Abstract
Background:
Liver is the main organ for metabolism of drugs and hepatotoxicity is a potential adverse effect for most drugs.
Aims:
This study was to study the frequency of drug-induced hepatotoxicity and to find the common drugs causing hepatotoxicity.
Materials and Methods:
The study was conducted at a tertiary care hospital in rural India. It is a study based on case series analysis. All patients with an abnormal liver function report, between July 2006 and July 2007, were included in the study
Results:
The study included 411 patients. Among them 141 patients were females and 270 males. The common cause for abnormal liver function was alcoholic liver disease (30.4%) followed by drug-induced hepatotoxicity (15.8%) and malaria (15.3%). Drug-induced hepatotoxicity was seen in 65 patients. It was common in males (55%) compared to females (44%). The mean age of the patients with drug-induced hepatotoxicity was 43±15.9. Antitubercular drugs were the commonly encountered drugs (44%) causing hepatotoxicity followed by lipid lowering agents (41%). The others drugs included antiretroviral drugs (6%),steroids (5%) and chlorpromazine (2%).
Conclusion:
A thorough history of drug intake must be taken in all patients presenting with abnormal hepatic function.
Keywords: Abnormal liver function, Alcoholic liver disease, Antiretroviral drugs, Antitubercular drugs, Drug, Drugs-induced liver damage, Hepatotoxicity, Hypolipidemic drugs, Lipid lowering agents, Rural India
Introduction
Liver, the largest organ in the body, is being evolved to maintain the body's internal milieu and also protects itself from the challenges it faces during its functioning.[1] It plays an important role in the metabolism, and storage of drugs; therefore it is susceptible to a high degree of toxicity. Hepatotoxicity implies liver injury caused due to chemicals. Certain drugs when administered at therapeutic dose and some in overdose may injure the liver. Early recognition of drug-induced liver damage is essential to minimize injury.
The incidence of drug-induced liver disease appears to be increasing, reflecting the increasing number of new agents that have been introduced into clinical use over the past several decades.[2] Thus, monitoring hepatic enzymes is considered appropriate, especially with those that lead to overt injury. Drugs have been reported to be responsible for approximately 10% of cases with hepatitis and less than 5% of hospital cases with jaundice.[3] Withdrawal of widely prescribed drugs from the market due to hepatotoxicity has attracted considerable attention among the medical fraternity.[4] To the best of our knowledge there are few studies regarding drug-induced hepatoxicity in rural south India, so this study was designed to analyze the frequency of drug-induced hepatotoxicity among patients with abnormal liver function, and to find the common drugs causing hepatotoxicity.
Materials and Methods
This study was conducted by the departments of pharmacology and biochemistry at a tertiary care hospital in rural south India. Ethical clearance was obtained from the ethical committee. This was a retrospective study based on case series analysis which was conducted from July 2006 to July 2007. All patients with abnormal liver function test were included in the study and constituted a sample size of 411. To identify drug-induced hepatotoxicity, the abnormal liver function test was done using the following criteria, either singly or in combination: (1) total bilirubin ≥1.5 mg/dl; direct bilirubin ≥0.5 mg/dl; Aspartate aminotransferase (AST) ≥80 IU/ml; Alanine amino transferase (ALT) ≥70 IU/ml.[5] Clinical history of drug intake was taken from case records. The patients were analyzed for age, gender, diagnosis, and history of drug intake. The data were collected in a proforma which was specially designed for the study. The data were analyzed using descriptive statistics.
Results
Over a period of 1 year, 3600 liver function tests (LFT) were done. Of these 411 patients had abnormal liver function. Among these patients 270 (66%) were males and 141 (34%) females.
The mean age of all the patients was 44±16 years, the mean age for females being 40.2±16 years and males 46±15.6 years.
As shown in Figure 1, we observed that the common cause for abnormal liver function was alcoholic liver disease, i.e., 125 patients (30.4%) followed by drug-induced hepatotoxicity, which was seen in 65 patients (15.8%) and then malaria which was seen in 63 patients (15.3%).
Figure 1.
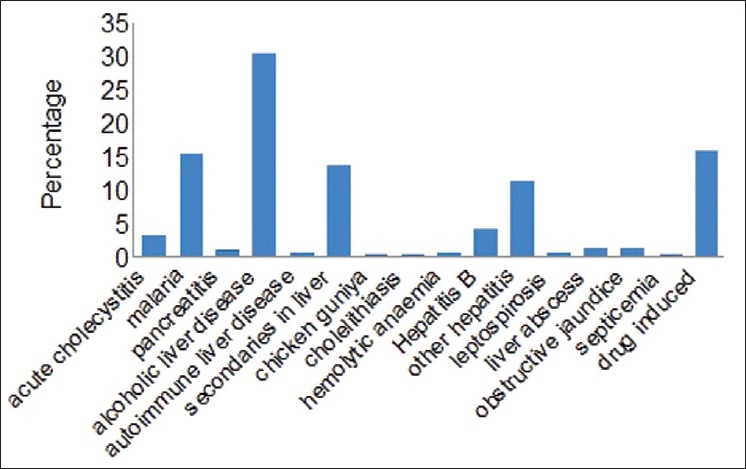
Causes for abnormal liver function test
Drug-induced hepatotoxicity was more common in males, i.e., 36 patients (56%), than in females, i.e., 29 patients (44%). The mean age of all these patients was 43±15.9 years.
As depicted in Figure 2, we found that antitubercular drugs (29 patients (44%)) were among the commonly prescribed drugs causing hepatotoxicity followed by lipid lowering agents, i.e., 27 patients (41%). In cases of antitubercular and antiretroviral drugs the single offending agent could not be identified as more than one drug was administered. In patients with tuberculosis Revised national tuberculosis control program (RNTCP) regimen was used. Atorvastatin was the drug found to induce toxicity in the case of lipid lowering agents. Among steroids, it was prednisolone.
Figure 2.
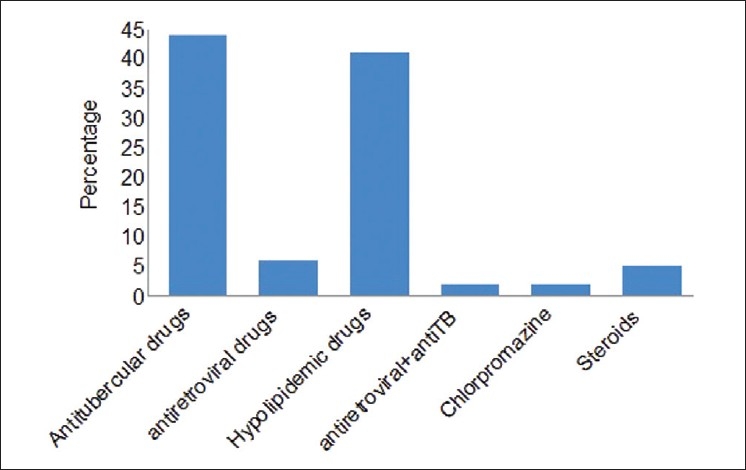
Drugs causing hepatotoxicity
Abnormal liver function was seen after 15 days, 1, 2, 6 months after starting treatment on antitubercular drugs, antiretroviral agents, hypolipidemic drugs, and steroids respectively. In all these cases both ALT and AST was raised and total bilirubin was elevated in most of the cases. One patient had abnormal liver function on admission due to consumption of chlorpromazine; this was a case of suicidal poisoning. Clinical history suggested that the liver function returned to normal after the offending drug was discontinued.
Discussion
The occurrence of 65 cases of drug-induced hepatotoxicity over a period of 1 year in one hospital emphasizes the seriousness of the problem. Drug reactions produce an array of hepatic lesions that mimic all known hepatobiliary diseases; this poses a diagnostic challenge for physicians and pathologists.[6]
We found that abnormal liver function as well as drug-induced hepatotoxicity was common in males than females, which was in accordance with the study conducted by Sgro et al., in France.[7] This could be because there were more male patients than females in our study. However there are other studies which contradict our finding.[8]
The mean age of patients with abnormal liver function and drug-induced hepatotoxicity was 44±16 years and 43±15.9 years respectively. This could be because with age progression there is increased risk of hepatic injury because of decreased clearance, decreased hepatic blood flow and low hepatic volume.[2]
In a study by Kshirsagar et al., it was found that the most common cause for abnormal liver function was ischemic heart disease,[5] but our study has shown alcoholic liver disease as the most common cause. Alcoholic liver disease is common in India due to increased incidence of alcohol addiction among the rural population, the reason being easy accessibility, availability, and lack of education on the hazards of uncontrolled alcohol consumption. Poverty and unemployment leads to consumption of low quality alcohol which could be adulterated, thus predisposing to increased risk of alcoholic liver disease. The district where the hospital is situated has been an endemic area for malaria. This could be the reason for malaria being the third most common cause among the study groups.
Drug-induced hepatotoxicity is underreported and underestimated in the rural population and is one of the important causes of acute liver failure in adults. Challenges exist in the clinical diagnosis of drug-induced liver injury and in obtaining information on hepatotoxicity in humans.[9]
In our survey we found antitubercular drugs as the common cause of drug-induced hepatotoxicity. But we were unable to identify an individual antitubercular drug as being responsible for hepatotoxicity as the patients received combination therapy. This is in concordance with the study conducted by Kshirsagar et al., where they found antitubercular drugs as the common cause for drug-induced hepatotoxicity.[5] The reason for this finding in our study could be the fact that tuberculosis is one of the common infections encountered in India, the reason being poverty and unhygienic environment, even more common in rural India. With emergence of HIV infection the incidence of tuberculosis as an opportunistic infection has doubled. A study conducted by Parasarthy et al., on south Indian patients, showed a high incidence of hepatotoxicity to antitubercular drugs.[10]
The use of highly active antiretroviral therapy has dramatically reduced HIV-associated morbidity and mortality. As a result, patients are often being treated longer and with more complex medical regimens than ever, increasing the risk for drug interactions and toxicities. In particular, hepatotoxicity caused by antiretroviral agents has become an increasingly appreciated potential complication of drug treatment. All classes of antiretrovirals have been reported to induce liver enzyme abnormalities. However, certain antiretrovirals appear much more likely to be associated with drug-induced hepatotoxicity.[11] We found one case of HIV with tuberculosis with an abnormal liver function test where we could not identify the drug causing hepatotoxicity as the patient was on an array of drugs which included antitubercular and antiretroviral drugs.
Cardiovascular diseases are one of the common causes of death all over the world. Hyperlipidemia is one of the major risk factors and its treatment has come to the forefront of primary and subspecialty care as a preventative strategy against cardiovascular morbidity and mortality. This has led to the increased use of hypolipidemic agents. Hence we found these agents also as one reason for drug-induced hepatotoxicity.
Drug-induced liver injury can mimic any form of acute or chronic liver diseases.[12] This warrants a need to investigate all cases of hepatotoxicity to rule out the use of drug as an impending cause for it. Assessment of risk to benefit ratios regarding a novel agent with hepatotoxicity requires considerable judgment and education on the part of prescribers and patients. Although a drug poses great danger to only a few patients, but its withdrawal leads to the loss of drug availability to many. For practicing physicians, drug-related hepatotoxicity is a liability risk, for the pharmaceutical industry it leads to financial losses, and from a regulatory perspective it is the common reason for regulatory actions on the part of the Food and Drug administration (FDA).[13] Hence a thorough evaluation of the drug should be done before it is made available to the patient.
Conclusion
Antitubercular drugs were the common drugs causing hepatotoxicity. A total of 15.8% of the total patients having abnormal liver function had drug-induced hepatotoxicity. To avoid this complication a baseline liver function test should be conducted before starting treatment on drugs susceptible to cause hepatotoxicity. Physicians must be vigilant in identifying drug-related liver injury because early detection can decrease the severity of hepatotoxicity if the drug is discontinued. The manifestations of drug-induced hepatotoxicity are highly variable, ranging from elevation of liver enzymes to fulminant hepatic failure. Knowledge of the commonly implicated agents and a high index of suspicion are essential in diagnosis.
Drug-induced hepatotoxicity will remain a problem that carries both clinical and regulatory significance as long as new drugs continue to enter the market. Future results from ongoing multicentric collaborative efforts may help contribute to our current understanding of hepatotoxicity associated with drugs.
Acknowledgments
I would like to thank all the patients who participated in the study. I would also like to thank the principal, Sri Devaraj Urs Medical College, Kolar, India, for giving us the permission to conduct this study. Last but not the least I would like to thank the Head of Pharmacology Dr. T N Kumar for his support and encouragement.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Kshirsagar A, Vetal Y, Purnima A, Bhosle P, Deepa I. Drug Induced Hepatotoxicity: A comprehensive review. Internet J Pharmacol. 2009;1:1–19. [Google Scholar]
- 2.Lewis JH. Drug-induced liver disease. Med Clin North Am. 2000;5:1275–311. doi: 10.1016/s0025-7125(05)70287-x. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman HJ. Drug induced liver disease: An overview. Semin Liver Dis. 1981;2:93–103. doi: 10.1055/s-2008-1040722. [DOI] [PubMed] [Google Scholar]
- 4.Maddrey WC. Drug Induced Hepatotoxicity. J Clin Gastroenterol. 2005;4:83–9. doi: 10.1097/01.mcg.0000155548.91524.6e. [DOI] [PubMed] [Google Scholar]
- 5.Ksirsagar NA, Karande SC, Potkar CN. A prospective study of drug induced hepatotoxicity in a large hospital. Indian J Gastroenterol. 1992;1:13–5. [PubMed] [Google Scholar]
- 6.Farell GC. Drug induced injury. J Gastroenterol Hepatol. 1997;9:242–50. doi: 10.1111/j.1440-1746.1997.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 7.Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug induced hepatic injuries: A French Population based study. Hepatology. 2002;2:451–5. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 8.Ostapowicz G, Fontana RJ, Schiodt FV, Larson V, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;12:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki A, Andrade RJ, Bjornsson E, Lucena MI, Lee WM, Yuen NA, et al. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse effects in vigibase: Unified list based on international collaborative work. Drug Saf. 2010;6:503–22. doi: 10.2165/11535340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Parasarthy R, Sarma GR, Janardhanam B, Ramachandran P, Santha T, Sivasubramanian S, et al. Hepatic toxicity in South Indian patients during treatment of tuberculosis with short term regimens containing isoniazid, rifampin and pyrazinamide. Tubercle. 1986;2:99–108. doi: 10.1016/0041-3879(86)90003-6. [DOI] [PubMed] [Google Scholar]
- 11.Kress KD. Antiretroviral-associated hepatotoxicity. Curr Inf Dis Rep. 2005;2:103–7. doi: 10.1007/s11908-005-0068-z. [DOI] [PubMed] [Google Scholar]
- 12.Kaplowitz N, Tak Yee AW, Francis RS, Andrew S. Drug-Induced hepatotoxicity. Ann Intern Med. 1986;6:826–39. doi: 10.7326/0003-4819-104-6-826. [DOI] [PubMed] [Google Scholar]
- 13.Victor JN, John RS. Drug related hepatotoxicity. N Engl J Med. 2006;7:731–9. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]


