Biospecimens are an essential resource for biomedical research, including research that aims to decipher the biology of cancer and improve its clinical management. Accordingly, cancer patients and others who contribute biospecimens for research are increasingly attentive to the results of studies conducted with their biological samples (1). They may seek aggregate research results as well as individual results, including incidental findings uncovered in the course of research (2-4). As advanced technologies for cancer research make more feasible the discovery of biomarkers for risk assessment, diagnosis, and prognosis, the research community is faced with the challenge of whether to share specific research results with biospecimen contributors.
Sharing research results, particularly genetics and genomics, with research participants has been at the forefront of many ethical and scientific discussions. Advocates of the process maintain that research participants should be offered the option of receiving potentially important information that could impact their health and life choices (1, 5-7). Moreover, investigators often feel motivated to share results that they perceive to be beneficial to research participants not only because of their potential clinical value but also to promote a trusting partnership with them. Opponents of sharing results assert that the purpose of research is to generate knowledge rather than provide clinical care and that research laboratories do not necessarily operate in accordance with clinical laboratory standards (1). In the United States, the Clinical Laboratory Improvement Amendments (CLIA) law (8) and implementing regulations (9) require a “laboratory” as defined in these authorities to obtain federal certification and accreditation before disclosing results for patient care. However, the legal definition of “laboratory” in the CLIA statute and implementing regulations limits this mandate to facilities that analyze human tissue for the purpose of clinical diagnosis, prevention, or treatment of disease, or impairment or assessment of health (8). Arguably, many research biobanks would not fit this definition since they do not analyze tissue for the purpose of clinical care. Furthermore, facilities only collecting or preparing specimens and not performing testing are expressly exempt from CLIA requirements (9). Accordingly, we postulate that CLIA may not require biobanks or research facilities to be CLIA-certified in order to share research results with participants, as long as such entities are not expressly constituted for the purpose of performing clinical laboratory testing. While there is controversy regarding the legal question of whether non-CLIA certified facilities may return research results, it has been recognized that results from non-CLIA certified labs can still have analytic validity, and that it may be appropriate as an ethical and policy matter for biobanks and studies to return such results labeled as “research” (4, 6).
Even for CLIA-certified laboratories, there is disagreement about the nature and appropriate scope of research results that can be shared with participants. Some clinical results can be irrefutably beneficial from the standpoint of clinical utility - e.g., informing research participants about a cancer predisposing genetic mutation with available preventive measures that can avert fatal disease. There is, however, less consensus regarding the return of results with unclear medical significance or where clinical intervention is unavailable. For example, sharing research results regarding genetic predisposition to early onset Alzheimer's disease might permit lifestyle adjustments and financial planning in anticipation of disease progression, but is not clinically actionable. These complex issues underlie the importance of developing overarching principles for the sharing of research results to guide the scientific community.
We advocate that attention be given to the conception of best practices for sharing research results during the planning and development of biobanks and for any research projects that involve the collection of biospecimens. These best practices should consider both scientific and ethical elements. Scientific elements include the objective of the research project, the type and identifiability of the biospecimens used, whether biospecimens are collected for specific or unspecified future research, the type of data generated (i.e., have clinical/health relevance), and the scientific validity and reproducibility of the data. Ethical elements include the nature of the informed consent process for the collection of biospecimens and the extent to which it offers clear options to research participants for receiving, declining, or being re-contacted about the receipt of research results. In addition, the ethical mandate to protect the welfare of human subjects (10) requires consideration of whether sharing research results will cause unwarranted assurance or unnecessary alarm.
To address some of these tangible issues, the National Cancer Institute (NCI) organized a workshop in 2010 entitled “Release of Research Results to Participants in Biospecimen Studies” with the objective of developing best practices about when and how to share biospecimen research findings with study participants. The workshop included a discussion group devoted to the conditions for sharing individual research results from biospecimens collected for/in the course of clinical studies (11). Herein, we describe a framework for decision making about whether and when to return research results from identifiable biospecimens that is based on recommendations from the NCI Workshop. This framework incorporates a set of operational criteria comprising a “smart filter” to guide the sharing of individual research results (11, Figure 1). These criteria include the following four core elements: 1) the research participant agreed in the consent process to receive research results; 2) the result is analytically valid; 3) the result is clinically significant or serious for the participant; and 4) the result is clinically actionable (11).
Figure 1.
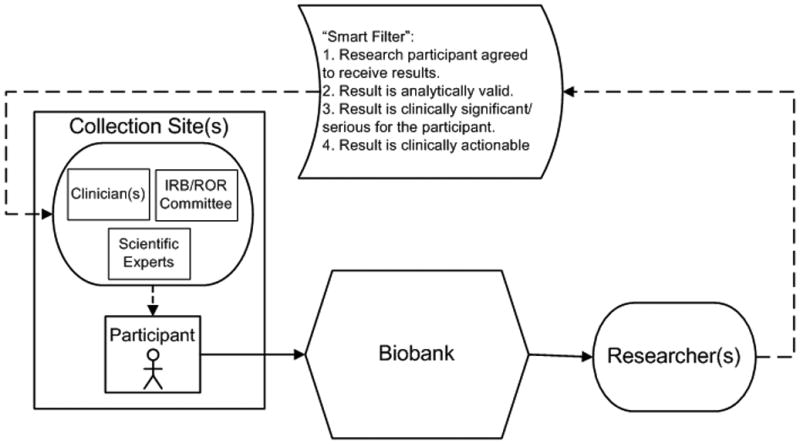
Schematic of application of the “smart filter” principles to a common biobanking model. In this model biospecimens (solid arrows) would be collected from a research participant at a collection site(s), sent to a biobank and then further distributed to a researcher(s). If a researcher found a research result that should be considered for sharing (dashed arrows), the smart filter would be applied to determine whether the result should be shared. If the research result was deemed to meet the smart filter criteria, the result would be reported to the collection site for possible sharing with the research participant. The responsibility for applying the “smart filter” may or may not lie with the biobank, depending on the model used and any agreements in place between parties. When considering whether to share results with the participant, the collection site may call upon groups with needed expertise such as clinicians, members of the institutional review board (IRB), or a return of results (ROR) committee, and scientific experts.
With regard to the first core element, the informed consent form should describe if research participants may choose to receive, or be re-contacted about, research results. The consent form is internationally recognized as the operational mechanism for ensuring informed and voluntary decisions to participate in research (12, 13). The NCI Workshop recommended that the informed consent document contain “language to describe the circumstances under which research participants may receive individual, clinically relevant research findings and the mechanisms for communicating such findings” (11). Yet, particularly in genome wide studies and research analyzing genetic databases in conjunction with medical records, there are inherent limitations to disclosing the full extent and nature of possible research findings. Considering the current suggestion of the HHS Office for Human Research Protections that one-time general consent for future research use of biospecimens be required (14), the universe of potential research results might be virtually indescribable. Moreover, increasingly sophisticated analytical platforms and informatics tools have transformed cancer research, complicating the common understanding of typical consent form descriptors such as “genetic research”. There is a great need for research to determine how participants interpret such consent form language. Given the ambiguities inherent in communicating the intention to share results, when a specific finding falls within the scope of the informed consent, participants should receive an additional opportunity to consider whether to receive or decline known information, particularly unexpected incidental findings. Investigators collecting biospecimens should consider during the protocol design stage whether it would be ethically appropriate to offer research participants the option to decline the receipt of research results with potential clinical utility, such as diagnostic discrepancies. Some have argued that in rare cases a “duty to rescue” exists requiring the return of research results that offer clear evidence of clinical utility and actionability (15).
With regard to the second core element, ensuring the analytical validity of research results, standards may not exist in every research laboratory for assessing the accuracy, precision, sensitivity, and specificity of the testing conducted and results generated. When research results are generated and interpreted in a non-CLIA approved laboratory, the NCI workshop recommended that such results be verified in a CLIA-approved laboratory unless the verification is not possible. In such cases, investigators should ascertain the analytical validity and safety of the performed test (11). At a minimum, any research results generated in a non-CLIA approved laboratory should clearly be labeled as research, and not clinical findings (4, 6). An issue to consider in the context of CLIA verification is who will cover the cost of re-testing.
The third core element requires an assessment that the research result is clinically significant or serious. Interpreting the parameters of this element can be highly subjective and therefore should be undertaken in consultation with an institutional review board (IRB) or other expert panel. Factors to consider include the relative risk of the disease or health condition associated with the information, the likelihood of fatality, and the level of morbidity. The NCI workshop did not reach consensus about whether to share results that are not clinically significant to the research participant but have reproductive implications or significance to family members.
Individual and societal values of the research participant and any financial constraints should be considered by biospecimen projects in deciding whether to broaden the scope of sharing to include these criteria.
The fourth core element restricts the sharing of individual research results to those that are “clinically actionable”. As with the third element, defining the parameters is subjective, and no clear consensus was reached by the NCI workshop. A more narrow view restricts returnable results to those that lead to therapeutic interventions designed to cure disease. This is consistent with a perceived duty of medical researchers to deliver information that clearly demonstrates the benefit of available remedies (15). A broader view would consider appropriate the sharing of information which, even if it would not support specific therapies, would help research participants manage their medical condition or plan for the future. Sharing the greater range of information would be more appropriate for biospecimen research projects that can provide access to genetic counselors and medical staff, or otherwise assist research participants with the process of interpreting and understanding research results.
Every project faces unique issues in determining how, when and by whom research results can best be shared. Biobanks housed in university hospitals, cancer centers, and community hospitals, and those specially created for multi-center clinical trials, rare disease research collaborations, or other specific studies, will differ significantly in infrastructure, scope of mission, and funding sources. Whenever possible, research results should be communicated by knowledgeable professionals who have ongoing relationships with participants. Such individuals are also most likely to be in possession of private identifiable information about participants and will therefore be in a position to contact participants with fewer legal constraints. Policies for sharing individual research results should be developed in consultation with local IRBs, advisory boards with representatives from the participant community or other representative groups.
In terms of who is responsible for communicating research results to participants, the NCI workshop recommended that the biospecimen collection sites be responsible for communicating results generated from primary research or from an internal review of biospecimens (e.g., pathology) at the biobank (11). In general, results can be linked to individual participants by the primary collecting site that maintains identifiable information or in some cases by the biobank, if it holds identifiable information. The NCI workshop also considered whether biobanks should assume the responsibility for sharing results generated in the course of secondary research studies. Workshop attendees recommended that biobanks coordinate the process by which secondary research results are communicated to human research participants with input from the primary and secondary institutions (11). In most cases, secondary researchers would not be able to link research results to individual participants as they would likely receive coded or de-identified samples. The NCI workshop discussed mechanisms such as the material transfer agreement as a means of clarifying the responsibilities of all participants in the research process (11).
In conclusion, limiting the scope of research result sharing to defined criteria such as those described in the “smart filter” demonstrates respect for research participants and protects them from needless harm that can be caused by preliminary or non-validated research findings. The recommendations generated by the NCI workshop (11) will be incorporated in a future iteration of the NCI Best Practices for Biospecimen Resources (16) to guide the scientific community. More research is needed to further the development of evidence-based criteria for research results that can be shared with research participants.
Acknowledgments
We thank all who participated in discussions at the National Cancer Institute sponsored workshop on Release of Research Results to Participants in Biospecimen Studies, held July 8-9, 2010. In particular, we thank the members of breakout group 2 that included:
Chair: Pearl O'Rourke, MD; Co-Chair: John M. Jessup, MD.
Members: Christine D. Berg, MD; Andrew Hruszkewycz, MD, PhD; Penny Keller; Irina Lubensky, MD; Elizabeth Mansfield; PhD; Karen Maschke, PhD; Jean E. McEwen, PhD, JD; Tracy L. McGregor, MD; Jane Perlmutter, Ph.D., MBA; Katherine Schneider, MPH, GCG; Richard Schwab, MD; Susan M. Wolf, JD.
Footnotes
Disclosure: This work was supported by general appropriations to the National Cancer Institute.
Conflict of Interest Notification Page: The authors declare no conflicts of interest
The meeting summary can be found at http://biospecimens.cancer.gov/resources/publications/workshop/rrra.asp
The contents of this publication do not reflect the views or policies of the Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute.
References
- 1.Dressler LG. Disclosure of research results from cancer genomic studies: state of the science. Clin Cancer Res. 2009;15(13):4270–76. doi: 10.1158/1078-0432.CCR-08-3067. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman D, Murphy J, Scott J, Hudson K. Subjects matter: a survey of public opinions about a large genetic cohort study. Genet Med. 2008;10:831–39. doi: 10.1097/GIM.0b013e31818bb3ab. [DOI] [PubMed] [Google Scholar]
- 3.Partridge AH, Winer EP. Informing clinical trial participants about study results. JAMA. 2002;288:363–5. doi: 10.1001/jama.288.3.363. [DOI] [PubMed] [Google Scholar]
- 4.Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics. 2008;36(2):219–48. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoppers BM, Joly Y, Simard J, Durocher F. The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet. 2006;14:1170–8. doi: 10.1038/sj.ejhg.5201690. [DOI] [PubMed] [Google Scholar]
- 6.Fabsitz RR, McGuire A, Sharp RR, Puggal M, et al. Ethical and Practical Guidelines for Reporting Genetic Research Results to Study Participants. Updated Guidelines From a National Heart, Lung, and Blood Institute Working Group. Circ Cardiovasc Genet. 2010;3:574–80. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf SM, Crock BN, Van Ness B, et al. Managing Incidental Findings & Research Results in Genomic Research Involving Biobanks & Archived Datasets. Genetics in Medicine. 2012;14(4) doi: 10.1038/gim.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical Laboratory Improvement Amendments of 1988. Pub. L. No. 100-578, 102 Stat. 2903
- 9.CLIA regulations at 42 CFR 493.2
- 10.The Belmont Report. Ethical Principles and Guidelines for the protection of human subjects of research. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. April 18, 1979. Available from: http://ohsr.od.nih.gov/guidelines/belmont.html
- 11.NCI Workshop on Release of Research Results to Participants in Biospecimen Studies. [accessed August 8, 2011]; http://biospecimens.cancer.gov/global/pdfs/NCI_Return_Research_Results_Summary_Final-508.pdf.
- 12.HHS Policy for Protection of Human Research Subjects at 45 Code of Federal Regulations (CFR) Part 46, subparts A-D. [accessed August 8, 2011]; Available from: http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html.
- 13.ISBER Working Groups: Informed Consent Procedures for the Collection of Biospecimens. [accessed August 8, 2011]; http://www.isber.org/wg/icp/index.cfm.
- 14.Proposed Rules. Human Subjects Research Protections: Enhancing Protections for Research Subjects and Reducing Burden, Delay, and Ambiguity for Investigators. Federal Register / Vol. 76, No. 143 / Tuesday, July 26, 2011, 44512-44531. Available from: http://www.gpo.gov/fdsys/pkg/FR-2011-07-26/pdf/2011-18792.pdf
- 15.Beskow LM, Burke W. Offering Individual Genetic Research Results: Context Matters. Sci Transl Med. 2010 doi: 10.1126/scitranslmed.3000952. 2:38cm20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute. Bethesda, Maryland: 2011. [accessed November 10, 2011]. Best Practices for Biospecimen Resources. Available at: http://biospecimens.cancer.gov/bestpractices/ [Google Scholar]


