Abstract
Smoking cessation is a process that unfolds over time and is characterized by intermittent lapses. Behavioral relapse prevention interventions commonly assume that lapse-relapse progression is driven by a set of psychological responses known as the Abstinence Violation Effect (AVE; Marlatt & Gordon, 1985), yet efforts to reduce the AVE have generally failed to affect clinical outcomes. We used parametric recurrent event survival analyses to better understand the dynamic relationship between a set of AVE responses to lapsing and subsequent lapse-relapse progression. Participants were 203 smokers who achieved abstinence and subsequently lapsed on one or more separate occasions. Using electronic diaries for ecological momentary assessment, participants responded to items assessing three core components of the AVE (internal attribution of self-blame for the lapse, abstinence self-efficacy and guilt) following a total of 1,001 smoking episodes in near real time. Contrary to hypothesis, neither self-blame, self-efficacy nor guilt following participants’ first lapse predicted relapse, and all three were overshadowed by responses to recurrent lapses that followed. Controlling for responses to their first lapse, responses to each additional lapse did prospectively predict lapse progression, such that drops in self-efficacy were associated with accelerated progression to a subsequent lapse (HR=1.09, CI=1.02–1.15), while increases in internal attributions of blame actually protected against lapsing (HR=0.98, CI=0.97–0.99). Treatment with nicotine patches slowed recurrent lapse progression (HR=0.58, CI=0.48–0.70), but this effect dissipated over multiple lapses, and was moderated by elevated ratings of post-lapse guilt (HR=1.08, CI=1.01–1.18), which predicted accelerated progression within the active patch group, while protecting against lapse in the placebo group. Results highlight the dynamic nature of lapse responses during smoking cessation, indicating that self-efficacy predicts progression from one lapse to the next, while attributions of self-blame and guilt did not influence progression as predicted by the RPM.
Keywords: Smoking, lapse, relapse, abstinence violation effect
Attempts to abstain from addictive behavior most often end in relapse. Relapse necessarily begins with an initial episode of use (i.e., a lapse), but it seldom occurs all at once (Brandon, Vidrine, & Litvin, 2007; Miller, 1996). This is particularly true in smoking cessation: smoking relapse is most often the end point of a process that unfolds over a period of days or weeks and is characterized by many intermittent lapses (Shiffman, 2006), even as the quitter attempts to maintain or reestablish abstinence. While approximately 85–95% of lapsers ultimately relapse (e.g., Brandon, Tiffany, & Baker, 1986; Kenford, Fiore, Jorenby, Smith, Wetter, & Baker, 1994), the dynamic process preceding relapse is highly variable between and within subjects (Tindle et al, 2006). As such, fine-grained analysis of the cessation process promises to reveal a great deal about individual differences and treatments for relapse prevention.
The relapse prevention model (RPM) developed by Marlatt was the first to establish an integrative framework for understanding the cognitive-behavioral processes that drive progression from lapses to relapse (Marlatt & Gordon, 1985), and has been prominent in clinical thinking about relapse. Nearly all other prominent models of addiction and relapse focus on the psychophysiological determinants of drug priming and reinforcement (e.g., Baker et al., 1986; Kalivas & Volkow, 2005; Koob & Le Moal, 1997; Robinson & Berridge, 2003). According to the RPM (Marlatt & Gordon, 1985; Witkiewitz & Marlatt, 2004), the primary determinants of whether an individual who has lapsed will progress towards relapse or towards reestablishing abstinence are that person’s explicit (i.e., subject to conscious awareness) cognitive and emotional responses to lapsing. Specifically, relapse is predicted to be more likely when lapses produce an abstinence violation effect (AVE), characterized by internal attribution of blame, reduced abstinence self-efficacy, and feelings of guilt. This constellation of responses, coupled with the subjective effects of drug ingestion, is posited to predispose the person to further lapses, thus driving the lapse-relapse process in an accelerating downward spiral (Marlatt & Gordon, 1985). Treatment components stemming from the RPM have been incorporated into behavioral interventions for relapse prevention (Brandon, Vidrine, & Litvin, 2007), not only for smoking cessation (Abrams et al., 2003), but also for other addictions and health-related behavior change targets (Marlatt & Donovan, 2005). A focus of relapse-prevention treatment has been on helping those who lapse manage the AVE and maintain or reestablish abstinence from the undesired behavior.
The relapse-prevention model does not assume that all lapse episodes will elicit an AVE response of equivalent magnitude. Instead, greater AVE responses are expected when the associated lapse is attributed to internal, stable, and global causal factors (Marlatt & Gordon, 1985). These causal attributions are thought to determine the way individuals interpret the meaning and implications of a lapse. Whereas a lapse that is attributed to a momentary, context-specific, external cause (e.g., an unavoidable stressor) would be expected to elicit only a minimal AVE, the model predicts that a lapse which is interpreted as reflecting a lack of will-power would trigger a more powerful AVE and thereby increase the likelihood of relapse. Marlatt (Marlatt & Gordon, 1985) likens this reaction to the experience of cognitive dissonance originally described by Festinger (1964). In both cases the individual is driven to resolve the perceived discrepancy between their intentions to maintain abstinence and their actual behavior (lapse), because this discrepancy produces an aversive cognitive and affective reaction.
Despite intuitive appeal and encouraging results in other areas, RPM interventions have generally failed to improve smoking cessation outcomes (Irvin et al., 1999; Lancaster et al., 2006), and the role of AVE in smoking cessation process remains unclear. Some studies suggest that more intense AVE responses are associated with relapse (Baer, Kamarck, Lichtenstein, & Ransom, 1989; Condiotte & Litchenstein, 1981; Curry, Marlatt, & Gordon, 1987; Garcia, Schmitz, & Doerfler, 1990; O’Connell & Martin, 1987; Schoeneman, Hollis, Stevens, Fischer, & Cheek, 1988), while others have found that initial AVE responses are unrelated to lapse-relapse outcomes (Brandon, Tiffany, Olefski, & Baker, 1990; Borland, 1990; Hall, Havassy, & Wasserman, 1990; Schoeneman, Stevens, Hollis, Cheek, & Fischer, 1988). The only prospective studies conducted to date, both of which came from an earlier smoking cessation trial conducted by our group, did not find a link between AVE responses to first lapse and relapse outcomes (Shiffman et al., 1996; Shiffman, Hickox, et al., 1997).
It is noteworthy that all studies of the AVE during smoking cessation have focused exclusively on the first lapse from abstinence, and that almost all AVE studies to date have used retrospective measures to assess momentary AVE reactions long after they occur. In addition to the documented inaccuracies of retrospective recall (e.g., Hammersley, 1994; Shiffman, Hufford, et al., 1997), especially after later events have shed light on the original event (e.g., a relapse after a lapse; Ross, 1989), it is assumed that this single AVE response has consequences for the entire lapse-relapse process, which may take weeks or months to resolve. This fails to fully test Marlatt et al.’s (1985 p.158) original account, which allows for the fact that the first lapse may elicit only a weak AVE response, while subsequent slips may drive further progression or intervening experiences (e.g., those that raise self-efficacy) may promote recovery. No study conducted to date has empirically examined the AVE in this way. Instead of restricting focus to responses associated with an initial lapse, the present project conceptualizes the AVE as a dynamic cascade of responses to a series of lapse events during a self-imposed attempt to maintain abstinence from smoking.
To the extent that the AVE is bound to a series of recurrent lapses, the timing, frequency and severity of each lapse should also synergistically influence AVE dynamics and lapse progression. During smoking cessation some quitters tend to experience rapidly occurring lapses, while others experience more isolated lapses spread over extended periods of time (e.g., Conklin et al., 2005; Hoeppner et al., 2008; Wileyto et al., 2005). Amount smoked during a lapse varies as well, ranging from a single puff to multiple cigarettes. The way pre-lapse abstinence duration and amount smoked might modulate recurrent AVEs and subsequent progression remains unclear. One possibility is that lapses occurring after longer periods of abstinence taint successful progress accumulated to that point, call the lapser’s ability to maintain abstinence into question, increase attributions of blame, as well as feelings of guilt. Under this scenario RPM would predict accelerated progression to additional lapses. Alternatively, longer pre-lapse abstinence time may actually increase perceptions of control over cessation, and may therefore protect against the AVE, mitigating the detrimental impact of lapses. This paper is the first to evaluate these alternative possibilities.
The overarching goal of the present research was to examine the way psychological responses to lapses influenced quitters’ ability to maintain abstinence. We used EMA measures of three core components of the AVE (internal attribution of self-blame for the lapse, abstinence self-efficacy and guilt) obtained at the time of lapse as smokers struggled to avert relapse over the course of 6 weeks after quitting. EMA captured the timing of lapses, the amount smoked during each lapse episode, and participants’ immediate AVE responses. Recurrent-event survival models were used to evaluate the extent to which AVE responses to each successive lapse influenced the hazard of an additional lapse. Based on relapse prevention theory (Marlatt & Gordon, 1985; Witkiewitz & Marlatt, 2004), it was hypothesized that the severity of the AVE response following each lapse would predict progression to a subsequent lapse, such that greater internal attributions of blame and guilt, along with reduced self-efficacy, would accelerate lapses. Recurrent lapses and AVE responses were thus expected to synergistically drive one another toward relapse, and our analysis attempts to capture and elucidate this cascading downward spiral driven by cognitive and affective responses to recurrent lapses during self-imposed abstinence.
Method
Design and Overview
The analysis was based on data from a randomized, double-blind, placebo-controlled clinical trial of high-dose nicotine patch for smoking cessation. Clinical outcomes have been reported elsewhere (Shiffman, Ferguson, & Gwaltney, 2006; Shiffman, Scharf, et al., 2006). Participant recruitment and data collection occurred between October 1997 and February 2000.
Participants
Participants were 305 smokers who quit for at least 24 hours while enrolled in a research smoking cessation clinic. Participants had to smoke at least 15 cigarettes per day, to have been smoking for at least 5 years, to be between the ages of 21 and 65. Smokers who were eligible, who passed a medical screening, and who signed an informed consent form were enrolled. The sample is described in more detail elsewhere (e.g., Shiffman, Scharf, et al., 2006).
To be eligible for inclusion in the present analyses, participants had to have achieved initial abstinence (24 hours without smoking) and subsequently experienced a lapse during the study; 203 smokers met these criteria. The sample was typical of a smoking cessation treatment cohort. Fifty-six percent of the participants were women and 84% were Caucasian. Participants averaged 38.94 years of age (SD=8.89) and had been smoking for 21.8 years (SD=9.0), smoking an average of 24.9 cigarettes per day (SD=8.9) at enrollment. Eighty-four percent reported at least one previous quit attempt, with an average 3 previous attempts (Table 1).
Table 1.
Participant Characteristics: Mean (SD) and N (%)
| Variable | Abstinent (n=305) |
Lapsed (n=203) 67% of Abstinent |
Resumed (n=92) 45% of Lapsed |
Relapsed (n=28) 30% of Resumed |
|---|---|---|---|---|
| Age | 39.34 (9.19) | 38.91 (8.88) | 39.53 (9.23) | 37.21 (8.35) |
| Cigarettes per Day | 24.29 (8.89) | 24.91 (8.90) | 26.13 (9.23) | 24.96 (8.07) |
| Years Smoking | 21.95 (9.42) | 21.86 (9.02) | 22.25 (9.65) | 20.79 (9.09) |
| Number of Previous Quits | 3.20 (3.85) | 2.89 (3.35) | 2.65 (3.28) | 1.68 (1.39) |
| FTND (0–10) | 5.95 (1.94) | 6.13 (1.80) | 6.34 (1.81) | 6.27 (1.64) |
| Gender: Female | 170 (52.50) | 110 (54.90) | 57 (61.96) | 21 (75.00) |
| Ethnicity: Caucasian | 277 (85.50) | 172 (84.71) | 81 (88.04) | 27 (96.43) |
| Education: Post HS | 213 (65.70) | 131 (64.54) | 58 (63.04) | 18 (64.29) |
| Married | 165 (50.90) | 96 (47.29) | 43 (46.74) | 10 (35.71) |
| Income: ≥ $40,000 | 176 (54.30) | 104 (51.24) | 48 (52.16) | 13 (46.43) |
| Treatment: Active Patch | 188 (58.00) | 113 (55.67) | 37 (40.22) | 6 (21.43) |
Procedure
During the first 6 weeks of a smoking cessation attempt, participants monitored their moment-to-moment experiences on palm-top computers, including their reactions to any smoking behavior. Cessation was biochemically validated, with participants seen at least weekly to verify their smoking status via carbon monoxide (CO) testing. Any participant who reported abstinence, but who had an expired air CO > 10 parts per million was considered a smoker in the analyses with smoking assigned to the first day after the last clean CO. During the “evening-report” that occurred at the end of each day, participants were offered the opportunity to “confess” to lapses that were not entered in real-time. Although we do not have data on AVE reactions following these (infrequent) unrecorded lapses, we did include them in the sequence of lapse events so that lapses observed in real-time are included in their rightful spot in the overall sequence of lapses for each subject. Compliance with the assessment protocol was high (participants responded to 92% of random prompts within two minutes; Shiffman et al., 2006). Participants completed an average of 4.36 ± 1.38 random prompts per day during the post-quit period. More details concerning the procedures used for this study can be found in Shiffman, Scharf, et al. (2006).
Treatment
Participants were randomized on the quit day to receive either transdermal nicotine patch (NP, n=113) or matched placebo (n=90); more subjects were randomized to active treatment in order to observe a similar number of lapses in each group, anticipating that NRT would reduce rates of lapsing. Randomization was stratified by baseline craving. Individuals randomized to active NP treatment received 35 mg for 3 weeks, 21 mg for the next 2 weeks, and only placebo NP for 1 final week. NP treatment assignment was entered as a control variable in all analyses.
Participants received two sessions of cognitive-behavioral treatment prior to quit day, one on the designated quit day, and three thereafter. Treatment took a behavioral-psychoeducational approach with strong emphasis on providing a supportive group environment (e.g., Brown, 2003). Treatment intervention specifically avoided discussion of the AVE.
Measures
Individual differences
Data on age, gender, ethnicity, education, and income were collected, as were measures of daily smoking rate, number of past quit attempts, and the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker & Fagerstrom, 1991). These measures were entered in the analyses as covariates.
Lapse and relapse outcomes
Any smoking after initial cessation, ranging from a single puff to multiple cigarettes, can be considered a lapse (Brownell et al., 1986; Shiffman et al., 1986). Yet smoking is only theorized to elicit an abstinence violation effect when it disrupts ongoing abstinence. Even when it remains below the level of full-blown relapse, smoking that is part of a routine pattern of daily use may not produce an AVE, because there is no abstinence to violate. To avoid data from periods when smoking had become routine, we limited the analysis to lapses that occurred before the onset of routine daily smoking. Daily resumption was defined as 3 or more consecutive days of smoking at any level, the last day of which marked the end of the initial abstinence attempt and the resumption of daily smoking. In line with previous work, the threshold for full-blown relapse was more stringent, operationalized as 3 consecutive days with at least five cigarettes a day, with the final lapse in the sequence marking relapse (Shiffman et al., 1996; 2006).
Lapse timing
EMA documented the timing and frequency of all lapse episodes. The amount of abstinence time preceding each lapse was used to evaluate the extent to which lapses occurring after longer periods of time were more or less likely to trigger AVE reactions.
Psychological responses to lapse
Items administered following a lapse were designed to capture the AVE responses, while being expressed in colloquial language subjects could understand (based on prior studies and pilot work that suggested more abstract inquiries were confusing). Elements of the AVE response included self-blame, self-efficacy, and guilt. Participants rated each item on a 0 to 10 scale. Assessments were completed on-screen, one item at a time. Internal attributions of blame (Self-blame). Participants rated the degree to which they were responsible for the lapse (“Was the slip your fault?”). Self-efficacy (SE). After each lapse, 3 items designed to measure aspects of self-efficacy were administered, one concerning confidence (“Confident in ability to abstain?”), one assessing discouragement (“Feel encouraged?”), and one assessing desire to give up entirely (“Feel like giving up?”). Guilt. Participants rated how guilty they felt following the lapse (“Feel Guilty?”). Because both Guilt and Self-blame values were clustered toward the top of the scale, both variables were reverse-scored before and after a log transformation to deal with negative skew while retaining the intended direction of the scale (Tabachnick & Fidell, 2006). We evaluated whether these AVE constructs should be combined to form a composite measure, but a factor analysis revealed that they did not cohere as a unitary construct, with only the items associated with self-efficacy reaching an acceptable level of inter-item reliability (Cronbach’s Alpha Reliability Coefficient = .71). We therefore treated self-blame, self-efficacy and guilt as separate constructs in all subsequent analyses. The reader should note that for clarity and brevity we use the plural “AVE responses” throughout the remainder of the paper to refer to these three separate AVE response types rather than to repeated measures of a single AVE composite at multiple time points, unless otherwise stated.
Data Analysis Strategy
Parametric survival analyses that allowed for recurrent events within-subjects treated each lapse episode as the beginning of an interval during which the participant was at risk for having another lapse, and examined how AVE responses to each lapse affected the likelihood of progression. Survival analysis assesses risk for an event by analyzing the incidence of the event over a specified period of time, referred to as the event’s hazard. Single-event survival analysis examines a single event, assuming that no further events are possible (it was originally developed to analyze death rates). In contrast, recurrent events survival analyses assess the hazard of events that can occur multiple times (e.g., lapses). Recurrent models incorporating both the timing and sequence of lapses made it possible to systematically examine the extent to which each successive AVE response prospectively accelerated lapses across the series, driving the process downward toward relapse.
To account for correlated observations due to repeated measures within subjects (i.e., recurrent lapse events), we used parametric shared-frailty models, the survival-data analog to mixed-effects (i.e., multilevel or hierarchical linear) regression models (Hougaard, 1999; Hosmer, Lemeshow, & May, 2008). These assume that there are individual differences in lapse risk, as well as differences attributable to within-subject variability (i.e., across lapse episodes). “Frailty effects” account for such individual differences in vulnerability, as distinct from factors that influence survival for each individual episode. We expected that individuals more prone to daily resumption and relapse would reach these milestones earlier and thereby drop from the sample of those at risk for an additional lapse.
When examining predictor variables that varied from lapse to lapse, we also included each participant’s average response value as a between-subject covariate in order to further differentiate within- from between-subject effects (Begg & Parides, 2003). Hazard ratios (HRs), representing the proportional increase in risk of lapse given a one-unit increase in a predictor variable were estimated, and their confidence intervals used to identify statistically significant effects. To probe significant effects that did emerge, improve interpretation of interactions, and clarify our results, we also present model-based predicted values in the text and graphics, representing the precise amount of prospective abstinence time associated with unit changes in each predictor. Analyses were performed in Stata (version 11; StataCorp, 2009).
Results
Lapse and Relapse Outcomes
The 203 participants who established at least 24-hrs abstinence and subsequently lapsed recorded a total of 1,001 lapse episodes. Participants experienced an average of 5.7 (SD=4.9; Median=4.0) episodes prior to either resumption of daily smoking or the end of observation. All who relapsed first resumed daily smoking. Ninety-two participants (45% of lapsers) resumed daily smoking during the observation period, and 28 of these (30% of daily resumers; 14% of lapsers) were observed to relapse (Table 1). Among those who did not relapse, resumption of daily smoking occurred after a median of 13.5 days (M=19.0; SD=14.5), whereas among those who did relapse, resumption of daily smoking occurred after a median of 12.7 days (M=17.9; SD=14.1). Relapse, when it occurred, happened about a week after daily resumption, following a median of 19.0 days post-quit (M=21.46; SD=10.13). The 64 participants who resumed daily smoking but did not relapse averaged 7.1 (SD = 5.0; Median=5.0) lapses prior to resumption, while the 28 who resumed daily smoking and then relapsed resumed daily smoking significantly faster (HR= 3.44, CI=2.12–5.59), averaging 6.1 (SD = 4.8; Median=5.0) lapses prior to resumption. The 111 (55%) lapsers who neither resumed daily smoking nor relapsed averaged 4.74 (SD = 4.68; Median=3.0) lapses each.
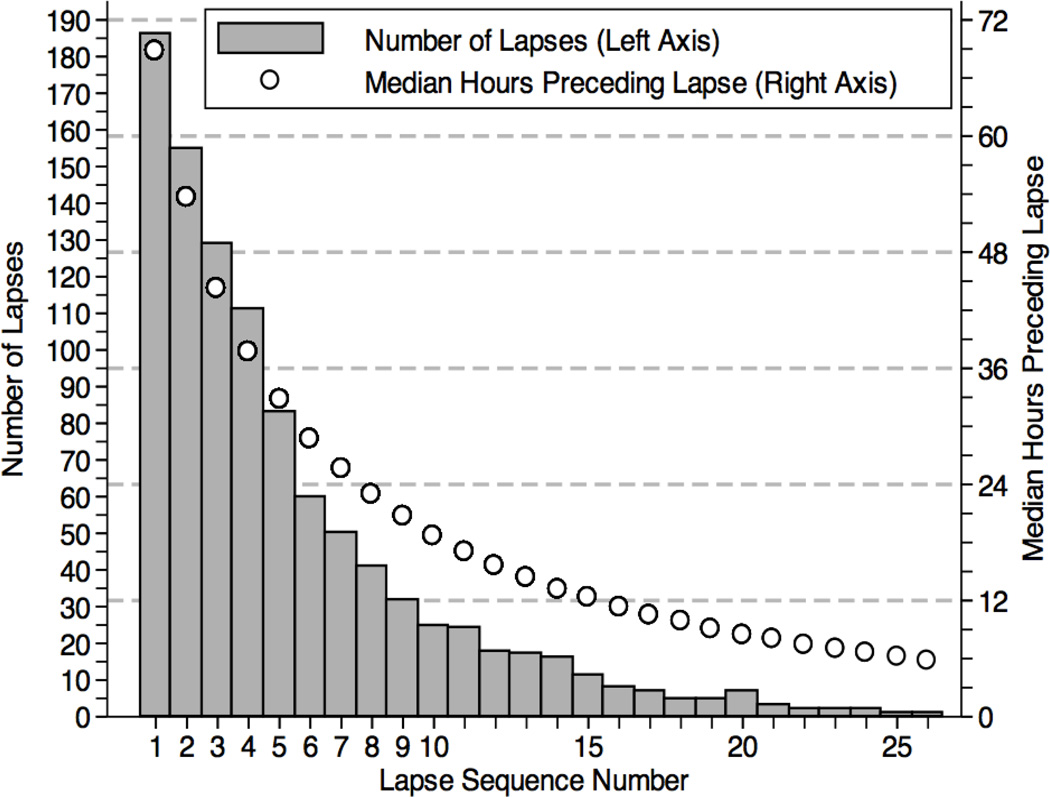
Figure 1 (bars, left axis) presents the distribution of lapse episodes in the sequence that they occurred, illustrating the number of first lapses (n=188), second lapses (n=157), and so forth. Consistent with the expectation that individuals more prone to daily resumption and relapse would reach these milestones early on and thereby drop from the sample of those at risk for an additional lapse, the total number of lapses recorded by all participants decreased across the sequence. Put another way, only those who successfully maintained abstinence for a longer period of time (an increasingly diminishing subset) were able to record additional lapses.
Figure 1.
Lapses distributed by the sequence they occurred (Left Axis), along with median hours of abstinence preceding each lapse (Right Axis).
Lapse timing
In addition to the quantity of lapses, Figure 1 presents the median hours of abstinence preceding each lapse (circles, right axis), as modeled by parametric shared-frailty recurrent-events regression. Including their initial 24-hours of abstinence, the observed median time to an initial lapse was 3.8 days (M=8.4 days; SD=9.7), ranging widely from 1.3 to 40.6 days, while the median time between each successive lapse was 2.8 days (M=5.9 days; SD=7.9). Within subjects, predicted values indicate that each successive lapse in the sequence occurred on average 8% faster than its predecessor (HR=1.08, CI=1.07–1.10), such that the next lapse occurred about 4.2 hours faster than the previous one. However, the addition of a non-linear (quadratic) lapse sequence parameter to the recurrent-events model indicated that acceleration to each additional lapse was not constant (linear) over the course of the observation period; rather, lapse intervals followed a curvilinear process (HR=0.99, CI=0.99–0.99). To probe the nature of this effect, model-based predicted values at each lapse in the series were calculated. These correspond to the pattern presented in Figure 1 (circles, right axis), confirming that early lapses in the sequence accelerated more quickly than later lapses. For example, model-based predictions indicate that while the 2nd successive lapse occurred a median 15.2 hours faster than the 1st lapse, and that the 5th lapse occurred a median 4.9 hours faster than the 4th, while the 8th lapse occurred a median 2.7 hours faster than the 7th lapse, and the 15th lapse only occurred 1.0 hours faster than the 14th. We controlled for these sequence and timing effects in all subsequent analyses, and evaluated the extent to which lapse sequence and/or timing moderated the effects of AVE responses on prospective lapse progression.
Amount smoked per lapse
Participants smoked a median 0.80 (M = 1.2) cigarettes during each lapse episode. Most lapse episodes (79%) included no more than a single cigarette, while 12% included 2 cigarettes, and 9% included 3 or more cigarettes. The amount smoked per lapse varied both between (z>7.9, p<.001) and within (z>29.2, p<.001) participants, with between-subject differences accounting for approximately 44% of variation in amount smoked per lapse. Amount smoked per lapse values were log transformed prior to analysis to deal with positive skew. We used recurrent-event survival analysis to test whether the amount smoked was related to the risk of progression to a subsequent lapse, controlling for the lapse sequence and timing. Confirming expectations, lapses in which subjects had smoked more were more likely to progress to an additional lapse (HR=1.17, CI=1.03–1.33). The amount smoked in each lapse was controlled for in all other analyses. Amount smoked did not vary across the sequence of lapses, nor according to each pre-lapse abstinence interval.
Nicotine replacement therapy
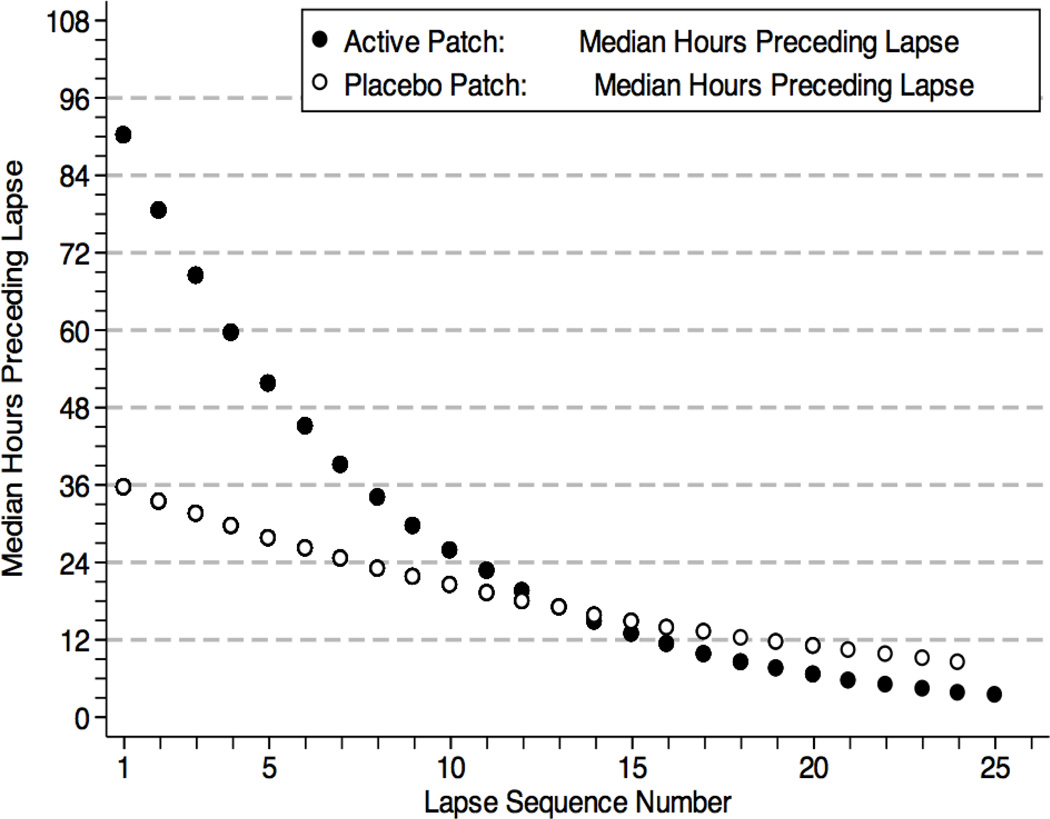
As reported elsewhere (Shiffman et al., 2006), active high-dose nicotine patch reduced the overall likelihood of relapse (among lapsers) relative to placebo (HR=0.22, CI=0.09–0.53). NRT treatment was associated with slower lapse progression, such that the high-dose nicotine patch group progressed from one lapse episode to another at nearly half the rate in the placebo patch group (HR=0.58, CI=0.48–0.70). While NRT treatment effects were not associated with the abstinence duration preceding each lapse (HR=1.02, CI=0.98–1.07), a significant interaction with lapse sequence number did emerge, indicating that the effect of active patch versus placebo was greater early in the sequence (HR=1.07, CI=1.04–1.10), such that active patch provided a significant benefit over placebo through lapse 8 in the sequence but not after (zs < 1.73, ps > 0.08). Figure 2 presents the median interval between lapses, across multiple lapses, for the NRT and placebo groups, illustrating that NRT is initially associated with very large increases in abstinence between lapses, but these intervals shrink over time, converging with the placebo group around the eighth to tenth lapse. Interestingly, this finding coincides with the fact that the median hours preceding a lapse initially dropped below 24 hours (our criterion for establishing abstinence initially) at around this point in the process (Figure 1). Observed main and sequence-related effects of NRT were controlled for in subsequent analyses.
Figure 2.
Model-based predicted median hours of prospective abstinence preceding each lapse, plotted as a function of Active versus Placebo NRT patch assignment.
Abstinence Violation Effects and Lapse-Relapse Progression
Initial AVE and Relapse
To investigate whether AVE responses to the very first lapse were associated with progression to relapse, we examined whether participants’ responses to the first lapse episode predicted time to relapse. None of participants’ initial AVE responses, self-blame, self-efficacy or guilt, were significant predictors of relapse (HRs 0.95–1.08, ps > .24).
Initial AVE and Resumption of Daily Smoking
We also examined whether participants’ responses to the first lapse episode predicted time to resumption of daily smoking. While initial post-lapse ratings of guilt (HR=0.98, CI=0.91–1.05) and self-blame (HR=1.06, CI=0.97 –1.15) did not predict daily resumption, initial lapse ratings of self-efficacy did do so (HR=1.11, CI=1.01–1.20), such that lower levels of self-efficacy following the first lapse predicted faster progression to daily resumption.
Recurrent AVEs and Lapse Progression
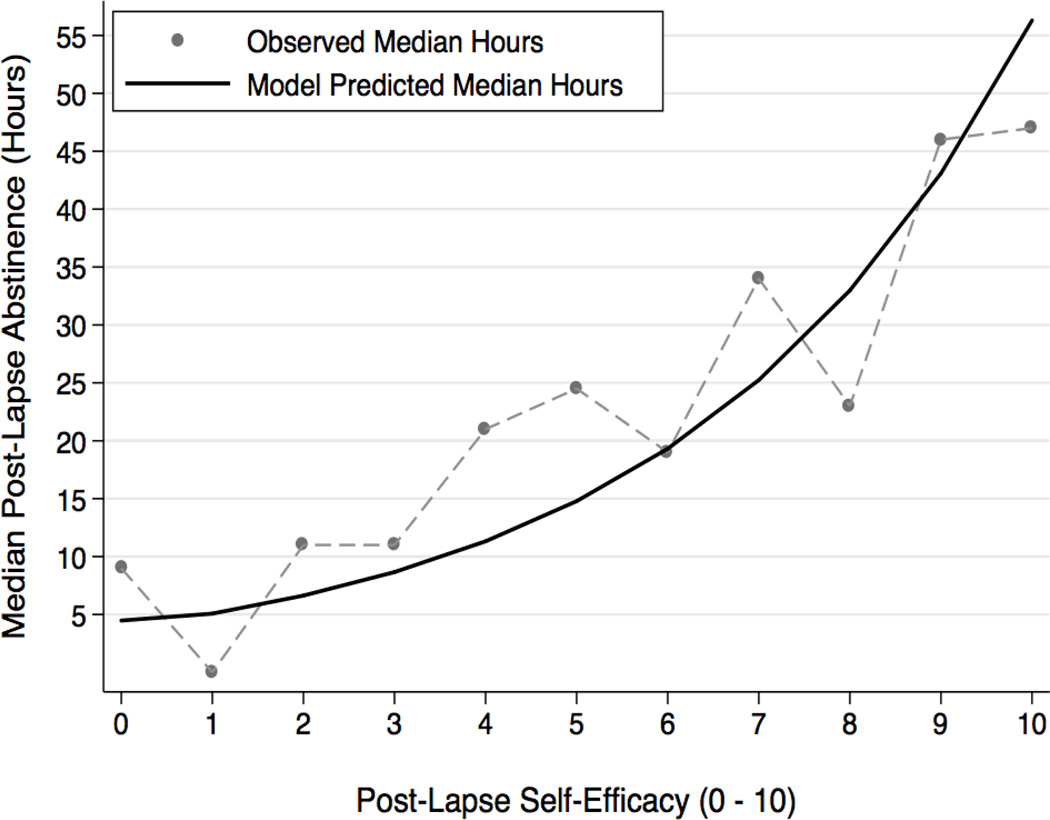
With responses to the initial lapse entered as covariates, we next investigated the extent to which AVE responses reported after each successive lapse were associated with progression to a subsequent lapse. In line with predictions, lower ratings of post-lapse self-efficacy were prospectively associated with faster progression to a subsequent lapse, such that each 1-unit decrease in self-efficacy predicted a 9% increase in the hazard of an additional lapse (HR=1.09, CI=1.02–1.15). To illustrate and improve interpretation of this effect, the previous model was used to calculate predicted values of the effect of self-efficacy on hours of abstinence preceding an additional lapse. With all other covariates fixed at their mean values, an average self-efficacy rating (M = 6.4; SD = 2.16), prospectively predicted an additional 25.07 median hours of abstinence before another lapse (95% CI = 20.31–29.84). Upwards from the mean self-efficacy value, each 1-unit increase in self-efficacy produced an increasing amount of prospective abstinence; smokers whose efficacy was at the maximum are predicted to be able to maintain abstinence for 56.29 hours (median; 95% CI = 19.14 – 93.45; Figure 3). Contrary to AVE theory, lapse-specific ratings of self-blame (HR=1.03, CI=0.98–1.08) and guilt (HR=1.00, CI=0.95–1.05), on their own, did not predict subsequent lapse progression.
Figure 3.
Additional hours of prospective abstinence time, plotted across each 1-unit change in post-lapse self-efficacy.
Duration of abstinence preceding lapse
In an initial step, we evaluated whether pre-lapse abstinence duration affected the severity of AVE responses. A mixed-effects regression of AVE severity on time preceding the associated lapse revealed that none of the three AVE responses was associated with pre-lapse abstinence duration (βs < 0.02, ps > 0.17). This finding indicates that AVE severity was not tied to pre-lapse abstinence duration, but leaves open the possibility that pre-lapse abstinence altered the implications of each lapse by moderating the association between the severity of each AVE response and prospective abstinence.
Next, we evaluated whether the amount of time preceding each lapse in the sequence affected the extent to which AVE responses predicted the hazard of an additional lapse. First, we found that higher self-efficacy was particularly protective against progression when the lapse had been preceded by a longer period of abstinence (HR=0.98, CI=0.96–0.99). To probe this effect, we calculated predicted values at different levels of pre-lapse abstinence; specifically, one-half day (12-hrs), 1-day, 2-days, and 3-days of pre-lapse abstinence. This analysis revealed that elevated self-efficacy ratings protected against subsequent progression when they followed at least 1-day of pre-lapse abstinence, and that this protective effect was magnified to the degree that the lapse followed greater amounts of abstinence. For instance, while self-efficacy ratings following 24-hours of abstinence were associated with up to 11.11 median hours of abstinence prior to another lapse, self-efficacy ratings following 3 or more days of abstinence conferred as much as 30.99 median hours of additional, prospective abstinence.
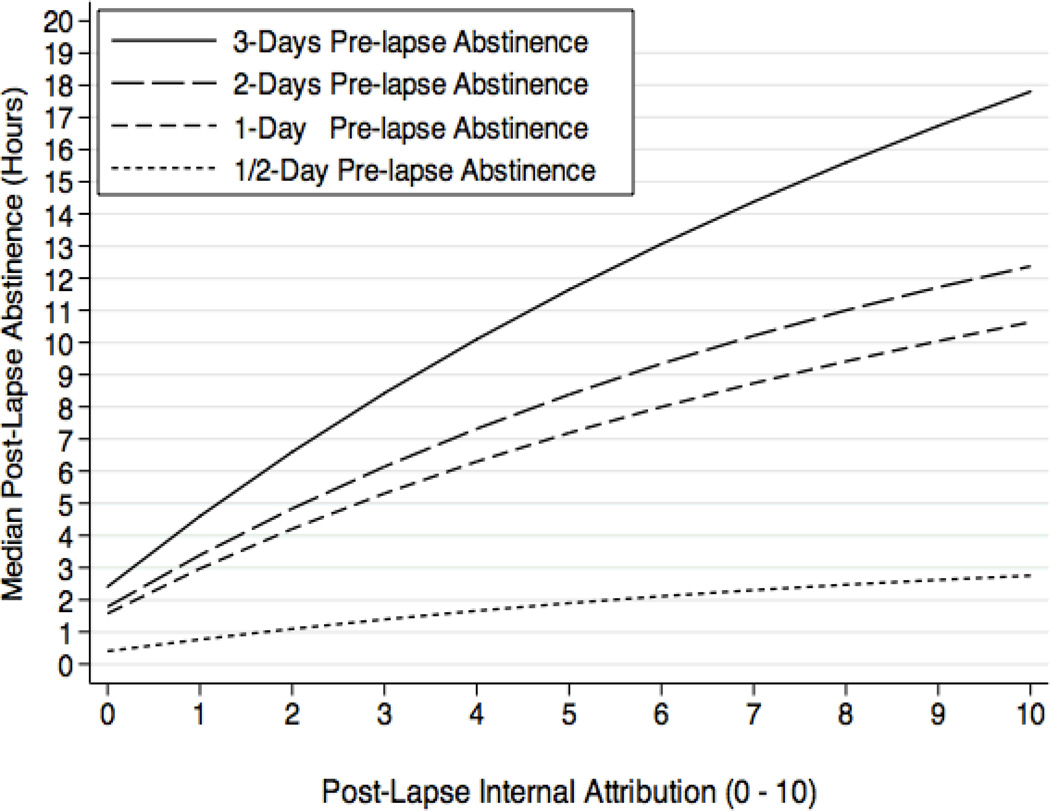
Interestingly, we observed a similar pattern for the interaction involving self-blame, such that greater pre-lapse abstinence followed by elevated self-blame was prospectively associated with lower risk or delay of lapse progression (HR=0.98, CI=0.97–0.99). To illustrate this effect we calculated marginal effects at the same 4-levels of pre-lapse abstinence (1/2 day, 1-day, 2-days, and 3-days), and plotted the median hours of post-lapse abstinence time associated with each 1-unit increase in self-blame (Figure 4). This graphic shows that greater self-blame is associated with longer latency to the next lapse, and that this effect is particularly strong when the lapse is preceded by longer periods of abstinence. Increased self-blame for a lapse following 3 or more days of abstinence can delay the next lapse by as much as 17.8 hours (median). Pre-lapse abstinence duration did not affect the prospective association between post-lapse guilt and subsequent lapse progression (HR=1.00, CI=0.99–1.01).
Figure 4.
Additional hours of prospective abstinence time across each 1-unit change in post-lapse internal attribution of blame, plotted as a function of abstinence duration (days) preceding the lapse.
Lapse sequence
Whether each AVE response occurred early or later in the sequence of lapses experienced by each subject did not affect the extent to which the response predicted the hazard of an additional lapse. Prospective effects of internal attributions of blame (HR=0.98, CI=0.93–1.04), abstinence self-efficacy (HR=0.99, CI=0.93–1.05), and guilt (HR=0.99, CI=0.95–1.03), were not moderated by the lapse sequence number associated with each rating, regardless of whether a linear or quadratic lapse sequence effect was evaluated. In a mixed-effect regression of AVE severity on lapse sequence number, AVE severity did not increase with increasing lapses (βs < 0.04, ps > 0.26), ruling out the possibility that the observed null effects of sequence number on progression were due to corresponding changes in AVE severity.
Nicotine replacement therapy
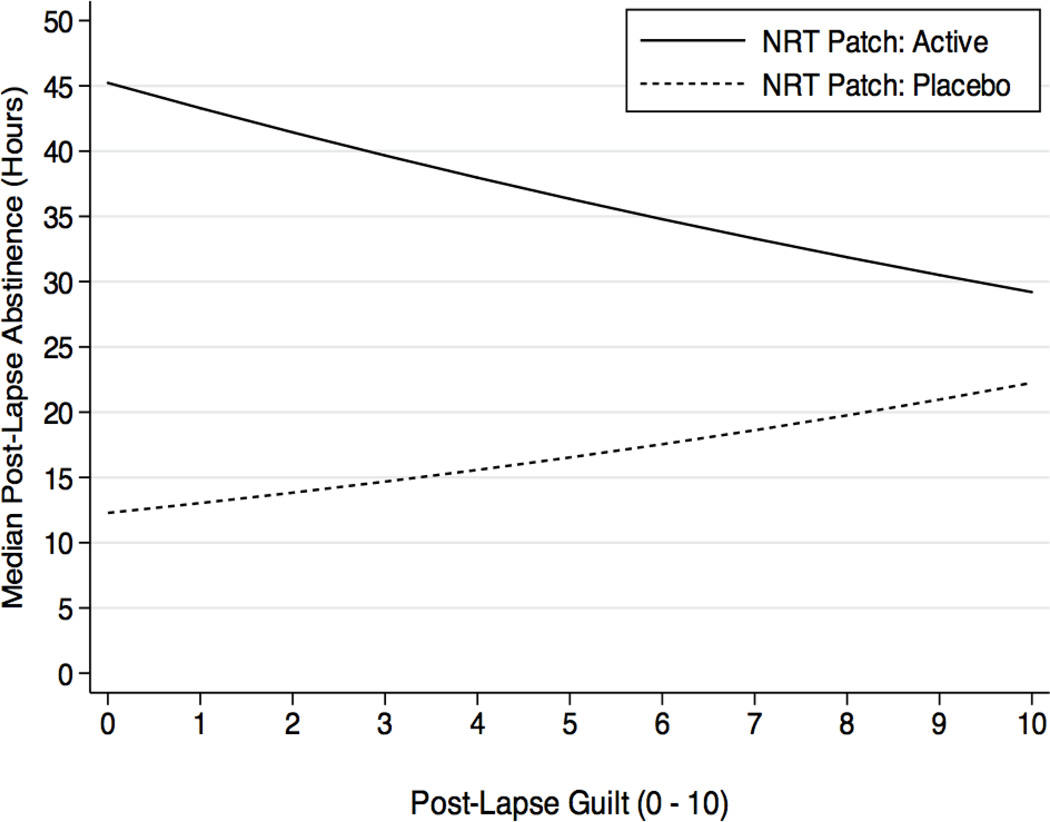
We examined whether the observed effects of active versus placebo patch assignment on lapse progression were also associated with AVE responses to each lapse. We found that ratings of guilt interacted with NRT assignment (HR=1.08, CI=1.01–1.18), but that guilt X NRT effects did not vary with lapse timing and/or sequence (HRs=0.99, ps > .31). Evaluation of the guilt X NRT interaction effect revealed that guilt had opposite effects depending on NRT treatment condition (Figure 5). In the active NRT patch group, increased guilt was associated with faster progression: each 1-unit increase in guilt was associated with a reduction of 1.48 hours to the next successive lapse. In contrast, in the placebo group, elevated levels of guilt were actually protective, such that each unit increase was associated with an additional 54.6 minutes of abstinence prior to the next lapse. Internal lapse attributions and self-efficacy did not interact with NRT assignment alone or in conjunction with sequence and/or timing (HRs=1.04, ps > .35).
Figure 5.
Additional hours of prospective abstinence time across each 1-unit change in post-lapse guilt, plotted separately for those assigned to Active versus Placebo NRT patch.
Discussion
Many smoking cessation studies have sought to identify factors that influence cessation success versus failure. These have typically defined failure as continued smoking at some distal time-point. Rather than focusing on binary and distal relapse outcomes, our analyses aimed to advance understanding of factors that influence the dynamic process of recurrent lapse episodes recorded as participants attempted to maintain abstinence from smoking. The analysis evaluated the way emotional and cognitive responses to smoking lapses prospectively affect subsequent lapse progression. We assessed the implications of Marlatt’s AVE concept, which holds that each lapse – not just the first – represents a pivotal situation after which the lapser will either become increasingly demoralized or remain confident and committed to cessation.
Past research on AVE effects focused exclusively on quitters’ initial lapse. Our results indicate that participants’ AVE responses to their initial lapse did not predict relapse outcomes, although greater initial abstinence self-efficacy was associated with slower resumption of daily low-level smoking. Analyses across all lapses in the sequence indicate that when a smoker’s post-lapse self-efficacy was lower the smoker progressed more rapidly to a subsequent lapse. This result suggests that when lapses degrade smokers’ confidence and make them feel like giving up, more lapses soon follow, perhaps because the next lapse seems inevitable, and avoiding it or coping with the temptation to lapse seems too difficult or pointless. These findings are consistent with predictions from RPM, and also from broader self-efficacy theory (Bandura, 1977), suggesting that clinical interventions should aim to maintain smokers’ morale and improve their abstinence self-efficacy following lapses, with the caveat that self-efficacy inflated well beyond smokers’ actual competence to resist lapsing could cause smokers to take risks that lead to lapses and relapse.
The role of pre-lapse abstinence appears to be more subtle, interacting with AVE responses in a way that influences progression to additional lapses. Rather than undermining self-efficacy after a lapse, results indicate that longer periods of pre-lapse abstinence potentiated the effect of self-efficacy in protecting against subsequent progression. In such instances, the individual’s feeling of confidence may be better grounded in real experience; i.e., their ability to maintain abstinence for a longer time before the lapse event. In contrast, high self-efficacy following a very short period of abstinence may be less realistic and more brittle in the face of challenge, and hence have a weaker association with subsequent behavior.
Similar dynamics emerged for self-blame. Elevated internal attributions of blame were protective to the extent that they followed increasingly longer periods of pre-lapse abstinence. This latter finding contradicts the RPM, as relapse prevention theory considers self-blame a maladaptive reaction that will accelerate progression to the extent that it is demoralizing and leads to a self-fulfilling prophecy of failure. However, considering a lapse one’s own fault may be both realistic and productive, if it generates renewed commitment to abstinence and activates attempts to correct the factors that led the smoker astray. Such efforts may be more successful when they follow a longer period of abstinence for three reasons. First, longer prior abstinence may reflect smokers’ actual ability to maintain abstinence when they are trying. In this scenario longer periods of pre-lapse abstinence may be associated with greater perceptions of control over abstinence, and greater perceptions of control may then translate to elevated ratings of self-blame when lapses occur. Second, perceptions of accumulated success with abstinence may offset the negative effects of a lapse. Despite any temporary cognitive or affective discouragement following a lapse, accumulated success may serve as a buffer that increases the likelihood that the person will re-establish and maintain abstinence. Third, engaging in self-blame may promote self-examination of both what one did wrong in the previous lapse, which may be easier and more accurate when a lapse is more isolated, and comes against a background of maintained abstinence. For example, smokers may be able to contrast what they did right to maintain abstinence with what they did wrong that allowed it to be disrupted. In any case, these findings suggest that, rather than trying to relieve smokers of self-blame and doubt, as current approaches often do, treatment should encourage realistic self-appraisal when smokers lapse in order to promote abstinence moving forward.
Nicotine Replacement Therapy
Our analyses also shed light on the role played by NRT assignment, demonstrating the extent to which nicotine patch treatment prevents progression across a series of repeated lapses. Shiffman, Scharf, et al (2006) showed that treatment with high-dose patch impeded overall progression from the first lapse to relapse. The present analysis provides additional detail, demonstrating that active patch slowed progression from each lapse to the next, but that this protective effect was limited to the first 8–10 lapses. This suggests that smokers should be encouraged to remain on treatment even after they have lapsed, at least through the first 8–10 lapses, while persisting in efforts to recover abstinence as soon as possible. Conversely, it also suggests when it may no longer be productive to persist in patch treatment in the face of an extended series of recurring lapses. We also observed that the effects of active patch assignment on progression were moderated by lapse-related guilt, such that elevated guilt accelerated progression among those on active patch, while it was protective among those on placebo. It is not clear why such psychological reactions should interact with pharmacological treatment. Further exploration of the interaction between guilt and NRT treatment – and, more broadly, between pharmacological and psychological factors in relapse – is warranted.
Amount smoked
Confirming our expectation that more smoking would beget more smoking, we found that the amount participants smoked in a lapse magnified the risk of progressing to the next lapse. This result extends a previous analyses limited to the first lapse (Shiffman et al., 2006) which found that more smoking was associated with faster progression to relapse. Smoking may beget smoking because of priming effects, which are well-documented in animal studies implementing experimental “reinstatement” models of relapse (Shaham et al., 2003). Re-engagement in smoking behavior may also re-activate other addiction-related processes, such as the association of rapid nicotine delivery and “spiked” nicotine in the brain (uniquely associated with smoking, and not patch wear; Benowitz, 2010).
Limitations and Strengths
The study suffered some limitations. Participants were motivated and confident heavy smokers who sought treatment, most of whom were Caucasian and of above average educational attainment, and may not be representative of all smokers. Although our behavioral treatment intervention specifically excluded discussion of the AVE, behavioral counseling could affect AVE responses in unanticipated ways, such as promoting an emphasis on complete abstinence, and these effects may be magnified in highly motivated samples. Performing the analyses in the context of a trial of smoking cessation medications may also limit the generalizability of results: the approximately one third of smokers who seek pharmacotherapy (Shiffman et al, 2008) may be different, and, although we controlled for treatment assignment, even treatment with placebo may have psychological effects (e.g., related to the expectation that treatment should protect against craving and lapses without much effort of their own) that cannot be evaluated in this design. Although participants recorded many lapses, it is possible that some were omitted, so the assessment of lapse-to-lapse intervals is likely imperfect. Biochemical verification could not guarantee accurate reporting of individual cigarettes, although it was helpful in verifying whether participants were being honest about their overall smoking status. The duration of the study was limited to 6-weeks post cessation, leaving us unable to evaluate responses to lapses that occurred after longer periods of time. Evaluation of lapse effects relative to pre- and post-lapse abstinence durations as quitters move further into the maintenance phase of cessation (e.g., Baker et al., 2010) represents an interesting avenue for future study.
Our measures of AVE responses did not correspond literally to the abstract constructs in the RPM, but they were derived from RPM, and did in fact demonstrate ability to predict progression from one lapse to the next. Another limitation is that our operational definition of relapse was necessarily arbitrary (Miller, 1996), and is more conservative than the 7-days’ smoking criterion used in other studies. Regardless, both of these relapse thresholds fall well short of resumption of participants’ pre-quit, “normal” smoking rates, which have been shown to take months and maybe years to reestablish (e.g., Conklin et al., 2005). We also supplemented our pre-specified relapse criterion with a criterion marking the resumption of low-level daily smoking. This provided a more sensitive measure of “routine” smoking that made it possible for us to improve our focus on true abstinence violations. Of note, alternative definitions of low-level resumption did not change the observed pattern of results.
The strengths of the study lie in its use of near-real-time EMA reports of AVE responses, recorded soon after each lapse, and the ability to use a stream of EMA reports over many lapses to characterize the prospective influence of AVE responses on progression to subsequent lapses. No study conducted to date has leveraged this methodology to empirically examine the AVE as a cascading phenomenon that affects lapse progression during the smoking cessation process.
Implications and Future Directions
The present project sought to move beyond distal end-points of smoking treatment to study the lapse-by-lapse association between psychological AVE responses and lapse progression. Findings provide mixed support for the RPM. Consistent with RPM and self-efficacy theory, we found that lapse-related decreases in abstinence self-efficacy predisposed quitters to additional lapses. Our prospective analyses also yielded findings that contradict the RPM. In particular, guilt seemed to play no role in progression in the sample as a whole, and in the placebo group, where the absence of pharmacological treatment represents the typical quit approach, increased guilt was actually associated with protection from progression, suggesting that guilt may help motivate corrective behavior following a lapse. Also directly contradicting AVE theory, internal attributions of blame following longer periods of abstinence were increasingly protective, challenging the idea that attributions of blame for a lapse are maladaptive. The protective effect of self-blame suggests that it may be misguided for treatment practitioners to focus on encouraging external, situational attributions among those who lapse; rather, quitters may benefit from non-judgmental discussion of their own role in lapses, with the goal of increasing personal responsibility and thereby effective action to maintain abstinence.
Methodologically, our results illustrate the utility of analyzing highly detailed ecological data to represent and thereby understand the complexity inherent to the process of behavior change over time. Our use of EMA methods avoided reliance of retrospective data, and allowed for detailed, lapse-by-lapse prospective analyses of how lapse responses affect smokers’ subsequent course. Future studies that incorporate fine-grained, ecologically valid measures with dynamic longitudinal analysis techniques may reveal much more about the subtle processes that drive the link between lapse and relapse outcomes. For example, our data demonstrate that the lapse progression process is highly variable both between individual participants and across momentary circumstances. This suggests that it would be useful to formally model this variation and thereby identify subgroups of subjects who followed similar recurrent survival trajectories. A corresponding extension of this approach would be to identify subgroups of subjects who followed similar AVE response trajectories; e.g., using latent mixture models to identify different trajectories towards relapse versus recovery. While the cross-sectional factor analyses reported here indicate that the items we used to assess abstinence self-efficacy did not hang together with either attributions of blame or guilt, this does not rule out the possibility of multivariate, lag-sequential or longitudinal associations between the AVE response types. Work of this sort may allow us to conceptualize the AVE as a multivariate, latent construct that evolves over the course of the lapse-relapse process.
Although contradicting some particular aspects of AVE theory, this work confirms the importance of psychological responses in the relapse process. Relapse prevention theory can be distinguished from most other prominent theories of lapse-relapse progression, all of which assume that the pharmacological effects of lapsing promote relapse more-or-less directly. For instance, neural sensitization models posit that priming doses can reinstate self-administration entirely non-consciously, via neurocognitive motivation circuits that underlie previously conditioned procedural drug-use action schemes (Baker et al., 2004; Robinson & Berridge, 2003; Kalivas & Volkow, 2005). A recent reformulation of the relapse prevention model presents a multi-disciplinary framework, retaining its emphasis on psychological responses to lapses while incorporating a greater role for pharmacologic factors such as nicotine withdrawal and reinforcement processes related to lapsing (Witkiewitz & Marlatt, 2004). While our findings suggest it may be useful to reconceptualize aspects of the AVE and its implications for treatment, they clearly support the notion that subjective psychological responses to lapsing during cessation are an important determinant of progression toward relapse. A transdisciplinary approach that incorporates genetic, pharmacologic and psychological variables would allow us to contrast theoretical models currently conceptualized at different levels of analysis, and may thereby improve our understanding of both treatment effects and the natural history of lapse-relapse progression during smoking cessation.
It is important to advance our understanding of the smoking relapse process, so that we might improve our ability to affect clinical outcomes. According to RPM, each lapse in the process represents a potential target for interventions designed to bolster coping resources and renew commitment to change. However, RPM interventions have generally failed to improve smoking cessation outcomes (Irvin et al., 1999; Lancaster et al., 2006). This may be because RPM interventions have focused on reducing what were seen as negative and counter-productive responses such as self-blame and guilt. The present findings indicate that such reactions can sometimes be productive, and suggest that clinicians should not focus on avoiding all “negative” reactions to lapses, but encourage realistic self-evaluation that emphasizes personal responsibility in the interest of bolstering appropriate efforts to avoid re-lapsing and get lapsed smokers back on a path towards abstinence.
Acknowledgments
This work was supported by National Institute on Drug Abuse Grant DA006084. Saul Shiffman is cofounder of invivodata, which provides electronic diaries for research, and serves as consultant to GlaxoSmithKline Consumer Healthcare exclusively regarding matters related to smoking cessation and also is developing new nicotine medications. Patches for the study were provided through an unrestricted grant from GlaxoSmithKline Consumer Healthcare.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn
References
- Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstein MG, Monti PM. The tobacco dependence treatment handbook: A guide to best practices. New York: Guilford Press; 2003. [Google Scholar]
- Baer JS, Kamarck R, Lichtenstein E, Ransom CC. Prediction of smoking relapse: Analysis of temptation and transgression after initial cessation. Journal of Consulting and Clinical Psychology. 1989;57:623–627. doi: 10.1037//0022-006x.57.5.623. [DOI] [PubMed] [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. Nebr. Symp. Motiv. 1986;34:257–323. [PubMed] [Google Scholar]
- Baker TB, Mermelstein R, Collins LM, et al. New methods for tobacco dependence treatment research. Annals of Behavioral Medicine. 2010 Dec 3;:2010. doi: 10.1007/s12160-010-9252-y. [E-publication ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Statistics in Medicine. 2003;22:2591–2602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- Benowitz N. Nicotine addiction. New England Journal of Medicine. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger N, Davis A, Rafaeli E. Diary methods: Capturing life as it is lived. Annual Review of Psychology. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- Borland R. Slip-ups and relapse in attempts to quit smoking. Addictive Behaviors. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany S, Baker TB. The process of smoking relapse. In: Leukefeld CG, editor. NIDA Research Monograph. Vol. 72. Washington D.C.: National Institute of Drug Abuse; 1986. [PubMed] [Google Scholar]
- Brandon TH, Tiffany S, Obremski K, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annual Review of Clinical Psychology. 2007;3:257–284. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Brown RA. Intensive behavioral treatment. In: Abrams DB, et al., editors. The tobacco dependence treatment handbook: A guide to best practices. New York: Guilford Press: 2003. pp. 118–177. [Google Scholar]
- Brownell KD, Marlatt GA, Lichtenstein E, Wilson GT. Understanding and preventing relapse. American Psychologist. 1986;41:765–782. doi: 10.1037//0003-066x.41.7.765. [DOI] [PubMed] [Google Scholar]
- Condiotte MM, Lichtenstein E. Self-efficacy and relapse in smoking cessation programs. Journal of Consulting and Clinical Psychology. 1981;49:648–658. doi: 10.1037//0022-006x.49.5.648. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA, Sheidow AJ, Jones BL, Levine MD, Marcus MD. The return to smoking:1-year relapse trajectories among female smokers. Nicotine and Tobacco Research. 2005;7(4):533–540. doi: 10.1080/14622200500185371. [DOI] [PubMed] [Google Scholar]
- Curry S, Marlatt GA, Gordon JR. Abstinence violation effect: Validation of an atributional construct with smoking cessation. journal of Consulting and Clinical Psychology. 1987;55:145–149. doi: 10.1037//0022-006x.55.2.145. [DOI] [PubMed] [Google Scholar]
- Festinger L. Conflict, decision, and dissonance. Stanford: Stanford University Press; 1964. [Google Scholar]
- Garcia ME, Schmitz JM, Doerfler LA. A fine-grained analysis of the role of self-efficacy in self-initiated attempts to quit smoking. Journal of Consulting and Clinical Psychology. 1990;58:317–322. doi: 10.1037//0022-006x.58.3.317. [DOI] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. Journal of Consulting and Clinical Psychology. 1990;58:175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- Hammersley R. A digest of memory phenomena for addiction research. Addiction. 1994;89:283–293. doi: 10.1111/j.1360-0443.1994.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hoeppner BB, Goodwin MS, Velicer W, Mooney M, Hatsukami D. Detecting longitudinal patterns of daily smoking following drastic cigarette reduction. Addictive Behaviors. 2008;33:623–639. doi: 10.1016/j.addbeh.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: Wiley; 2008. [Google Scholar]
- Hougaard P. Analysis of Multivariate Survival Data. New York: Springer; 2000. [Google Scholar]
- Irvin JE, Bowers CA, Dunn ME, Wang MC. Efficacy of relapse prevention: a meta-analytic review. Journal of Consulting and Clinical Psychology. 1999;67:563–70. doi: 10.1037//0022-006x.67.4.563. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby D, Smith S, Welter D, Baker TB. Predicting smoking cessation: Who will quit with and without nicotine patch. JAMA. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Hajek P, Stead L, West R, Jarvis M. Prevention of relapse after quitting smoking: A systematic review of trials. Archives of Internal Medicine. 2006;166:828–835. doi: 10.1001/archinte.166.8.828. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. 2nd ed. New York: Guilford; 2005. [Google Scholar]
- Marlatt GA, Gordon JR, editors. Relapse prevention: maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 1985. [Google Scholar]
- Miller WR. What is relapse? Fifty ways to leave the wagon. Addiction. 1996;91:S15–S27. [PubMed] [Google Scholar]
- O'Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. Journal of Consulting and Clinical Psychology. 1987;55:367–371. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Ross M. Relation of implicit theories to the construction of personal histories. Psychological Review. 1989;96:341–357. [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Schoeneman TJ, Hollis JF, Stevens VJ, Fischer K, Cheek PR. Recovering stride versus letting it slide: Attributions for "slips" following smoking cessation treatment. Psychology and Health. 1988;2:335–347. [Google Scholar]
- Schoeneman TJ, Stevens VJ, Hollis JF, Cheek PR, Fischer K. Attribution, affect, and expectancy following smoking cessation treatment. Basic and Applied Social Psychology. 1988;9:173–184. [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Reflections on smoking relapse research. Drug and Alcohol Review. 2006;25:15–20. doi: 10.1080/09595230500459479. [DOI] [PubMed] [Google Scholar]
- Shiffman, et al. Use of smoking cessation treatments in the US. American Journal of Preventative Medicine. 2008;34:102–111. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: Relationship to smoking relapse, and effects on nicotine replacement therapy. Psychopharmacology. 2006;184:608–618. doi: 10.1007/s00213-005-0175-4. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel J, Richards T. Progression from a smoking lapse to relapse: Prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. Journal of Consulting and Clinical Psychology. 1996;64:993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel J, Richards T. The abstinence violation effect following smoking lapses and temptations. Cognitive Therapy and Research. 1997;21:497–523. [Google Scholar]
- Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel J. Remember that? A comparison of real-time versus retrospective recall of smoking lapses. Journal of Consulting and Clinical Psychology. 1997;65(2):292–300. doi: 10.1037/0022-006x.65.2.292.a. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, et al. Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology. 2006;74:276–285. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shumaker SA, Abrams DB, Cohen S, Garvey A, Grunberg NE, et al. Models of smoking relapse. Health Psychology. 1986;5:13–27. [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Annals of Behavioral Medicine. 1994;16:199–202. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th Ed. New York: Allen & Bacon; 2006. [Google Scholar]
- Tennen H, Affleck G, Armeli S. Daily processes in health and illness. In: Suls J, Wallston K, editors. Social Psychological Foundations of Health. Malden, MA: Blackwell; 2003. [Google Scholar]
- Tindle H, Shiffman S, Paty JA, Dang Q. Relapsing in real time: A prospective assessment of the natural history of abstinence failure. Poster presented at the Annual Meeting of the Society for Research on Nicotine and Tobacco; Orlando, FL. 2006. Feb, [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J, et al. Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine and Tobacco Research. 2005;7:257–268. doi: 10.1080/14622200500055673. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems. American Psychologist. 2004;59:1–12. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]