Abstract
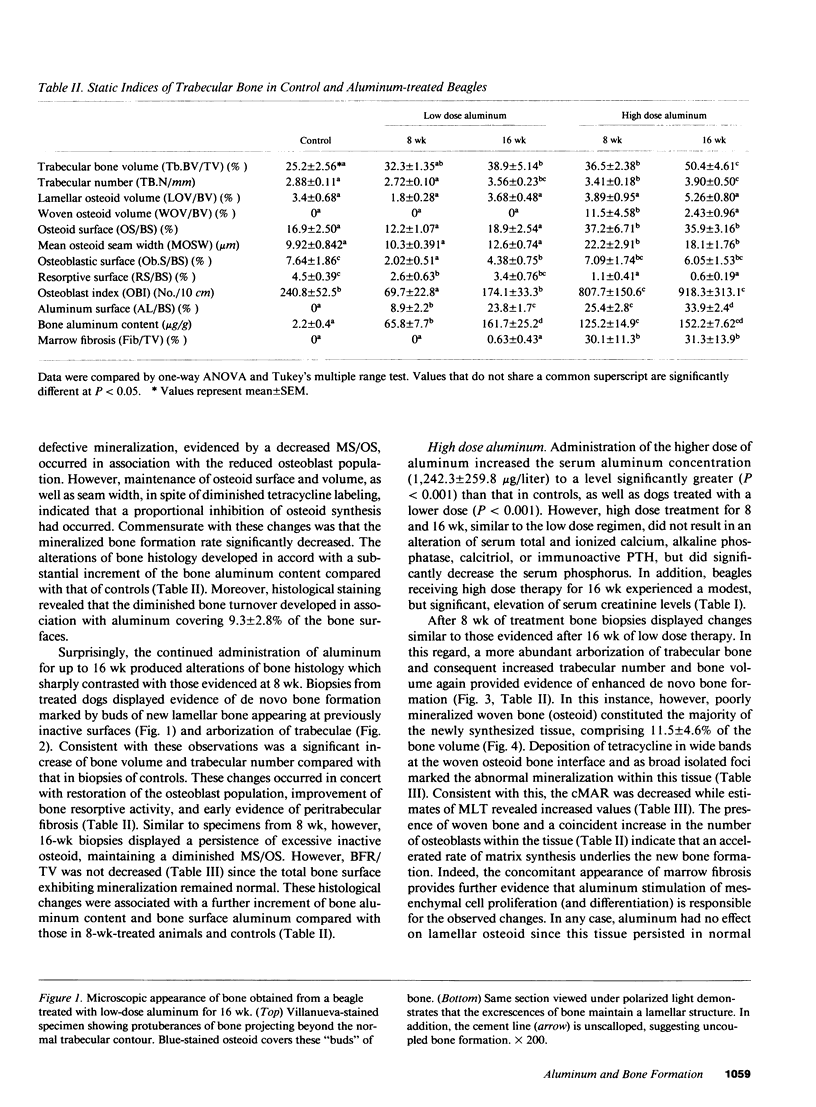
To define the primary effects of aluminum on bone in the mammalian species, we examined the dose/time-dependent actions of aluminum in normal beagles. Administration of low dose aluminum (0.75 mg/kg) significantly elevated the serum aluminum (151.7 +/- 19.9 micrograms/liter) compared with that in controls (4.2 +/- 1.35 micrograms/liter) but did not alter the calcium, creatinine, or parathyroid hormone. After 8 wk of therapy, bone biopsies displayed reduced bone resorption (2.6 +/- 0.63 vs. 4.5 +/- 0.39%) and osteoblast covered bone surfaces (2.02 +/- 0.51 vs. 7.64 +/- 1.86%), which was indicative of low turnover. In contrast, prolonged treatment resulted in increased bone volume and trabecular number (38.9 +/- 1.35 vs. 25.2 +/- 2.56% and 3.56 +/- 0.23 vs. 2.88 +/- 0.11/mm) which was consistent with uncoupled bone formation. Administration of higher doses of aluminum (1.20 mg/kg) increased the serum aluminum further (1242.3 +/- 259.8 micrograms/liter) but did not affect calcium, creatinine, or parathyroid hormone. However, after 8 wk of treatment, bone biopsies displayed changes similar to those after long-term, low-dose therapy. In this regard, an increased trabecular number (3.41 +/- 0.18/mm) and bone volume (36.5 +/- 2.38%) again provided evidence of uncoupled bone formation. In contrast, in this instance poorly mineralized woven bone contributed to the enhanced bone volume. High-dose treatment for 16 wk further enhanced bone volume (50.4 +/- 4.61%) and trabecular number (3.90 +/- 0.5/mm). These observations illustrate that aluminum may stimulate uncoupled bone formation and induce a positive bone balance. This enhancement of bone histogenesis contrasts with the effects of pharmacologic agents that alter the function of existing bone remodeling units.
Full text
PDF

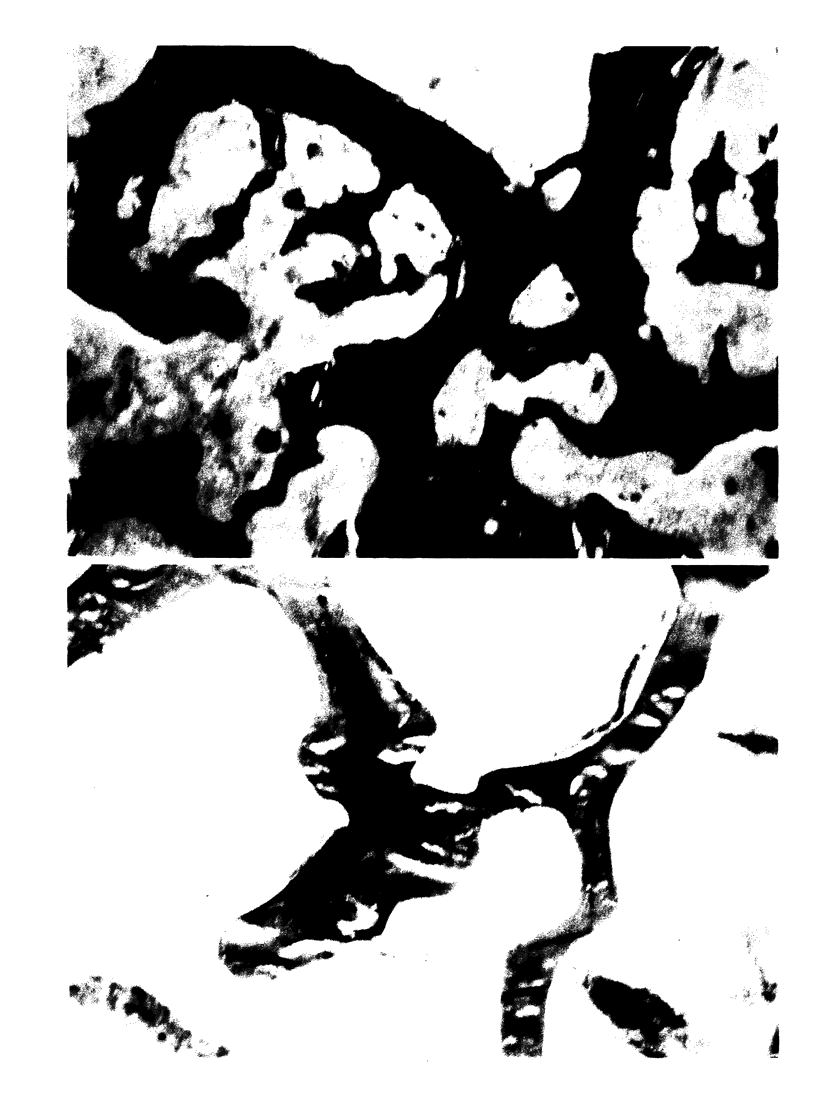

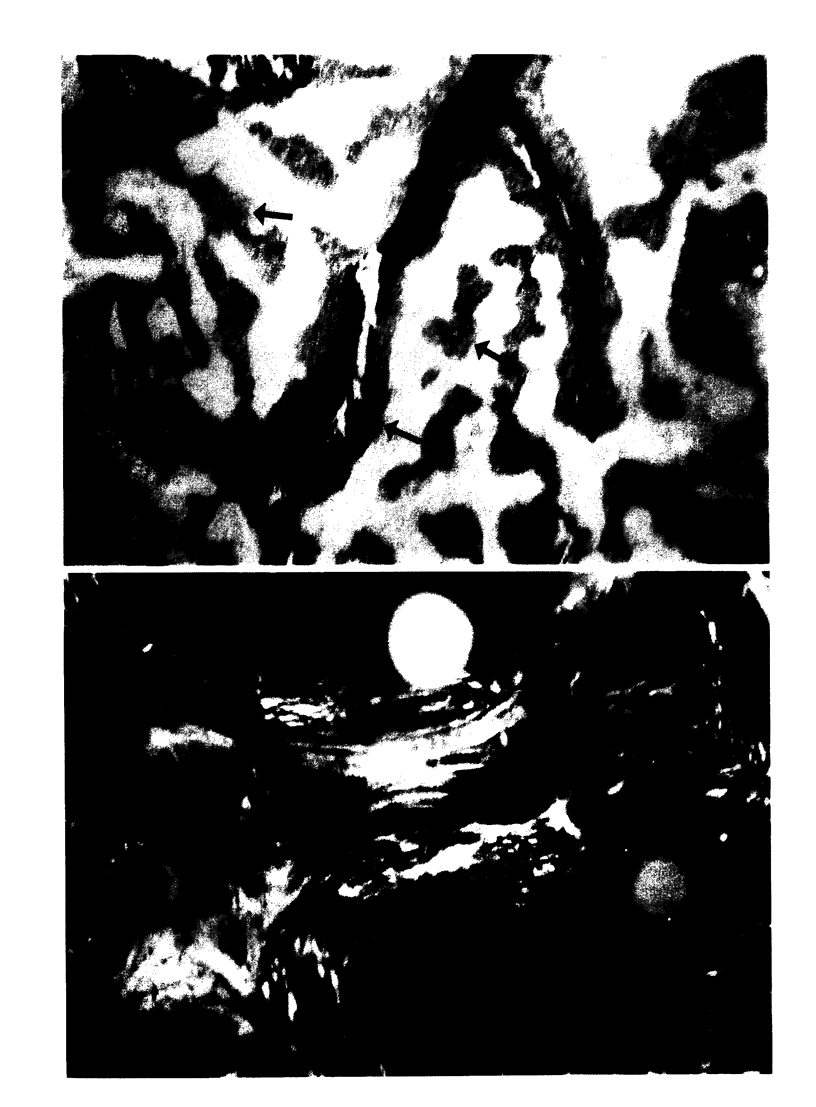



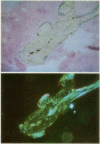
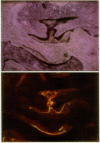
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderman F. R., Gitelman H. J. Improved electrothermal determination of aluminum in serum by atomic absorption spectroscopy. Clin Chem. 1980 Feb;26(2):258–260. [PubMed] [Google Scholar]
- Andress D. L., Maloney N. A., Endres D. B., Sherrard D. J. Aluminum-associated bone disease in chronic renal failure: high prevalence in a long-term dialysis population. J Bone Miner Res. 1986 Oct;1(5):391–398. doi: 10.1002/jbmr.5650010503. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Alfrey A. C., Posen S., Lissner D., Hills E., Dunstan C. R., Evans R. A. Effect of aluminum on normal and uremic rats: tissue distribution, vitamin D metabolites, and quantitative bone histology. Calcif Tissue Int. 1983 May;35(3):344–351. doi: 10.1007/BF02405056. [DOI] [PubMed] [Google Scholar]
- Ellis H. A., McCarthy J. H., Herrington J. Bone aluminium in haemodialysed patients and in rats injected with aluminium chloride: relationship to impaired bone mineralisation. J Clin Pathol. 1979 Aug;32(8):832–844. doi: 10.1136/jcp.32.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman W. G., Gilligan J., Horst R. Short-term aluminum administration in the rat. Effects on bone formation and relationship to renal osteomalacia. J Clin Invest. 1984 Jan;73(1):171–181. doi: 10.1172/JCI111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman W. G., Henry D. A., Horst R., Nudelman R. K., Alfrey A. C., Coburn J. W. Parenteral aluminum administration in the dog: II. Induction of osteomalacia and effect on vitamin D metabolism. Kidney Int. 1984 Feb;25(2):370–375. doi: 10.1038/ki.1984.26. [DOI] [PubMed] [Google Scholar]
- Hodsman A. B., Sherrard D. J., Alfrey A. C., Ott S., Brickman A. S., Miller N. L., Maloney N. A., Coburn J. W. Bone aluminum and histomorphometric features of renal osteodystrophy. J Clin Endocrinol Metab. 1982 Mar;54(3):539–546. doi: 10.1210/jcem-54-3-539. [DOI] [PubMed] [Google Scholar]
- Jee W. S., Ueno K., Deng Y. P., Woodbury D. M. The effects of prostaglandin E2 in growing rats: increased metaphyseal hard tissue and cortico-endosteal bone formation. Calcif Tissue Int. 1985 Mar;37(2):148–157. doi: 10.1007/BF02554834. [DOI] [PubMed] [Google Scholar]
- Klein G. L., Sedman A. B., Heyman M. B., Marathe G., Battifora H. A., Worrall J. L., Horst R. L., Brewer G. J., Miller N. L., Alfrey A. C. Hepatic abnormalities associated with aluminum loading in piglets. JPEN J Parenter Enteral Nutr. 1987 May-Jun;11(3):293–297. doi: 10.1177/0148607187011003293. [DOI] [PubMed] [Google Scholar]
- Kragstrup J., Richards A., Fejerskov O. Experimental osteo-fluorosis in the domestic pig: a histomorphometric study of vertebral trabecular bone. J Dent Res. 1984 Jun;63(6):885–889. doi: 10.1177/00220345840630061401. [DOI] [PubMed] [Google Scholar]
- Lieberherr M., Grosse B., Cournot-Witmer G., Hermann-Erlee M. P., Balsan S. Aluminum action on mouse bone cell metabolism and response to PTH and 1,25(OH)2D3. Kidney Int. 1987 Mar;31(3):736–743. doi: 10.1038/ki.1987.60. [DOI] [PubMed] [Google Scholar]
- Malluche H. H., Sherman D., Meyer W., Massry S. G. A new semiautomatic method for quantitative static and dynamic bone histology. Calcif Tissue Int. 1982 Sep;34(5):439–448. doi: 10.1007/BF02411282. [DOI] [PubMed] [Google Scholar]
- Maloney N. A., Ott S. M., Alfrey A. C., Miller N. L., Coburn J. W., Sherrard D. J. Histological quantitation of aluminum in iliac bone from patients with renal failure. J Lab Clin Med. 1982 Feb;99(2):206–216. [PubMed] [Google Scholar]
- McClure J., Fazzalari N. L., Fassett R. G., Pugsley D. J. Bone histoquantitative findings and histochemical staining reactions for aluminium in chronic renal failure patients treated with haemodialysis fluids containing high and low concentrations of aluminium. J Clin Pathol. 1983 Nov;36(11):1281–1287. doi: 10.1136/jcp.36.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz W. A., Schenk R. K. Quantitative structural analysis of human cancellous bone. Acta Anat (Basel) 1970;75(1):54–66. doi: 10.1159/000143440. [DOI] [PubMed] [Google Scholar]
- Ott S. M., Maloney N. A., Coburn J. W., Alfrey A. C., Sherrard D. J. The prevalence of bone aluminum deposition in renal osteodystrophy and its relation to the response to calcitriol therapy. N Engl J Med. 1982 Sep 16;307(12):709–713. doi: 10.1056/NEJM198209163071202. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M., Mathews C. H., Villanueva A. R., Kleerekoper M., Frame B., Rao D. S. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983 Oct;72(4):1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A. M. Quantum concept of bone remodeling and turnover: implications for the pathogenesis of osteoporosis. Calcif Tissue Int. 1979 Aug 24;28(1):1–5. doi: 10.1007/BF02441211. [DOI] [PubMed] [Google Scholar]
- Pierides A. M., Edwards W. G., Jr, Cullum U. X., Jr, McCall J. T., Ellis H. A. Hemodialysis encephalopathy with osteomalacic fractures and muscle weakness. Kidney Int. 1980 Jul;18(1):115–124. doi: 10.1038/ki.1980.117. [DOI] [PubMed] [Google Scholar]
- Platts M. M., Goode G. C., Hislop J. S. Composition of the domestic water supply and the incidence of fractures and encephalopathy in patients on home dialysis. Br Med J. 1977 Sep 10;2(6088):657–660. doi: 10.1136/bmj.2.6088.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles L. D., Dennis V. W., Gitelman H. J., Harrelson J. M., Drezner M. K. Aluminum deposition at the osteoid-bone interface. An epiphenomenon of the osteomalacic state in vitamin D-deficient dogs. J Clin Invest. 1985 May;75(5):1441–1447. doi: 10.1172/JCI111846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A., Felsenfeld A. J., Haygood C. C., Wilson P., Clarke C., Llach F. Animal model of aluminum-induced osteomalacia: role of chronic renal failure. Kidney Int. 1983 Feb;23(2):327–335. doi: 10.1038/ki.1983.23. [DOI] [PubMed] [Google Scholar]
- Sedman A. B., Alfrey A. C., Miller N. L., Goodman W. G. Tissue and cellular basis for impaired bone formation in aluminum-related osteomalacia in the pig. J Clin Invest. 1987 Jan;79(1):86–92. doi: 10.1172/JCI112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B. Aluminum ions stimulate DNA synthesis in quiescent cultures of Swiss 3T3 and 3T6 cells. J Cell Physiol. 1984 Mar;118(3):298–304. doi: 10.1002/jcp.1041180313. [DOI] [PubMed] [Google Scholar]
- Snow G. R., Anderson C. Short-term chronic fluoride administration and trabecular bone remodeling in beagles: a pilot study. Calcif Tissue Int. 1986 Apr;38(4):217–221. doi: 10.1007/BF02556713. [DOI] [PubMed] [Google Scholar]
- Villanueva A. R. A bone stain for osteoid seams in fresh, unembedded, mineralized bone. Stain Technol. 1974 Jan;49(1):1–8. doi: 10.3109/10520297409116928. [DOI] [PubMed] [Google Scholar]
- Walker G. S., Aaron J. E., Peacock M., Robinson P. J., Davison A. M. Dialysate aluminium concentration and renal bone disease. Kidney Int. 1982 Feb;21(2):411–415. doi: 10.1038/ki.1982.37. [DOI] [PubMed] [Google Scholar]
- Ward M. K., Feest T. G., Ellis H. A., Parkinson I. S., Kerr D. N. Osteomalacic dialysis osteodystrophy: Evidence for a water-borne aetiological agent, probably aluminium. Lancet. 1978 Apr 22;1(8069):841–845. doi: 10.1016/s0140-6736(78)90191-5. [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Miura F., Suda T. Prostaglandin as a mediator of bone resorption induced by experimental tooth movement in rats. J Dent Res. 1980 Oct;59(10):1635–1642. doi: 10.1177/00220345800590101301. [DOI] [PubMed] [Google Scholar]