET13, MET14, MET16, MET17, MET22, MET28 |
| Branched chain (Leu, Ile, Val) | ILV1, ILV2, ILV3, ILV6, LEU1, LEU4, BAT1, BAT2, LEU3 |
| Alanine | ALT1 |
| TCA cycle | ACO2, IDP1, CIT3, RTG3 |
| NCR | GLN3, GAT1 |
Data are from (Natarajan et al. (2001). Genes in boldface type encode transcription factors.
The transcriptional activator Gcn4, which binds to promoters of genes possessing the consensus UASGCRE sequence motif GAGTCA, mediates GAAC. GCN4 expression is induced in starved cells at the translational level by a reinitiation mechanism involving four short upstream open reading frames (uORFs) (Mueller and Hinnebusch 1986). The analysis of how the GAAC pathway controls GCN4 expression has defined the central mechanisms governing the initiation of protein synthesis in eukaryotes and has provided the basis for understanding translational control of gene expression (Hinnebusch 2005; Altmann and Linder 2010). The mechanistic details of GAAC regulation have advanced to a very precise level of understanding (Sonenberg and Hinnebusch 2009), and a detailed discussion is out of the scope of this review. However, in subsequent sections, the role of GAAC as integrated into the overall metabolic adjustments in growing and nonstarved cells will be discussed, as will its role in the transcriptional regulation of genes associated with amino acid biosynthesis.
An outline of the GAAC pathway and the global consequence of the induced expression of Gcn4 in metabolic regulation is presented in Figure 4. Briefly, upon amino acid starvation, multiple tRNAs become deacylated (Zaborske et al. 2009, 2010). Gcn2 has an auto-inhibited kinase domain that is allosterically activated in starved cells through binding of uncharged tRNAs to an adjacent histidyl-tRNA synthetase-like domain (Wek et al. 1989; Dong et al. 2000). The activated Gcn2 kinase phosphorylates the α-subunit of eIF2, leading to reduced levels of ternary complex (TC; eIF2-GTP-Met-tRNAiMet). The diminished levels of TC decrease the efficiency of scanning ribosomes to reinitiate translation, increasing the proportion of ribosomes that reinitiate translation at GCN4. In addition to translational control, GCN4 transcription is induced under conditions that derepress NCR (Godard et al. 2007), and starvation leads to decreased degradation of Gcn4 by the proteasome (Kornitzer et al. 1994).
Nitrogen utilization and amino acid biosynthetic pathways are coordinately regulated
The comparisons of the transcriptional response in exponentially growing cells adapted to growth in the presence of a single nitrogen source make the global analyses carried out by Godard et al. (2007) unique. Specifically, the absence of gross experimental impositions, such as a shift in nitrogen source, mutational inactivation of genes, or use of inhibitors, provides novel insight as to how multiple general modes of regulation, i.e. UPR and GAAC, are integrated with NCR to coordinate the pathways regulating nitrogen source utilization. The UPR is required to modulate processes promoting efficient protein folding in the endoplasmic reticulum (ER); in response to increased levels of folding intermediates or the presence of misfolded proteins in the ER, the UPR is activated to restore folding homeostasis (reviewed in Bernales et al. 2006). Accordingly, UPR activation in cells using preferred nitrogen sources presumably reflects an increased demand for ER lumenal chaperones to support the higher rates of protein synthesis in rapidly growing cells. Finally, this study demonstrated that SPS-sensor-controlled gene expression is induced in the presence of high concentrations of most amino acids. This latter finding indicates that cells maintain the expression of broad-specificity amino acid permeases to ensure an enhanced amino acid uptake capacity.
The derepression of GAAC gene expression in cells conditioned for growth using nonpreferred nitrogen sources is significant and clearly supports the notion that GAAC is not merely a starvation response, but is integral to the proper regulation of the amino acid biosynthetic capacity of growing cells. The pronounced activation of GAAC in cells grown in amino acids classified as class B nonpreferred nitrogen sources (Table 1) (Godard et al. 2007) suggested that Gcn2 is activated. This was tested by examining the growth of strains lacking GCN2, and consistently, the gcn2Δ strains exhibited reduced growth in the presence of nonpreferred nitrogen sources. These results clearly indicate that Gcn2 is important to achieving a balanced logarithmic mode of growth on these nitrogen sources. Consistently, it has been demonstrated that shifting cells from ammonium to nonpreferred nitrogen sources leads to alterations in the pools sizes of charged tRNA, leading to Gcn2-dependent phosphorylation of eIF2α (Staschke et al. 2010). Finally, GCN4 mRNA abundance also correlated with derepression of NCR-sensitive genes (Godard et al. 2007). Thus, cells using nonpreferred nitrogen sources modulate Gcn4 levels at both the transcriptional and the translational levels in a manner that synergistically increases NCR- and GAAC- regulated gene expression. In the absence of a preferred nitrogen source, the combined derepression of NCR- and GAAC-regulated gene expression reflects the ability of cells to reprogram patterns of gene expression to optimize the catabolic utilization of nonpreferred amino acids and to simultaneously modulate protein synthesis in a manner that prioritizes processes leading to increased amino acid uptake and biosynthesis.
Integration of general and specific modes of regulation
The metabolic pathways in yeast are tuned to nutrient availability. Amino acid utilization and biosynthetic pathways rely on multiple modes of regulation to coordinate metabolic events. Here aspects of the arginine, lysine, methionine, and serine metabolic pathways will be discussed as paradigm examples to illustrate important concepts and the diversity of regulatory mechanisms. In these pathways, both general (NCR and GAAC) and specific modes of transcriptional control operate and function together to achieve nuanced responses. Integration of signals to generate the correct transcriptional response often occurs at the level of the promoters of regulated genes and requires the ordered combinatory assembly of multisubunit complexes. Arginine metabolism provides good examples of end product (arginine) inhibition at the enzyme (allosteric control), transcriptional (direct binding to the ArgR/Mcm1 transcription factor), and translational (CPA1 expression) levels. Lysine biosynthesis illustrates how end-product inhibition coupled with the direct transcriptional regulation by the pathway intermediate α-aminoadipate semialdehyde (α-AAS) permits the fine-tuning of biosynthetic pathways. Recent advances regarding the regulation of methionine biosynthesis indicates that polyubiquitylation is not necessarily linked to metabolite-induced degradation of the transcriptional activator Met4. Finally, the expression of SER3, encoding an enzyme in the serine biosynthetic pathway, provides an example of the recently uncovered role of small noncoding RNAs in governing gene expression.
Arginine metabolism:
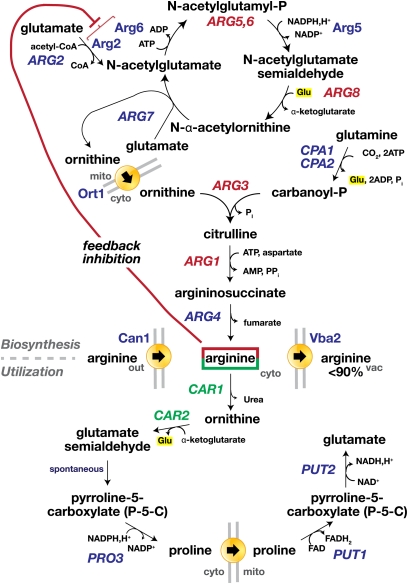
The arginine utilization (Cooper 1982b) and biosynthetic pathways (Jones and Fink 1982) are outlined and described in Figure 5. If available, arginine is transported into cells by the arginine permease Can1 (Hoffmann 1985), after which it is transported into the vacuole by the Vba2 transporter (Ohsumi and Anraku 1981; Sato et al. 1984; Shimazu et al. 2005). Greater than 90% of free arginine within cells, i.e., arginine not incorporated in protein, is compartmentalized within the vacuole (Messenguy et al. 1980; Kitamoto et al. 1988; Ohsumi et al. 1988). The mechanisms enabling cells to properly coordinate and regulate levels of arginine in cytoplasmic pools are not well understood. However, it is well established that noncompartmentalized arginine exerts a positive effect on the expression of genes encoding enzymes required for arginine utilization and a repressive effect on genes required for arginine biosynthesis (Dubois et al. 1978).
Figure 5 .
Arginine metabolic network. Arginine is primarily transported into cells by the arginine permease Can1 (Table 4), and once internalized, the bulk of arginine is transported into the vacuole by the Vba2 transporter (Table 6). Cytoplasmic arginine exerts positive (green) and negative (red) effects on gene expression encoding enzymes required for arginine utilization and catabolism, respectively. Both positive and negative regulation relies on the ArgM/Mcm1 complex, which in an arginine-dependent manner participates in activating the expression of the genes in green and repressing the genes in red. (Arginine utilization; bottom) Arginine is degraded to form glutamate. Arginine is initially degraded in the cytoplasm to form proline; this requires the concerted action of arginase (CAR1) and ornithine aminotransferase (CAR2) to form glutamate γ-semialdehyde, which spontaneously converts to Δ1-pyrroline-5-carboxylate (P5C). P5C is converted to proline by the PRO3 gene product. Cytoplasmic proline is transported into the mitochondria where it is converted back to P5C by proline oxidase (PUT1). Finally, the mitochondrial P5C is converted to glutamate by the PUT2 gene product. Whereas CAR1 and CAR2 are positively regulated by the presence of arginine (discussed below), the expression of PUT1 and PUT2 is induced by proline (Marczak and Brandriss 1989; Siddiqui and Brandriss 1989). Proline binds directly to the transcription factor Put3, a member of the well-studied Zn(II)2Cys6 binuclear cluster family of transcriptional regulators (Des Etages et al. 2001). The activation of Put3 requires no additional components and can be induced by certain proline analogs with an unmodified pyrrolidine ring (Sellick and Reece 2003). Detailed structural analysis indicates that proline directly controls the regulatory properties of transcriptional activator, providing a clear demonstration of how metabolite recognition and transcriptional control can be directly coupled (Sellick and Reece 2005). (Arginine biosynthesis; top) The first five steps of biosynthesis take place in the mitochondria (ARG2, ARG5,-6, ARG8, ARG7) and result in the synthesis of ornithine. ARG5,-6 encode the enzymes that catalyze the second and third steps and are translated into a pre-protein that is imported into mitochondria, where it is cleaved, resulting in separate proteins, i.e., N-acetylglutamate kinase (Arg6) and N-acetylglutamyl-phosphate reductase (Arg5) (Boonchird et al. 1991). The first two enzymes in the pathway, N-acetylglutamate synthase (Arg2) and N-acetylglutamate kinase (Arg6), bind each other, forming a complex that is necessary for their stability and for feedback inhibition by arginine (Abadjieva et al. 2000, 2001; Pauwels et al. 2003). The ornithine synthesized in mitochondria is transported to the cytoplasm via the mitochondrial carrier protein Ort1 (Table 5), and the remaining steps are carried out in the cytoplasm. Carbomoyl phosphate reacts with ornithine to form arginine in three steps (ARG3, ARG1, ARG4). Carbomoyl phosphate is synthesized from CO2, ATP, and the amide nitrogen of glutamine in a reaction catalyzed by the arginine-specific carbomoyl phosphate synthetase, a heterodimeric enzyme composed of a small regulatory subunit (CPA1) and a catalytic subunit (CPA2).
The positive and negative effects on arginine-dependent gene expression are mediated by the multimeric ArgR/Mcm1 complex that binds upstream regulatory motifs in the promoters of arginine-sensitive promoters. The ArgR/Mcm1 complex minimally consists of Arg80, Arg81, Arg82, and Mcm1. Arg80 and Mcm1 are MADS box proteins that are known to coregulate gene expression by facilitating the cooperative binding of diverse sequence-specific factors to cognate promoters (Messenguy and Dubois 2003). Mcm1 and Arg80 form heterodimers that bind arginine-regulated promoters more efficiently than Mcm1 or Arg80 homodimers (Amar et al. 2000). The N-terminal domain of Arg81 contains a region related to bacterial arginine repressors, and mutations affecting the conserved residues alter the arginine concentration required for DNA-binding activity of ArgR/Mcm1. Consequently, Arg81 is a good candidate for being the arginine sensor that regulates the activity of the complex (Amar et al. 2000). Consistent with a sensor/regulator function, Arg81 facilitates the arginine-dependent recruitment of the Arg80/Mcm1 dimers to promoters. Arg82 is thought to stabilize Arg80/Mcm1 dimers. Arg82 exhibits inositol polyphosphate kinase activity; however, this activity is dispensable for proper ArgR-dependent repression of ARG genes (Dubois et al. 2000). In contrast, the kinase activity of Arg82 is required for proper Gat1-mediated derepression of NCR-sensitive genes and for phosphate-mediated repression of PHO genes (El Alami et al. 2003).
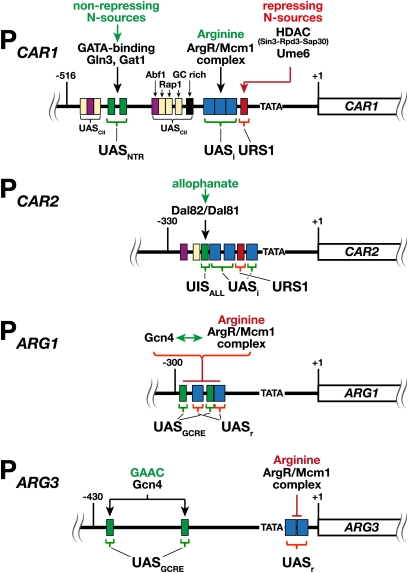
The promoters of four arginine-sensitive promoters are schematically depicted in Figure 6; CAR1 (PCAR1) and CAR2 (PCAR2) are positively controlled, whereas ARG1 (PARG1) and ARG3 (PARG3) are negatively controlled by arginine. PCAR1 contains up to 14 different cis-acting promoter elements, of which at least 11 are functionally active and contribute to regulate CAR1 expression (Smart et al. 1996; Dubois and Messenguy 1997). Only three of these promoter elements will be discussed, i.e., the repressing URS1 motif, the UASi required for arginine induction, and the UASNTR required for NCR-controlled transcription. Expression of CAR1 is largely dependent on overcoming the strongly repressing effect of URS1; mutations that modify the URS1 lead to constitutive expression in the absence of arginine induction. Under non-inducing conditions, Ume6 binding to URS1 recruits the components of the histone deacetylase complex Sin3–Rpd3–Sap30, which results in a repressed state (Messenguy et al. 2000). Upon nitrogen starvation, the repression at URS1 is released and Ume6 interacts with the ArgR/Mcm1 complex, presumably enhancing the binding of this complex to the three UASi motifs in a manner that facilitates induced expression. To achieve full derepression of CAR1, Gln3 and Gat1 binding to GATA sequences in the UASNTR elements is required (Smart et al. 1996). Finally, CAR1 expression is nonspecifically induced by the addition of micromolar amounts of the amino acids (Dubois and Wiame 1976; Godard et al. 2007). Although the precise mechanism responsible for this effect has not been established, mutations inactivating the SPS-sensing pathway prevent this nonspecific induction (Klasson et al. 1999). The regulatory mechanisms controlling CAR2 expression appear similar to CAR1 (Figure 6); however, PCAR2 is not under NCR control (Deschamps et al. 1979), but rather is responsive to allophanate, a degradation product of urea. Consistently, PCAR2 lacks an UASNTR, but instead has an upstream inducing sequence motif (UISALL) required for allophanate induction (Park et al. 1999). The allophanate-regulated factor Dal82 and its coactivator Dal81 bind the UISALL.
Figure 6 .
Schematic diagram of the arginine-sensitive promoters PCAR1, PCAR2, PARG1, and PARG3. PCAR1 and PCAR2 are induced, whereas PARG1 and PARG3 are repressed by arginine in an ArgR/Mcm1-dependent manner. The promoter elements, i.e., the sites for specific DNA-binding proteins, are color coded as follows: red, URS1 (Ume6 binding); blue, UASi and UASr (ArgR/MCM1 binding); black, GC rich; light yellow, Rap1; purple, Abf1; green, UASNTR (PCAR1; GATA factor Gln3 and Gat1 binding), UISALL (PCAR2; Dal82/Dal81 binding), or UASGCRE (PAGR1 and PARG3; Gcn4 binding). Coordinates are relative to the translation start sites.
Regulation of the functional expression of the arginine biosynthetic pathway genes is complex. The transcription of ARG1, ARG3, ARG4, ARG5, 6, and ARG8 is repressed by arginine via the ArgR/Mcm1 complex (Messenguy and Dubois 2003; Godard et al. 2007), and in addition, nine of the genes (i.e., ARG1–ARG8, CPA1, and CPA2) are targets of GAAC (Natarajan et al. 2001). Chromatin-immunoprecipitation experiments, used to probe ArgR/Mcm1 repressor binding to the ARG1 promoter PARG1 (Figure 6), have revealed that Gcn4 binding to PARG1 strongly enhances the subsequent arginine-dependent assembly of ArgR/Mcm1 repressor complexes (Yoon et al. 2004). Arg80/Mcm1 heterodimers lacking Arg81 and Arg82 are efficiently recruited to PARG1 in a Gcn4-dependent and an Arg81-independent manner either in the presence or the absence of exogenous arginine. The presence of arginine stimulates the recruitment of Arg81 and Arg82. These findings suggest that Gcn4 facilitates the binding of an Arg80/Mcm1 heterodimer to UASr motifs and that, under conditions of arginine excess, arginine binding promotes the subsequent assembly of a functional holo-ArgR/Mcm1 repressor complex. Conversely, during arginine starvation Mcm1 exerts a positive role in ARG1 transcription. Mcm1 binding to PARG1 enhances Gcn4 binding and recruitment of the positively acting SWI/SNF ATP-dependent chromatin-remodeling complex (Yoon and Hinnebusch 2009; Hong and Yoon 2011). In summary, Gcn4 and Mcm1 function cooperatively, and arginine availability controls the repressor or activator functions of the ArgR/Mcm1 complex at PARG1.
The role of Gcn4 binding at PARG1 under GAAC-inducing conditions in the absence of arginine has been intensively studied (Govind et al. 2005; Kim et al. 2005). In response to amino acid starvation, the binding of Gcn4 to the PARG1 UASGCRE is facilitated by its interactions with the Cyc8–Tup1 complex (Kim et al. 2005). Gcn4 binding initiates the nearly simultaneous recruitment of SAGA histone acetylase (HAT), SWI/SNF, and Mediator components [Mediator facilitates interactions between specific coactivation complexes and RNA polymerase II (Pol II)] (Govind et al. 2005). These coactivators, together with RSC (ATP-dependent chromatin-remodeling complex), coordinate the rapid recruitment of TATA-binding protein (TBP)–TFIID and Pol II to the promoter, stimulating preinitiation complex assembly and elongation through ARG1 (see Roeder 2005 for a review on transcriptional coactivators). The finding that amino acid starvation-induced Gcn4 binding results in the rapid recruitment of coactivators suggests cooperative or synergistic interactions between these factors (Govind et al. 2005). Consistent with this notion, the SAGA HAT subunit Gcn5 is required for wild-type kinetics of SWI/SNF recruitment, and RSC function is needed for optimal SAGA recruitment. Also, Mediator is strongly required for activator recruitment of both SAGA and SWI/SNF.
Deletion of CYC8 confers sensitivity to metsulfuron methyl, an inhibitor of isoleucine/valine biosynthesis, suggesting that Cyc8-Tup1 is broadly required in facilitating Gcn4-dependent activation of GAAC-controlled genes. The positive role of Cyc8 in GAAC is consistent with its requirement in activating Rtg3-dependent CIT2 transcription in response to mitochondrial dysfunction (Conlan et al. 1999). In the absence of Cyc8, the diminished binding of Gcn4 to PARG1 is severe enough to reduce the recruitment of SAGA, Mediator, TBP, and RNA Pol II (Kim et al. 2005). The overexpression of GCN4 does not suppress these defects, raising the possibility that Gcn4 may enhance its own binding to the UASGCRE by recruiting Cyc8-Tup1. Together, these findings clearly demonstrate the important role of Cyc8 in the induction of amino acid biosynthetic gene expression.
Two sequence motifs involved in arginine-mediated repression of ARG3 lie immediately downstream of the TATA box in PARG3 (de Rijcke et al. 1992). To mediate ArgR/Mcm1-dependent repression, these motifs must be located close to the transcription initiation start site; however, they remain functional even when the TATA box is moved to a downstream location. The placement of the arginine response element in PARG3 is consistent with ArgR/Mcm1 binding interfering with the assembly (or functioning) of the transcriptional preinitiation complex. Interestingly, the displacement of the UASr to a far upstream position 5′ of the most proximal UASGCRE abolishes its repressive effect. In this new context, the ArgR/Mcm1-binding motif serves to enhance ARG3 expression in the presence of arginine, but only in the absence of Gcn4. These findings are consistent with the more recent studies analyzing the arginine-sensitive promoters discussed above.
Carbomoyl phosphate is required during the synthesis of arginine (Figure 5). This intermediate is derived from CO2, ATP, and the amide nitrogen of glutamine in a reaction catalyzed by the arginine-specific carbomoyl phosphate synthetase, a heterodimeric enzyme composed of a small regulatory subunit (CPA1) and catalytic subunit (CPA2). The expression of CPA1 is regulated at the level of translation in manner that is fundamentally distinct from the mechanism controlling the translation of GCN4 (Werner et al. 1987; Delbecq et al. 1994; Gaba et al. 2001). The CPA1 mRNA has a 250-nt leader that encodes a uORF composed of 25 codons, termed the arginine attenuator peptide. In the absence of arginine, ribosomes are able to reach the downstream start codon of the Cpa1-coding sequence by scanning past the uORF. However, in the presence of high levels of cytoplasmic arginine, ribosomes synthesizing the uORF polypeptide stall at its termination codon in an arginine attenuator peptide sequence-dependent manner. As a consequence of stalled ribosomes, CPA1 mRNA is degraded by the induction of nonsense-mediated mRNA decay (Gaba et al. 2005). Thus, the translational regulation of CPA1 occurs by impairing ribosome scanning and not by affecting reinitiation, as is the case of the translational control of GCN4 expression (Hood et al. 2009).
Lysine metabolism:
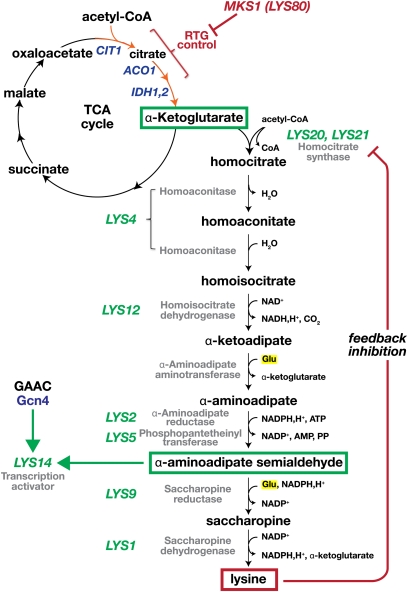
Lysine is synthesized from α-ketoglutarate via the fungal-specific α-aminoadipate (AAA) pathway (Figure 7) (Xu et al. 2006). This pathway is composed of eight enzymatic steps involving nine gene products; with the exception of the gene encoding the α-aminoadipate aminotransferase, catalyzing the fourth step of the pathway, all LYS genes have been defined. The first step of the pathway (homocitrate synthase) is catalyzed by either Lys20 or Lys21. The homocitrate synthase activity of both Lys20 and Lys21 is feedback-inhibited by lysine (Andi et al. 2005). These proteins are 90% identical (Chen et al. 1997), and although Lys20 activity accounts for ∼70% of the flux through the pathway, both Lys20 and Lys21 can individually support wild-type growth in the absence of the other during fermentative growth on glucose (Feller et al. 1999; Quezada et al. 2008). However, during respiratory growth, e.g., using ethanol as carbon source, inactivating mutations in LYS21 but not LYS20 impair growth. Under these conditions, the levels of Lys20 are reduced post-transcriptionally, as LYS20 mRNA is unaffected by carbon source. Together, these findings suggest that, during respiratory growth, cells control the activity of Lys20 to avoid diverting α-ketoglutarate into lysine biosynthesis (Quezada et al. 2008). Interestingly, in cells grown in the absence of exogenously added lysine, under conditions where LYS gene expression is derepressed, Lys20 and Lys21 specifically localize to the nucleus (Chen et al. 1997); the functional significance of their nuclear localization remains unknown. The next two steps of the pathway from homocitrate to α-ketoadipate (LYS4 and LYS12), and the final three steps from α-aminoadipate to lysine (LYS2/LYS5, LYS9, and LYS1) are thought to take place in the mitochondria and cytosol, respectively (Xu et al. 2006). Several mitochondrial carrier family members (Odc1, Odc2, and Ctp1) are implicated in the transport of AAA intermediates across the mitochondrial inner membrane (Table 5) (Breitling et al. 2002; Palmieri et al. 2006).
Figure 7 .
Lysine biosynthetic pathway. LYS gene expression is controlled in response to the levels of α-AAS. This pathway intermediate binds and activates the pathway-specific transcription factor Lys14. As a consequence of a pathway intermediate controlling Lys14, conditions that increase or decrease the flux through the pathway, positively or negatively, affect LYS gene expression, respectively. The pathway is stimulated by the precursor α-ketoglutarate and consistently activated in cells lacking MKS1. Conversely, due to feedback inhibition of the first step of the pathway (catalyzed by either Lys20 or Lys21), excess lysine reduces the production of α-AAS and causes apparent repression of the LYS genes. LYS14 is subject to GAAC regulation, which suggests that derepression of all eight LYS genes under amino acid starvation conditions is mediated through Gcn4-induced LYS14 expression (Natarajan et al. 2001).
Table 5. Mitochondrial carrier family: transporters directly coupled to amino acid metabolism.
| Systematic name | Gene name | Description (substrate specificity) | Transport in/out organelle | Reference |
|---|---|---|---|---|
| YPR021c | AGC1a | Aspartate-glutamate transport (Asp, Glu) | aspartate → out, in ← glutamate | Cavero et al. (2003) |
| YPL134c | ODC1 | Lysine and glutamate biosynthesis/lysine catabolism/nitrogen assimilation (α-ketoglutarate, α-ketoadipate) | α-ketoadipate → out, in ← α-ketoglutarate | Palmieri et al. (2001) |
| YOR222w | ODC2a | Lysine and glutamate biosynthesis/lysine catabolism/nitrogen assimilation (α-ketoglutarate, α-ketoadipate) | α-ketoadipate → out, in ← α-ketoglutarate | Palmieri et al. (2001) |
| YOR130c | ORT1a (ARG11) | Arginine biosynthesis (ornithine) | ornithine → out, in ← H+ | Crabeel et al. (1996); Palmieri et al. (1997); Soetens et al. (1998) |
| YBR291c | CTP1 | Lysine biosynthesis - Lys14 regulated; suggested role in peroxisome membrane (citrate, malate) | citrate → out, in ← malate | Breitling et al. (2002); Kaplan et al. (1995) |
| YKL120w | OAC1a | Leucine biosynthesis (oxalacetate, α-isopropyl malate) | α-isopropylmalate → out, in ← oxalacetate | Marobbio et al. (2008) |
| YBL089w | YMH2 | Arginine biosynthesis (citrate, α-ketoglutarate) | citrate → out, in → α-ketoglutarate | Castegna et al. (2010) |
| YHR002w | LEU5 | Leucine biosynthesis - CoA transport (cofactor A) | in | Prohl et al. (2001) |
| YPR058w | YMC1a | Lysine and glutamate biosynthesis (α-ketoglutarate) | n.d. | Trotter et al. (2005) |
| YBR104w | YMC2a | Lysine and glutamate biosynthesis (α-ketoglutarate) | n.d. | Trotter et al. (2005) |
| YNL003c | SAM5 (PET8) | Methionine biosynthesis (S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAHC)) | In ← SAM, SAHC → out | Marobbio et al. (2003) |
| ThPP = Thiamine pyrophosphate | ||||
| TMP = Thiamine monophosphate |
GAAC-regulated expression (Natarajan et al. 2001)
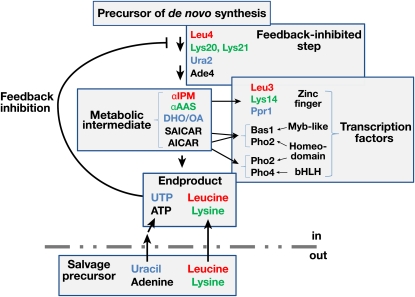
A fundamental feature of this pathway is that expression of the LYS genes is controlled in response to the levels of α-AAS. This pathway intermediate binds and activates the pathway-specific transcription factor Lys14 (Becker et al. 1998; El Alami et al. 2002). Lys14 is a member of the Zn(II)2Cys6 binuclear cluster family of transcriptional regulators, which are constitutively nuclear and found associated with promoter sequences of the genes they regulate (reviewed in Campbell et al. 2008). As a consequence of a pathway intermediate controlling the capacity of Lys14 to activate gene expression, conditions that increase or decrease the flux through the pathway, positively or negatively, affect LYS gene expression. The pathway is stimulated by the precursor α-ketoglutarate, which accounts for the observed activation of the pathway in cells lacking MKS1 (LYS80) (Feller et al. 1997). Inactivation of MKS1 leads to increased α-ketoglutarate levels due to activation of the retrograde response, which induces Rgt1/Rtg3-dependent transcription of genes encoding the TCA cycle enzymes citrase synthase (CIT1), aconitase (ACO1), and isocitrate dehydrogenase (IDH1,-2) (Liu et al. 2003, 2005). Consequently, the increased flux in the pathway results in elevated production of α-AAS, turning on Lys14-dependent expression of all LYS genes (Dilova et al. 2002). Conversely, due to feedback inhibition of the first step of the pathway (catalyzed by either Lys20 or Lys21) (Andi et al. 2005), excess lysine reduces the production of α-AAS and causes apparent repression of the LYS genes. Similar regulatory schemes integrating both the final product concentration and the flux in the pathway (sensed via the concentration of strategic metabolic intermediates) are found in the leucine, purine, and pyrimidine synthesis pathways (Figure 8) (Flynn and Reece 1999). This dual mechanism permits fine-tuning of biosynthetic pathways.
Figure 8 .
Transcriptional regulation of biosynthetic pathways by metabolic intermediates. The expression of genes encoding catalytic components in the lysine (green), leucine (red), pyrimidine (blue), and purine (black) is controlled by pathway-specific transcription factors that induce transcription upon binding a metabolic intermediate of the pathway. In these pathways, feedback inhibition by the end product of the first and committing step of the pathway provides the means to decrease the production of the inducer and cause the apparent repression of the pathway. This dual-sensing mechanism permits fine-tuning of biosynthetic pathways by integrating both the final end-product concentration, whether synthesized or transported into cells via salvage mechanisms, and the flux in the pathway (as sensed via the concentration of strategic metabolic intermediates).
Finally, LYS gene expression is coordinately induced in cells lacking functional peroxisomes, suggesting that α-AAS is normally sequestered within this organelle (Breitling et al. 2002). This latter finding raises the possibility that one or more steps of basal lysine biosynthesis may occur within peroxisomes, which would restrict α-AAS from entering the nucleus and preventing improper induction of Lys14-dependent gene expression.
Methionine metabolism:
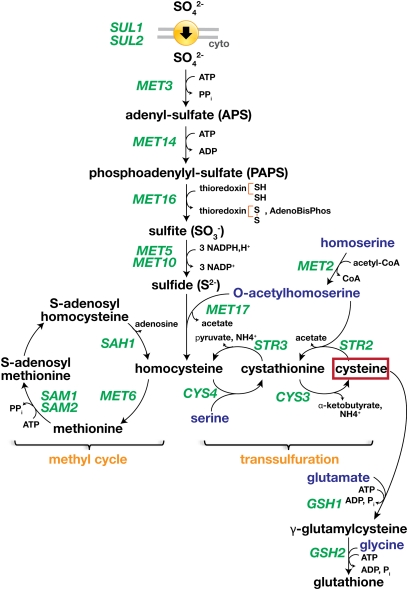
The synthesis of the sulfur-containing amino acids methionine and cysteine has been extensively studied (reviewed in Thomas and Surdin-Kerjan 1997). The synthetic pathways of these amino acids also provide cells with glutathione and S-adenosylmethionine (SAM) (Figure 9). Glutathione functions as a major redox buffer maintaining the reducing environment of the cytoplasm and is required for cell survival under cadmium and arsenic stress (Dormer et al. 2000; Baudouin-Cornu and Labarre 2006). SAM serves as a methyl donor and is an important precursor for the synthesis of polyamines, vitamins, phospholipids, and modified nucleotides.
Figure 9 .
Sulfur metabolic network. Three major branches of the sulfur metabolic network have been defined. First, sulfate is transported into cells via the sulfate permeases (SUL1 and SUL2) and is reduced to sulfide (MET3, MET14, MET16, MET5, and MET10). Second, sulfide is incorporated in the formation of homocysteine (MET17) from O-acetylhomoserine that is derived from homoserine (MET2). Third, homocysteine is converted to methionine and SAM in the methyl cycle (MET6, SAM1, SAM2, SAH1) or converted to cysteine in the two steps of the trans-sulfuration pathway (CYS4 and CYS3). Glutathione is synthesized from cysteine (GSH1, GSH2). The sulfur-containing compounds are written in black. The levels of cysteine negatively control the activity of Met4-dependent transcription. The genes under positive control by Met4 are indicated in green.
The expression of the majority of genes encoding enzymes of the sulfur metabolic network requires the transcriptional activator Met4 (Thomas and Surdin-Kerjan 1997; Lee et al. 2010). Although the C-terminal region of Met4 contains a dimerization/DNA-binding domain of the basic-leucine zipper family, Met4 lacks DNA-binding activity. Hence the ability of Met4 to activate transcription depends on interactions with DNA-binding factors that act as dedicated adaptors for recruiting Met4 to promoters. Met4 interacts directly with either of two highly similar zinc-finger proteins, Met31 and Met32, or with the basic-helix-loop-helix protein Cbf1. In a recent transcriptome analysis, 45 core Met4-dependent promoters were identified, and each contained a Met31/Met32-binding site that consisted of a CTGTGGC motif; in 24 of these promoters, a Cbf1 motif with an invariant sequence of CACGTGA is present (Lee et al. 2010). Thus, the association of Cbf1 and Met31/32 with their respective DNA elements in MET promoters appears to provide platforms for recruiting and interacting with Met4. An additional cofactor, Met28, which also lacks DNA-binding activity, is thought to stabilize DNA-bound Met4 complexes (Kuras et al. 1997; Blaiseau and Thomas 1998). Under sulfur-limiting conditions, these interactions enable Met4 to activate transcription through recruitment of the SAGA histone acetyltransferase and Mediator coactivator complexes (Kuras et al. 2002; Leroy et al. 2006).
The activity of Met4 is tightly controlled according to the sulfur status of the cell; the intracellular level of cysteine provides the major regulatory signals for MET gene expression (Hansen and Johannesen 2000). Under repressing conditions when cysteine is abundant, the activation potential of Met4 is negatively controlled by SCFMet30 (Kaiser et al. 2000; Rouillon et al. 2000; Kuras et al. 2002; Flick et al. 2004; Chandrasekaran et al. 2006; Menant et al. 2006; Ouni et al. 2010). Met30 is the substrate recognition subunit of the essential Skp1/Cdcd53/F-box protein Met30 (SCFMet30) ubiquitin ligase complex. In striking contrast to most studied instances in which SCF-complex ubiquitylation targets substrates for degradation by the 26S proteasome (reviewed in Jonkers and Rep 2009), the ubiquitylation of Met4 by SCFMet30 is not strictly linked to its immediate degradation (Kaiser et al. 2000; Flick et al. 2004). In the context of its association with SCFMet30, polyubiquitylated Met4 is stabilized by interactions with its cofactors Cbf1, Met31, and Met32 (Chandrasekaran et al. 2006; Chandrasekaran and Skowyra 2008). Conversely, the cofactors associated with Met4-SCFMet30 are polyubiquitylated and targeted for degradation (Ouni et al. 2010). Under cysteine-limiting conditions, due to inhibition of SCFMet30, perhaps linked to the dissociation of the Met30 (Barbey et al. 2005), Met4 and its bound cofactors are not ubiquitylated, and MET gene expression is induced (Kuras et al. 2002; Leroy et al. 2006). The pools of free Cbf1, Met31, and Met32 not associated with Met4 are degraded in a constitutive manner (Ouni et al. 2010).
The importance of SCFMet30 control of Met4 activity is clearly demonstrated by the finding that the lethality resulting from the inactivation of MET30, leading to unbridled Met4 activation function, can be bypassed by deletion of the activation domain of Met4 or the deletion of MET32, but not of CBF1, MET28, or MET31 (Patton et al. 2000; Su et al. 2005). Consistent with Met32 having an important role in MET gene expression, a truncated version of Met32 (Met32Δ145–192) acts as a dominant suppressor of met30 null mutations by interfering with the recruitment of Met4 to both Cbf1 and Met31/32-dependent promoters (Su et al. 2008).
The transcription of MET30 is itself regulated in response to the sulfur status of cells and is dependent upon Met4. Consequently, Met4 appears to reciprocally control its own levels through a feedback-like mechanism that regulates the amount of assembled SCFMet30 ubiquitin ligase (Rouillon et al. 2000). Finally, although GAAC is thought to have a limited role in MET gene expression under methionine-limiting conditions, starvation for histidine or tryptophan results in strong Gcn4-dependent induction of several MET genes, including MET1, MET10, MET13, MET16, MET17, MET22, MET28, SUL1, and SUL2 (Natarajan et al. 2001). The fact that Gcn4 induces MET28 suggests that GAAC may indirectly activate MET genes by facilitating the stability of pathway-specific activation complexes. In addition, and perhaps in a synergistic manner, SAM1 and SAM2, which encode SAM synthetases, have been reported to be repressed two- to fivefold under GAAC-inducing conditions. Thus, it is possible that GAAC-dependent repression of SAM synthetase decreases the SAM pool and activates MET gene transcription by reducing SCFMet30-mediated degradation of Met4 (Natarajan et al. 2001).
Serine biosynthesis:
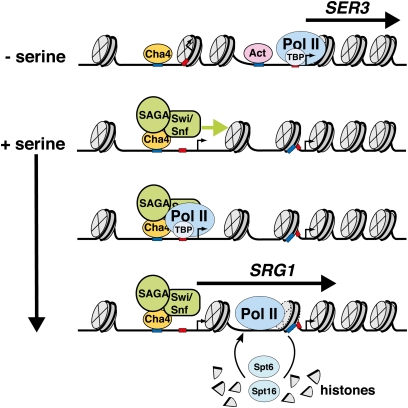
SER3 encodes phosphoglycerate dehydrogenase, which catalyzes the first step in serine biosynthesis from 3-phosphoglycerate (Figure 2). SER3 expression is negatively regulated by serine availability by a newly discovered mechanism that involves the expression of SRG1, a small noncoding RNA (Martens et al. 2004). High serine levels induce transcription of SRG1, and its expression is associated with repositioning of nucleosomes in a region that overlaps the SER3 promoter, which consequently represses SER3 (Figure 10) (Hainer et al. 2011). Expression of SRG1 is activated by the well-characterized transcription factor Cha4 (Martens et al. 2005), a member of the Zn(II)2Cys6 binuclear cluster family of transcriptional regulators (Holmberg and Schjerling 1996). In a serine-dependent manner, Cha4 recruits the SAGA and Swi/Snf coactivator complexes to the SRG1 promoter, events also required for SER3 repression. Importantly, Cha4 binds to UASCHA elements in the promoter of genes required for serine/threonine catabolism and, in response to serine or threonine induction, activates their expression, e.g., CHA1 encoding the catabolic serine/threonine deaminase (Holmberg and Schjerling 1996). Taken together, these findings demonstrate that serine repression of SER3 transcription occurs by activating SRG1 intergenic transcription. Thus, yeast uses the same transcription factor to simultaneously activate and repress opposing pathways to regulate serine biosynthesis and catabolism.
Figure 10 .
Model for the repression of SER3 by SRG1 intergenic transcription. In the absence of serine, the Cha4 activator is bound to the SRG1 promoter but is unable to initiate transcription. The SER3 promoter is depleted of nucleosomes allowing proteins, either an as-yet-unknown sequence-specific activator or general transcription factors, to bind and activate SER3 transcription. In response to serine, Cha4 recruits SAGA and Swi/Snf to reposition the nucleosomes at the 5′ end of SRG1 toward the SER3 promoter, permitting initiation of SRG1 transcription. These repositioned nucleosomes are then disassembled ahead of the transcribing RNA Pol II and reassembled after passage of RNA Pol II by the Spt6 and Spt16 histone chaperones. The nucleosomes being maintained by SRG1 transcription occlude the SER3 promoter, preventing the binding of transcription factors and SER3 transcription. This figure and legend, orginally published in Pruneski and Martens (2011), are reproduced in accordance with Landes Bioscience policy, the publishers of Cell Cycle, with permission of the authors.
SPS-sensor signaling: extracellular amino acid-induced nitrogen source uptake
During the past 10 years, it has become clear that yeast cells possess and use plasma membrane-localized sensing systems to obtain information regarding concentrations of nutrients in the extracellular environment, including the availability of amino acids, ammonium, and glucose (reviewed in Forsberg and Ljungdahl 2001b; Zaman et al. 2008; Rubio-Texeira et al. 2010). Several of these newly discovered nutrient sensors have components that are members of protein families of well-characterized nutrient transporters. Interestingly, the ability of these transporter homologs to transduce nutrient (ligand)-induced signals across the plasma membrane appears to be independent of nutrient uptake, and thus these sensor components apparently function analogously to traditional ligand-activated receptors.
Growing yeast cells respond to the presence of micromolar amounts of extracellular amino acids by inducing the expression of genes required for their uptake. This nutrient-induced response is mediated by the SPS-sensing pathway (reviewed in Ljungdahl 2009). This pathway derives its name from the three core components of the plasma membrane-localized SPS sensor, i.e., Ssy1, Ptr3, and Ssy5 (Forsberg and Ljungdahl 2001a). The SPS sensor regulates gene expression by controlling the activity of two transcription factors, Stp1 and Stp2 (Figure 11A) (Andréasson and Ljungdahl 2002). These factors are synthesized as latent cytoplasmic proteins with N-terminal regulatory domains that function as nuclear exclusion determinants (Andréasson and Ljungdahl 2004). Upon induction by extracellular amino acids, the SPS sensor catalyzes an endoproteolytic processing event that cleaves the regulatory N-terminal domains. The shorter forms of Stp1 and Stp2 efficiently target to the nucleus where they bind promoters of a limited set of genes, including a subset of broad-specificity amino acid permeases (cluster 1, Table 4) and the peptide transporter Ptr2 (Didion et al. 1996, 1998; de Boer et al. 1998, 2000; Iraqui et al. 1999; Klasson et al. 1999; Wielemans et al. 2010; Tumusiime et al. 2011).
Figure 11 .
Schematic diagram of the SPS-sensing pathway of extracellular amino acids. (A) In cells grown in the absence of inducing amino acids (left), the SPS sensor of extracellular amino acids is present in the plasma membrane (PM) in its preactivation conformation (Forsberg and Ljungdahl 2001a), and the transcription of SPS-sensor-regulated genes, i.e., amino acid permeases (AAP), occurs at basal levels, and cells exhibit low rates of amino acid uptake. The transcription factors Stp1 and Stp2 (DNA-binding motifs, green boxes) are synthesized as inactive precursors that localize to the cytosol due to the presence of their N-terminal regulatory domain (anchor) that prevents them from efficiently entering the nucleus. Low levels of full-length Stp1 and Stp2 that escape cytoplasmic retention (dashed arrow, left panel) are prevented from derepressing AAP gene expression due to activity of the Asi complex (Asi1–Asi2–Asi3) (Boban et al. 2006; Zargari et al. 2007). In the presence of extracellular amino acids (right panel), the SPS (Ssy1-Ptr3-Ssy5) sensor activates the intrinsic proteolytic activity of the Ssy5 protease, resulting in the endoproteolytic processing of Stp1 and Stp2 (scissors). The shorter activated forms of Stp1 and Stp2 lacking regulatory domains are targeted to the nucleus where, together with Dal81, they bind SPS-sensor-regulated promoters (UASaa) and induce transcription (Abdel-Sater et al. 2004b; Boban and Ljungdahl 2007). The increased transcription of AAP genes results in increased rates of amino uptake. AAPs are cotranslationally inserted into the ER membrane, which is contiguous with the outer nuclear membrane. Movement of AAPs to the PM (represented by the dashed arrow, right panel) requires the ER membrane-localized chaperone Shr3 (Ljungdahl et al. 1992; Kota and Ljungdahl 2005; Kota et al. 2007). (B) Transporter-based model for Ssy1 amino acid receptor function (Wu et al. 2006). Similar to canonical transporters, Ssy1 can attain four conformational states. However, in contrast to transporters, interconversion between the outward-facing ligand bound state and the inward-facing ligand bound state (reaction 3) is prevented by a ligand-induced reaction barrier. The outward-facing conformations of the Ssy1 sensor are thought to be signaling (green), and the inward-facing conformations are nonsignaling (red). (C) Multistep regulation of the Ssy5 endoprotease. Ssy5 is expressed as an inactive zymogen (left) composed of a prodomain that assists the folding of the Cat domain. Ssy5 is auto-processed when the Cat domain attains an active conformation. The noncovalently attached prodomain remains bound to the Cat domain, forming an inactive but catalytically competent “primed” protease complex. Primed Ssy5 is incorporated as a subcomplex of the SPS sensor via protein–protein interactions involving Ptr3, where it binds, but does not cleave, its substrates Stp1 and Stp2. In the absence of extracellular amino acids, i.e., under non-inducing conditions (left), the basal level of phosphorylation of a phosphodegron in the prodomain is likely to be determined by counteracting activities of casein kinase I (Yck1 and Yck2) and the phosphatase PP2A with its regulatory subunit Rts1 (Eckert-Boulet et al. 2006). In the presence of extracellular amino acids (right), the primary amino acid sensor Ssy1 is stabilized in a conformation that triggers intracellular signaling. This conformation increases the level of phosphorylation of the prodomain phosphodegron (Omnus et al. 2011), presumably by increasing the accessibility of Yck1 or Yck2. An increased level of phosphorylation within the degron provides the requisite surface recognized by the SCFGrr1 complex and subsequent polyubiquitylation of lysine residues of the degron. Concomitant with being ubiquitylated, the prodomain is directly targeted for degradation by the 26S proteasome, unfettering the Stp1 and Stp2 processing activity of the Cat domain.
Ssy1 is a unique member of the amino acid permease family of proteins (Table 4). Ssy1 does not catalyze measurable amino acid uptake (Didion et al. 1998; Iraqui et al. 1999; Klasson et al. 1999), but instead functions as a receptor of extracellular amino acids (Figure 11B) (Wu et al. 2006). In addition to a core membrane transporter-like domain composed of 12 hydrophobic membrane-spanning segments, Ssy1 has an extended cytoplasmically oriented N-terminal domain that is not present in other amino acid permeases. Consistent with being a receptor, Ssy1 exhibits marked substrate (ligand) preferences; nonpolar amino acids (leucine, isoleucine, methionine, phenylalanine, and tryptophan) and polar uncharged amino acids (tyrosine, threonine) are strong inducers, whereas valine, cysteine, alanine, serine, and even citrulline induce intermediate levels, and arginine, lysine, and proline are poor inducers (Iraqui et al. 1999; Gaber et al. 2003). Ssy1 monitors the ratio of external vs. internal amino acids across the plasma membrane by undergoing transporter-like conformational changes between an outward-facing (signaling) and an inward-facing (nonsignaling) conformation (Wu et al. 2006; Poulsen et al. 2008). Thus, in contrast to functional transporters, but in accordance with a receptor function, amino acid binding to a single substrate-binding site appears to impose a reaction barrier that inhibits the conversion from an outward- to an inward-facing conformation. Consequently, Ssy1 signaling is sensitive to both external and internal levels of amino acids, and the SPS sensor induces gene expression only when the levels of external amino acids are higher than the levels of free amino acids in cytoplasmic pools. This model elegantly accounts for the accumulated experimental data and also provides a framework for understanding the transceptor concept as applied to active nutrient carriers that combine nutrient transporter and receptor functions, including Gap1 and Pho84 (Thevelein and Voordeckers 2009; van Zeebroeck et al. 2009; Popova et al. 2010; Rubio-Texeira et al. 2010).
The extended N-terminal domain of Ssy1 functions as a scaffold for binding of Ptr3 and Ssy5 (Bernard and André 2001; Forsberg and Ljungdahl 2001b; Liu et al. 2008). Ssy5 exhibits homology to chymotrypsin-like serine proteases and is expressed as a zymogen (Abdel-Sater et al. 2004a; Andréasson et al. 2006; Poulsen et al. 2006). After translation, and concomitantly with folding to achieve catalytic competence, Ssy5 cleaves itself into an N-terminal prodomain and a C-terminal catalytic (Cat) domain. The prodomain remains noncovalently attached with the Cat domain, forming a primed, but inactive, protease subcomplex within the SPS sensor that associates with Stp1 and Stp2 (Andréasson et al. 2006). The prodomain functions as the inhibitory subunit of the SPS sensor, and its amino acid-induced degradation via the 26S proteasome correlates with the signal-propagating, endoproteolytic processing of Stp1 and Stp2 (Pfirrmann et al. 2010; Omnus et al. 2011).
Consistent with 26S proteasome involvement, amino acid-induced Stp1 and Stp2 processing requires the SCFGrr1 ubiquitin ligase complex (Abdel-Sater et al. 2004a; Andréasson and Ljungdahl 2004; Spielewoy et al. 2004; Liu et al. 2008). SCF complexes achieve specificity through association with exchangeable F-box proteins (Jonkers and Rep 2009). Grr1 is one of the best-characterized F-box proteins and is required for Ssy5 prodomain downregulation and Cat domain activation (Andréasson et al. 2006). Also, casein kinase I-dependent phosphorylation (either Yck1 or Yck2) is required for SPS-sensor signaling (Abdel-Sater et al. 2004a; Spielewoy et al. 2004; Liu et al. 2008). It has been shown that Yck1/2-dependent Ssy5 prodomain phosphorylation precedes SCFGrr1-dependent ubiquitylation (Abdel-Sater et al. 2011). In other signaling pathways, Yck1/2-catalyzed phosphorylation has been shown to lead to Grr1-dependent polyubiquitylation and subsequent degradation of the modified substrates by the 26S proteasome (Moriya and Johnston 2004; Spielewoy et al. 2004). Hence, the SPS sensor coordinates the activity of general signaling components to achieve a highly specific output (Omnus et al. 2011) (Figure 11C).
The constitutive nuclear factor Dal81 has an important and synergistic role in amplifying the induced expression of SPS-sensor genes (Boban and Ljungdahl 2007). Dal81 is also required for several other well-characterized nitrogen source utilization pathways, including urea, allantoin, and GABA. Dal81 functions together with an inducer-specific transcription factor, i.e., Uga3 (Jacobs et al. 1981; Turoscy and Cooper 1982; Coornaert et al. 1991), Dal82 (Vissers et al. 1990), or Stp1 and Stp2 (Iraqui et al. 1999; Abdel-Sater et al. 2004b; Boban and Ljungdahl 2007) to activate target genes. The fact that these pathways share a common coactivator enables cells to integrate the signals derived from these different nitrogen sources due to competitive binding of the specific factors to Dal81 (Abdel-Sater et al. 2004b). A distinct hierarchy exists, and the induced targeting of activated forms of Stp1 and Stp2 overrides allantoate- and GABA-induced signals, enabling cells to take advantage of the availability of amino acids as preferred nitrogen sources. Integration appears to be achieved by the preferential recruitment of Dal81 to SPS-sensor-regulated promoters. Accordingly, in an SPS-sensor-dependent manner, the presence of extracellular amino acids impair Dal82-dependent expression of DAL5, encoding allantoate/ureidosuccinate permease (Cai et al. 2007), and Uga3-dependent gene expression of UGA4, the GABA permease (Cardillo et al. 2010).
Overlapping transcriptional regulatory networks contribute to the expression of several SPS-sensor-controlled genes. The promoter region of AGP1 encoding a broad-specificity amino acid permease is under NCR control; however, its expression is strictly dependent on inducing signals mediated by the SPS sensor (Iraqui et al. 1999; Abdel-Sater et al. 2004b; Wielemans et al. 2010). Stp1 binding to UASaa motifs in the promoters of SPS-sensor-regulated genes is insensitive to the nitrogen status of the cell and to factors controlling NCR, i.e., Gln3, Ure2, and Gzf3 (Godard et al. 2007). In cells grown with the preferred nitrogen source ammonium, the addition of amino acids leads to the rapid induction of AGP1 expression. In cells lacking a functional SPS-sensing pathway, AGP1 expression is not induced, and the PAGP1 promoter is unresponsive to mutations that inactivate Ure2. Conversely, amino acid-induced expression of AGP1 is greatly augmented in cells grown in the presence of nonpreferred nitrogen sources (Abdel-Sater et al. 2004b; Godard et al. 2007). Thus, in contrast to other permease genes responsive to NCR control (e.g., GAP1, DAL5, and MEP2), Gln3 is not able to activate AGP1 expression in the absence of synergistic signals initiated by the presence of extracellular amino acids that are transduced via the SPS-sensing pathway.
The analysis of dipeptide uptake in yeast also provides a clear demonstration of how SPS-sensor-induced signals are integrated with other nutritionally regulated activities. Amino acid-induced and SPS-sensor-mediated activation of dipeptide transporter PTR2 expression is a requisite for dipeptide uptake (Island et al. 1987; Barnes et al. 1998; Hauser et al. 2001); however, full induction of peptide uptake requires an additional activation step (Byrd et al. 1998; Turner et al. 2000; Hwang and Varshavsky 2008; Xia et al. 2008). Peptides entering the cell that contain N-terminal amino acids recognized according to the N-end rule as destabilizing (Dohmen et al. 1994; Varshavsky 2008), allosterically activate Ubr1, an E3 ubiquitin ligase, which in turn accelerates degradation of the transcriptional repressor Cup9. A decreased level of Cup9 gives rise to fully derepressed PTR2 expression and dipeptide transport. Although Ubr1 and Cup9 are absolutely required for full induction of PTR2 transcription, they do not affect PAGP1-lacZ (Bernard and André 2001) or BAP2 (Alagramam et al. 1995) expression.
Membrane transporter systems and compartmentalization
The compartmentalized eukaryotic cell architecture is a complicating factor in evaluating the flow of metabolites associated with amino acid and nucleotide biosynthesis. To fully understand metabolic regulation, the dynamic distribution of compartmentalized metabolites will eventually have to be taken into account. Although this was already pointed out by Cooper (1982b) and Jones and Fink (1982), it remains a substantial challenge. Great progress has been made in understanding how metabolites are transported into cells and into or out of organelles. Here full-genome sequence data have contributed greatly to the identification of families of transport proteins (Saier 2000; Brohee et al. 2010). Subsequent cell biological analyses have established the intracellular localization of these transporters, and in many cases purification and reconstitution experiments have provided precise mechanistic understanding of their function.
Membrane transport systems of nitrogenous compounds relevant to amino acid metabolism are schematically depicted in Figure 12. Transporters and permeases that function at the plasma membrane and primarily facilitate metabolite uptake into cells are listed in Table 4. Transport across the plasma membrane is facilitated via H+-symport energized by the plasma membrane H+-ATPase Pma1 (Serrano et al. 1986; Horák 1997). A fundamental theme that has emerged from the functional analysis of plasma membrane transport is that, in many instances, individual substrates are transported by several different systems, which, although displaying different kinetic specificities, function redundantly (Regenberg et al. 1999). The expression of redundant systems with differing catalytic properties allows yeast cells to extract necessary nutrients from a great variety of environments.
Figure 12 .
Membrane transport systems of nitrogenous compounds relevant to amino acid metabolism. Plasma membrane-localized permeases/transporters are shown with their corresponding substrates. The expression of transport proteins in green text is under nitrogen regulation (NCR). The expression of transport proteins in blue text is transcriptionally controlled by the SPS sensor of extracellular amino acids. Transporters thought to be involved in the excretion of amino acids, either functioning in the late secretory pathway or at the plasma membrane, are shown with red outwardly pointing arrows. Transporters localized to intracellular organelle membranes, i.e., mitochondria (M), peroxisome (P), and vacuole (V), are depicted; the arrows indicate the direction of the transport catalyzed.
Transport across the inner mitochondrial membrane is catalyzed by the mitochondrial carrier protein (MCP) family; a selected subset of MCPs that are intimately linked to amino acid metabolism is listed in Table 5 (Palmieri et al. 2006). The MCPs exhibit a variety of transport mechanisms, including uniport, symport, and antiport, and the transport can occur in an electroneutral, proton-mediated, or electrophoretic manner. In the case of bidirectional antiport exchange, the direction of transport is determined by the relative concentrations of substrates in the cytoplasmic and mitochondrial substrate.
The vacuole is an organelle with a well-established role in amino acid homeostasis (Matile and Wiemken 1967; Cooper 1982a; Kitamoto et al. 1988; Klionsky et al. 1990; Jacquemin-Faure et al. 1994; Sekito et al. 2008). The vacuolar transport systems are listed in Table 6 (Sekito et al. 2008). Metabolite transport across the vacuolar membrane is energized by the oligomeric vacuolar V0V1 H+-ATPase (Uchida et al. 1985; Anraku et al. 1992), and movement of substrates across the membrane occurs by either H+-antiport (In) or H+-symport (Out). Importantly, the vacuole is a major storage compartment for amino acids, and cells have discrete pools of amino acids; i.e., the basic amino acids (His, Arg, and Lys) are sequestered in the vacuole, whereas the acid amino acids (Asp and Glu) are selectively excluded.
Table 6. Vacuole-localized amino acid transport proteins.
| Systematic name | Gene name | Substrate specificity | Transport in/out of organelle | Reference |
|---|---|---|---|---|
| AVT subfamily | ||||
| YJR001w | AVT1a | Ile, Leu, Asn, Gln, Tyr | In | Russnak et al. (2001) |
| YEL064c | AVT2 | Not known, may localize to ER | — | Russnak et al. (2001) |
| YKL146w | AVT3b | Ile, Leu, Asn, Gln, Tyr | Out | Russnak et al. (2001); Yang et al. (2006) |
| YNL101w | AVT4a,b | Ile, Leu, Asn, Gln, Tyr | Out | Russnak et al. (2001); Yang et al. (2006) |
| YBL089w | AVT5 | Not known | — | Russnak et al. (2001) |
| YER119c | AVT6b | Asp, Glu | Out | Chahomchuen et al. (2009); Russnak et al. (2001) |
| YIL088c | AVT7a | Not known | — | Russnak et al. (2001) |
| VBA subfamily | ||||
| YMR088c | VBA1a | His, Lys | In | Shimazu et al. (2005) |
| YBR293w | VBA2 | Arg, His, Lys, Tyr | In | Shimazu et al. (2005) |
| YCL069w | VBA3 | His, Lys | In | Shimazu et al. (2005) |
| YDR119w | VBA4 | Not known | — | Shimazu et al. (2005) |
| YKR105c | VBA5 | Not known | — | Shimazu et al. (2005) |
| Other transport proteins | ||||
| YCL038c | ATG22 | Ile, Leu, Tyr | Out | Sychrova and Chevallier (1994); Yang et al. (2006) |
| YEL063c | UGA4 | GABA, putrescine | In | Uemura et al. (2004) |
| YNL268w | ERS1 | Cys | Out | Gao et al. (2005) |
NCR-sensitive expression.
Required for viability upon nitrogen starvation.
Interestingly, efforts to understand TORC1 signaling appear to be narrowing in on intracellular trafficking of membrane proteins, including the general amino acid permease (GAP1). TORC1 localizes to the vacuolar/endosomal membrane (Wedaman et al. 2003), which in essence is a major crossroad for protein sorting (Nickerson et al. 2009). The membrane-anchored EGO–GSE complex, found associated with the late endosome and vacuolar membranes, is required for TORC1 localization and activation (Dubouloz et al. 2005; Binda et al. 2009) and for proper Gap1 trafficking from the endosome to the plasma membrane during amino acid limitation (Gao and Kaiser 2006). Consistent with a link to vacuole function, null alleles of genes encoding class C-VPS components, e.g., PEP3, exhibit synthetic lethality with a tor1Δ null allele (Zurita-Martinez et al. 2007). The presence of glutamate or glutamine suppresses the synthetic lethality of a pep3tor1 mutant, indicating that nitrogen metabolism and vacuolar function are intimately intertwined. Class C-VPS mutants do not have identifiable vacuolar structures (Bowers and Stevens 2005), have low levels of intracellular pools of amino acids, and are unable to survive nitrogen starvation (Zurita-Martinez et al. 2007). Proper TOR signaling apparently requires a vacuole/endosomal membrane platform The central role of the vacuole in nitrogen metabolism may explain the nitrogen-related phenotypes associated with rapamycin treatment.
Nucleotides
Nucleotides are critical players in a multitude of very different cellular processes. Purines and pyrimidines are the basic components of nucleic acids, and ATP is the central cellular energy supply. In addition, GTP and modified nucleotides such as cyclic AMP are signaling molecules. Finally, nucleotides are incorporated in cofactors (e.g., NAD and coenzyme A) and serve as precursors (e.g., UDP-glucose and GDP-mannose). Purine and pyrimidine synthesis occurs through distinct metabolic pathways highly conserved among both prokaryotic and eukaryotic species. These pathways (Figure 13 and Figure 15; see also Table 7 and Table 8) combine de novo synthesis from amino acids and sugar with nucleotide recycling from precursors available in the growth medium or provided via degradation of macromolecules. The purine and pyrimidine pathways ensure net synthesis of nucleotides but also allow interconversion of nucleobases, nucleosides, and nucleotides, thus permitting a proper balance of the final products to be achieved. Intracellular concentrations of purine and pyrimidine nucleotides are presented in Table 9.
Figure 13 .
Pyrimidine synthesis and salvage pathways. DHO, dihydro-orotate; OA, orotic acid; USA, ureidosuccinic acid. Gene names are italicized. Regulatory molecules are shown in red.
Figure 15 .
Purine and histidine pathways in yeast. Ado, adenosine; AICAR, 5′-phosphoribosyl-5-amino-4-imidazole carboxamide; Ino, inosine; guo, guanosine; IMP, inosine 5′-monophosphate; PRPP, 5-phosphoribosyl-1-pyrophosphate; SAICAR, 5′-phosphoribosyl-4-(N-succinocarboxamide)-5-amino-imidazole. Gene names are italicized. Regulatory molecules are shown in red.
Table 7. Pyrimidine metabolism genes.
| Gene | ORF | Activity in UTP synthesis | References |
|---|---|---|---|
| URA2 | YJL130C | Bifunctional: carbamoylphosphate synthetase - aspartate transcarbamylase | Denis-Duphil (1989); Nagy et al. (1989); Souciet et al. (1987) |
| URA4 | YLR420W | Dihydroorotase | Guyonvarch et al. (1988) |
| URA1 | YKL216W | Dihydroorotate dehydrogenase | Roy (1992) |
| URA5 | YML106W | Major orotate phosphoribosyltransferase isozyme | de Montigny et al. (1989) |
| URA10 | YMR271C | Minor orotate phosphoribosyltransferase isozyme | de Montigny et al. (1990) |
| URA3 | YEL021W | Orotidine-5′-phosphate decarboxylase | Storms et al. (1979) |
| URA6 | YKL024C | Uridylate kinase | Liljelund and Lacroute (1986) |
| YNK1 | YKL067W | Nucleoside diphosphate kinase | Fukuchi et al. (1993) |
| Activity in CTP synthesis | |||
| URA7 | YBL039C | Major CTP synthase isozyme | Ozier-Kalogeropoulos et al. (1991) |
| URA8 | YJR103W | Minor CTP synthase isozyme | Ozier-Kalogeropoulos et al. (1994) |
| Activity in salvage | |||
| FCY1 | YPR062W | Cytosine deaminase | Erbs et al. (1997) |
| FUR1 | YHR128W | Uracil phosphoribosyltransferase | de Montigny et al. (1990) |
| SDT1 | YGL224C | Pyrimidine nucleotidase | Nakanishi and Sekimizu (2002) |
| URK1 | YNR012W | Uridine kinase | Kern (1990) |
| URH1 | YDR400W | Uridine-cytidine N-ribohydrolase | Kurtz et al. (2002) |
| CDD1 | YLR245C | Cytidine deaminase | Kurtz et al. (1999) |
Table 8. Purine metabolism genes.
| Gene | ORF | Activity in IMP synthesis | References |
|---|---|---|---|
| ADE4 | YMR300C | Phosphoribosyl-pyrophosphate amidotransferase | Mantsala and Zalkin (1984) |
| ADE5,-7 | YGL234W | glycinamide Glycinamide ribotide synthetase | Henikoff (1986) |
| ADE8 | YDR408C | Phosphoribosyl-glycinamide transformylase | White et al. (1988) |
| ADE6 | YGR061C | Formylglycinamidine-ribonucleotide -synthetase | Giani et al. (1991) |
| ADE5,-7 | YGL234W | Amino-imidazole ribotide synthetase | Henikoff (1986) |
| ADE2 | YOR128C | Phosphoribosyl-amino-imidazole carboxylase | Stotz and Linder (1990) |
| ADE1 | YAR015W | N-succinyl-5-aminoimidazole-4-carboxamide ribotide synthetase | Crowley and Kaback (1984) |
| ADE13 | YLR359W | Adenylosuccinate lyase | Guetsova et al. (1997) |
| ADE16 | YLR028C | Isoform of 5-aminoimidazole-4-carboxamide ribonucleotide transformylase and inosine monophosphate cyclohydrolase | Tibbetts and Appling (1997) |
| ADE17 | YMR120C | Isoform of 5-aminoimidazole-4-carboxamide ribonucleotide transformylase and inosine monophosphate cyclohydrolase | Tibbetts and Appling (1997) |
| Activity in ADP synthesis | |||
| ADE12 | YNL220W | Adenylosuccinate synthase | Andreichuk Iu et al. (1995) |
| ADE13 | YLR359W | Adenylosuccinate lyase | Guetsova et al. (1997) |
| ADK1 | YDR226W | Adenylate kinase | Konrad (1988); Magdolen et al. (1987) |
| Activity in GDP synthesis | |||
| IMD1 | YAR073W | IMP dehydrogenase isoform, probable pseudogene | Escobar-Henriques and Daignan-Fornier (2001); Hyle et al. (2003) |
| IMD2 | YHR216W | IMP dehydrogenase isoform | Escobar-Henriques and Daignan-Fornier (2001); Hyle et al. (2003) |
| IMD3 | YLR432W | IMP dehydrogenase isoform | Escobar-Henriques and Daignan-Fornier (2001); Hyle et al. (2003) |
| IMD4 | YML056C | IMP dehydrogenase isoform | Escobar-Henriques and Daignan-Fornier (2001); Hyle et al. (2003) |
| GUA1 | YMR217W | GMP synthase | Dujardin et al. (1994) |
| GUK1 | YDR454C | Guanylate kinase | Konrad (1992) |
| Activity in purine salvage | |||
| AAH1 | YNL141W | Adenine deaminase | Ribard et al. (2003) |
| AMD1 | YML035C | AMP deaminase | Meyer et al. (1989) |
| APT1 | YML022W | Adenine phosphoribosyltransferase | Alfonzo et al. (1999) |
| HPT1 | YDR399W | Hypoxanthine-guanine phosphoribosyltransferase | Guetsova et al. (1997) |
| XPT1 | YJR133W | Xanthine phosphoribosyl transferase | Alfonzo et al. (1999) |
| ADO1 | YJR105W | Adenosine kinase | Lecoq et al. (2001a) |
| ISN1 | YOR155C | Inosine 5′-monophosphate (IMP)-5′-nucleotidase | Itoh et al. (2003) |
| PNP1 | YLR209C | Inosine and guanosine phosphorylase | Lecoq et al. (2001b) |
| GUD1 | YDL238C | Guanine deaminase | Saint-Marc and Daignan-Fornier (2004) |
Table 9. Intracellular nucleotide concentrations.
| Nucleotide | Concentration (mM) |
|---|---|
| ATP | 4.6 ± 0.6 |
| GTP | 1.3 ± 0.2 |
| UTP | 1.6 ± 0.1 |
| CTP | 0.73 ± 0.03 |
| ADP | 0.82 ± 0.081 |
| GDP | 0.39 ± 0.036 |
| UDP | 0.093 ± 0.007 |
| AMP | 0.049 ± 0.012 |
| GMP | 0.12 ± 0.011 |
| UMP | 0.394 ± 0.061 |
| CMP | 0.017 ± 0.006 |
| IMP | 0.178 ± 0.019 |
| dATP | 0.031 ± 0.008 |
| dGTP | 0.016 ± 0.005 |
| dCTP | 0.023 ± 0.004 |
| dTTP | 0.022 ± 0.007 |
FY4 prototrophic strain was grown in SD CASA (0.5% ammonium sulfate, 0.17% yeast nitrogen base, and 2% glucose, supplemented with 0.2% casamino acids) (Benoît Pinson, personal communication).
Regulation of pyrimidine metabolism
The de novo pyrimidine pathway is regulated at the enzymatic level through feedback inhibition of the first enzyme, Ura2, by the final product UTP (reviewed in Jones and Fink 1982). Moreover, several genes of the pathway are also upregulated at the transcriptional level in response to pyrimidine starvation (Jones and Fink 1982). The elegant genetic work of Lacroute and coworkers in the early eighties allowed identification of Ppr1 as a zinc-finger transcription factor required for expression of the URA1 and URA3 genes (Loison et al. 1980; Losson and Lacroute 1981; Kammerer et al. 1984). Ppr1 is also likely to positively regulate URA4 and URA10 on the basis of data from in vitro binding to promoter sequences (Roy et al. 1990; Roy 1992). However, the role of Ppr1 in transcription has not been evaluated genome-wide, which could reveal interesting cross-pathway regulation.
The early observation that yeast mutants accumulating orotic acid (OA) and dihydro-orotate (DHO) show increased DHO dehydrogenase (Ura1) and orotidine 5′-monophosphate decarboxylase (Ura3) activity (Lacroute 1968) suggested that these metabolic intermediates could play a direct role in the pyrimidine pathway regulation. In this model, a specific pathway intermediate functions as a cofactor for the transcriptional activator, and the abundance of the metabolite is downregulated by feedback inhibition (Figure 8). This assumption was tested directly using purified Ppr1 in vitro (Flynn and Reece 1999), where addition of either DHO or OA to the reaction stimulated Ppr1-dependent transcription (Flynn and Reece 1999). This reconstitution experiment established that Ppr1 directly senses the levels of pyrimidine biosynthesis intermediates and modulates pyrimidine enzyme synthesis in response to the flux in the pathway. Interestingly, it was also found that efficient binding of Ppr1 to DNA required a yet-unidentified small molecule (Flynn and Reece 1999). Identification of this molecule in the future could uncover new aspects of pyrimidine biosynthesis regulation.
Transcription of URA2, encoding the first committed step of UTP synthesis, is also upregulated in response to pyrimidine depletion (Exinger and Lacroute 1992; Kwapisz et al. 2008). However, expression of URA2 is not Ppr1 dependent (Losson and Lacroute 1981; Kwapisz et al. 2008), but is regulated by a novel mechanism recently described. This mechanism involves alternative transcription start sites that lead to the synthesis of small “cryptic unstable transcripts” (CUTs) (Thiebaut et al. 2008) but also to a downstream T-rich region (Kwapisz et al. 2008). “Nonproductive” transcription from the upstream sites downregulates expression of the URA2 mRNA while uracil deprivation activates selection of the functional URA2 mRNA start site by a mechanism that does not involve lower expression of the CUTs (Thiebaut et al. 2008) (Figure 14). Interestingly, mechanisms involving noncoding RNAs appear to regulate other nucleotide genes such as URA8, IMD2, and ADE12 (Davis and Ares 2006; Steinmetz et al. 2006; Kuehner and Brow 2008; Kwapisz et al. 2008; Thiebaut et al. 2008). Strikingly, these four genes encode the first committed steps of UTP, CTP, GTP, and ATP synthesis (see Figures 13 and 15). Although not fully understood at the molecular level, these regulatory processes apparently involve direct sensing of nucleotide concentration by the transcription machinery and thus efficiently connect individual nucleotide synthesis to its actual availability.
Figure 14 .
Organization of the URA2 and IMD2 promoter regions. The transcription start sites are shown, and their respective distances from the mRNA start site are indicated. Unstable transcripts are shown in gray.
Regulation of the purine de novo synthesis pathway
The transcription factors responsible for activation of purine de novo pathway genes were identified while studying the histidine pathway regulation. The pioneering work by Fink and coworkers on the transcriptional regulation of the HIS4 gene revealed the important role of the Myb-related Bas1 and homeodomain Bas2 transcription factors (Arndt et al. 1987; Tice-Baldwin et al. 1989). Knockout of these genes was found to result in adenine bradytrophy, suggesting that they could also regulate purine biosynthesis genes. This hypothesis was correct, and Bas1 and Bas2 were found to be required for expression of several ADE genes in vivo and to bind to their promoter in vitro (Daignan-Fornier and Fink 1992). On the basis of proteome, transcriptome, and gene-by-gene analysis, it was found that Bas1 and Bas2 activate the expression of all 10 AMP-biosynthesis genes except ADE16 (Daignan-Fornier and Fink 1992; Denis et al. 1998) and bind to a specific promoter region of these genes (Daignan-Fornier and Fink 1992; Rolfes et al. 1997; Pinson et al. 1998). Furthermore, Bas1/Bas2 also mediate adenine-repressible transcriptional activation of genes of other pathways metabolically connected to the purine pathway, such as histidine (HIS1, HIS4, and HIS7), glutamine (GLN1), or one-carbon-unit (SHM2, MTD1) metabolism genes (Figure 15) (Arndt et al. 1987; Springer et al. 1996; Denis and Daignan-Fornier 1998). Bas1 binding to the promoter region of most of these genes has been confirmed in vivo by ChIP-CHIP analysis (Mieczkowski et al. 2006). Importantly, Bas2 is also known as Pho2, a major regulator of phosphate utilization in yeast (see Purine phosphate connection: more signal molecules) (Arndt et al. 1987). In general, both Bas1 and Pho2 are required for transcriptional activation although one-carbon-unit metabolism genes are much more dependent on Bas1 than on Bas2/Pho2 (Denis and Daignan-Fornier 1998; Subramanian et al. 2005).
All the genes activated by Bas1 and Bas2/Pho2 also respond to extracellular adenine, their expression being low when adenine is abundant in the growth medium (Tice-Baldwin et al. 1989; Daignan-Fornier and Fink 1992; Springer et al. 1996; Denis et al. 1998; Denis and Daignan-Fornier 1998). To get an insight into the molecular mechanisms linking adenine availability to transcriptional activation, Rolfes et al. (1997) used chimeras between LexA and either Bas1 or Pho2. They found that lexA-Pho2 could activate transcription independently of Bas1 and in an adenine-independent way, while lexA-Bas1 activation was both Pho2 dependent and adenine responsive (Zhang et al. 1997). They proposed that adenine limitation favors formation of a complex between Bas1 and Pho2, thus unmasking the Bas1 activation domain (Zhang et al. 1997). Consistently, a Bas1–Pho2 fusion chimera activated expression of the ADE genes in an adenine-independent way (Pinson et al. 2000). Further analyses using Bas1 and Pho2 fused to the transcription activation domain of VP16 suggested that Bas1 binds to the ADE gene promoters and recruits Pho2 (Pinson et al. 2000). This was further demonstrated by chromatin immunoprecipitation (ChIP) analysis (Som et al. 2005).
How does adenine limitation stimulate Bas1–Pho2 interaction? A genetic analysis of mutants unresponsive to adenine has shown that adenine needs to be taken up and metabolized to ADP to exert its regulatory role (Guetsova et al. 1997; Rébora et al. 2001). This is consistent with the observation that extracellular hypoxanthine downregulates expression of an ADE1-lacZ fusion while guanine has little effect (Guetsova et al. 1997) because hypoxanthine can be converted to adenylic nucleotides while guanine cannot (Figure 15). Strikingly, most of the deregulated mutants affected the ADE13 gene encoding adenylosuccinate lyase, suggesting a key role for this step of the pathway (Figure 15) (Rébora et al. 2001). Further genetic analyses revealed that AICAR (5′-phosphoribosyl-5-amino-4-imidazole carboxamide) and SAICAR (succinyl-AICAR), the metabolic intermediates just downstream and upstream of Ade13, respectively, play a pivotal role by promoting interactions between Bas1 and Pho2 (Rébora et al. 2001, 2005). Several lines of evidence supported this idea. First, a direct correlation was found between intracellular AICAR concentrations, measured by HPLC, and ADE gene expression (Pinson et al. 2009). Second, a Bas1–Pho2 fusion makes expression of the ADE genes (S)AICAR independent (Rébora et al. 2001; Pinson et al. 2009). Finally, two-hybrid studies revealed that the Bas1–Pho2 interaction is stimulated under conditions where (S)AICAR accumulates (Rébora et al. 2001; Pinson et al. 2009), and consistently, these conditions stimulate Bas1-dependent recruitment of Pho2 to the ADE5, 7 and ADE17 promoters, as determined by ChIP (Pinson et al. 2009). Affinity chromatography revealed that AICAR binds Pho2 but not Bas1 in vitro strongly, suggesting that Pho2 could be an AICAR sensor through direct interaction with the small molecule (Pinson et al. 2009). Importantly AICAR, but not SAICAR, also affects interaction of Pho2 with another transcription factor, Pho4, and thus modulates expression of phosphate utilization genes (see Purine phosphate connection: more signal molecules and Figure 8) (Pinson et al. 2009). Together, these results suggest that somehow AICAR binding to Pho2 potentiates this transcription factor by stimulating the interaction of Pho2 with its partners.
The link between exogenous adenine and (S)AICAR synthesis is thought to occur through enzymatic regulation of the first step of the pathway, catalyzed by Ade4, which is downregulated by ADP and ATP in vitro (Rébora et al. 2001). Indeed, the intracellular concentration of ADP and ATP is clearly higher in adenine-replete conditions (Gauthier et al. 2008), while the (S)AICAR concentration decreases (Hurlimann et al. 2011). Thus, both ATP, the final product of the pathway, and a metabolic intermediate, (S)AICAR, are required for proper transcriptional regulation of adenylic nucleotides. Although the mechanisms are different, the general scheme is very close to that of the pyrimidine pathway, where UTP feedback inhibits Ura2, the first enzyme of the pathway, thus modulating the synthesis of orotate and dihydroorotate, two metabolic intermediates that directly stimulate the transcription factor Ppr1 (see Regulation of pyrimidine metabolism). Dual-sensing mechanisms are well suited for regulation of nonlinear pathways, such as the purine de novo pathway, which can be fed on the side by the histidine pathway and which branches to allow ATP and GTP synthesis (Figure 15).
Regulation of GTP synthesis
Expression of genes required for GTP synthesis is not coregulated with that of genes for AMP synthesis. The IMD genes and GUA1 do not respond to adenine as ADE genes do (Escobar-Henriques and Daignan-Fornier 2001). In fact, IMD2/3/4 genes are strongly downregulated when guanine is added to the growth medium, while GUA1 expression is unaffected (Escobar-Henriques and Daignan-Fornier 2001). This effect of guanine is abolished in the fcy2, hpt1, and guk1 guanine utilization mutants, indicating that guanine metabolism is required for GTP to exert its regulatory role (Escobar-Henriques and Daignan-Fornier 2001). In contrast, inhibitors of IMPDH activity, such as mycophenolic acid (MPA) or 6-azauracil (6AU) that lower GTP pools (Exinger and Lacroute 1992), strongly induce IMDs genes (Shaw and Reines 2000; Escobar-Henriques and Daignan-Fornier 2001; Saint-Marc et al. 2009).
An analysis of the IMD2 promoter, aimed at deciphering the molecular mechanisms leading to transcriptional regulation, revealed several unusual features. First, a regulatory element lying >200 bp upstream of the transcription start, carries a typical TATA box motif and is required for binding of the TATA-box-binding protein on the IMD2 promoter (Escobar-Henriques and Daignan-Fornier 2001; Escobar-Henriques et al. 2003a). Deletion of this TATA box motif resulted in constitutive expression of IMD2 mRNA at a level that was intermediary between fully repressed and fully induced levels (Shaw et al. 2001; Escobar-Henriques et al. 2003b; Kuehner and Brow 2008). A repressive element was also identified between the TATA box and the transcriptional start site (Shaw et al. 2001) and was shown to have transcription terminator properties (Jenks et al. 2008).
Recently, two groups studying RNA stability and transcription termination at the genome-wide scale observed unusual features at the IMD2 locus. These studies revealed multiple unstable transcripts (CUTs) produced from the IMD2 promoter region (Figure 14) (Davis and Ares 2006; Steinmetz et al. 2006). Importantly, abundance of the CUTs appears inversely correlated to that of IMD2 mRNA (Davis and Ares 2006; Steinmetz et al. 2006). Since the CUTs always start with a G while IMD2 mRNA starts with an A, Brow and coworkers proposed that somehow the transcription machinery senses GTP concentration and responds to it via differential start-site utilization (Steinmetz et al. 2006). This model was globally supported by an analysis of various mutations in the IMD2 promoter (Kuehner and Brow 2008). The precise mechanism is not yet understood, and it could involve transcription elongation factors, since the corresponding mutants poorly induce IMD2 expression in the presence of MPA or 6AU and are hypersensitive to these drugs (Shaw and Reines 2000; Desmoucelles et al. 2002; Riles et al. 2004).
Nucleotide balance
Proper nucleotide balance appears to result from individual nucleotide sensing and adjustment. However, since CTP is synthesized from UTP, and since both GTP and ATP are made from IMP, the synthesis of individual nucleotides does not occur independently. Yet, synthesis of each nucleotide appears to be finely regulated by nucleotide-dependent transcriptional regulations involving noncoding RNAs and specific responses to limitation in each given nucleotide. Enzymatic mechanisms are also involved in ensuring the nucleotide balance; for example, GMP synthesis requires ATP at the Gua1-catalyzed step, while AMP synthesis requires GTP for Ade12-dependent activity (Figure 15). What happens if the nucleotide balance is disturbed? To tackle this question, specific mutations disrupting the purine nucleotide balance that result in lower ATP or GTP concentration (Gauthier et al. 2008; Saint-Marc et al. 2009; Iglesias-Gato et al. 2011) or that strongly increase the GTP pool (Breton et al. 2008) were constructed. While these mutations had a strong impact on yeast growth and resulted in general transcriptional and translational responses, no evidence for a common specific cellular response to defective nucleotide balance emerged. It thus seems that yeast cells do not have an integrated response to nucleotide imbalance, most probably because regulatory mechanisms ensuring proper nucleotide balance are highly robust.
Regulation in response to growth phase
As building blocks, nucleotides are required mainly during growth and division. Their synthesis should therefore be much lower in quiescent cells, in which efficient recycling should fulfill most requirements. Indeed, most purine biosynthesis genes are downregulated at the transcriptional level upon entry into stationary phase. Interestingly, in Bacillus subtilis, diminution of intracellular GTP concentration is required for proper entry into stationary phase (Ratnayake-Lecamwasam et al. 2001). In yeast, expression of IMPDH-coding genes (IMDs) is strongly affected by growth phase (Shaw et al. 2001; Escobar-Henriques et al. 2003a). A mutagenesis of the IMD2 gene revealed that the sequence involved in growth phase regulation lies in the coding region of the gene (Escobar-Henriques et al. 2003a). Somehow this sequence affects recruitment of the TATA-box-binding protein to the upstream IMD2 promoter regions (Escobar-Henriques et al. 2003a), but the precise mechanisms have not yet been elucidated.
For adenine deaminase (Aah1), regulation upon entry into stationary phase occurs both at the transcriptional and the post-transcriptional levels. A genetic screen for mutants abolishing this regulation revealed that transcriptional regulation involves the kinase Ssn3 and its cyclin Ssn8 (Escusa et al. 2006), which together are involved in phosphorylation of the RNA polymerase II C-terminal domain. The post-transcriptional regulation occurs through degradation of Aah1 by the proteasome and is mediated by a SCF complex involving the F-box protein Saf1, which is itself upregulated in stationary phase (Escusa et al. 2006, 2007). However, a saf1 mutant can enter and exit stationary phase normally, and the role of nucleotide synthesis in stationary-phase establishment is still unclear.
Several purine biosynthesis enzymes (Ade4, Ade5,7, Ade17, Ade12) were found to form punctate cytoplasmic foci in cells grown to stationary phase (Narayanaswamy et al. 2009). For Ade4-GFP, these foci could be reversed by addition of adenine or hypoxanthine to the growth medium (Narayanaswamy et al. 2009). This intriguing property is reminiscent of that of purine biosynthesis enzymes in HeLa cells that tend to cluster upon purine limitation (An et al. 2008). The existence of such complexes in yeast remains to be established.
Phosphate
Identification of phosphate-responsive genes
Phosphate is an abundant molecule that is incorporated into ATP and transferred from ATP to a large number of small biomolecules including nucleotides, sugars, and lipids, but also macromolecules such as proteins. In yeast, the total phosphate concentration is in the hundred-millimolar range. Phosphate, like carbon, nitrogen, or sulfur, is required for progression through the cell cycle, and cells starved for phosphate arrest in G1 (Saldanha et al. 2004). Yeast cells have developed complex responses to adapt to phosphate scarcity, and the physiological response to inorganic phosphate availability has been studied for a long time. Most of the work in the past was based on the expression of excreted phosphatases that were detected using a chromogenic substrate (reviewed in Johnston and Carlson 1992). More recent transcriptomic approaches have provided an overview of the phosphate response in yeast.
In pioneering work, Brown and coworkers used microarray analysis to identify phosphate-responsive genes that are induced when cells are shifted from high to low inorganic phosphate (Pi) medium (Ogawa et al. 2000). Subsequently, Piper and coworkers used chemostat cultures to analyze phosphate-responsive genes and identified a set of genes specifically up- or downregulated in cells grown under low-phosphate conditions (Boer et al. 2003). These studies gave very consistent results, but also revealed sets of genes induced only upon starvation or only when cells are adapted to growth on low Pi (Table 10). Some of these differences could be due to differences in Pi concentration in the two experiments. Indeed, it is known that intermediate phosphate concentration (low Pi vs. starvation) may differentially affect expression of the PHO genes (Lam et al. 2008).
Table 10. Phosphate-responsive genes.
| Starvationa | Chemostatb | Both experiments | |
|---|---|---|---|
| Phosphate utilization and regulation | PHO5 PHO12 PHO8 | PHO3 | SPL2 PHO89 PHO84 PHO11, PHO81 PHO86 |
| Polyphosphate metabolism | PPN1 VTC2 | VTC1 VTC3 VTC4 | |
| Phospholipid metabolism | GPD1 PLB1 | PLB3 INM1 GIT1 KCS1 DDP1 TAX4 | HOR2 GDE1 PHM8 VIP1 SUR1 |
| Sugar metabolism | GLK1 PGM2 TSL1 TPS2 GSY1GLC3 CIT2 GRE3 | PYK2 NTH2 | |
| Amino acid and nucleotide metabolism | HIS1 MET6 CHA1 | PRS4 PPR1 SDT1 IMD4 | |
| Cell wall | DAN1 DAN4 TIR1 TIR3 KRE2 RCR1 | ||
| Other | KRE29 PLM2 CTF19 RTC3 MGA1 PTK2 MAF1 DDR48 EMI2 ERG28 LAS1 TMA10 RTS3 AMS1 PMC1 YCR007C RCN1 CMK2 SPC110 HSP42 SSA4 YPK2 ZRT1 CTT1 DIA1 YNL208W MSC1 PHM7 AIM17 YJR061W YOR385WYLR149C YOR289W YMR291W YMR007W YBR051W YJL107C | ALR1 TRK2 ERC1 AST1 UFO1 ZAP1 APG2 AUT4 BUD23 FLO9 YLH47 PMU1 SHE9 YDL109C QDR1 DML1 SQS1 ZPS1 COS10 GFD2 YHR210C YGR079W YER186C YLR346C YNR014W YNL046W YBL070C YOR343C YMR279C | ICY1 PHM6 YNL217W YAR069C YJL119C |
Phosphate-responsive genes belong to several functional categories: organic phosphate utilization genes (phosphatases and transporters), polyphosphate metabolism, regulatory factors (PHO81, SPL2), and other metabolic pathways (histidine, trehalose, glycogen, inositol phosphate) (Table 10). While upregulation of organic phosphate utilization genes in response to phosphate limitation is not surprising, induction of polyphosphate synthesis genes or cross-pathway regulations is less intuitive and will be discussed individually in the following sections.
Phosphorylation of Pho4 and subcellular localization in response to phosphate availability
On the basis of thorough genetic analysis of the phosphate response using acid phosphatase activity as a readout, three positive regulators (PHO2, PHO4, PHO81) and two negative regulators (PHO80 and PHO85) have been identified. Epistasis studies carried out in the early 1970s by the Oshima group indicated that these genes function within a linear regulatory cascade (Figure 16) (Johnston and Carlson 1992). The most downstream effectors of the cascade, Pho2 and Pho4, are transcription factors (Johnston and Carlson 1992) that are modulated by the proteins in the upper part of the cascade through complex molecular mechanisms that were only recently uncovered. The elegant work in the mid-1990s by the O’Shea laboratory provided a clear breakthrough in our understanding of how yeast cells regulate phosphate utilization. Pho85 and Pho80 were shown to be a protein kinase and a cyclin, respectively, that co-immunoprecipitated as a complex (Kaffman et al. 1994). Furthermore, the Pho80–Pho85 complex was found to co-immunoprecipitate with Pho4, could phosphorylate Pho4 in vitro (Kaffman et al. 1994), and is required for Pho4 hyperphosphorylation in vivo (Kaffman et al. 1994). Five phosphorylated peptides dependent on the Pho80–Pho85 complex were identified (Kaffman et al. 1994), and the cognate-phosphorylated serine residues were identified by mutagenesis and phosphopeptide mapping (O’Neill et al. 1996). Importantly, phosphorylation of Pho4 by Pho80–Pho85 impedes nuclear localization; Pho4 is localized in the nucleus under low phosphate conditions or when the phospho-acceptor serines are mutated, and conversely, Pho4 is cytosolic in the presence of high phosphate when Pho80–Pho85 is active (Figure 6) (O’Neill et al. 1996). Phosphorylation of two serine residues is specifically required for proper export of Pho4 to the cytosol in response to high phosphate via interaction of Pho4 with the exportin Msn5 (Komeili and O’Shea 1999). Phosphorylation of a third serine residue inhibited interaction of Pho4 with the Pse1 importin and Pho4 nuclear import (Komeili and O’Shea 1999). Finally, a fourth serine residue was found to modulate interaction of Pho4 with Pho2 (Komeili and O’Shea 1999). Conversely, a mutation in a serine residue in Pho2, which can be phosphorylated in vitro by Cdc28, affects its interaction with Pho4 (Liu et al. 2000). However, there is no evidence that this potential modification of Pho2 could be responsive to the phosphate switch in vivo. Since both Pho2 and Pho4 are required for expression of the phosphate-responsive genes because their interaction is reduced by phosphate as revealed by two-hybrid experiments (Hirst et al. 1994) and since the two proteins bind cooperatively in vitro to the promoters of the target genes (Barbaric et al. 1996), their ability to interact probably limits their transcriptional activation capacity. Importantly, a Pho4 mutant lacking all the Pho80–Pho85 phosphorylation sites is fully derepressed for Pho5 expression under high-phosphate conditions, indicating that phosphorylation of Pho4 is the main way to regulate PHO5 expression (Komeili and O’Shea 1999). It should be stressed that the regulation of Pho4 activity by phosphorylation is not an on/off mechanism and that intermediary situations leading to partial phosphorylation of Pho4 and specific enrichment of the transcription factor at specific promoters as revealed by ChIP exist (Springer et al. 2003). A study of Pho4 binding, utilizing genomic nucleotide arrays to analyze DNA sequences that coprecipitate (ChIP on CHIP), revealed that Pho4 is associated with promoters under high-phosphate conditions, indicating that phosphorylation of Pho4 does not fully exclude it from the nucleus (Nishizawa et al. 2008).
Figure 16 .
Regulation of phosphate utilization. The phosphate regulatory cascade is shown. New genes recently identified as important for regulation of phosphate utilization are shown in red. Question marks designate the steps for which no molecular mechanism has been documented yet.
Role of an intermediate metabolite (IP7) in the regulation of Pho81
While it became clear that subcellular localization of Pho4 was critical for the phosphate response, two major questions remained to be answered: what is the nature of the molecular signal generated in response to inorganic phosphate availability? And how is it transmitted to the Pho80–Pho85 complex? On the basis of epistasis studies, Pho81, the regulator just upstream of the Pho80–Pho85 complex, is considered to be the best candidate as a phosphate sensor.
Pho81 is a positive regulator of the PHO pathway that acts through negative regulation of Pho80–Pho85 (Ueda et al. 1975) (Figure 16). Indeed, the kinase–cyclin complex could be co-immunoprecipitated with Pho81 and immunoprecipitated Pho80–Pho85 activity was higher in a strain lacking PHO81 while it was inhibited in vitro by addition of purified Pho81 (Schneider et al. 1994). A small region of Pho81 has similarity to CDK inhibitors, and this region is sufficient to inhibit kinase activity although with a much higher IC50 than the entire Pho81 protein (Schneider et al. 1994). Several studies aimed at identifying functional domains in the large Pho81 protein have allowed narrowing down the minimal regulatory domain (Ogawa et al. 1995; Huang et al. 2001); however, the roles of the other parts of the protein are not clearly established. Still, a regulatory role of the Pho81 amino-terminus is expected, as there are four different point mutations in this region, leading to its constitutive activation (Creasy et al. 1993; Ogawa et al. 1995). Pho81 is found mainly in the nucleus although it is also detected in the cytosol and at the plasma membrane (Huang et al. 2001). It is clear that nuclear localization of Pho81 is not regulated by inorganic phosphate availability (Huang et al. 2001). Interestingly, PHO81 itself is a phosphate-responsive gene, thus resulting in a positive feedback loop.
The molecular nature of the signal received by Pho81 was recently identified using an in vitro assay based on the formation of the Pho81–Pho80–Pho85 complex (Lee et al. 2007). These authors observed that a cellular extract from cells grown in low phosphate was sufficient to inhibit the kinase activity. Further fractionation revealed that the inhibitor is a small molecule that was identified as α-myo-d-inositol heptakisphosphate (IP7) by NMR and mass spectrometry and was further confirmed using the synthetic compound (Lee et al. 2007). Consistently, IP7 was more abundant in cells under low-phosphate conditions, and Pho4 localization was reduced or elevated, respectively, by mutations impairing IP7 synthesis (vip1) or degradation (ddp1) (Figure 6) (Lee et al. 2007). Since Pho81 constitutively interacts with the Pho80–Pho85 kinase complex, IP7 is thought to reversibly change Pho81 conformation and affect accessibility of the kinase substrate (Lee et al. 2008). Although several earlier reports, including transcriptome analyses, had linked insositol phosphate metabolism with the phosphate response (Flick and Thorner 1998; El Alami et al. 2003; Steger et al. 2003; Auesukaree et al. 2005), the results were not entirely consistent. Mutations in genes of the inositol polyphosphate pathway—PLC1, ARG82, or KCS1 (Figure 6)—constitutively express several genes of the PHO regulon (El Alami et al. 2003), suggesting that their regulatory role is not limited to synthesis of IP7 under low-phosphate conditions. In another report, Plc1 and Arg82 were found necessary for chromatin remodeling of the PHO5 promoter upon induction in a pho80ts mutant (Steger et al. 2003). These studies have incontestably uncovered a complex interplay between inositol polyphosphate metabolism and regulation of phosphate-responsive genes.
Thus the current model of phosphate response is as follows: under low-phosphate conditions, IP7 becomes more abundant and binding of IP7 to Pho81 results in inhibition of the protein kinase activity of Pho80–Pho85. The resulting lower phosphorylation of Pho4 favors its nuclear localization and its interaction with Pho2 and attendant transcriptional activation of the phosphate-responsive genes. At this point, the molecular mechanism leading to increased IP7 concentration in response to phosphate starvation is not elucidated. In this perspective, the regulation of the inositol phosphate pathway genes in response to phosphate limitation appears interesting (Table 10). Kcs1 and Ddp1 that contribute to diminish IP7 concentration (by degradation or competition for the IP7 synthesis substrate) are induced only in the chemostat experiment (Boer et al. 2003) while Vip1, which synthesizes IP7, is induced immediately after Pi limitation (Ogawa et al. 2000) and maintained high in the chemostat experiment (Boer et al. 2003). It is tempting to speculate that transcriptional regulation of these genes could contribute to finely tune IP7 concentration upon Pi limitation.
Phosphate uptake and sensing
A lot of recent work has been devoted to understanding how yeast cells sense phosphate. Do yeast cells sense external and/or internal phosphate concentration? How is it converted into a transduction signal? As for other nutrients such as glucose or amino acids (Forsberg and Ljungdahl 2001b), phosphate uptake and sensing appear intimately connected.
Inorganic phosphate uptake in Saccharomyces cerevisiae involves several transporters able to ensure phosphate uptake over a wide range of Pi concentrations. Two high-affinity transporters were identified as Pho84 and Pho89 (Bun-Ya et al. 1991; Martínez and Persson 1998). A Pho84–GFP fusion was found at the plasma membrane (Petersson et al. 1999). Both PHO84 and PHO89 were among the most highly inducible genes upon phosphate limitation (Ogawa et al. 2000). While Pho84 cotransports phosphate with H+, Pho89 is a phosphate/Na+ symporter and works most efficiently under basic conditions, which are not usual physiological conditions for yeast cells (Zvyagilskaya et al. 2008). Indeed, on the basis of knockout experiments, Pho84 appears to be the major high-affinity phosphate transporter (Pattison-Granberg and Persson 2000).
Importantly, mutations in the PHO84 gene result in constitutive expression of PHO5, and epistasis studies have placed PHO84 upstream of all the regulatory components of the PHO pathway (Lenburg and O’Shea 1996). Phosphate uptake was significantly reduced in the pho84 mutant even at high-orthophosphate concentrations, thus suggesting that constitutive expression of PHO5 in the pho84 mutant could be due to decreased internal phosphate concentration (Wykoff and O’Shea 2001); This assumption was further supported by direct measurement of internal phosphate concentration by 31P NMR (Auesukaree et al. 2004; Pinson et al. 2004). This regulatory defect of PHO5 expression in the pho84 mutant could be compensated by overexpressing low-affinity phosphate transporters, and thus it is unlikely that Pho84 acts as a critical phosphate sensor in the phosphate regulatory pathway (Wykoff and O’Shea 2001). However, in work based on mutant analysis and use of agonists such as glycerol-3-phosphate, Pho84 was found to be involved in phosphate signaling to the protein kinase A pathway (Popova et al. 2010). Importantly, a good correlation was observed between internal phosphate concentration and PHO5 expression in several mutants (Auesukaree et al. 2004), thus suggesting that there is an internal phosphate-concentration-sensing mechanism. How it is connected to Pho81 regulation of the Pho80-85 kinase via an IP7-dependent and/or -independent mechanism remains to be determined.
Three low-affinity phosphate transporters—Pho87, Pho90 and Pho91—were characterized and found to be strictly required in the absence of high-affinity transporters (Wykoff and O’Shea 2001). However, more recently, Pho91 was shown to be a vacuolar phosphate transporter (Hurlimann et al. 2007). Mutations in the low-affinity phosphate transporter genes led to upregulation of phosphate-regulated genes (Auesukaree et al. 2003; Pinson et al. 2004). Importantly, this transcriptional response was not associated with a lower intracellular phosphate concentration, suggesting that these transporters contribute to phosphate sensing independently of internal phosphate concentration (Pinson et al. 2004). This result suggests the existence of an extracellular phosphate-sensing mechanism that could be mediated by the low-affinity transporters. Strikingly, the low-affinity phosphate transporters carry an hydrophilic amino-terminal extension that is reminiscent of the carboxy-terminus extension found in the well-described glucose sensors Snf3 and Rgt2 (Özcan et al. 1996). More recently, the amino-terminal regions of the Pho87 and Pho90 transporters (named SPX domains) and the small Spl2 protein were found to negatively regulate phosphate uptake by these transporters (Wykoff et al. 2007; Hurlimann et al. 2009). The effect of Spl2 overexpression on phosphate uptake was dependent on the presence of the SPX domain, and consistently Spl2 was found to interact with the SPX domain in split ubiquitin and co-immunoprecipitation assays (Hurlimann et al. 2009). Spl2 is a phosphate-responsive gene (Ogawa et al. 2000) initially isolated as a suppressor of a phospholipase C mutant (Flick and Thorner 1998). Under low-phosphate conditions, induction of Spl2 expression leads to downregulation of low-affinity transport, which is thought to result in lower intracellular phosphate and attendant activation of the PHO regulon (Wykoff et al. 2007) although this aspect has not yet been addressed experimentally. This feedback loop is required for the bistable properties of the system that result in heterogeneous (either low or high) expression of the PHO84 in individual cells of a clonal population, as revealed by flow cytometry using a GFP construct driven by the PHO84 promoter (Wykoff et al. 2007).
It should be stressed that SPX domains are found in 10 yeast proteins (PHO81, PHO87, PHO90, PHO91, VTC2, VTC3, VTC4, GDE1, SYG1, and YDR089W), of which 8 are closely linked to phosphate metabolism; whether all the SPX domains are regulated by Spl2 is not known. Importantly, in plants, the SPX domain occurs as well in proteins involved in maintaining phosphate homeostasis (Duan et al. 2008), suggesting that it could be directly involved in phosphate sensing.
Purine phosphate connection: more signal molecules
Systematic high-throughput screens for mutants constitutively expressing PHO5 revealed, among many mutants, two purine metabolism mutants: ado1 and adk1 (Auesukaree et al. 2005; Huang and O’Shea 2005). ADO1 and ADK1, respectively, encode adenosine and adenylate kinase, which successively phosphorylate adenosine to AMP and then ADP (Figure 15). The corresponding mutants affect the phosphate response through different pathways. The effect of ado1 is abolished in a vip1 but not in a spl2 background, whereas the adk1 effect is vip1 independent and spl2 dependent (Gauthier et al. 2008). While the role of Ado1 in the phosphate response is unclear, Adk1 effects could be due to lower ATP concentrations in the mutant strain. Indeed, other conditions resulting in lower ATP, such as growth in the absence of adenine, lead to upregulation of PHO84 expression (Gauthier et al. 2008). However, it should be stressed that this presumed regulatory role for ATP is based on coincidental observations and that no molecular mechanism linking ATP concentration to Spl2-dependent regulation has been identified yet. Whether direct or not, such a central role for ATP could reflect the fact that incorporation of phosphate into biomolecules always requires ATP at some point.
More surprisingly, an important role for the purine pathway metabolic intermediate AICAR in the regulation of PHO genes was revealed by transcriptome analysis of mutants accumulating various amounts of AICAR (Pinson et al. 2009). AICAR, but not SAICAR, was found to promote interaction between Pho2 and Pho4 in vivo in a two-hybrid assay, and a Pho2-Pho4 chimera was not responsive to AICAR (Figure 16) (Pinson et al. 2009). Both Pho2 and Pho4 bound an AICAR column in vitro, indicating that the effect of AICAR on these transcription factors could be direct (Pinson et al. 2009). Because AICAR is a major regulator of purine biosynthesis genes, it is possible that coregulation of the two pathways reflects the fact that purine synthesis significantly contributes to phosphate consumption. It is noteworthy that HIS1, which encodes the first committed step in the histidine pathway, is upregulated at the transcriptional level under low Pi conditions (Ogawa et al. 2000). The induction of HIS1 was not observed in the chemostat experiment (Table 10), suggesting that this response is transient and that AICAR could be used to boost the transcriptional response.
Importantly, the above mentioned upregulation of PHO84 in the adk1 mutant is independent of the AICAR response. Indeed, PHO84 is still upregulated in an adk1ade8his1 mutant unable to synthesize AICAR as testified by the inability of this triple mutant to upregulate ADE17 expression (Gauthier et al. 2008). Thus, upregulation of the PHO genes in the AICAR-accumulating mutant ade16ade17 or in the adk1 mutant most likely occurs through different means, thereby illustrating the complex regulatory network between purine and phosphate metabolism.
Polyphosphates as a means to save and buffer intracellular phosphate
While it is still unclear whether intracellular orthophosphate is directly sensed and used as a signal, the situation is even more complicated due to compartmentalization and storage of inorganic polyphosphate (polyP). PolyP is composed of linear phosphate polymers, which can represent >30 mM equivalent Pi while free cytosolic orthophosphate concentrations are ∼20 mM (Pinson et al. 2004). Most polyP is stored in the vacuole, although some is found in other cellular compartments (e.g., nucleus and mitochondria) (Urech et al. 1978; Saito et al. 2005).
Genome-wide analysis of phosphate-responsive genes has allowed the identification of several genes involved in polyP metabolism (Ogawa et al. 2000). Importantly, measurement of phosphate uptake in the polyP synthesis mutants revealed that polyP accumulation acts as a phosphate sink required for sustained Pi uptake (Ogawa et al. 2000). This led to the hypothesis that polyP acts as a buffer that can be mobilized when extracellular phosphate is transiently limiting (Thomas and O’Shea 2005) or when intracellular consumption fluctuates along the cell cycle (Neef and Kladde 2003).
A systematic search for knockout mutants affecting polyP content revealed 255 genes connected to this process (Freimoser et al. 2006), although the reasons why most of these mutants affect polyP concentration are largely unknown.
Regulation by noncoding RNAs
While the idea of pervasive transcription is emerging, it is striking that transcription of noncoding RNAs has been reported at several yeast loci connected to phosphate metabolism where they were suspected to play important regulatory roles. Antisense transcripts have been detected at the PHO5 and PHO84 loci (Camblong et al. 2007; Uhler et al. 2007). These transcripts, which are degraded by the nuclear exosome, are clearly more abundant in exosome mutants (Camblong et al. 2007; Uhler et al. 2007). In the case of PHO5, antisense transcription appears to affect the speed of chromatin remodeling during transcription activation (Uhler et al. 2007). For PHO84, antisense transcription was observed during chronological aging (Camblong et al. 2007). In this case, antisense transcription was found to result in the recruitment of the histone deacetylase Hda1 and thereby downregulate the authentic PHO84 promoter (Camblong et al. 2007). Importantly, antisense at PHO84 was found to act both in cis and in trans, as shown by ectopic expression of the antisense and use of a ribozyme inserted in the antisense sequence to cleave the antisense after its synthesis (Camblong et al. 2009). In a third study carried out on the KCS1 locus, both antisense and intragenic transcripts were detected (Nishizawa et al. 2008). Importantly, expression of the noncoding transcripts was dependent on the transcription factor Pho4 and responded to phosphate availability (Nishizawa et al. 2008). However, since the abundance of these transcripts did not significantly affect KCS1 expression (Nishizawa et al. 2008), it is not yet clear whether these transcripts play a regulatory role such as the one established in the case of PHO84 (Camblong et al. 2007). Further roles for noncodingRNAs in regulation of phosphate utilization are waiting to be discovered.
Future Directions
The cellular components required for amino acid, nucleotide, and phosphate metabolism in yeast can be subdivided in four interrelated rudimental categories: (1) the permeases in the plasma membrane that facilitate uptake and secretion of metabolites; (2) the metabolic sensors that directly control enzymatic activity or indirectly regulate metabolic pathways by altering patterns of gene expression; (3) the enzymes catalyzing the synthesis, catabolism, and interconversion of metabolites; and (4) the intracellular organelles, including the vacuole, mitochondria, and peroxisome that compartmentalize metabolic processes and also serve as storage compartments. Although great progress has been made in understanding these rudiments, major holes in understanding remain, in particular how individual components are coordinated to function in synchrony.
The compartmentalized structure of eukaryotic cells requires intracellular targeting mechanisms to ensure correct localization of transport systems. Cell biological approaches continue to provide insight into the intracellular traffic of nutrient permeases/transporters in the early (ER) and late (Golgi and post-Golgi) stages of the secretory pathway. In particular, an increased understanding of the routing mechanisms may provide answers as to how cells sense their overall metabolic state. For example, it is intriguing to consider the general amino acid permease (Gap1) as an example. Gap1 is known to function as a transceptor in amino acid-starved cells that responds to the reintroduction of amino acids and initiates signals activating the cAMP-protein kinase pathway (Thevelein and Voordeckers 2009; van Zeebroeck et al. 2009; Popova et al. 2010; Rubio-Texeira et al. 2010). Proper routing of Gap1 from endosomal compartments to the plasma membrane depends on it being functionally active (Gao and Kaiser 2006; Risinger et al. 2006); i.e., to be sorted correctly, Gap1 must be able to switch its conformation between an outward facing (lumenal) and an inward facing (cytoplasm) conformation (Cain and Kaiser 2011). The combination of these findings raises the possibility that, as Gap1 progresses through the secretory pathway, it may have the capacity to sample the amino acid levels within cells. Hence, in similarity to the amino acid receptor of the external amino acid Ssy1 (Wu et al. 2006; Poulsen et al. 2008), transceptors may be the ultimate source of internal nutrient-based signals affecting important signaling components, e.g., TORC1.
Finally, there is a growing appreciation of the importance of metabolites in regulating many biological processes. In the future, we expect that the ability to correlate data obtained from metabolomic and classical gene expression analyses will be highly informative and critical for making progress toward a more complete understanding. For example, in the case of the cellular response to phosphate limitation, it is clear that transcriptome data obtained from batch (phosphate starvation) and from chemostat cultures (adapted to phosphate limitation) reveal different, albeit overlapping, sets of genes. The differences in gene expression are likely due to differences in the metabolic state of cells; consequently, metabolomic analysis should provide crucial information to help decipher the molecular nature of the phosphate limitation signal(s). Also, the great expandsion of whole-genome information on many different fungi enables sophisticated comparisons to be carried out, which will likely yield interesting insights regarding metabolic regulation and may reveal precise information of general features and specific adaptations.
Acknowledgments
We thank members of the Ljungdahl and Daignan-Fornier laboratories for fruitful discussions and patience. J. A. Martens (University of Pittsburgh) is gratefully acknowledged for granting permission to reproduce the schematic diagram depicted in Figure 10. The vastness of the subject matter and space limitations have precluded referencing all relevant papers, and undoubtedly we have failed to cite some papers of equal or greater value than the ones cited; we apologize for the inadvertent omission of uncited work. Original research in our laboratories is supported by the Swedish Research Council (P.O.L.) and Conseil Régional d’Aquitaine and Agence Nationale de la Recherche grant no. BLAN06-1_136912 (B.D.-F.).
Footnotes
Communicating editor: A. Hinnebusch
Literature Cited
- Abadjieva A., Hilven P., Pauwels K., Crabeel M., 2000. The yeast ARG7 gene product is autoproteolyzed to two subunit peptides, yielding active ornithine acetyltransferase. J. Biol. Chem. 275: 11361–11367 [DOI] [PubMed] [Google Scholar]
- Abadjieva A., Pauwels K., Hilven P., Crabeel M., 2001. A new yeast metabolon involving at least the two first enzymes of arginine biosynthesis: acetylglutamate synthase activity requires complex formation with acetylglutamate kinase. J. Biol. Chem. 276: 42869–42880 [DOI] [PubMed] [Google Scholar]
- Abdel-Sater F., El Bakkoury M., Urrestarazu A., Vissers S., André B., 2004a Amino acid signaling in yeast: casein kinase I and the Ssy5 endoprotease are key determinants of endoproteolytic activation of the membrane-bound Stp1 transcription factor. Mol. Cell. Biol. 24: 9771–9785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Sater F., Iraqui I., Urrestarazu A., André B., 2004b The external amino acid signaling pathway promotes activation of Stp1 and Uga35/Dal81 transcription factors for induction of the AGP1 gene in Saccharomyces cerevisiae. Genetics 166: 1727–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Sater F., Jean C., Merhi A., Vissers S., André B., 2011. Amino-acid signalling in yeast: activation of the Ssy5 protease is associated with its phosphorylation-induced ubiquitylation. J. Biol. Chem. 286: 12006–12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam K., Naider F., Becker J. M., 1995. A recognition component of the ubiquitin system is required for peptide transport in Saccharomyces cerevisiae. Mol. Microbiol. 15: 225–234 [DOI] [PubMed] [Google Scholar]
- Alfonzo J. D., Crother T. R., Guetsova M. L., Daignan-Fornier B., Taylor M. W., 1999. APT1, but not APT2, codes for a functional adenine phosphoribosyltransferase in Saccharomyces cerevisiae. J. Bacteriol. 181: 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann M., Linder P., 2010. Power of yeast for analysis of eukaryotic translation initiation. J. Biol. Chem. 285: 31907–31912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar N., Messenguy F., El Bakkoury M., Dubois E., 2000. ArgRII, a component of the ArgR-Mcm1 complex involved in the control of arginine metabolism in Saccharomyces cerevisiae, is the sensor of arginine. Mol. Cell. Biol. 20: 2087–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S., Kumar R., Sheets E. D., Benkovic S. J., 2008. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 320: 103–106 [DOI] [PubMed] [Google Scholar]
- Andi B., West A. H., Cook P. F., 2005. Regulatory mechanism of histidine-tagged homocitrate synthase from Saccharomyces cerevisiae. I. Kinetic studies. J. Biol. Chem. 280: 31624–31632 [DOI] [PubMed] [Google Scholar]
- André B., Hein C., Grenson M., Jauniaux J. C., 1993. Cloning and expression of the UGA4 gene coding for the inducible GABA-specific transport protein of Saccharomyces cerevisiae. Mol. Gen. Genet. 237: 17–25 [DOI] [PubMed] [Google Scholar]
- Andréasson C., Ljungdahl P. O., 2002. Receptor-mediated endoproteolytic activation of two transcription factors in yeast. Genes Dev. 16: 3158–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréasson C., Ljungdahl P. O., 2004. The N-terminal regulatory domain of Stp1p is modular and, fused to an artificial transcription factor, confers full Ssy1p-Ptr3p-Ssy5p sensor control. Mol. Cell. Biol. 24: 7503–7513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréasson C., Neve E. P. A., Ljungdahl P. O., 2004. Four permeases import proline and the toxic proline analogue azetidine-2-carboxylate into yeast. Yeast 21: 193–199 [DOI] [PubMed] [Google Scholar]
- Andréasson C., Heessen S., Ljungdahl P. O., 2006. Regulation of transcription factor latency by receptor-activated proteolysis. Genes Dev. 20: 1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreichuk Iu V., Shabes A. V., Ryzhova T. A., Kotova I. A., Domkin V. D., 1995. Saccharomyces cerevisiae ADE12 gene, coding for adenylosuccinate synthetase (EC 6.3.4.4). Cloning, sequencing, expression, and superproduction. [Translated from Russian.] Mol. Gen. Mikrobiol. Virusol. No. 1, 21–28 [PubMed] [Google Scholar]
- Anraku Y., Hirata R., Wada Y., Ohya Y., 1992. Molecular genetics of the yeast vacuolar H(+)-ATPase. J. Exp. Biol. 172: 67–81 [DOI] [PubMed] [Google Scholar]
- Aoki-Kinoshita K. F., Kanehisa M., 2007. Gene annotation and pathway mapping in KEGG. Methods Mol. Biol. 396: 71–91 [DOI] [PubMed] [Google Scholar]
- Aouida M., Leduc A., Poulin R., Ramotar D., 2005. AGP2 encodes the major permease for high affinity polyamine import in Saccharomyces cerevisiae. J. Biol. Chem. 280: 24267–24276 [DOI] [PubMed] [Google Scholar]
- Aouida M., Khodami-Pour A., Ramotar D., 2009. Novel role for the Saccharomyces cerevisiae oligopeptide transporter Opt2 in drug detoxification. Biochem. Cell Biol. 87: 653–661 [DOI] [PubMed] [Google Scholar]
- Arndt K. T., Styles C., Fink G. R., 1987. Multiple global regulators control HIS4 transcription in yeast. Science 237: 874–880 [DOI] [PubMed] [Google Scholar]
- Auesukaree C., Homma T., Kaneko Y., Harashima S., 2003. Transcriptional regulation of phosphate-responsive genes in low-affinity phosphate-transporter-defective mutants in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 306: 843–850 [DOI] [PubMed] [Google Scholar]
- Auesukaree C., Homma T., Tochio H., Shirakawa M., Kaneko Y., et al. , 2004. Intracellular phosphate serves as a signal for the regulation of the PHO pathway in Saccharomyces cerevisiae. J. Biol. Chem. 279: 17289–17294 [DOI] [PubMed] [Google Scholar]
- Auesukaree C., Tochio H., Shirakawa M., Kaneko Y., Harashima S., 2005. Plc1p, Arg82p, and Kcs1p, enzymes involved in inositol pyrophosphate synthesis, are essential for phosphate regulation and polyphosphate accumulation in Saccharomyces cerevisiae. J. Biol. Chem. 280: 25127–25133 [DOI] [PubMed] [Google Scholar]
- Avendano A., Deluna A., Olivera H., Valenzuela L., Gonzalez A., 1997. GDH3 encodes a glutamate dehydrogenase isozyme, a previously unrecognized route for glutamate biosynthesis in Saccharomyces cerevisiae. J. Bacteriol. 179: 5594–5597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram S., Simon E., Gildor T., Glaser F., Kornitzer D., 2008. Autophosphorylation-induced degradation of the Pho85 cyclin Pcl5 is essential for response to amino acid limitation. Mol. Cell. Biol. 28: 6858–6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajmoczi M., Sneve M., Eide D. J., Drewes L. R., 1998. TAT1 encodes a low-affinity histidine transporter in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 243: 205–209 [DOI] [PubMed] [Google Scholar]
- Barbaric S., Munsterkotter M., Svaren J., Horz W., 1996. The homeodomain protein Pho2 and the basic-helix-loop-helix protein Pho4 bind DNA cooperatively at the yeast PHO5 promoter. Nucleic Acids Res. 24: 4479–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey R., Baudouin-Cornu P., Lee T. A., Rouillon A., Zarzov P., et al. , 2005. Inducible dissociation of SCF(Met30) ubiquitin ligase mediates a rapid transcriptional response to cadmium. EMBO J. 24: 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Lai W., Breslav M., Naider F., Becker J. M., 1998. PTR3, a novel gene mediating amino acid-inducible regulation of peptide transport in Saccharomyces cerevisiae. Mol. Microbiol. 29: 297–310 [DOI] [PubMed] [Google Scholar]
- Baudouin-Cornu P., Labarre J., 2006. Regulation of the cadmium stress response through SCF-like ubiquitin ligases: comparison between Saccharomyces cerevisiae, Schizosaccharomyces pombe and mammalian cells. Biochimie 88: 1673–1685 [DOI] [PubMed] [Google Scholar]
- Beck T., Hall M. N., 1999. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402: 689–692 [DOI] [PubMed] [Google Scholar]
- Becker B., Feller A., el Alami M., Dubois E., Pierard A., 1998. A nonameric core sequence is required upstream of the LYS genes of Saccharomyces cerevisiae for Lys14p-mediated activation and apparent repression by lysine. Mol. Microbiol. 29: 151–163 [DOI] [PubMed] [Google Scholar]
- Belenky P. A., Moga T. G., Brenner C., 2008. Saccharomyces cerevisiae YOR071C encodes the high affinity nicotinamide riboside transporter Nrt1. J. Biol. Chem. 283: 8075–8079 [DOI] [PubMed] [Google Scholar]
- Bernales S., Papa F. R., Walter P., 2006. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 22: 487–508 [DOI] [PubMed] [Google Scholar]
- Bernard F., André B., 2001. Genetic analysis of the signalling pathway activated by external amino acids in Saccharomyces cerevisiae. Mol. Microbiol. 41: 489–502 [DOI] [PubMed] [Google Scholar]
- Bertram P. G., Choi J. H., Carvalho J., Ai W., Zeng C., et al. , 2000. Tripartite regulation of Gln3p by TOR, Ure2p, and phosphatases. J. Biol. Chem. 275: 35727–35733 [DOI] [PubMed] [Google Scholar]
- Binda M., Peli-Gulli M. P., Bonfils G., Panchaud N., Urban J., et al. , 2009. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol. Cell 35: 563–573 [DOI] [PubMed] [Google Scholar]
- Blaiseau P. L., Thomas D., 1998. Multiple transcriptional activation complexes tether the yeast activator Met4 to DNA. EMBO J. 17: 6327–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boban M., Ljungdahl P. O., 2007. Dal81 enhances Stp1- and Stp2-dependent transcription necessitating negative modulation by inner nuclear membrane protein Asi1 in Saccharomyces cerevisiae. Genetics 176: 2087–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boban M., Zargari A., Andréasson C., Heessen S., Thyberg J., et al. , 2006. Asi1 is an inner nuclear membrane protein that restricts promoter access of two latent transcription factors. J. Cell Biol. 173: 695–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckstaens M., André B., Marini A. M., 2007. The yeast ammonium transport protein Mep2 and its positive regulator, the Npr1 kinase, play an important role in normal and pseudohyphal growth on various nitrogen media through retrieval of excreted ammonium. Mol. Microbiol. 64: 534–546 [DOI] [PubMed] [Google Scholar]
- Boer V. M., de Winde J. H., Pronk J. T., Piper M. D., 2003. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J. Biol. Chem. 278: 3265–3274 [DOI] [PubMed] [Google Scholar]
- Bomeke K., Pries R., Korte V., Scholz E., Herzog B., et al. , 2006. Yeast Gcn4p stabilization is initiated by the dissociation of the nuclear Pho85p/Pcl5p complex. Mol. Biol. Cell 17: 2952–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonchird C., Messenguy F., Dubois E., 1991. Determination of amino acid sequences involved in the processing of the ARG5/ARG6 precursor in Saccharomyces cerevisiae. Eur. J. Biochem. 199: 325–335 [DOI] [PubMed] [Google Scholar]
- Bowers K., Stevens T. H., 2005. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1744: 438–454 [DOI] [PubMed] [Google Scholar]
- Breitling R., Sharif O., Hartman M. L., Krisans S. K., 2002. Loss of compartmentalization causes misregulation of lysine biosynthesis in peroxisome-deficient yeast cells. Eukaryot. Cell 1: 978–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton A., Pinson B., Coulpier F., Giraud M. F., Dautant A., et al. , 2008. Lethal accumulation of guanylic nucleotides in Saccharomyces cerevisiae HPT1-deregulated mutants. Genetics 178: 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohee S., Barriot R., Moreau Y., André B., 2010. YTPdb: a wiki database of yeast membrane transporters. Biochim. Biophys. Acta 1798: 1908–1912 [DOI] [PubMed] [Google Scholar]
- Bun-Ya M., Nishimura M., Harashima S., Oshima Y., 1991. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol. Cell. Biol. 11: 3229–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd C., Turner G. C., Varshavsky A., 1998. The N-end rule pathway controls the import of peptides through degradation of a transcriptional repressor. EMBO J. 17: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Hauser M., Naider F., Becker J. M., 2007. Differential regulation and substrate preferences in two peptide transporters of Saccharomyces cerevisiae. Eukaryot. Cell 6: 1805–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain N. E., Kaiser C. A., 2011. Transport activity-dependent intracellular sorting of the yeast general amino acid permease. Mol. Biol. Cell 22: 1919–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camblong J., Iglesias N., Fickentscher C., Dieppois G., Stutz F., 2007. Antisense RNA stabilization induces transcriptional gene silencing via histone deacetylation in S. cerevisiae. Cell 131: 706–717 [DOI] [PubMed] [Google Scholar]
- Camblong J., Beyrouthy N., Guffanti E., Schlaepfer G., Steinmetz L. M., et al. , 2009. Trans-acting antisense RNAs mediate transcriptional gene cosuppression in S. cerevisiae. Genes Dev. 23: 1534–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. N., Leverentz M. K., Ryan L. A., Reece R. J., 2008. Metabolic control of transcription: paradigms and lessons from Saccharomyces cerevisiae. Biochem. J. 414: 177–187 [DOI] [PubMed] [Google Scholar]
- Cardenas M. E., Cutler N. S., Lorenz M. C., Di Como C. J., Heitman J., 1999. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 13: 3271–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo S. B., Bermudez Moretti M., Correa Garcia S., 2010. Uga3 and Uga35/Dal81 transcription factors regulate UGA4 transcription in response to gamma-aminobutyric acid and leucine. Eukaryot. Cell 9: 1262–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho J., Bertram P. G., Wente S. R., Zheng X. F., 2001. Phosphorylation regulates the interaction between Gln3p and the nuclear import factor Srp1p. J. Biol. Chem. 276: 25359–25365 [DOI] [PubMed] [Google Scholar]
- Castegna A., Scarcia P., Agrimi G., Palmieri L., Rottensteiner H., et al. , 2010. Identification and functional characterization of a novel mitochondrial carrier for citrate and oxoglutarate in Saccharomyces cerevisiae. J. Biol. Chem. 285: 17359–17370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavero S., Vozza A., del Arco A., Palmieri L., Villa A., et al. , 2003. Identification and metabolic role of the mitochondrial aspartate-glutamate transporter in Saccharomyces cerevisiae. Mol. Microbiol. 50: 1257–1269 [DOI] [PubMed] [Google Scholar]
- Chahomchuen T., Hondo K., Ohsaki M., Sekito T., Kakinuma Y., 2009. Evidence for Avt6 as a vacuolar exporter of acidic amino acids in Saccharomyces cerevisiae cells. J. Gen. Appl. Microbiol. 55: 409–417 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran S., Skowyra D., 2008. The emerging regulatory potential of SCFMet30-mediated polyubiquitination and proteolysis of the Met4 transcriptional activator. Cell Div. 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran S., Deffenbaugh A. E., Ford D. A., Bailly E., Mathias N., et al. , 2006. Destabilization of binding to cofactors and SCFMet30 is the rate-limiting regulatory step in degradation of polyubiquitinated Met4. Mol. Cell 24: 689–699 [DOI] [PubMed] [Google Scholar]
- Chen S., Brockenbrough J. S., Dove J. E., Aris J. P., 1997. Homocitrate synthase is located in the nucleus in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 272: 10839–10846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasova V., Qiu H., Hinnebusch A. G., 2010. Snf1 promotes phosphorylation of the alpha subunit of eukaryotic translation initiation factor 2 by activating Gcn2 and inhibiting phosphatases Glc7 and Sit4. Mol. Cell. Biol. 30: 2862–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman J. A., Rai R., Cunningham T., Svetlov V., Cooper T. G., 1996. Gat1p, a GATA family protein whose production is sensitive to nitrogen catabolite repression, participates in transcriptional activation of nitrogen-catabolic genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman J. A., Rai R., Loprete D. M., Cunningham T., Svetlov V., et al. , 1997. Cross regulation of four GATA factors that control nitrogen catabolic gene expression in Saccharomyces cerevisiae. J. Bacteriol. 179: 3416–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan R. S., Gounalaki N., Hatzis P., Tzamarias D., 1999. The Tup1-Cyc8 protein complex can shift from a transcriptional co-repressor to a transcriptional co-activator. J. Biol. Chem. 274: 205–210 [DOI] [PubMed] [Google Scholar]
- Cooper T., 1982a Nitrogen metabolism in Saccharomyces cerevisiae, pp. 39–100 The Molecular Biology of the Yeast Saccharomyces: Metabolism and Gene Expression, edited by Strathern J. N., Jones E. W., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Cooper T., 1982b Transport in Saccharomyces cerevisiae, pp. 399–461 The Molecular Biology of the Yeast Saccharomyces: Metabolism and Gene Expression, edited by Strathern J. N., Jones E. W., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Cooper T. G., 2002. Transmitting the signal of excess nitrogen in Saccharomyces cerevisiae from the Tor proteins to the GATA factors: connecting the dots. FEMS Microbiol. Rev. 26: 223–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coornaert D., Vissers S., André B., 1991. The pleiotropic UGA35(DURL) regulatory gene of Saccharomyces cerevisiae: cloning, sequence and identity with the DAL81 gene. Gene 97: 163–171 [DOI] [PubMed] [Google Scholar]
- Coschigano P. W., Magasanik B., 1991. The URE2 gene product of Saccharomyces cerevisiae plays an important role in the cellular response to the nitrogen source and has homology to glutathione s-transferases. Mol. Cell. Biol. 11: 822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne W. E., Magasanik B., 1988. Regulation of nitrogen assimilation in Saccharomyces cerevisiae: roles of the URE2 and GLN3 genes. J. Bacteriol. 170: 708–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., Tate J. J., Cooper T. G., 2002. Cytoplasmic compartmentation of Gln3 during nitrogen catabolite repression and the mechanism of its nuclear localization during carbon starvation in Saccharomyces cerevisiae. J. Biol. Chem. 277: 37559–37566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., Kulkarni A., Tate J. J., Cooper T. G., 2004. Gln3 phosphorylation and intracellular localization in nutrient limitation and starvation differ from those generated by rapamycin inhibition of Tor1/2 in Saccharomyces cerevisiae. J. Biol. Chem. 279: 10270–10278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabeel M., Soetens O., De Rijcke M., Pratiwi R., Pankiewicz R., 1996. The ARG11 gene of Saccharomyces cerevisiae encodes a mitochondrial integral membrane protein required for arginine biosynthesis. J. Biol. Chem. 271: 25011–25018 [DOI] [PubMed] [Google Scholar]
- Creasy C. L., Madden S. L., Bergman L. W., 1993. Molecular analysis of the PHO81 gene of Saccharomyces cerevisiae. Nucleic Acids Res. 21: 1975–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo J. L., Helliwell S. B., Wiederkehr C., Demougin P., Fowler B., et al. , 2004. NPR1 kinase and RSP5–BUL1/2 ubiquitin ligase control GLN3-dependent transcription in Saccharomyces cerevisiae. J. Biol. Chem. 279: 37512–37517 [DOI] [PubMed] [Google Scholar]
- Crowley J. C., Kaback D. B., 1984. Molecular cloning of chromosome I DNA from Saccharomyces cerevisiae: isolation of the ADE1 gene. J. Bacteriol. 159: 413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham T. S., Cooper T. G., 1991. Expression of the DAL80 gene, whose product is homologous to the GATA factors and is a negative regulator of multiple nitrogen catabolic genes in Saccharomyces cerevisiae, is sensitive to nitrogen catabolite repression. Mol. Cell. Biol. 11: 6205–6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daignan-Fornier B., Fink G. R., 1992. Coregulation of purine and histidine biosynthesis by the transcriptional activators BAS1 and BAS2. Proc. Natl. Acad. Sci. USA 89: 6746–6750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. A., Ares M., Jr, 2006. Accumulation of unstable promoter-associated transcripts upon loss of the nuclear exosome subunit Rrp6p in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 103: 3262–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer M., Bebelman J. P., Gonçalves P. M., Maat J., van Heerikhuizen H., et al. , 1998. Regulation of expression of the amino acid transporter gene BAP3 in Saccharomyces cerevisiae. Mol. Microbiol. 30: 603–613 [DOI] [PubMed] [Google Scholar]
- de Boer M., Nielsen P. S., Bebelman J. P., Heerikhuizen H., Andersen H. A., et al. , 2000. Stp1p, Stp2p and Abf1p are involved in regulation of expression of the amino acid transporter gene BAP3 of Saccharomyces cerevisiae. Nucleic Acids Res. 28: 974–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecq P., Werner M., Feller A., Filipkowski R. K., Messenguy F., et al. , 1994. A segment of mRNA encoding the leader peptide of the CPA1 gene confers repression by arginine on a heterologous yeast gene transcript. Mol. Cell. Biol. 14: 2378–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuna A., Avendano A., Riego L., Gonzalez A., 2001. NADP-glutamate dehydrogenase isoenzymes of Saccharomyces cerevisiae. Purification, kinetic properties, and physiological roles. J. Biol. Chem. 276: 43775–43783 [DOI] [PubMed] [Google Scholar]
- de Montigny J., Belarbi A., Hubert J. C., Lacroute F., 1989. Structure and expression of the URA5 gene of Saccharomyces cerevisiae. Mol. Gen. Genet. 215: 455–462 [DOI] [PubMed] [Google Scholar]
- de Montigny J., Kern L., Hubert J. C., Lacroute F., 1990. Cloning and sequencing of URA10, a second gene encoding orotate phosphoribosyl transferase in Saccharomyces cerevisiae. Curr. Genet. 17: 105–111 [DOI] [PubMed] [Google Scholar]
- Denis V., Daignan-Fornier B., 1998. Synthesis of glutamine, glycine and 10-formyl tetrahydrofolate is coregulated with purine biosynthesis in Saccharomyces cerevisiae. Mol. Gen. Genet. 259: 246–255 [DOI] [PubMed] [Google Scholar]
- Denis V., Boucherie H., Monribot C., Daignan-Fornier B., 1998. Role of the myb-like protein bas1p in Saccharomyces cerevisiae: a proteome analysis. Mol. Microbiol. 30: 557–566 [DOI] [PubMed] [Google Scholar]
- Denis-Duphil M., 1989. Pyrimidine biosynthesis in Saccharomyces cerevisiae: the ura2 cluster gene, its multifunctional enzyme product, and other structural or regulatory genes involved in de novo UMP synthesis. Biochem. Cell Biol. 67: 612–631 [DOI] [PubMed] [Google Scholar]
- de Rijcke M., Seneca S., Punyammalee B., Glansdorff N., Crabeel M., 1992. Characterization of the DNA target site for the yeast ARGR regulatory complex, a sequence able to mediate repression or induction by arginine. Mol. Cell. Biol. 12: 68–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps J., Dubois E., Wiame J. M., 1979. L-Ornithine transaminase synthesis in Saccharomyces cerevisiae: regulation by inducer exclusion. Mol. Gen. Genet. 174: 225–232 [DOI] [PubMed] [Google Scholar]
- Des Etages S. A., Saxena D., Huang H. L., Falvey D. A., Barber D., et al. , 2001. Conformational changes play a role in regulating the activity of the proline utilization pathway-specific regulator in Saccharomyces cerevisiae. Mol. Microbiol. 40: 890–899 [DOI] [PubMed] [Google Scholar]
- Desmoucelles C., Pinson B., Saint-Marc C., Daignan-Fornier B., 2002. Screening the yeast “disruptome” for mutants affecting resistance to the immunosuppressive drug, mycophenolic acid. J. Biol. Chem. 277: 27036–27044 [DOI] [PubMed] [Google Scholar]
- Didion T., Grauslund M., Kielland-Brandt M. C., Andersen H. A., 1996. Amino acids induce expression of BAP2, a branched-chain amino acid permease in Saccharomyces cerevisiae. J. Bacteriol. 178: 2025–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didion T., Regenberg B., Jørgensen M. U., Kielland-Brandt M. C., Andersen H. A., 1998. The permease homologue Ssy1p controls the expression of amino acid and peptide transporter genes in Saccharomyces cerevisiae. Mol. Microbiol. 27: 643–650 [DOI] [PubMed] [Google Scholar]
- Dilova I., Chen C. Y., Powers T., 2002. Mks1 in concert with TOR signaling negatively regulates RTG target gene expression in S. cerevisiae. Curr. Biol. 12: 389–395 [DOI] [PubMed] [Google Scholar]
- Dohmen R. J., Wu P., Varshavsky A., 1994. Heat-inducible degron: a method for constructing temperature-sensitive mutants. Science 263: 1273–1276 [DOI] [PubMed] [Google Scholar]
- Dong J., Qiu H., Garcia-Barrio M., Anderson J., Hinnebusch A. G., 2000. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell 6: 269–279 [DOI] [PubMed] [Google Scholar]
- Dormer U. H., Westwater J., McLaren N. F., Kent N. A., Mellor J., et al. , 2000. Cadmium-inducible expression of the yeast GSH1 gene requires a functional sulfur-amino acid regulatory network. J. Biol. Chem. 275: 32611–32616 [DOI] [PubMed] [Google Scholar]
- Drillien R., Lacroute F., 1972. Ureidosuccinic acid uptake in yeast and some aspects of its regulation. J. Bacteriol. 109: 203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Yi K., Dang L., Huang H., Wu W., et al. , 2008. Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J. 54: 965–975 [DOI] [PubMed] [Google Scholar]
- Dubois E., Messenguy F., 1997. Integration of the multiple controls regulating the expression of the arginase gene CAR1 of Saccharomyces cerevisiae in response to different nitrogen signals: role of Gln3p, ArgRp-Mcm1p, and Ume6p. Mol. Gen. Genet. 253: 568–580 [DOI] [PubMed] [Google Scholar]
- Dubois E., Hiernaux D., Grennon M., Wiame J. M., 1978. Specific induction of catabolism and its relation to repression of biosynthesis in arginine metabolism of Saccharomyces cerevisiae. J. Mol. Biol. 122: 383–406 [DOI] [PubMed] [Google Scholar]
- Dubois E., Dewaste V., Erneux C., Messenguy F., 2000. Inositol polyphosphate kinase activity of Arg82/ArgRIII is not required for the regulation of the arginine metabolism in yeast. FEBS Lett. 486: 300–304 [DOI] [PubMed] [Google Scholar]
- Dubois E. L., Wiame J.-M., 1976. Non specific induction of arginase in Saccharomyces cerevisiae. Biochimie 58: 207–211 [DOI] [PubMed] [Google Scholar]
- Dubouloz F., Deloche O., Wanke V., Cameroni E., De Virgilio C., 2005. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol. Cell 19: 15–26 [DOI] [PubMed] [Google Scholar]
- Dujardin G., Kermorgant M., Slonimski P. P., Boucherie H., 1994. Cloning and sequencing of the GMP synthetase-encoding gene of Saccharomyces cerevisiae. Gene 139: 127–132 [DOI] [PubMed] [Google Scholar]
- Eckert-Boulet N., Larsson K., Wu B., Poulsen P., Regenberg B., et al. , 2006. Deletion of RTS1, encoding a regulatory subunit of protein phosphatase 2A, results in constitutive amino acid signaling via increased Stp1p processing. Eukaryot. Cell 5: 174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Alami M., Feller A., Pierard A., Dubois E., 2002. The proper folding of a long C-terminal segment of the yeast Lys14p regulator is required for activation of LYS genes in response to the metabolic effector. Mol. Microbiol. 43: 1629–1639 [DOI] [PubMed] [Google Scholar]
- El Alami M., Messenguy F., Scherens B., Dubois E., 2003. Arg82p is a bifunctional protein whose inositol polyphosphate kinase activity is essential for nitrogen and PHO gene expression but not for Mcm1p chaperoning in yeast. Mol. Microbiol. 49: 457–468 [DOI] [PubMed] [Google Scholar]
- El Berry H. M., Majumdar M. L., Cunningham T. S., Sumrada R. A., Cooper T. G., 1993. Regulation of the urea active transporter gene (DUR3) in Saccharomyces cerevisiae. J. Bacteriol. 175: 4688–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjo F., Nosaka K., Ogata M., Iwashima A., Nishimura H., 1997. Isolation and characterization of a thiamin transport gene, THI10, from Saccharomyces cerevisiae. J. Biol. Chem. 272: 19165–19170 [DOI] [PubMed] [Google Scholar]
- Erbs P., Exinger F., Jund R., 1997. Characterization of the Saccharomyces cerevisiae FCY1 gene encoding cytosine deaminase and its homologue FCA1 of Candida albicans. Curr. Genet. 31: 1–6 [DOI] [PubMed] [Google Scholar]
- Escobar-Henriques M., Daignan-Fornier B., 2001. Transcriptional regulation of the yeast gmp synthesis pathway by its end products. J. Biol. Chem. 276: 1523–1530 [DOI] [PubMed] [Google Scholar]
- Escobar-Henriques M., Collart M. A., Daignan-Fornier B., 2003a Transcription initiation of the yeast IMD2 gene is abolished in response to nutrient limitation through a sequence in its coding region. Mol. Cell. Biol. 23: 6279–6290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Henriques M., Daignan-Fornier B., Collart M. A., 2003b The critical cis-acting element required for IMD2 feedback regulation by GDP is a TATA box located 202 nucleotides upstream of the transcription start site. Mol. Cell. Biol. 23: 6267–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escusa S., Camblong J., Galan J. M., Pinson B., Daignan-Fornier B., 2006. Proteasome- and SCF-dependent degradation of yeast adenine deaminase upon transition from proliferation to quiescence requires a new F-box protein named Saf1p. Mol. Microbiol. 60: 1014–1025 [DOI] [PubMed] [Google Scholar]
- Escusa S., Laporte D., Massoni A., Boucherie H., Dautant A., et al. , 2007. Skp1-Cullin-F-box-dependent degradation of Aah1p requires its interaction with the F-box protein Saf1p. J. Biol. Chem. 282: 20097–20103 [DOI] [PubMed] [Google Scholar]
- Exinger F., Lacroute F., 1992. 6-Azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr. Genet. 22: 9–11 [DOI] [PubMed] [Google Scholar]
- Feller A., Ramos F., Pierard A., Dubois E., 1997. Lys80p of Saccharomyces cerevisiae, previously proposed as a specific repressor of LYS genes, is a pleiotropic regulatory factor identical to Mks1p. Yeast 13: 1337–1346 [DOI] [PubMed] [Google Scholar]
- Feller A., Ramos F., Pierard A., Dubois E., 1999. In Saccharomyces cerevisae, feedback inhibition of homocitrate synthase isoenzymes by lysine modulates the activation of LYS gene expression by Lys14p. Eur. J. Biochem. 261: 163–170 [DOI] [PubMed] [Google Scholar]
- Feller A., Boeckstaens M., Marini A. M., Dubois E., 2006. Transduction of the nitrogen signal activating Gln3-mediated transcription is independent of Npr1 kinase and Rsp5-Bul1/2 ubiquitin ligase in Saccharomyces cerevisiae. J. Biol. Chem. 281: 28546–28554 [DOI] [PubMed] [Google Scholar]
- Flick J. S., Thorner J., 1998. An essential function of a phosphoinositide-specific phospholipase C is relieved by inhibition of a cyclin-dependent protein kinase in the yeast Saccharomyces cerevisiae. Genetics 148: 33–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick K., Ouni I., Wohlschlegel J. A., Capati C., McDonald W. H., et al. , 2004. Proteolysis-independent regulation of the transcription factor Met4 by a single Lys 48-linked ubiquitin chain. Nat. Cell Biol. 6: 634–641 [DOI] [PubMed] [Google Scholar]
- Flynn P. J., Reece R. J., 1999. Activation of transcription by metabolic intermediates of the pyrimidine biosynthetic pathway. Mol. Cell. Biol. 19: 882–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg H., Ljungdahl P. O., 2001a Genetic and biochemical analysis of the yeast plasma membrane Ssy1p-Ptr3p-Ssy5p sensor of extracellular amino acids. Mol. Cell. Biol. 21: 814–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg H., Ljungdahl P. O., 2001b Sensors of extracellular nutrients in Saccharomyces cerevisiae. Curr. Genet. 40: 91–109 [DOI] [PubMed] [Google Scholar]
- Freimoser F. M., Hurlimann H. C., Jakob C. A., Werner T. P., Amrhein N., 2006. Systematic screening of polyphosphate (poly P) levels in yeast mutant cells reveals strong interdependence with primary metabolism. Genome Biol. 7: R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi T., Nikawa J., Kimura N., Watanabe K., 1993. Isolation, overexpression and disruption of a Saccharomyces cerevisiae YNK gene encoding nucleoside diphosphate kinase. Gene 129: 141–146 [DOI] [PubMed] [Google Scholar]
- Gaba A., Wang Z., Krishnamoorthy T., Hinnebusch A. G., Sachs M. S., 2001. Physical evidence for distinct mechanisms of translational control by upstream open reading frames. EMBO J. 20: 6453–6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaba A., Jacobson A., Sachs M. S., 2005. Ribosome occupancy of the yeast CPA1 upstream open reading frame termination codon modulates nonsense-mediated mRNA decay. Mol. Cell 20: 449–460 [DOI] [PubMed] [Google Scholar]
- Gaber R. F., Ottow K., Andersen H. A., Kielland-Brandt M. C., 2003. Constitutive and hyperresponsive signaling by mutant forms of Saccharomyces cerevisiae amino acid sensor Ssy1. Eukaryot. Cell 2: 922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Kaiser C. A., 2006. A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nat. Cell Biol. 8: 657–667 [DOI] [PubMed] [Google Scholar]
- Gao X. D., Wang J., Keppler-Ross S., Dean N., 2005. ERS1 encodes a functional homologue of the human lysosomal cystine transporter. FEBS J. 272: 2497–2511 [DOI] [PubMed] [Google Scholar]
- Gauthier S., Coulpier F., Jourdren L., Merle M., Beck S., et al. , 2008. Co-regulation of yeast purine and phosphate pathways in response to adenylic nucleotide variations. Mol. Microbiol. 68: 1583–1594 [DOI] [PubMed] [Google Scholar]
- Georis I., Tate J. J., Cooper T. G., Dubois E., 2008. Tor pathway control of the nitrogen-responsive DAL5 gene bifurcates at the level of Gln3 and Gat1 regulation in Saccharomyces cerevisiae. J. Biol. Chem. 283: 8919–8929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georis I., Feller A., Tate J. J., Cooper T. G., Dubois E., 2009a Nitrogen catabolite repression-sensitive transcription as a readout of Tor pathway regulation: the genetic background, reporter gene and GATA factor assayed determine the outcomes. Genetics 181: 861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georis I., Feller A., Vierendeels F., Dubois E., 2009b The yeast GATA factor Gat1 occupies a central position in nitrogen catabolite repression-sensitive gene activation. Mol. Cell. Biol. 29: 3803–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georis I., Tate J. J., Feller A., Cooper T. G., Dubois E., 2011. Intranuclear function for protein phosphatase 2A: Pph21 and Pph22 are required for rapamycin-induced GATA factor binding to the DAL5 promoter in yeast. Mol. Cell. Biol. 31: 92–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani S., Manoni M., Breviario D., 1991. Cloning and transcriptional analysis of the ADE6 gene of Saccharomyces cerevisiae. Gene 107: 149–154 [DOI] [PubMed] [Google Scholar]
- Godard P., Urrestarazu A., Vissers S., Kontos K., Bontempi G., et al. , 2007. Effect of 21 different nitrogen sources on global gene expression in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 27: 3065–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind C. K., Yoon S., Qiu H., Govind S., Hinnebusch A. G., 2005. Simultaneous recruitment of coactivators by Gcn4p stimulates multiple steps of transcription in vivo. Mol. Cell. Biol. 25: 5626–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grauslund M., Didion T., Kielland-Brandt M. C., Andersen H. A., 1995. BAP2, a gene encoding a permease for branched-chain amino acids in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1269: 275–280 [DOI] [PubMed] [Google Scholar]
- Guaragnella N., Butow R. A., 2003. ATO3 encoding a putative outward ammonium transporter is an RTG-independent retrograde responsive gene regulated by GCN4 and the Ssy1-Ptr3-Ssy5 amino acid sensor system. J. Biol. Chem. 278: 45882–45887 [DOI] [PubMed] [Google Scholar]
- Guetsova M. L., Lecoq K., Daignan-Fornier B., 1997. The isolation and characterization of Saccharomyces cerevisiae mutants that constitutively express purine biosynthetic genes. Genetics 147: 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonvarch A., Nguyen-Juilleret M., Hubert J. C., Lacroute F., 1988. Structure of the Saccharomyces cerevisiae URA4 gene encoding dihydroorotase. Mol. Gen. Genet. 212: 134–141 [DOI] [PubMed] [Google Scholar]
- Hainer S. J., Pruneski J. A., Mitchell R. D., Monteverde R. M., Martens J. A., 2011. Intergenic transcription causes repression by directing nucleosome assembly. Genes Dev. 25: 29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Johannesen P. F., 2000. Cysteine is essential for transcriptional regulation of the sulfur assimilation genes in Saccharomyces cerevisiae. Mol. Gen. Genet. 263: 535–542 [DOI] [PubMed] [Google Scholar]
- Hardwick J. S., Kuruvilla F. G., Tong J. K., Shamji A. F., Schreiber S. L., 1999. Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc. Natl. Acad. Sci. USA 96: 14866–14870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser M., Narita V., Donhardt A. M., Naider F., Becker J. M., 2001. Multiplicity and regulation of genes encoding peptide transporters in Saccharomyces cerevisiae. Mol. Membr. Biol. 18: 105–112 [PubMed] [Google Scholar]
- Hazelwood L. A., Daran J. M., van Maris A. J., Pronk J. T., Dickinson J. R., 2008. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 74: 2259–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., 1986. The Saccharomyces cerevisiae ADE5,7 protein is homologous to overlapping Drosophila melanogaster Gart polypeptides. J. Mol. Biol. 190: 519–528 [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., 1992. General and pathway-specific regulatory mechanisms controlling the synthesis of amino acid biosynthetic enzymes in Saccharomyces cerevisae, pp. 319–414 The Molecular and Cellular Biology of the Yeast Saccharomyces, edited by Jones E. W., Pringle J. R., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Hinnebusch A. G., 2005. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 59: 407–450 [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., Natarajan K., 2002. Gcn4p, a master regulator of gene expression, is controlled at multiple levels by diverse signals of starvation and stress. Eukaryot. Cell 1: 22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst K., Fisher F., McAndrew P. C., Goding C. R., 1994. The transcription factor, the Cdk, its cyclin and their regulator: directing the transcriptional response to a nutritional signal. EMBO J. 13: 5410–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W., 1985. Molecular characterization of the CAN1 locus in Saccharomyces cerevisiae. A transmembrane protein without N-terminal hydrophobic signal sequence. J. Biol. Chem. 260: 11831–11837 [PubMed] [Google Scholar]
- Holmberg S., Schjerling P., 1996. Cha4p of Saccharomyces cerevisiae activates transcription via serine/threonine response elements. Genetics 144: 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong E. L., Balakrishnan R., Dong Q., Christie K. R., Park J., et al. , 2008. Gene Ontology annotations at SGD: new data sources and annotation methods. Nucleic Acids Res. 36: D577–D581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Yoon S., 2011. Mcm1p binding sites in the ARG1 promoter positively regulate ARG1 transcription and S. cerevisiae growth in the absence of arginine and Gcn4p. Amino Acids 40: 623–631 [DOI] [PubMed] [Google Scholar]
- Hood H. M., Neafsey D. E., Galagan J., Sachs M. S., 2009. Evolutionary roles of upstream open reading frames in mediating gene regulation in fungi. Annu. Rev. Microbiol. 63: 385–409 [DOI] [PubMed] [Google Scholar]
- Horák J., 1997. Yeast nutrient transporters. Biochim. Biophys. Acta 1331: 41–79 [DOI] [PubMed] [Google Scholar]
- Huang S., O’Shea E. K., 2005. A systematic high-throughput screen of a yeast deletion collection for mutants defective in PHO5 regulation. Genetics 169: 1859–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Jeffery D. A., Anthony M. D., O’Shea E. K., 2001. Functional analysis of the cyclin-dependent kinase inhibitor Pho81 identifies a novel inhibitory domain. Mol. Cell. Biol. 21: 6695–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlimann H. C., Stadler-Waibel M., Werner T. P., Freimoser F. M., 2007. Pho91 is a vacuolar phosphate transporter that regulates phosphate and polyphosphate metabolism in Saccharomyces cerevisiae. Mol. Biol. Cell 18: 4438–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlimann H. C., Pinson B., Stadler-Waibel M., Zeeman S. C., Freimoser F. M., 2009. The SPX domain of the yeast low-affinity phosphate transporter Pho90 regulates transport activity. EMBO Rep. 10: 1003–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlimann H. C., Laloo B., Simon-Kayser B., Saint-Marc C., Coulpier F., et al. , 2011. Physiological and toxic effects of the purine intermediate 5-amino-4-imidazolecarboxamide Ribonucleotide (AICAR) in yeast. J. Biol. Chem. 286: 30994–31002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C. S., Varshavsky A., 2008. Regulation of peptide import through phosphorylation of Ubr1, the ubiquitin ligase of the N-end rule pathway. Proc. Natl. Acad. Sci. USA 105: 19188–19193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyle J. W., Shaw R. J., Reines D., 2003. Functional distinctions between IMP dehydrogenase genes in providing mycophenolate resistance and guanine prototrophy to yeast. J. Biol. Chem. 278: 28470–28478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Gato D., Martin-Marcos P., Santos M. A., Hinnebusch A. G., Tamame M., 2011. Guanine nucleotide pool imbalance impairs multiple steps of protein synthesis and disrupts GCN4 translational control in Saccharomyces cerevisiae. Genetics 187: 105–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraqui I., Vissers S., Bernard F., de Craene J. O., Boles E., et al. , 1999. Amino acid signaling in Saccharomyces cerevisiae: a permease-like sensor of external amino acids and F-Box protein Grr1p are required for transcriptional induction of the AGP1 gene, which encodes a broad-specificity amino acid permease. Mol. Cell. Biol. 19: 989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Island M. D., Naider F., Becker J. M., 1987. Regulation of dipeptide transport in Saccharomyces cerevisiae by micromolar amino acid concentrations. J. Bacteriol. 169: 2132–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Island M. D., Perry J. R., Naider F., Becker J. M., 1991. Isolation and characterization of S. cerevisiae mutants deficient in amino acid-inducible peptide transport. Curr. Genet. 20: 457–463 [DOI] [PubMed] [Google Scholar]
- Isnard A. D., Thomas D., Surdin-Kerjan Y., 1996. The study of methionine uptake in Saccharomyces cerevisiae reveals a new family of amino acid permeases. J. Mol. Biol. 262: 473–484 [DOI] [PubMed] [Google Scholar]
- Itoh R., Saint-Marc C., Chaignepain S., Katahira R., Schmitter J. M., et al. , 2003. The yeast ISN1 (YOR155c) gene encodes a new type of IMP-specific 5′-nucleotidase. BMC Biochem. 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E., Dubois E., Hennaut C., Wiame J. M., 1981. Positive regulatory elements involved in urea amidolyase and urea uptake induction in Saccharomyces cerevisiae. Curr. Genet. 4: 13–18 [DOI] [PubMed] [Google Scholar]
- Jacquemin-Faure I., Thomas D., Laporte J., Cibert C., Surdin-Kerjan Y., 1994. The vacuolar compartment is required for sulfur amino acid homeostasis in Saccharomyces cerevisiae. Mol. Gen. Genet. 244: 519–529 [DOI] [PubMed] [Google Scholar]
- Jauniaux J. C., Grenson M., 1990. GAP1, the general amino acid permease gene of Saccharomyces cerevisiae. Nucleotide sequence, protein similarity with the other bakers yeast amino acid permeases, and nitrogen catabolite repression. Eur. J. Biochem. 190: 39–44 [DOI] [PubMed] [Google Scholar]
- Jauniaux J. C., Vandenbol M., Vissers S., Broman K., Grenson M., 1987. Nitrogen catabolite regulation of proline permease in Saccharomyces cerevisiae. Cloning of the PUT4 gene and study of PUT4 RNA levels in wild-type and mutant strains. Eur. J. Biochem. 164: 601–606 [DOI] [PubMed] [Google Scholar]
- Jenks M. H., O’Rourke T. W., Reines D., 2008. Properties of an intergenic terminator and start site switch that regulate IMD2 transcription in yeast. Mol. Cell. Biol. 28: 3883–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Carlson M., 1992. Regulation of carbon and phosphate utilization, pp. 193–281 The Molecular and Cellular Biology of the Yeast Saccharomyces, edited by Jones E. W., Pringle J. R., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Jones E. W., Fink G. R., 1982. Regulation of amino acid and nucleotide biosynthesis in yeast, pp. 181–299 The Molecular Biology of the Yeast Saccharomyces: Metabolism and Gene Expression, edited by Strathern J. N., Jones E. W., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Jonkers W., Rep M., 2009. Lessons from fungal F-box proteins. Eukaryot. Cell 8: 677–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jund R., Weber E., Chevallier M. R., 1988. Primary structure of the uracil transport protein of Saccharomyces cerevisiae. Eur. J. Biochem. 171: 417–424 [DOI] [PubMed] [Google Scholar]
- Kaffman A., Herskowitz I., Tjian R., O’Shea E. K., 1994. Phosphorylation of the transcription factor PHO4 by a cyclin-CDK complex, PHO80–PHO85. Science 263: 1153–1156 [DOI] [PubMed] [Google Scholar]
- Kaiser P., Flick K., Wittenberg C., Reed S. I., 2000. Regulation of transcription by ubiquitination without proteolysis: Cdc34/SCF(Met30)-mediated inactivation of the transcription factor Met4. Cell 102: 303–314 [DOI] [PubMed] [Google Scholar]
- Kammerer B., Guyonvarch A., Hubert J. C., 1984. Yeast regulatory gene PPR1. I. Nucleotide sequence, restriction map and codon usage. J. Mol. Biol. 180: 239–250 [DOI] [PubMed] [Google Scholar]
- Kaplan R. S., Mayor J. A., Gremse D. A., Wood D. O., 1995. High level expression and characterization of the mitochondrial citrate transport protein from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 270: 4108–4114 [DOI] [PubMed] [Google Scholar]
- Kaur J., Bachhawat A. K., 2007. Yct1p, a novel, high-affinity, cysteine-specific transporter from the yeast Saccharomyces cerevisiae. Genetics 176: 877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern L., 1990. The URK1 gene of Saccharomyces cerevisiae encoding uridine kinase. Nucleic Acids Res. 18: 5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J., Swanson M. J., Qiu H., Govind C. K., Hinnebusch A. G., 2005. Activator Gcn4p and Cyc8p/Tup1p are interdependent for promoter occupancy at ARG1 in vivo. Mol. Cell. Biol. 25: 11171–11183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto K., Yoshizawa K., Ohsumi Y., Anraku Y., 1988. Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J. Bacteriol. 170: 2683–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson H., Fink G. R., Ljungdahl P. O., 1999. Ssy1p and Ptr3p are plasma membrane components of a yeast system that senses extracellular amino acids. Mol. Cell. Biol. 19: 5405–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D., 1990. The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 54: 266–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeili A., O’Shea E. K., 1999. Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science 284: 977–980 [DOI] [PubMed] [Google Scholar]
- Konrad M., 1988. Analysis and in vivo disruption of the gene coding for adenylate kinase (ADK1) in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 263: 19468–19474 [PubMed] [Google Scholar]
- Konrad M., 1992. Cloning and expression of the essential gene for guanylate kinase from yeast. J. Biol. Chem. 267: 25652–25655 [PubMed] [Google Scholar]
- Kornitzer D., Raboy B., Kulka R. G., Fink G. R., 1994. Regulated degradation of the transcription factor Gcn4. EMBO J. 13: 6021–6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi A., Koizumi Y., Yanagida F., Udaka S., 2001. MUP1, high affinity methionine permease, is involved in cysteine uptake by Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 65: 728–731 [DOI] [PubMed] [Google Scholar]
- Kota J., Ljungdahl P. O., 2005. Specialized membrane-localized chaperones prevent aggregation of polytopic proteins in the ER. J. Cell Biol. 168: 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J., Gilstring C. F., Ljungdahl P. O., 2007. Membrane chaperone Shr3 assists in folding amino acid permeases preventing precocious ERAD. J. Cell Biol. 176: 617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner J. N., Brow D. A., 2008. Regulation of a eukaryotic gene by GTP-dependent start site selection and transcription attenuation. Mol. Cell 31: 201–211 [DOI] [PubMed] [Google Scholar]
- Kulkarni A., Buford T. D., Rai R., Cooper T. G., 2006. Differing responses of Gat1 and Gln3 phosphorylation and localization to rapamycin and methionine sulfoximine treatment in Saccharomyces cerevisiae. FEMS Yeast Res. 6: 218–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras L., Barbey R., Thomas D., 1997. Assembly of a bZIP-bHLH transcription activation complex: formation of the yeast Cbf1-Met4-Met28 complex is regulated through Met28 stimulation of Cbf1 DNA binding. EMBO J. 16: 2441–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras L., Rouillon A., Lee T., Barbey R., Tyers M., et al. , 2002. Dual regulation of the met4 transcription factor by ubiquitin-dependent degradation and inhibition of promoter recruitment. Mol. Cell 10: 69–80 [DOI] [PubMed] [Google Scholar]
- Kurtz J. E., Exinger F., Erbs P., Jund R., 1999. New insights into the pyrimidine salvage pathway of Saccharomyces cerevisiae: requirement of six genes for cytidine metabolism. Curr. Genet. 36: 130–136 [DOI] [PubMed] [Google Scholar]
- Kurtz J. E., Exinger F., Erbs P., Jund R., 2002. The URH1 uridine ribohydrolase of Saccharomyces cerevisiae. Curr. Genet. 41: 132–141 [DOI] [PubMed] [Google Scholar]
- Kwapisz M., Wery M., Despres D., Ghavi-Helm Y., Soutourina J., et al. , 2008. Mutations of RNA polymerase II activate key genes of the nucleoside triphosphate biosynthetic pathways. EMBO J. 27: 2411–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroute F., 1968. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J. Bacteriol. 95: 824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam F. H., Steger D. J., O’Shea E. K., 2008. Chromatin decouples promoter threshold from dynamic range. Nature 453: 246–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwers E., Erpapazoglou Z., Haguenauer-Tsapis R., André B., 2010. The ubiquitin code of yeast permease trafficking. Trends Cell Biol. 20: 196–204 [DOI] [PubMed] [Google Scholar]
- Lecoq K., Belloc I., Desgranges C., Daignan-Fornier B., 2001a Role of adenosine kinase in Saccharomyces cerevisiae: identification of the ADO1 gene and study of the mutant phenotypes. Yeast 18: 335–342 [DOI] [PubMed] [Google Scholar]
- Lecoq K., Belloc I., Desgranges C., Konrad M., Daignan-Fornier B., 2001b YLR209c encodes Saccharomyces cerevisiae purine nucleoside phosphorylase. J. Bacteriol. 183: 4910–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. A., Jorgensen P., Bognar A. L., Peyraud C., Thomas D., et al. , 2010. Dissection of combinatorial control by the Met4 transcriptional complex. Mol. Biol. Cell 21: 456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Mulugu S., York J. D., O’Shea E. K., 2007. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316: 109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Huang K., Quiocho F. A., O’Shea E. K., 2008. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat. Chem. Biol. 4: 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenburg M. E., O’Shea E. K., 1996. Signaling phosphate starvation. Trends Biochem. Sci. 21: 383–387 [PubMed] [Google Scholar]
- Leroy C., Cormier L., Kuras L., 2006. Independent recruitment of mediator and SAGA by the activator Met4. Mol. Cell. Biol. 26: 3149–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljelund P., Lacroute F., 1986. Genetic characterization and isolation of the Saccharomyces cerevisiae gene coding for uridine monophosphokinase. Mol. Gen. Genet. 205: 74–81 [DOI] [PubMed] [Google Scholar]
- Liu C., Yang Z., Yang J., Xia Z., Ao S., 2000. Regulation of the yeast transcriptional factor PHO2 activity by phosphorylation. J. Biol. Chem. 275: 31972–31978 [DOI] [PubMed] [Google Scholar]
- Liu Z., Sekito T., Spirek M., Thornton J., Butow R. A., 2003. Retrograde signaling is regulated by the dynamic interaction between Rtg2p and Mks1p. Mol. Cell 12: 401–411 [DOI] [PubMed] [Google Scholar]
- Liu Z., Spirek M., Thornton J., Butow R. A., 2005. A novel degron-mediated degradation of the RTG pathway regulator, Mks1p, by SCFGrr1. Mol. Biol. Cell 16: 4893–4904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Thornton J., Spirek M., Butow R. A., 2008. Activation of the SPS amino acid-sensing pathway in Saccharomyces cerevisiae correlates with the phosphorylation state of a sensor component, Ptr3. Mol. Cell. Biol. 28: 551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl P. O., 2009. Amino-acid-induced signalling via the SPS-sensing pathway in yeast. Biochem. Soc. Trans. 37: 242–247 [DOI] [PubMed] [Google Scholar]
- Ljungdahl P. O., Gimeno C. J., Styles C. A., Fink G. R., 1992. SHR3: a novel component of the secretory pathway specifically required for the localization of amino acid permeases in yeast. Cell 71: 463–478 [DOI] [PubMed] [Google Scholar]
- Llorente B., Dujon B., 2000. Transcriptional regulation of the Saccharomyces cerevisiae DAL5 gene family and identification of the high affinity nicotinic acid permease TNA1 (YGR260w). FEBS Lett. 475: 237–241 [DOI] [PubMed] [Google Scholar]
- Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., et al. , 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10: 457–468 [DOI] [PubMed] [Google Scholar]
- Loison G, Losson R., Lacroute F., 1980. Constitutive mutants for orotidine 5 phosphate decarboxylase and dihydroorotic acid dehydrogenase in Saccharomyces cerevisiae. Curr. Genet. 2: 39–44 [DOI] [PubMed] [Google Scholar]
- Lorenz M. C., Heitman J., 1998. The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 17: 1236–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losson R., Lacroute F., 1981. Cloning of a eukaryotic regulatory gene. Mol. Gen. Genet. 184: 394–399 [DOI] [PubMed] [Google Scholar]
- Magasanik B., 1992. Regulation of nitrogen utilization, pp. 283–317 The Molecular and Cellular Biology of the Yeast Saccharomyces: Gene Expression, edited by Jones E. W., Pringle J. R., Broach J. R. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Magasanik B., 2003. Ammonia assimilation by Saccharomyces cerevisiae. Eukaryot. Cell 2: 827–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., Kaiser C. A., 2002. Nitrogen regulation in Saccharomyces cerevisiae. Gene 290: 1–18 [DOI] [PubMed] [Google Scholar]
- Magdolen V., Oechsner U., Bandlow W., 1987. The complete nucleotide sequence of the gene coding for yeast adenylate kinase. Curr. Genet. 12: 405–411 [DOI] [PubMed] [Google Scholar]
- Mantsala P., Zalkin H., 1984. Glutamine nucleotide sequence of Saccharomyces cerevisiae ADE4 encoding phosphoribosylpyrophosphate amidotransferase. J. Biol. Chem. 259: 8478–8484 [PubMed] [Google Scholar]
- Marczak J. E., Brandriss M. C., 1989. Isolation of constitutive mutations affecting the proline utilization pathway in Saccharomyces cerevisiae and molecular analysis of the PUT3 transcriptional activator. Mol. Cell. Biol. 9: 4696–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini A. M., Vissers S., Urrestarazu A., André B., 1994. Cloning and expression of the MEP1 gene encoding an ammonium transporter in Saccharomyces cerevisiae. EMBO J. 13: 3456–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini A. M., Soussi-Boudekou S., Vissers S., André B., 1997. A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 17: 4282–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marobbio C. M., Vozza A., Harding M., Bisaccia F., Palmieri F., et al. , 2002. Identification and reconstitution of the yeast mitochondrial transporter for thiamine pyrophosphate. EMBO J. 21: 5653–5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marobbio C. M., Agrimi G., Lasorsa F. M., Palmieri F., 2003. Identification and functional reconstitution of yeast mitochondrial carrier for S-adenosylmethionine. EMBO J. 22: 5975–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marobbio C. M., Giannuzzi G., Paradies E., Pierri C. L., Palmieri F., 2008. α-Isopropylmalate, a leucine biosynthesis intermediate in yeast, is transported by the mitochondrial oxalacetate carrier. J. Biol. Chem. 283: 28445–28453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens J. A., Laprade L., Winston F., 2004. Intergenic transcription is required to repress the Saccharomyces cerevisiae SER3 gene. Nature 429: 571–574 [DOI] [PubMed] [Google Scholar]
- Martens J. A., Wu P. Y., Winston F., 2005. Regulation of an intergenic transcript controls adjacent gene transcription in Saccharomyces cerevisiae. Genes Dev. 19: 2695–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez P., Persson B. L., 1998. Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol. Gen. Genet. 258: 628–638 [DOI] [PubMed] [Google Scholar]
- Matile P., Wiemken A., 1967. The vacuole as the lysosome of the yeast cell. Arch. Mikrobiol. 56: 148–155 [DOI] [PubMed] [Google Scholar]
- Menant A., Barbey R., Thomas D., 2006. Substrate-mediated remodeling of methionine transport by multiple ubiquitin-dependent mechanisms in yeast cells. EMBO J. 25: 4436–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenguy F., Dubois E., 2003. Role of MADS box proteins and their cofactors in combinatorial control of gene expression and cell development. Gene 316: 1–21 [DOI] [PubMed] [Google Scholar]
- Messenguy F., Colin D., ten Have J. P., 1980. Regulation of compartmentation of amino acid pools in Saccharomyces cerevisiae and its effects on metabolic control. Eur. J. Biochem. 108: 439–447 [DOI] [PubMed] [Google Scholar]
- Messenguy F., Vierendeels F., Scherens B., Dubois E., 2000. In Saccharomyces cerevisiae, expression of arginine catabolic genes CAR1 and CAR2 in response to exogenous nitrogen availability is mediated by the Ume6 (CargRI)-Sin3 (CargRII)-Rpd3 (CargRIII) complex. J. Bacteriol. 182: 3158–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S. L., Kvalnes-Krick K. L., Schramm V. L., 1989. Characterization of AMD, the AMP deaminase gene in yeast. Production of amd strain, cloning, nucleotide sequence, and properties of the protein. Biochemistry 28: 8734–8743 [DOI] [PubMed] [Google Scholar]
- Mieczkowski P. A., Dominska M., Buck M. J., Gerton J. L., Lieb J. D., et al. , 2006. Global analysis of the relationship between the binding of the Bas1p transcription factor and meiosis-specific double-strand DNA breaks in Saccharomyces cerevisiae. Mol. Cell. Biol. 26: 1014–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minehart P. L., Magasanik B., 1991. Sequence and expression of GLN3, a positive nitrogen regulatory gene of Saccharomyces cerevisiae encoding a protein with a putative zinc finger DNA-binding domain. Mol. Cell. Biol. 11: 6216–6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. P., Magasanik B., 1984. Regulation of glutamine-repressible gene products by the GLN3 function in Saccharomyces cerevisiae. Mol. Cell. Biol. 4: 2758–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya H., Johnston M., 2004. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc. Natl. Acad. Sci. USA 101: 1572–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G., 1986. Multiple upstream AUG codons mediate translational control of GCN4. Cell 45: 201–207 [DOI] [PubMed] [Google Scholar]
- Nagy M., Le Gouar M., Potier S., Souciet J. L., Herve G., 1989. The primary structure of the aspartate transcarbamylase region of the URA2 gene product in Saccharomyces cerevisiae. Features involved in activity and nuclear localization. J. Biol. Chem. 264: 8366–8374 [PubMed] [Google Scholar]
- Nakanishi T., Sekimizu K., 2002. SDT1/SSM1, a multicopy suppressor of S-II null mutant, encodes a novel pyrimidine 5′-nucleotidase. J. Biol. Chem. 277: 22103–22106 [DOI] [PubMed] [Google Scholar]
- Narayanaswamy R., Levy M., Tsechansky M., Stovall G. M., O’Connell J. D., et al. , 2009. Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc. Natl. Acad. Sci. USA 106: 10147–10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K., Meyer M. R., Jackson B. M., Slade D., Roberts C., et al. , 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol. Cell. Biol. 21: 4347–4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef D. W., Kladde M. P., 2003. Polyphosphate loss promotes SNF/SWI- and Gcn5-dependent mitotic induction of PHO5. Mol. Cell. Biol. 23: 3788–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen B., De Wachter R., Goffeau A., 1997. Classification of all putative permeases and other membrane plurispanners of the major facilitator superfamily encoded by the complete genome of Saccharomyces cerevisiae. FEMS Microbiol. Rev. 21: 113–134 [DOI] [PubMed] [Google Scholar]
- Nickerson D. P., Brett C. L., Merz A. J., 2009. Vps-C complexes: gatekeepers of endolysosomal traffic. Curr. Opin. Cell Biol. 21: 543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger P., Miozzari G., Hütter R., 1981. Biological role of the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1: 584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikawa J., Tsukagoshi Y., Yamashita S., 1986. Cloning of a gene encoding choline transport in Saccharomyces cerevisiae. J. Bacteriol. 166: 328–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Komai T., Katou Y., Shirahige K., Ito T., et al. , 2008. Nutrient-regulated antisense and intragenic RNAs modulate a signal transduction pathway in yeast. PLoS Biol. 6: 2817–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N., DeRisi J., Brown P. O., 2000. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell 11: 4309–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N., Noguchi K., Sawai H., Yamashita Y., Yompakdee C., et al. , 1995. Functional domains of Pho81p, an inhibitor of Pho85p protein kinase, in the transduction pathway of Pi signals in Saccharomyces cerevisiae. Mol. Cell. Biol. 15: 997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y., 1981. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 256: 2079–2082 [PubMed] [Google Scholar]
- Ohsumi Y., Kitamoto K., Anraku Y., 1988. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J. Bacteriol. 170: 2676–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omnus D. J., Pfirrmann T., Andréasson C., Ljungdahl P. O., 2011. A phosphodegron controls nutrient-induced proteasomal activation of the signaling protease Ssy5. Mol. Biol. Cell 22: 2754–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill E. M., Kaffman A., Jolly E. R., O’Shea E. K., 1996. Regulation of PHO4 nuclear localization by the PHO80–PHO85 cyclin-CDK complex. Science 271: 209–212 [DOI] [PubMed] [Google Scholar]
- Ouni I., Flick K., Kaiser P., 2010. A transcriptional activator is part of an SCF ubiquitin ligase to control degradation of its cofactors. Mol. Cell 40: 954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan S., Dover J., Rosenwald A. G., Wölfl S., Johnston M., 1996. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc. Natl. Acad. Sci. USA 93: 12428–12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozier-Kalogeropoulos O., Fasiolo F., Adeline M. T., Collin J., Lacroute F., 1991. Cloning, sequencing and characterization of the Saccharomyces cerevisiae URA7 gene encoding CTP synthetase. Mol. Gen. Genet. 231: 7–16 [DOI] [PubMed] [Google Scholar]
- Ozier-Kalogeropoulos O., Adeline M. T., Yang W. L., Carman G. M., Lacroute F., 1994. Use of synthetic lethal mutants to clone and characterize a novel CTP synthetase gene in Saccharomyces cerevisiae. Mol. Gen. Genet. 242: 431–439 [DOI] [PubMed] [Google Scholar]
- Palkova Z., Devaux F., Icicova M., Minarikova L., Le Crom S., et al. , 2002. Ammonia pulses and metabolic oscillations guide yeast colony development. Mol. Biol. Cell 13: 3901–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F., Agrimi G., Blanco E., Castegna A., Di Noia M. A., et al. , 2006. Identification of mitochondrial carriers in Saccharomyces cerevisiae by transport assay of reconstituted recombinant proteins. Biochim. Biophys. Acta 1757: 1249–1262 [DOI] [PubMed] [Google Scholar]
- Palmieri L., De Marco V., Iacobazzi V., Palmieri F., Runswick M. J., et al. , 1997. Identification of the yeast ARG-11 gene as a mitochondrial ornithine carrier involved in arginine biosynthesis. FEBS Lett. 410: 447–451 [DOI] [PubMed] [Google Scholar]
- Palmieri L., Agrimi G., Runswick M. J., Fearnley I. M., Palmieri F., et al. , 2001. Identification in Saccharomyces cerevisiae of two isoforms of a novel mitochondrial transporter for 2-oxoadipate and 2-oxoglutarate. J. Biol. Chem. 276: 1916–1922 [DOI] [PubMed] [Google Scholar]
- Park H. D., Scott S., Rai R., Dorrington R., Cooper T. G., 1999. Synergistic operation of the CAR2 (ornithine transaminase) promoter elements in Saccharomyces cerevisiae. J. Bacteriol. 181: 7052–7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison-Granberg J., Persson B. L., 2000. Regulation of cation-coupled high-affinity phosphate uptake in the yeast Saccharomyces cerevisiae. J. Bacteriol. 182: 5017–5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton E. E., Peyraud C., Rouillon A., Surdin-Kerjan Y., Tyers M., et al. , 2000. SCF(Met30)-mediated control of the transcriptional activator Met4 is required for the G(1)-S transition. EMBO J. 19: 1613–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels K., Abadjieva A., Hilven P., Stankiewicz A., Crabeel M., 2003. The N-acetylglutamate synthase/N-acetylglutamate kinase metabolon of Saccharomyces cerevisiae allows co-ordinated feedback regulation of the first two steps in arginine biosynthesis. Eur. J. Biochem. 270: 1014–1024 [DOI] [PubMed] [Google Scholar]
- Petersson J., Pattison J., Kruckeberg A. L., Berden J. A., Persson B. L., 1999. Intracellular localization of an active green fluorescent protein-tagged Pho84 phosphate permease in Saccharomyces cerevisiae. FEBS Lett. 462: 37–42 [DOI] [PubMed] [Google Scholar]
- Pfirrmann T., Heessen S., Omnus D. J., Andreasson C., Ljungdahl P. O., 2010. The prodomain of Ssy5 protease controls receptor-activated proteolysis of transcription factor Stp1. Mol. Cell. Biol. 30: 3299–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalip V., Kuhn I., Lemoine Y., Jeltsch J. M., 1999. Characterization of the biotin biosynthesis pathway in Saccharomyces cerevisiae and evidence for a cluster containing BIO5, a novel gene involved in vitamer uptake. Gene 232: 43–51 [DOI] [PubMed] [Google Scholar]
- Pinson B., Sagot I., Borne F., Gabrielsen O. S., Daignan-Fornier B., 1998. Mutations in the yeast Myb-like protein Bas1p resulting in discrimination between promoters in vivo but not in vitro. Nucleic Acids Res. 26: 3977–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson B., Kongsrud T. L., Ording E., Johansen L., Daignan-Fornier B., et al. , 2000. Signaling through regulated transcription factor interaction: mapping of a regulatory interaction domain in the Myb-related Bas1p. Nucleic Acids Res. 28: 4665–4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson B., Merle M., Franconi J. M., Daignan-Fornier B., 2004. Low affinity orthophosphate carriers regulate PHO gene expression independently of internal orthophosphate concentration in Saccharomyces cerevisiae. J. Biol. Chem. 279: 35273–35280 [DOI] [PubMed] [Google Scholar]
- Pinson B., Vaur S., Sagot I., Coulpier F., Lemoine S., et al. , 2009. Metabolic intermediates selectively stimulate transcription factor interaction and modulate phosphate and purine pathways. Genes Dev. 23: 1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova Y., Thayumanavan P., Lonati E., Agrochao M., Thevelein J. M., 2010. Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc. Natl. Acad. Sci. USA 107: 2890–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P., Lo Leggio L., Kielland-Brandt M. C., 2006. Mapping of an internal protease cleavage site in the Ssy5p component of the amino acid sensor of Saccharomyces cerevisiae and functional characterization of the resulting pro- and protease domains by gain-of-function genetics. Eukaryot. Cell 5: 601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen P., Gaber R. F., Kielland-Brandt M. C., 2008. Hyper- and hyporesponsive mutant forms of the Saccharomyces cerevisiae Ssy1 amino acid sensor. Mol. Membr. Biol. 25: 164–176 [DOI] [PubMed] [Google Scholar]
- Prohl C., Pelzer W., Diekert K., Kmita H., Bedekovics T., et al. , 2001. The yeast mitochondrial carrier Leu5p and its human homologue Graves’ disease protein are required for accumulation of coenzyme A in the matrix. Mol. Cell. Biol. 21: 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneski J. A., Martens J. A., 2011. Transcription of intergenic DNA deposits nucleosomes on promoter to silence gene expression. Cell Cycle 10: 1021–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puria R., Cardenas M. E., 2008. Rapamycin bypasses vesicle-mediated signaling events to activate Gln3 in Saccharomyces cerevisiae. Commun. Integr. Biol. 1: 23–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puria R., Zurita-Martinez S. A., Cardenas M. E., 2008. Nuclear translocation of Gln3 in response to nutrient signals requires Golgi-to-endosome trafficking in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 105: 7194–7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H., Dong J., Hu C., Francklyn C. S., Hinnebusch A. G., 2001. The tRNA-binding moiety in GCN2 contains a dimerization domain that interacts with the kinase domain and is required for tRNA binding and kinase activation. EMBO J. 20: 1425–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada H., Aranda C., DeLuna A., Hernandez H., Calcagno M. L., et al. , 2008. Specialization of the paralogue LYS21 determines lysine biosynthesis under respiratory metabolism in Saccharomyces cerevisiae. Microbiology 154: 1656–1667 [DOI] [PubMed] [Google Scholar]
- Rai R., Genbauffe F., Lea H. Z., Cooper T. G., 1987. Transcriptional regulation of the DAL5 gene in Saccharomyces cerevisiae. J. Bacteriol. 169: 3521–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnayake-Lecamwasam M., Serror P., Wong K. W., Sonenshein A. L., 2001. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 15: 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rébora K., Desmoucelles C., Borne F., Pinson B., Daignan-Fornier B., 2001. Yeast AMP pathway genes respond to adenine through regulated synthesis of a metabolic intermediate. Mol. Cell. Biol. 21: 7901–7912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rébora K., Laloo B., Daignan-Fornier B., 2005. Revisiting purine-histidine cross-pathway regulation in Saccharomyces cerevisiae: a central role for a small molecule. Genetics 170: 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenberg B., Holmberg S., Olsen L. D., Kielland-Brandt M. C., 1998. Dip5p mediates high-affinity and high-capacity transport of L-glutamate and L-aspartate in Saccharomyces cerevisiae. Curr. Genet. 33: 171–177 [DOI] [PubMed] [Google Scholar]
- Regenberg B., During-Olsen L., Kielland-Brandt M. C., Holmberg S., 1999. Substrate specificity and gene expression of the amino-acid permeases in Saccharomyces cerevisiae. Curr. Genet. 36: 317–328 [DOI] [PubMed] [Google Scholar]
- Ribard C., Rochet M., Labedan B., Daignan-Fornier B., Alzari P., et al. , 2003. Sub-families of alpha/beta barrel enzymes: a new adenine deaminase family. J. Mol. Biol. 334: 1117–1131 [DOI] [PubMed] [Google Scholar]
- Riles L., Shaw R. J., Johnston M., Reines D., 2004. Large-scale screening of yeast mutants for sensitivity to the IMP dehydrogenase inhibitor 6-azauracil. Yeast 21: 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger A. L., Cain N. E., Chen E. J., Kaiser C. A., 2006. Activity-dependent reversible inactivation of the general amino acid permease. Mol. Biol. Cell 17: 4411–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G., 2005. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 579: 909–915 [DOI] [PubMed] [Google Scholar]
- Rolfes R. J., Zhang F., Hinnebusch A. G., 1997. The transcriptional activators BAS1, BAS2, and ABF1 bind positive regulatory sites as the critical elements for adenine regulation of ADE5,7. J. Biol. Chem. 272: 13343–13354 [DOI] [PubMed] [Google Scholar]
- Rouillon A., Surdin-Kerjan Y., Thomas D., 1999. Transport of sulfonium compounds. Characterization of the S-adenosylmethionine and S-methylmethionine permeases from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274: 28096–28105 [DOI] [PubMed] [Google Scholar]
- Rouillon A., Barbey R., Patton E. E., Tyers M., Thomas D., 2000. Feedback-regulated degradation of the transcriptional activator Met4 is triggered by the SCF(Met30)complex. EMBO J. 19: 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowen D. W., Esiobu N., Magasanik B., 1997. Role of GATA factor Nil2p in nitrogen regulation of gene expression in Saccharomyces cerevisiae. J. Bacteriol. 179: 3761–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., 1992. Nucleotide sequence of the URA1 gene of Saccharomyces cerevisiae. Gene 118: 149–150 [DOI] [PubMed] [Google Scholar]
- Roy A., Exinger F., Losson R., 1990. cis- and trans-acting regulatory elements of the yeast URA3 promoter. Mol. Cell. Biol. 10: 5257–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Texeira M., van Zeebroeck G., Voordeckers K., Thevelein J. M., 2010. Saccharomyces cerevisiae plasma membrane nutrient sensors and their role in PKA signaling. FEMS Yeast Res. 10: 134–149 [DOI] [PubMed] [Google Scholar]
- Russnak R., Konczal D., McIntire S. L., 2001. A family of yeast proteins mediating bidirectional vacuolar amino acid transport. J. Biol. Chem. 276: 23849–23857 [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, 2000. Families of transmembrane transporters selective for amino acids and their derivatives. Microbiology 146(Pt. 8): 1775–1795 [DOI] [PubMed] [Google Scholar]
- Saint-Marc C., Daignan-Fornier B., 2004. GUD1 (YDL238c) encodes Saccharomyces cerevisiae guanine deaminase, an enzyme expressed during post-diauxic growth. Yeast 21: 1359–1363 [DOI] [PubMed] [Google Scholar]
- Saint-Marc C., Pinson B., Coulpier F., Jourdren L., Lisova O., et al. , 2009. Phenotypic consequences of purine nucleotide imbalance in Saccharomyces cerevisiae. Genetics 183: 529–538, 521SI–527SI [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Ohtomo R., Kuga-Uetake Y., Aono T., Saito M., 2005. Direct labeling of polyphosphate at the ultrastructural level in Saccharomyces cerevisiae by using the affinity of the polyphosphate binding domain of Escherichia coli exopolyphosphatase. Appl. Environ. Microbiol. 71: 5692–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha A. J., Brauer M. J., Botstein D., 2004. Nutritional homeostasis in batch and steady-state culture of yeast. Mol. Biol. Cell 15: 4089–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J. M., Barre P., 1998. Improvement of nitrogen assimilation and fermentation kinetics under enological conditions by derepression of alternative nitrogen-assimilatory pathways in an industrial Saccharomyces cerevisiae strain. Appl. Environ. Microbiol. 64: 3831–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y., 1984. An arginine/histidine exchange transport system in vacuolar-membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 259: 11509–11511 [PubMed] [Google Scholar]
- Schmidt A., Hall M. N., Köller A., 1994. Two FK506 resistance-conferring genes in Saccharomyces cerevisiae, TAT1 and TAT2, encode amino acid permeases mediating tyrosine and tryptophan uptake. Mol. Cell. Biol. 14: 6597–6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Beck T., Koller A., Kunz J., Hall M. N., 1998. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 17: 6924–6931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K. R., Smith R. L., O’Shea E. K., 1994. Phosphate-regulated inactivation of the kinase PHO80–PHO85 by the CDK inhibitor PHO81. Science 266: 122–126 [DOI] [PubMed] [Google Scholar]
- Schreve J. L., Sin J. K., Garrett J. M., 1998. The Saccharomyces cerevisiae YCC5 (YCL025c) gene encodes an amino acid permease, Agp1, which transports asparagine and glutamine. J. Bacteriol. 180: 2556–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekito T., Fujiki Y., Ohsumi Y., Kakinuma Y., 2008. Novel families of vacuolar amino acid transporters. IUBMB Life 60: 519–525 [DOI] [PubMed] [Google Scholar]
- Sellick C. A., Reece R. J., 2003. Modulation of transcription factor function by an amino acid: activation of Put3p by proline. EMBO J. 22: 5147–5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick C. A., Reece R. J., 2005. Eukaryotic transcription factors as direct nutrient sensors. Trends Biochem. Sci. 30: 405–412 [DOI] [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R., 1986. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+/K+), K+- and Ca2+-ATPases. Nature 319: 689–693 [DOI] [PubMed] [Google Scholar]
- Shaw R. J., Reines D., 2000. Saccharomyces cerevisiae transcription elongation mutants are defective in PUR5 induction in response to nucleotide depletion. Mol. Cell. Biol. 20: 7427–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. J., Wilson J. L., Smith K. T., Reines D., 2001. Regulation of an IMP dehydrogenase gene and its overexpression in drug-sensitive transcription elongation mutants of yeast. J. Biol. Chem. 276: 32905–32916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer R., Meimoun A., Holtzman T., Kornitzer D., 2002. Regulation of the transcription factor Gcn4 by Pho85 cyclin PCL5. Mol. Cell. Biol. 22: 5395–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu M., Sekito T., Akiyama K., Ohsumi Y., Kakinuma Y., 2005. A family of basic amino acid transporters of the vacuolar membrane from Saccharomyces cerevisiae. J. Biol. Chem. 280: 4851–4857 [DOI] [PubMed] [Google Scholar]
- Siddiqui A. H., Brandriss M. C., 1989. The Saccharomyces cerevisiae PUT3 activator protein associates with proline-specific upstream activation sequences. Mol. Cell. Biol. 9: 4706–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart W. C., Coffman J. A., Cooper T. G., 1996. Combinatorial regulation of the Saccharomyces cerevisiae CAR1 (arginase) promoter in response to multiple environmental signals. Mol. Cell. Biol. 16: 5876–5887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetens O., Crabeel M., El Moualij B., Duyckaerts C., Sluse F., 1998. Transport of arginine and ornithine into isolated mitochondria of Saccharomyces cerevisiae. Eur. J. Biochem. 258: 702–709 [DOI] [PubMed] [Google Scholar]
- Som I., Mitsch R. N., Urbanowski J. L., Rolfes R. J., 2005. DNA-bound Bas1 recruits Pho2 to activate ADE genes in Saccharomyces cerevisiae. Eukaryot. Cell 4: 1725–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Hinnebusch A. G., 2009. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136: 731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souciet J. L., Potier S., Hubert J. C., Lacroute F., 1987. Nucleotide sequence of the pyrimidine specific carbamoyl phosphate synthetase, a part of the yeast multifunctional protein encoded by the URA2 gene. Mol. Gen. Genet. 207: 314–319 [DOI] [PubMed] [Google Scholar]
- Soussi-Boudekou S., Vissers S., Urrestarazu A., Jauniaux J. C., André B., 1997. Gzf3p, a fourth GATA factor involved in nitrogen-regulated transcription in Saccharomyces cerevisiae. Mol. Microbiol. 23: 1157–1168 [DOI] [PubMed] [Google Scholar]
- Spielewoy N., Flick K., Kalashnikova T. I., Walker J. R., Wittenberg C., 2004. Regulation and recognition of SCFGrr1 targets in the glucose and amino acid signaling pathways. Mol. Cell. Biol. 24: 8994–9005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer C., Kunzler M., Balmelli T., Braus G. H., 1996. Amino acid and adenine cross-pathway regulation act through the same 5′-TGACTC-3′ motif in the yeast HIS7 promoter. J. Biol. Chem. 271: 29637–29643 [DOI] [PubMed] [Google Scholar]
- Springer M., Wykoff D. D., Miller N., O’Shea E. K., 2003. Partially phosphorylated Pho4 activates transcription of a subset of phosphate-responsive genes. PLoS Biol. 1: E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staschke K. A., Dey S., Zaborske J. M., Palam L. R., McClintick J. N., et al. , 2010. Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast. J. Biol. Chem. 285: 16893–16911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger D. J., Haswell E. S., Miller A. L., Wente S. R., O’Shea E. K., 2003. Regulation of chromatin remodeling by inositol polyphosphates. Science 299: 114–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz E. J., Warren C. L., Kuehner J. N., Panbehi B., Ansari A. Z., et al. , 2006. Genome-wide distribution of yeast RNA polymerase II and its control by Sen1 helicase. Mol. Cell 24: 735–746 [DOI] [PubMed] [Google Scholar]
- Stolz J., Sauer N., 1999. The fenpropimorph resistance gene FEN2 from Saccharomyces cerevisiae encodes a plasma membrane H+-pantothenate symporter. J. Biol. Chem. 274: 18747–18752 [DOI] [PubMed] [Google Scholar]
- Stolz J., Vielreicher M., 2003. Tpn1p, the plasma membrane vitamin B6 transporter of Saccharomyces cerevisiae. J. Biol. Chem. 278: 18990–18996 [DOI] [PubMed] [Google Scholar]
- Stolz J., Hoja U., Meier S., Sauer N., Schweizer E., 1999. Identification of the plasma membrane H+-biotin symporter of Saccharomyces cerevisiae by rescue of a fatty acid-auxotrophic mutant. J. Biol. Chem. 274: 18741–18746 [DOI] [PubMed] [Google Scholar]
- Storms R. K., McNeil J. B., Khandekar P. S., An G., Parker J., et al. , 1979. Chimeric plasmids for cloning of deoxyribonucleic acid sequences in Saccharomyces cerevisiae. J. Bacteriol. 140: 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz A., Linder P., 1990. The ADE2 gene from Saccharomyces cerevisiae: sequence and new vectors. Gene 95: 91–98 [DOI] [PubMed] [Google Scholar]
- Streckfuss-Bomeke K., Schulze F., Herzog B., Scholz E., Braus G. H., 2009. Degradation of Saccharomyces cerevisiae transcription factor Gcn4 requires a C-terminal nuclear localization signal in the cyclin Pcl5. Eukaryot. Cell 8: 496–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N. Y., Flick K., Kaiser P., 2005. The F-box protein Met30 is required for multiple steps in the budding yeast cell cycle. Mol. Cell. Biol. 25: 3875–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N. Y., Ouni I., Papagiannis C. V., Kaiser P., 2008. A dominant suppressor mutation of the met30 cell cycle defect suggests regulation of the Saccharomyces cerevisiae Met4-Cbf1 transcription complex by Met32. J. Biol. Chem. 283: 11615–11624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M., Qiao W. B., Khanam N., Wilkins O., Der S. D., et al. , 2005. Transcriptional regulation of the one-carbon metabolism regulon in Saccharomyces cerevisiae by Bas1p. Mol. Microbiol. 57: 53–69 [DOI] [PubMed] [Google Scholar]
- Sychrova H., Chevallier M. R., 1993. Cloning and sequencing of the Saccharomyces cerevisiae gene LYP1 coding for a lysine-specific permease. Yeast 9: 771–782 [DOI] [PubMed] [Google Scholar]
- Sychrova H., Chevallier M. R., 1994. APL1, a yeast gene encoding a putative permease for basic amino acids. Yeast 10: 653–657 [DOI] [PubMed] [Google Scholar]
- Tachihara K., Uemura T., Kashiwagi K., Igarashi K., 2005. Excretion of putrescine and spermidine by the protein encoded by YKL174c (TPO5) in Saccharomyces cerevisiae. J. Biol. Chem. 280: 12637–12642 [DOI] [PubMed] [Google Scholar]
- Tanaka J., Fink G. R., 1985. The histidine permease gene (HIP1) of Saccharomyces cerevisiae. Gene 38: 205–214 [DOI] [PubMed] [Google Scholar]
- Tate J. J., Rai R., Cooper T. G., 2005. Methionine sulfoximine treatment and carbon starvation elicit Snf1-independent phosphorylation of the transcription activator Gln3 in Saccharomyces cerevisiae. J. Biol. Chem. 280: 27195–27204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate J. J., Rai R., Cooper T. G., 2006. Ammonia-specific regulation of Gln3 localization in Saccharomyces cerevisiae by protein kinase Npr1. J. Biol. Chem. 281: 28460–28469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate J. J., Georis I., Feller A., Dubois E., Cooper T. G., 2009. Rapamycin-induced Gln3 dephosphorylation is insufficient for nuclear localization: Sit4 and PP2A phosphatases are regulated and function differently. J. Biol. Chem. 284: 2522–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate J. J., Georis I., Dubois E., Cooper T. G., 2010. Distinct phosphatase requirements and GATA factor responses to nitrogen catabolite repression and rapamycin treatment in Saccharomyces cerevisiae. J. Biol. Chem. 285: 17880–17895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., Voordeckers K., 2009. Functioning and evolutionary significance of nutrient transceptors. Mol. Biol. Evol. 26: 2407–2414 [DOI] [PubMed] [Google Scholar]
- Thiebaut M., Colin J., Neil H., Jacquier A., Seraphin B., et al. , 2008. Futile cycle of transcription initiation and termination modulates the response to nucleotide shortage in S. cerevisiae. Mol. Cell 31: 671–682 [DOI] [PubMed] [Google Scholar]
- Thomas D., Surdin-Kerjan Y., 1997. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 61: 503–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. R., O’Shea E. K., 2005. An intracellular phosphate buffer filters transient fluctuations in extracellular phosphate levels. Proc. Natl. Acad. Sci. USA 102: 9565–9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts A. S., Appling D. R., 1997. Saccharomyces cerevisiae expresses two genes encoding isozymes of 5-aminoimidazole-4-carboxamide ribonucleotide transformylase. Arch. Biochem. Biophys. 340: 195–200 [DOI] [PubMed] [Google Scholar]
- Tice-Baldwin K., Fink G. R., Arndt K. T., 1989. BAS1 has a Myb motif and activates HIS4 transcription only in combination with BAS2. Science 246: 931–935 [DOI] [PubMed] [Google Scholar]
- Tomitori H., Kashiwagi K., Sakata K., Kakinuma Y., Igarashi K., 1999. Identification of a gene for a polyamine transport protein in yeast. J. Biol. Chem. 274: 3265–3267 [DOI] [PubMed] [Google Scholar]
- Trotter P. J., Adamson A. L., Ghrist A. C., Rowe L., Scott L. R., et al. , 2005. Mitochondrial transporters involved in oleic acid utilization and glutamate metabolism in yeast. Arch. Biochem. Biophys. 442: 21–32 [DOI] [PubMed] [Google Scholar]
- Tumusiime S., Zhang C., Overstreet M. S., Liu Z., 2011. Differential regulation of transcription factors Stp1 and Stp2 in the Ssy1-Ptr3-Ssy5 amino acid sensing pathway. J. Biol. Chem. 286: 4620–4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. C., Du F., Varshavsky A., 2000. Peptides accelerate their uptake by activating a ubiquitin-dependent proteolytic pathway. Nature 405: 579–583 [DOI] [PubMed] [Google Scholar]
- Turoscy V., Cooper T. G., 1982. Pleiotropic control of five eucaryotic genes by multiple regulatory elements. J. Bacteriol. 151: 1237–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida E., Ohsumi Y., Anraku Y., 1985. Purification and properties of H+-translocating, Mg2+-adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J. Biol. Chem. 260: 1090–1095 [PubMed] [Google Scholar]
- Ueda Y., To E. A., Oshima Y., 1975. Isolation and characterization of recessive, constitutive mutations for repressible acid phosphatase synthesis in Saccharomyces cerevisiae. J. Bacteriol. 122: 911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Tomonari Y., Kashiwagi K., Igarashi K., 2004. Uptake of GABA and putrescine by UGA4 on the vacuolar membrane in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 315: 1082–1087 [DOI] [PubMed] [Google Scholar]
- Uemura T., Tachihara K., Tomitori H., Kashiwagi K., Igarashi K., 2005. Characteristics of the polyamine transporter TPO1 and regulation of its activity and cellular localization by phosphorylation. J. Biol. Chem. 280: 9646–9652 [DOI] [PubMed] [Google Scholar]
- Uemura T., Kashiwagi K., Igarashi K., 2007. Polyamine uptake by DUR3 and SAM3 in Saccharomyces cerevisiae. J. Biol. Chem. 282: 7733–7741 [DOI] [PubMed] [Google Scholar]
- Uhler J. P., Hertel C., Svejstrup J. Q., 2007. A role for noncoding transcription in activation of the yeast PHO5 gene. Proc. Natl. Acad. Sci. USA 104: 8011–8016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urech K., Durr M., Boller T., Wiemken A., Schwencke J., 1978. Localization of polyphosphate in vacuoles of Saccharomyces cerevisiae. Arch. Microbiol. 116: 275–278 [DOI] [PubMed] [Google Scholar]
- van Roermund C. W., Hettema E. H., van den Berg M., Tabak H. F., Wanders R. J., 1999. Molecular characterization of carnitine-dependent transport of acetyl-CoA from peroxisomes to mitochondria in Saccharomyces cerevisiae and identification of a plasma membrane carnitine transporter, Agp2p. EMBO J. 18: 5843–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeebroeck G., Bonini B. M., Versele M., Thevelein J. M., 2009. Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor. Nat. Chem. Biol. 5: 45–52 [DOI] [PubMed] [Google Scholar]
- Varshavsky A., 2008. The N-end rule at atomic resolution. Nat. Struct. Mol. Biol. 15: 1238–1240 [DOI] [PubMed] [Google Scholar]
- Velasco I., Tenreiro S., Calderon I. L., André B., 2004. Saccharomyces cerevisiae Aqr1 is an internal-membrane transporter involved in excretion of amino acids. Eukaryot. Cell 3: 1492–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers S., André B., Muyldermans F., Grenson M., 1990. Induction of the 4-aminobutyrate and urea-catabolic pathways in Saccharomyces cerevisiae. Specific and common transcriptional regulators. Eur. J. Biochem. 187: 611–616 [DOI] [PubMed] [Google Scholar]
- Wagner R., de Montigny J., de Wergifosse P., Souciet J. L., Potier S., 1998. The ORF YBL042 of Saccharomyces cerevisiae encodes a uridine permease. FEMS Microbiol. Lett. 159: 69–75 [DOI] [PubMed] [Google Scholar]
- Watson T. G., 1976. Amino-acid pool composition of Saccharomyces cerevisiae as a function of growth rate and amino acid nitrogen source. J. Gen. Microbiol. 96: 263–268 [DOI] [PubMed] [Google Scholar]
- Weber E., Rodriguez C., Chevallier M. R., Jund R., 1990. The purine-cytosine permease gene of Saccharomyces cerevisiae: primary structure and deduced protein sequence of the FCY2 gene product. Mol. Microbiol. 4: 585–596 [DOI] [PubMed] [Google Scholar]
- Wedaman K. P., Reinke A., Anderson S., Yates J., III, McCaffery J. M., et al. , 2003. Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol. Biol. Cell 14: 1204–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Jackson B. M., Hinnebusch A. G., 1989. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc. Natl. Acad. Sci. USA 86: 4579–4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M., Feller A., Messenguy F., Pierard A., 1987. The leader peptide of yeast gene CPA1 is essential for the translational repression of its expression. Cell 49: 805–813 [DOI] [PubMed] [Google Scholar]
- White J. H., DiMartino J. F., Anderson R. W., Lusnak K., Hilbert D., et al. , 1988. A DNA sequence conferring high postmeiotic segregation frequency to heterozygous deletions in Saccharomyces cerevisiae is related to sequences associated with eucaryotic recombination hotspots. Mol. Cell. Biol. 8: 1253–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiame J. M., Grenson M., Arst H. N., Jr, 1985. Nitrogen catabolite repression in yeasts and filamentous fungi. Adv. Microb. Physiol. 26: 1–88 [DOI] [PubMed] [Google Scholar]
- Wielemans K., Jean C., Vissers S., André B., 2010. Amino acid signaling in yeast: post-genome duplication divergence of the Stp1 and Stp2 transcription factors. J. Biol. Chem. 285: 855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Ottow K., Poulsen P., Gaber R. F., Albers E., et al. , 2006. Competitive intra- and extracellular nutrient sensing by the transporter homologue Ssy1p. J. Cell Biol. 173: 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff D. D., O’Shea E. K., 2001. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159: 1491–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff D. D., Rizvi A. H., Raser J. M., Margolin B., O’Shea E. K., 2007. Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol. Cell 27: 1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Turner G. C., Hwang C. S., Byrd C., Varshavsky A., 2008. Amino acids induce peptide uptake via accelerated degradation of Cup9, the transcriptional repressor of the PTR2 peptide transporter. J. Biol. Chem. 283: 28958–28968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Andi B., Qian J., West A. H., Cook P. F., 2006. The alpha-aminoadipate pathway for lysine biosynthesis in fungi. Cell Biochem. Biophys. 46: 43–64 [DOI] [PubMed] [Google Scholar]
- Yang Z., Huang J., Geng J., Nair U., Klionsky D. J., 2006. Atg22 recycles amino acids to link the degradative and recycling functions of autophagy. Mol. Biol. Cell 17: 5094–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H. S., Cunningham T. S., Cooper T. G., 1992. The allantoin and uracil permease gene sequences of Saccharomyces cerevisiae are nearly identical. Yeast 8: 997–1006 [DOI] [PubMed] [Google Scholar]
- Yoon S., Hinnebusch A. G., 2009. Mcm1p binding sites in ARG1 positively regulate Gcn4p binding and SWI/SNF recruitment. Biochem. Biophys. Res. Commun. 381: 123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S., Govind C. K., Qiu H., Kim S. J., Dong J., et al. , 2004. Recruitment of the ArgR/Mcm1p repressor is stimulated by the activator Gcn4p: a self-checking activation mechanism. Proc. Natl. Acad. Sci. USA 101: 11713–11718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborske J. M., Narasimhan J., Jiang L., Wek S. A., Dittmar K. A., et al. , 2009. Genome-wide analysis of tRNA charging and activation of the eIF2 kinase Gcn2p. J. Biol. Chem. 284: 25254–25267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborske J. M., Wu X., Wek R. C., Pan T., 2010. Selective control of amino acid metabolism by the GCN2 eIF2 kinase pathway in Saccharomyces cerevisiae. BMC Biochem. 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman S., Lippman S. I., Zhao X., Broach J. R., 2008. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 42: 27–81 [DOI] [PubMed] [Google Scholar]
- Zargari A., Boban M., Heessen S., Andréasson C., Thyberg J., et al. , 2007. Inner nuclear membrane proteins Asi1, Asi2, and Asi3 function in concert to maintain the latent properties of transcription factors Stp1 and Stp2. J. Biol. Chem. 282: 594–605 [DOI] [PubMed] [Google Scholar]
- Zhang F., Kirouac M., Zhu N., Hinnebusch A. G., Rolfes R. J., 1997. Evidence that complex formation by Bas1p and Bas2p (Pho2p) unmasks the activation function of Bas1p in an adenine-repressible step of ADE gene transcription. Mol. Cell. Biol. 17: 3272–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Garrett J., Schreve J., Michaeli T., 1996. GNP1, the high-affinity glutamine permease of S. cerevisiae. Curr. Genet. 30: 107–114 [DOI] [PubMed] [Google Scholar]
- Zurita-Martinez S. A., Puria R., Pan X., Boeke J. D., Cardenas M. E., 2007. Efficient Tor signaling requires a functional class C Vps protein complex in Saccharomyces cerevisiae. Genetics 176: 2139–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvyagilskaya R. A., Lundh F., Samyn D., Pattison-Granberg J., Mouillon J. M., et al. , 2008. Characterization of the Pho89 phosphate transporter by functional hyperexpression in Saccharomyces cerevisiae. FEMS Yeast Res. 8: 685–696 [DOI] [PubMed] [Google Scholar]