Abstract
Background:
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the widely used drugs and are often used by pregnant women. However, they can have significant teratogenic effects. The aim of the study was to investigate pregnant women's knowledge about NSAIDs use during pregnancy and their perception and consumption pattern.
Materials and Methods:
The study was a cross sectional study on women waiting for a consultation in the selected maternity hospitals in Addis Ababa, Ethiopia. The pregnant women were selected randomly and then interviewed by using standardized questionnaires.
Result:
A total of 224 pregnant women were involved in the study. Out of those, 203 (90.6%) of them have taken NSAIDs since the beginning of their pregnancy. About 201 (89.7%), 198 (88.4%) and 189 (84.4%) of the pregnant women considered that ibuprofen, diclofenac and aspirin are not NSAIDs respectively. Regarding analgesic effect of NSAIDs, 97 (43.3%) of the pregnant women believed that NSAIDs are effective for treating pain. Acetaminophen was considered as the most effective treatment for pain by 84 (37.50%) of the patients.
Conclusion:
Acetaminophen is the most common analgesic that was taken by most pregnant women. The knowledge of pregnant women about NSAIDs is poor.
Keywords: Ethiopia, NSAIDs, Pregnancy, Self-medication
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are chemically heterogeneous groups of compounds which nevertheless share certain common therapeutic actions and adverse effects.[1] The most commonly used NSAIDs are aspirin, acetaminophen, Ibuprofen, diclofenac, indometacin and naproxen.[2] All NSAIDs share antipyretic, analgesic and anti-inflammatory actions with the exception of acetaminophen which is antipyretic and analgesic but is largely devoid of anti-inflammatory activity.[1–3] Non-steroidal anti-inflammatory drugs are among the widely used drugs[4] and are often used by pregnant women[5] especially during first trimester of pregnancy.[6]
Virtually, all currently available NSAIDs can have significant unwanted effects.[2] Several previous studies[7–9] showed that NSAIDs use on late pregnancy can be associated with severe adverse neonatal outcomes including increased risks of premature closure, persistent fetal pulmonary hypertension, increased risk of congenital heart defects, intracranial hemorrhages, renal toxicity in fetus, and orofacial clefts.
However, it is frequently observed that medical practitioners prescribe these drugs for pregnant women and also pregnant women by themselves purchase easily over the counter (OTC) NSAIDs to relieve their discomfort such as headache, fever, and arthritis which are very common during pregnancy. In addition, there is a perception that OTC drugs are safe since they are accessible without prescription.[10] So far in Ethiopia, the study about pregnant women's knowledge of NSAIDs was not conducted and documented.
In this study we sought to determine the non-steroidal anti-inflammatory drug consumption pattern of pregnant women since the beginning of their pregnancy, to investigate pregnant women's knowledge of drugs used for pain, to evaluate pregnant women's perception of the risks of NSAIDs in pregnancy and to determine the sources of drug information on risks in pregnancy and potential risks of the drug for the fetus.
Materials and Methods
Study setting
This study was conducted in selected governmental hospitals (Gandhi Memorial Hospital, Yekatit 12 Hospital, Balcha Hospital and Police Referral Hospital) and Private hospitals including Denberu Gynecology and Obestrics Hospital and Behtezata Hospital in Addis Ababa Ethiopia from January 21 to March 4, 2010.
Study design
The study was a cross sectional study on women waiting for a consultation in the selected maternity hospitals. They were selected randomly by using simple random sampling technique (lottery method). Each pregnant women waiting for a consultation at the hospital's waiting room, who fulfilled the inclusion criteria (who came for a consultation at the time of data collection and willing to be interviewed) were assigned a unique number and selected blindly and then interviewed by using standardized questionnaires. Pregnant women with other comorbid condition, chronically ill patients, or who were on emergency situation (ready for delivery) and those unwilling to participate in the study were excluded
Data collection
A questionnaire was prepared and the validity of the questionnaires was assessed through in-depth discussion with health professionals. The standardized questionnaire has been pre-tested on 15 women before actual data collection. The contents of the questionnaire were women's socio-demographic information, drug consumption pattern (when began using, reason for using, self medication issues), knowledge about NSAIDs (purpose, efficacy, contraindication, adverse effect), NSAIDs perception (safety and effectiveness) and source of information on risk in pregnancy. Then the interview takes place. At the end of the interview, general information about the NSAIDs safety during pregnancy was given.
Ethics
The study was approved by the Ethics Committee of Jimma University. The health professionals and pregnant women were informed about the importance of the study and requested for their cooperation during the study period. The confidentiality of the data obtained was assured and the name and address of the patient was omitted from the questionnaire.
Statistical analysis
The edited data was entered in Epi Info Version 6.0. All data collected were then analyzed using the Statistical Package for the Social Sciences (SPSS), version 16.0 software. Descriptive statistics such as mean, frequencies and percentages were used to describe and summarize the data. Bivariate associations between women characteristics (age, and educational status) and the answers to the questions concerning NSAIDs were tested using the χ2 and the Fisher tests and a P value of <0.05 were considered as statistically significant.
Results
About 282 women were contacted and 224 were involved in the study. The mean age was 31.6±8 years (19-43). Average number of children per women was 2. The percentage of women who had graduated from secondary school was 50% and from higher institution was 39.3%. Most of pregnant ladies (95.10%) were married. Forty one percent of the pregnant women were unemployed.
Drug consumption pattern during pregnancy
A total of 90.6% of pregnant women responded that they took NSAIDs since the beginning of their pregnancy. Among these pregnant women, 67% of them got drugs from prescription by medical doctors and about 21.2% practiced self-medication. Out of these pregnant women 48.8% of them had a prescription of acetaminophen. About 37% of the pregnant women practiced self-medication at least one time during their pregnancy [Table 1].
Table 1.
Non-steroidal anti-inflammatory drugs consumption pattern before and during pregnancy
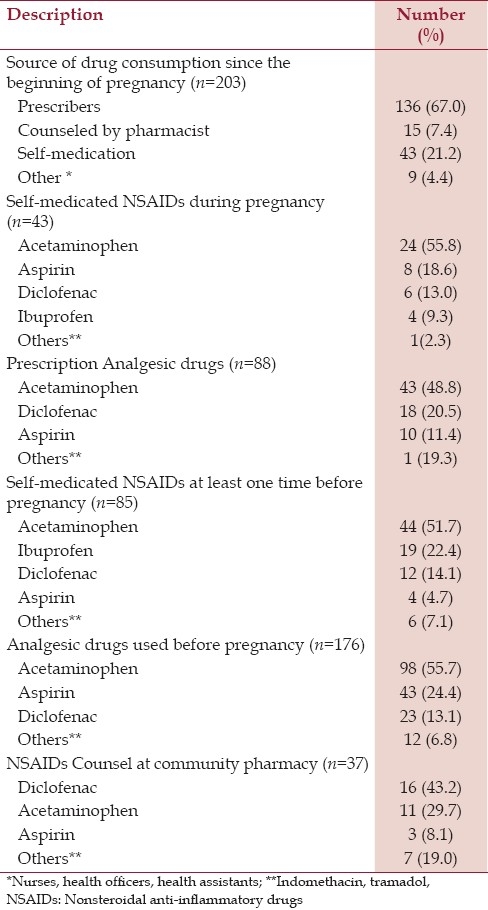
Analgesic drug use pattern before pregnancy
Concerning analgesic drug use before pregnancy, 176 (78.6%) of the pregnant women responded that they usually took NSAIDS. Ninety nine (55.7%), 43 (24.4%) and 23 (13.6%) of those pregnant women (n=176), said that they took acetaminophens, diclofenac and aspirin before pregnancy to relieve different types of pain. A total of 16.5% of the pregnant women had a counsel from community pharmacy about NSAIDs use. Thirty seven (43.2%) of the pregnant women said that they used mainly diclofenac from community pharmacy [Table 1].
Knowledge of pregnant women about NSAIDs
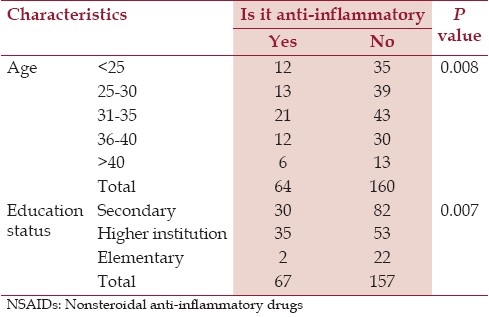
From the overall pregnant women, 76.8% of the women stated that acetaminophen is an NSAID. In other case, 89.7, 88.4 and 84.4% of the pregnant women thought that ibuprofen, diclofenac and aspirin are not NSAIDs respectively. Table 2 shows the association of pregnant women's knowledge, age and education status of the participants.
Table 2.
Association of age and education status with knowledge of pregnant women about NSAIDs
When asked about the untoward effects of NSAIDs, 37.1% of the pregnant women said that acetaminophen could induce allergic reaction, 14.7% of the women thought that gastric ulcer is the main adverse effect of aspirin and also 37.5, 9.8 and 8% responded that they fear stomach pain after taking aspirin, diclofenac and Ibuprofen respectively. Nineteen percent of the pregnant women thought aspirin has nausea, vomiting and diarrhea effect more than the any other NSAIDs. Thirty eight women (16.9%) have awareness on NSAIDs contraindication during pregnancy. In other case, 38.6, 32.4 and 16.2 of the pregnant women stated that NSAIDs are used for headache, fever and rheumatoid arthritis respectively. The remaining 7.6 and 5.2% women thought that the NSAIDs are used for menstrual pain and other types of discomforts associated with specific disease.
NSAIDs perception
Regarding analgesic effect of NSAIDs, 43.3% of the pregnant women thought that NSAIDs are effective for treating pain. Acetaminophen was considered as the most effective treatment for pain by 37.5% of the respondents. The safest medication during pregnancy, according to the participants, was acetaminophen (70.5%) [Table 3].
Table 3.
Safety and effectiveness perception of NSAIDs during pregnancy
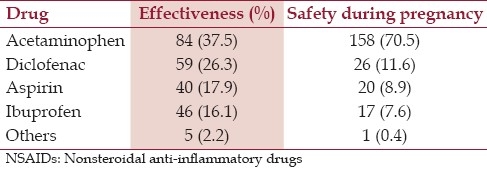
Two hundred nine (93.3%), 97 (43.3%), 25 (11.2%) and 10.2% of the pregnant women thought that it is possible to take acetaminophen, aspirin and diclofenac on late pregnancy respectively.
Risk factors awareness and sources of information on risk during pregnancy
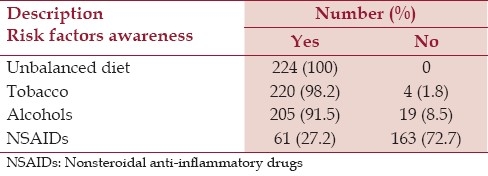
Only 27.2% of the pregnant women responded that they become aware of the risks of NSAIDs intake during pregnancy while 91.5, 98.2 and all of the pregnant women know the possible risk of alcohol, tobacco and unbalanced diet respectively [Table 4]. Regarding sources of information on risk during pregnancy, 55.8% of the women got information from physicians. Other information mediators were books, magazines and media (24.9%), midwives (10.2%), health officers (8.9%) and pharmacist (3.6%).
Table 4.
Risk factors awareness among pregnant women
Discussion
Although use of NSAID during pregnancy is prevalent, epidemiologic studies on the teratogenic risks are relatively sparse.[11] In this study, over ninety percent of the pregnant women have taken a drug since the beginning of their pregnancy. The majority of analgesic drugs were prescription drugs. This agrees with study done in France[10] and Canada[12] which exhibited that NSAIDs belong to the most common prescription drugs in pregnancy.
Most of the pregnant women also cited prescription from physicians as their main source of NSAIDs in which acetaminophen is the most prescribed drug. This is comparable with similar other study[13] which showed that 60% of NSAIDs were prescription drugs and 35% were over the counter drugs. This indicates that there is insufficient knowledge of NSAIDs risks by both prescribers and pregnant women in those study areas.
In the present study, over 35% of the pregnant women practiced self medication at least one time during pregnancy and about half of those women said that acetaminophen is their drug of choice for self-medication to relieve pain and other discomforts. This value is greater than the finding obtained from the studies conducted in France.[10,14] However, it is lower than the other study conducted in USA.[5]
Most of the pregnant women did not know that Ibuprofen, diclofenac and aspirin are anti-inflammatory drugs. Damase-Michel et al.,[10] showed that 79 and 66% of the pregnant women did not know whether aspirin and ibuprofen are anti-inflammatory drugs. The fact that a majority of pregnant women are unable to identify medications that contain a NSAID indicates that it is not enough to tell a woman that she must not take NSAID on late pregnancy. Additional information regarding different brand products should be provided to avoid confusion. It indicates that it is essential to give her the brand names of the drugs which are dangerous during pregnancy. In this study, it was found that age (P<0.05) and higher scholar level (P<0.05) have a role on knowledge on anti-inflammatory drugs. Older and educated women are more knowledgeable about NSAIDs.
In addition, majority of the pregnant women thought that NSAIDs are safe and it is possible to take them on late pregnancy. In study conducted in France,[10] pregnant women thought, that Ibuprofen and aspirin are OTC drugs and women could estimate that an OTC drug that can be given to children cannot induce adverse drug reaction in adults. Other study in USA[15] also showed comparable finding where Ibuprofen was the most frequently used OTC drug from 29 to 60% of exclusive OTC users were neither aware of nor believed they were at risk for ADRs.[16] However; majority of the pregnant women didn’t know whether it is possible to take NSAIDs on late pregnancy. As it was observed acetaminophen was thought by most pregnant women as the safest NSAID to be taken during pregnancy. The knowledge of pregnant women on possibility to take NSAIDs on late pregnancy is also associated with age and education status. Women at higher age have better knowledge on NSAIDs contra-indication during pregnancy (P<0.05). A similar association was observed for women at higher scholastic level (P<0.05)
In this study, compared to Alcohols, Tobacco and unbalanced diet, a small number of pregnant women are aware of the risks of NSAIDs intake during pregnancy. Around 56% of pregnant women said that they were informed about risks in pregnancy by their physician and only small number of pregnant women has been informed by pharmacists. Thus it would be necessary to inform women of child bearing age who are used to taking analgesic that some OTC analgesic drugs can be dangerous during pregnancy and that acetaminophen must be preferred as compared with other NSAIDs.
The findings of this study should be interpreted with some limitations. As it was cross-sectional study, it is susceptible to bias due to low response and misclassification due to recall bias, unable to measure incidence, or associations identified may be difficult to interpret. Social desirability bias is also the possible bias which may have been encountered in this study. Because it was conducted at a single city, the findings may not be generalizable to the whole country. Despite the above limitations, the study addressed an important issue regarding knowledge of pregnant women about non-steroidal anti-inflammatory drugs and their perception and drug consumption pattern during pregnancy in Ethiopia.
Conclusion
Drug intake during pregnancy is common by most pregnant women. Acetaminophen is the most common analgesic that is taken by most pregnant women. The overall knowledge of pregnant women about NSAIDs is poor. Adequate information must be provided to pregnant women about pregnancy risk category of NSAIDs.
Acknowledgment and Funding
This study was financed by Students Research Project (SRP) of Jimma University. The authors are grateful to patients who participated in the study.
Footnotes
Source of Support: This study was financed by Students Research Project (SRP) of Jimma University.
Conflict of Interest: None declared.
References
- 1.Laurence L, Brunton J, John S, Keit L, editors. Non-steroidal anti-inflammatory drugs. 11th ed. McGraw Hill; 2006. Goodman and Gill's. The pharmacologic basis of therapeutics; pp. 673–706. [Google Scholar]
- 2.Rang HP, Dale MM, Rtter JM, Moore PK. Pharmacology. In: Laurence H, editor. Anti- Inflammatory and immunosuppressant drugs. 5th ed. Vol. 47. Churchill Livingston; 2003. pp. 244–60. [Google Scholar]
- 3.Vane JR, Botting RM. Mechanism of action of aspirin-like drugs. Semin Arthritis Rheum. 1997;26:2–10. doi: 10.1016/s0049-0172(97)80046-7. [DOI] [PubMed] [Google Scholar]
- 4.Brooks P. Use and benefits of non steroidal anti-inflammatory drugs. Am J Med. 1998;104:9–13S. doi: 10.1016/s0002-9343(97)00204-0. [DOI] [PubMed] [Google Scholar]
- 5.Glover DD, Amonkar M, Rybeck BF, Tracy TS. Prescription, over-thecounter, and herbal medicine use in a rural, obstetric population. Am J Obstet Gynecol. 2003;188:1039–45. doi: 10.1067/mob.2003.223. [DOI] [PubMed] [Google Scholar]
- 6.Olesen C, Steffensen FH, Nielsen GL, de Jong-van den Berg L, Olsen J, Sørensen HT. Drug use in first pregnancy and lactation: A population-based survey among Danish women.The EUROMAP group. Eur J Pharmacol. 1999;55:139–44. doi: 10.1007/s002280050608. [DOI] [PubMed] [Google Scholar]
- 7.Ericson A, Kallen BA. Nonsteroidal anti-inflammatory drugs in early pregnancy. Reprod Toxicol. 2001;15:371–5. doi: 10.1016/s0890-6238(01)00137-x. [DOI] [PubMed] [Google Scholar]
- 8.Chacón Aguilar R, Menéndez Hernando C, Chimenti Camacho P, Franco Sánchez ML, Sánchez Luna M. Persistent pulmonary hypertension of the new born following ingestion of NSAIDs during pregnancy. An Pediatr (Barc) 2008;68:357–60. doi: 10.1157/13117706. [DOI] [PubMed] [Google Scholar]
- 9.Akil M, Amos RS, Stewart P. Infertility may sometimes be associated with NSAID consumption. Br J Rheumatol. 1996;35:76–8. doi: 10.1093/rheumatology/35.1.76. [DOI] [PubMed] [Google Scholar]
- 10.Damase-Michel C, Christaud J, Berrebi A, Lacroix I, Montastruc JL. What do pregnant women know about non-steroidal anti-inflammatory drugs? Pharmacoepidemiol Drug Saf. 2009;18:1034–8. doi: 10.1002/pds.1817. [DOI] [PubMed] [Google Scholar]
- 11.van Gelder M, Roeleveld N, Nordeng H. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and the risk of selected birth defects: A prospective Cohort study. PLoS One. 2011;6:e22174. doi: 10.1371/journal.pone.0022174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wen SW, Yang T, Krewski D, Yang Q, Nimrod C, Garner P, et al. Patterns of pregnancy exposure to prescription FDA C, D and X drugs in a Canadian population. J Perinatol. 2008;28:324–9. doi: 10.1038/jp.2008.6. [DOI] [PubMed] [Google Scholar]
- 13.Vroom F, van den Berg PB, de Jong-van den Berg LT. Prescribing of NSAIDS and ASA during pregnancy: Do we need to be more careful? Br J Clin Pharmacol. 2008;65:275–6. doi: 10.1111/j.1365-2125.2007.02994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikou S, Buire AC, Trenque T. Automédication chez la femmeenceinte. Thérapie. 2008;63:415–8. doi: 10.2515/therapie/2008064. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox CM, Cryer B, Triadafilopoulos G. Patterns of use and public perception of over-the counter pain relievers: Focus on non-steroidal anti-inflammatory drugs. J Rheumatol. 2005;32:2218–24. [PubMed] [Google Scholar]
- 16.Werler MM, Mitchell AA, Shapiro S. The relation of aspirin use during the first trimester of pregnancy to congenital cardiac defects. N Engl J Med. 1989;321:1639–42. doi: 10.1056/NEJM198912143212404. [DOI] [PubMed] [Google Scholar]


