Abstract
Carotid body chemoreceptors transduce a decrease in arterial oxygen tension into increased sinus nerve action potential (AP) activity which undergoes a maturational increase in the post-natal period. MaxiK-channels channels are proposed to play a major role in organ function based on their maturation-dependent expression in glomus cells and inhibition by acute hypoxia. To better resolve the role of this channel, single-unit AP activity was recorded of rat chemoreceptor neurons, in vitro, during a progressive decrease in oxygen from normoxia (~150 torr) to moderate hypoxia (~60 torr). Blockade of MaxiK channels with charybdotoxin (100 nM) in both older (P16-P18) and younger (P2-P3) animals resulted in no significant change in AP activity, but increased nerve conduction speed in the older animals. In dissociated glomus cells, charybdotoxin slightly enhanced the intracellular calcium response to acute hypoxia at both ages. We conclude that MaxiK channels play little or no role in mediating the response to acute, moderate hypoxia, either in the newborn or older animal.
Keywords: carotid body, chemoreceptors, hypoxia, calcium, hyperoxia
1. INTRODUCTION
Carotid body chemoreceptors transduce a decrease in arterial oxygen tension into increased afferent nerve activity on the carotid sinus nerve, a branch of the glossopharyngeal nerve, and this activity evokes a number of protective reflexes, including increased drive to breathe and arousal from sleep. These receptors are relatively immature at birth as evidenced by a reduced rate of action potential (AP) activity for a given hypoxic stimulus, and maturation to an adult response occurs over the first two weeks of life in rats. The mechanism of hypoxia transduction within the carotid body is not resolved, but it is generally accepted that the transduction site is the glomus cell, a secretory cell presynaptic to the afferent nerve fibers. Hypoxia causes depolarization of glomus cells (Eyzaguirre et al., 1989), leading to calcium influx (Buckler and Vaughan-Jones, 1994) and release of neurotransmitters/neuromodulators which alter the excitability of the afferent nerve endings (Gonzalez et al., 1994).
Several channel mechanisms have been proposed to explain the coupling between hypoxia and glomus cell depolarization. Three O2-sensitive potassium-selective channels which close in response to hypoxia have been identified in glomus cells. The first, identified in 1988, is a voltage sensitive, “A” type transient K+ current (Lopez-Barneo et al., 1988; Lopez-Lopez et al., 1989; Lopez-Lopez et al., 1993). It is proposed to play a role in establishing resting membrane potential, but appears to be only present in the rabbit and therefore is not an hypoxia sensor across species. The second, described in 1990, is a specific isoform of the “BK-like” K+ channel (MaxiK) which is activated by increases in intracellular calcium and cell depolarization and is inhibited by hypoxia (Peers, 1990; Peers and O’Donnell, 1990; Ross et al., 2011). This current is poorly expressed in the immediate newborn period and expression increases over the period of organ maturation, suggesting causality between the two (Hatton et al., 1997). The third, described in 1997, is a leak (i.e., non-voltage dependent) K+ channel whose open probability is reduced during hypoxia (Buckler, 1997; Buckler et al., 2000; Williams and Buckler, 2004).
Despite the unequivocal demonstration that MaxiK channels are present in glomus cells, pharmacologic blockade of MaxiK activity causes little change in organ function in normoxia or at low levels of oxygen tension. This was initially observed in early experiments which utilized tetraethylammonium (TEA) as a pharmacologic antagonist of nicotinic receptors, but TEA was subsequently identified as a blocker of voltage-dependent K+ channels. Administration of TEA, in vivo, had no discernable effect on chemoreceptor function (Moe et al., 1948).
In more recent targeted work, administration of charybdotoxin, a specific blocker of MaxiK channels, and TEA failed to increase baseline nerve activity (Cheng and Donnelly, 1995) or catecholamine secretion (Doyle and Donnelly, 1994; Gomez-Nino et al., 2009), a proxy for transmitter release from the glomus cell. Similarly, MaxiK blockade failed to enhance the release rate of catecholamine during stimulation with hypoxia (2-10% O2) (Gomez-Nino et al., 2009). These later authors interpreted the results as consistent with little MaxiK activity during normoxia (hence little effect of pharmacologic blockade) and fully inhibited MaxiK activity during hypoxia (hence little effect of pharmacologic blockade). This leaves a potential role for MaxiK channels at levels of oxygen above that producing full inhibition of MaxiK activity. Accordingly, one purpose of the present study is to examine the effects of MaxiK channel inhibition on chemoreceptor afferent activity under conditions where the level of oxygen was slowly decreased from normoxia in order to determine the potential importance of MaxiK at higher levels of oxygen tension.
Previous work demonstrated that hypoxia caused significantly less inhibition of MaxiK current of 4d old rat glomus cells compared to cells from 10 day old and adult rats (Hatton et al., 1997). This suggests that MaxiK currents, being less inhibited by hypoxia in the newborn, could play a role in postnatal development of glomus cell O2 responsiveness by damping the depolarization response of the newborn to hypoxia. To examine the potential role of MaxiK channels during development, identical experiments were undertaken on chemoreceptors harvested from newborn animals at which time MaxiK O2 sensitivity is low (Hatton et al., 1997).
2. METHODS
Experiments were undertaken with the approval of the Yale Animal Care and Use Committee and Animal Care and Use Committee of the University of Arkansas.
2.1 Afferent nerve recording
Carotid bodies intact with the petrosal ganglia were harvested from rats of two ages: P2-3 (immature) and P18-21 (mature). The response of the afferent nerve and the calcium response of glomus cells have an adult form by P14-18 (Bamford et al., 1999; Kholwadwala and Donnelly, 1992; Wasicko et al., 1999).
Prior to tissue harvest, rats were deeply anesthetized by placement in a closed chamber whose atmosphere was gradually replaced with 100% CO2. While anesthetized, the animals were removed and decapitated. Following a midline neck incision, the trachea was retracted rostrally and the carotid arteries dissected free past the carotid bifurcation. After cutting the internal and external carotid arteries and removal of the superior cervical ganglion, the vagus nerve was dissected free, centrally, past its junction with the glossopharyngeal nerve and the combined nerve was cut near its entrance to the brainstem. The ganglion was reflected over the carotid bifurcation and the tissue removed and placed in oxygenated (95% O2/ 5% CO2) saline solution (in mM: 120 NaCl, 5 KCl, 2 CaCl2, 1 Na2HPO4, 1 MgSO4, 24 NaHCO3 and 5 glucose). In the bath, the vagus nerve and carotid arteries were dissected free from the glossopharyngeal nerve and carotid body. To aid in tissue cleaning, the complex (carotid body/sinus nerve/glossopharyngeal nerve and ganglia) was transferred to a chamber filled with saline containing collagenase (0.1%, Roche Diagnostics type P) and protease (0.02% Sigma type XIV) at 37°C for 30min with gentle agitation. The complex was further cleaned and transferred to a perfusion chamber (Warner Instruments RC-22C, chamber volume approximately 140μL) mounted on the stage of an inverted microscope equipped with Hoffman contrast optics (Zeiss Axiovert 10). The complex was superfused with oxygenated (21% O2/5% CO2/balance N2) saline controlled at 37°C by an in-line heater (TC344 temperature controller; Warner Instruments). Perfusion rate was approximately 3ml/min and was limited by the flow resistance of the perfusion tubing (stainless steel, 0.030” I.D. × 5ft. Upchurch Scientific). Greater details regarding dissection and cleaning were previously given (Donnelly and Rigual, 2000). Chamber oxygen tension was measured by phosphorescence quenching (Oxy-micro with PST-1 probe, World Precision Instruments).
Single-unit activity was recorded using a suction electrode advanced into the petrosal ganglion. Electrode tip size was approximately 30μm in diameter which allowed individual ganglion cells to enter the tip. The pipette potential was amplified with an extracellular amplifier (BAK Instruments, MDA5), filtered (0-5kHz), displayed on an oscilloscope, digitized (10kHz sample rate) and stored on computer (Axoscope, Axon Instruments). Unit chemoreceptor activity was discriminated and timed, post-hoc, using an event detection program which identified the timing and magnitude of action potential events (CLAMPFIT9.0, Axon Instruments). The number of individual spikes per second were measured every 5 sec and associated with chamber oxygen tension.
As an aid for identification of nerve fibers projecting to the carotid body, a stimulus electrode (pipette filled with 1M NaCl; 0.2 MΩ impedance) was advanced into the carotid body. A constant-current stimulus (200μA)(BSI-2, BAK Instruments) was delivered at 1Hz × 0.1msec duration (BAK Instruments, BPG-1) and the success of the stimulus in initiating an orthodromic action potential was determined in the post-stimulus period.
2.2 Experimental protocol for chemoreceptor nerve recordings
After an initial stabilization period, the response to acute, progressive hypoxia was elicited by switching the perfusate to a small (60ml) reservoir, initially equilibrated with 21% O2/5% CO2/bal N2 and residing in a heated (37°C) water bath. The reservoir PO2 was decreased by switching the gas source for bubbling to 0% O2/5% CO2/bal N2 resulting in a slow decrease in PO2. The hypoxic stimulus was terminated upon reaching 60 torr or when an obvious decrease in AP rate was observed, indicating a maximal response had already been achieved. For charybdotoxin administration, the perfusate was switched to a reservoir containing the drug and initially equilibrated with 21% O2/5% CO2/bal N2. A 10 min drug equilibration period was allowed and the response to hypoxia elicited by bubbling the same reservoir with 5% CO2/bal N2. After the hypoxia trial in the presence of drug, the perfusate was switched to a reservoir which was free of drug for 10 min and again bubbled with 5% CO2/bal N2 to produce a graded hypoxic stimulus. This protocol was repeated using a higher dosage of charybdotoxin (500nM, N=2) and using TEA (5mM, N=2) in place of the lower dosage charybdotoxin.
2.3 Intracellular calcium measurements in dissociated CB glomus cells
Carotid bodies were harvested from rats of two ages: P0-1 (immature) and P14-16 (mature). Rat pups were anesthetized with isoflurane, decapitated and the carotid bifurcations removed and placed in ice-cold saline. The carotid bodies were dissected free, cut in half and placed in saline containing 1mg/ml trypsin (Sigma) and 1mg/ml collagenase (Worthington Biochemical, type 1). Cells were dispersed by gentle trituration and pelleted for 4min at 2000g. Cells were resuspended in Ham’s F12 with 10% fetal calf serum and plated on coverslips coated with poly-D-lysine. A more detailed description of the dissociation protocol was given previously (Wasicko et al., 2006). Cells were loaded with the calcium-sensitive dye, FURA-2 by incubation with 4mM FURA-2 acetoxymethyl ester for 30 min at 37 °C in saline equilibrated with 21% O2/5% CO2/bal N2. FURA-2 fluorescent emission was measured every 8 seconds at ~510 nm in response to alternating excitation at 340 and 380 nm. Images were acquired and stored using a Nikon TE300 inverted microscope and cooled CCD camera (Photometrics) under computer control (Metafluor, Universal Imaging, West Chester, PA, USA).
2.4 Experimental protocol for calcium measurements of isolated glomus cells
After loading with fura-2, the coverslip was placed in a closed microscope imaging chamber (0·1 ml total volume) and perfused with a bicarbonate-buffered balanced salt solution (BSS) containing (mM): 118 NaCl, 23 NaHCO3, 3 KCl, 2 KH2PO4, 1·2 CaCl2, 1 MgCl2 and 10 glucose. The saline was initially bubbled in a heated reservoir with 21% O2/5% CO2/bal N2. The response to increased K+ was elicited by switching the perfusate for ~ 1 min to a reservoir containing 20 mM K+ (equimolar substitution of Na+). Cells were allowed to recover in normal BSS for 5 min and then challenged with hypoxia by switching the perfusate to BSS equilibrated with 5% CO2/bal N2 for 2 min (0% O2). Cells were then exposed to charybdotoxin (100 nM) in perfusate equilibrated with 21% O2/5% CO2/bal N2 for 5 min and then challenged with hypoxic BSS containing charybdotoxin (100 nM) equilibrated with 5% CO2/bal N2. The drug was washed out for 5 min and the cells re-challenged with saline equilibrated with 5% CO2/bal N2. At each age, control experiments were performed consisting of exposure of cells to increased K+ and three hypoxia challenges as described above, but without exposure to charybdotoxin.
2.5 Data analysis
Unit spiking rates before, during and following drug applications were recorded from the best-fit exponential line of the action potential frequency vs PO2 graph. Values were measured at normoxia (~ 150 torr) and every 10 torr down to 80 torr. Since the threshold for hypoxia-induced increase in chemoreceptor activity was variable among units, likely due to varying depth of the sensor site, the control AP frequency at each PO2 was subtracted from that measured during drug perfusion. AP rates at each level of O2 were considered as repeated measures and drug treatment (sham/charybdotoxin) was a grouping variable. If ANOVA was significant for drug effects or drug * O2 effects then a post-hoc comparison at each level of O2 was made using Student’s t-test with Holm-Bonferroni correction to the critical p-value.
For calcium measurements, areas of interest were selected over cells which demonstrated a rapid rise in intracellular calcium during superfusion with increased K+. These cells were considered as presumptive glomus cells. Results from cells in a cluster were averaged and considered as a single observation, and both single cells and clusters were included in the analysis. The [Ca2+]i response to increased K+ or to hypoxia was taken as the maximum (peak) [Ca2+]i value observed during the challenge period. Responses to hypoxia at each age were compared using repeated measures analysis of variance with Bonferroni correction for post-hoc multiple comparisons between the three hypoxia challenges. Cells that responded to charybdotoxin alone were considered outliers and not included in the analysis. This group was less than 5% of the recorded cells. For all statistical testing, significance was established at p<0.05. All results are expressed as mean±SEM.
3. RESULTS
3.1 Afferent nerve responses to MaxiK channel blockade
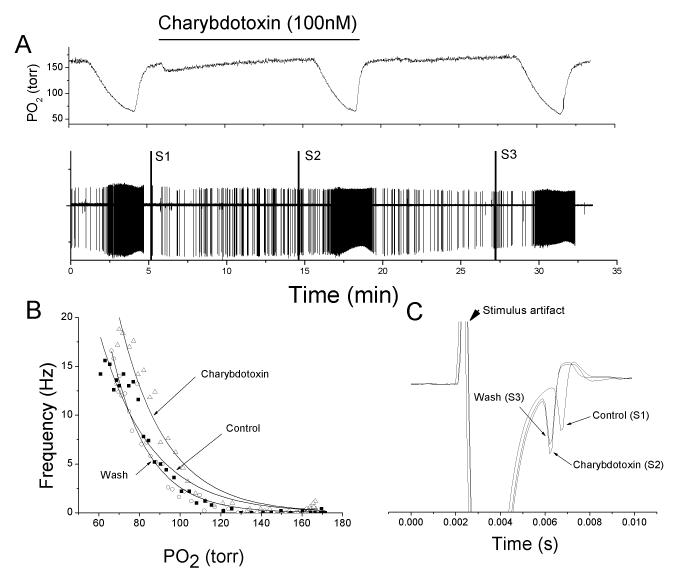
The unit response to progressive hypoxia was elicited by switching the source of gas source bubbling through the perfusion reservoir from 21% O2/5% CO2/bal N2 to 0% O2/5% CO2/bal-N2 (Fig 1A). This resulted in a slow decrease in chamber oxygen tension and the rate of descent could be regulated by the bubbling rate. Unit activity was quantified in 5 sec bins, graphed against chamber O2 tension and best-fit to a single exponential function (Fig 1B). At the end of the first progressive-hypoxia challenge, the perfusate was switched to one containing the sham-drug treatment for 10min, and the response to progressive was repeated (Fig 1A). Washout of the sham drug treatment occurred over the subsequent 10min and the response to progressive hypoxia was repeated.
Fig 1.
Charybdotoxin (MaxiK channel blocker) minimally alters the chemoreceptor response to hypoxia in mature (P18) chemoreceptor. A: polygraphic recording of experimental run. Top trace: chamber O2 tension. Bar indicates period of sham-drug perfusion. Bottom trace: raw recording of action potential (AP) activity recorded from a petrosal chemoreceptor neuron with time on the abscissa and voltage on the ordinate. S1, S2 and S3 are stimulus artifacts during electrical stimulation to the carotid body for orthodromic AP initiation. B: frequency/PO2 response relationship for chemoreceptor before (open circle), during (open triangle) and following (filled square) sham drug perfusion. Exponential line fits using least-square methods. C: expanded overlaid traces during orthodromic stimulation from S1 to S3 showing no change in conduction time.
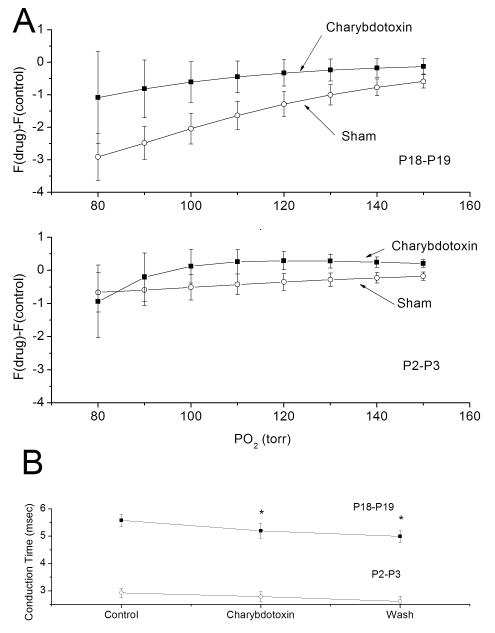
MaxiK channel blockade had little effect on the response of the mature carotid body (Fig 1). In general, the best-fit line for the exponential increase in AP activity rate for a progressive hypoxic challenge was slightly right shifted by charybdotoxin which reversed following washout (Fig 1B). AP rates were generally higher in the presence of charybdotoxin (Fig 2A), but the difference was slight. The change in AP rate (F(charybdotoxin) – F(control)) was not different across O2 levels (p=0.5, N=15) and values were not different than zero (p=0.41). Using drug treatment as a grouping variable, no O2 effect (p=0.21), drug effect (p=0.21) or O2 × Drug (p=0.99) effect was detected. Nerve conduction time was significantly shortened (p<0.05, N=7) by charybdotoxin treatment (Fig 2B), but this did not reverse in the washout period.
Fig 2.
MaxiK channel blockade does not change the AP activity level at moderate levels of chemoreceptor stimulation in either the newborn or mature rat but increases conduction velocity in the mature. A: Average change in afferent AP rates between hypoxia response in the presence of charybdotoxin or sham-drug and the control response from chemoreceptors of mature (upper traces) and newborn (lower traces) rats (F(drug)-F(control)). Thus, the ordinate data are the differences between successive trials. On average, AP activity was slightly higher in the presence of charybdotoxin, but the magnitude change was small and failed to reach statistical significance in either the mature or newborn. B: Conduction time was measured before, during and following charybdotoxin exposure and analyzed us ANOVA with repeated measures. Conduction time was significantly different across the three measures for the mature and charybdotoxin conduction time was shorter in the presence of charybdotoxin compared to control (paired t-test with Bonferroni correction). Nerve conduction time was not significantly changed for the newborn.
As with the mature, MaxiK channel blockade had little effect on the response in the newborn. On average, AP rate was slightly higher in charybdotoxin treatment compared to sham drug treatment (Fig 2A), but the change was small and did not reach statistical significance. The change in AP rate did not change significantly across O2 levels (p=0.64, N=9) and regressive slope was not significantly different than zero (p=0.32). Using drug treatment (sham/charybdotoxin) as the grouping variable for ANOVA, O2 level had no statistically significant effect on F(charybdotoxin)-F(control) (p=0.88) and O2 x drug was not significant (p=0.97) (Fig 4A). In addition, nerve conduction time was not altered across the three trials (Fig 2B).
Fig 4.
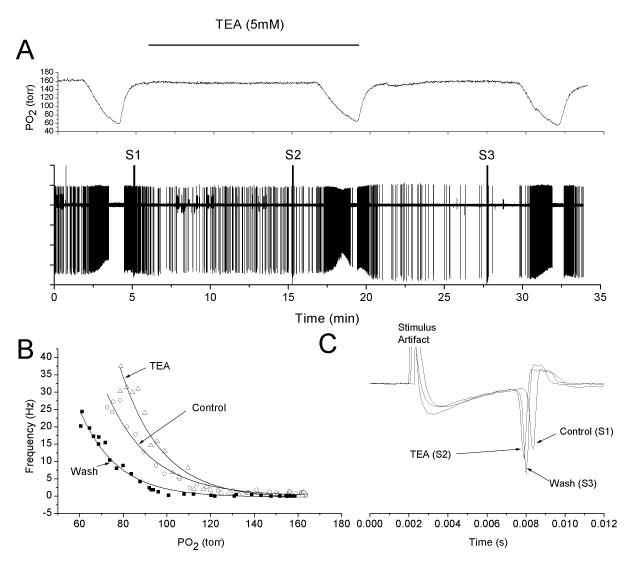
TEA, a small molecule K+ channel blocker which also blocks MaxiK channels, slightly enhances the afferent nerve response to hypoxia. Panels are as in figure 1. Like charybdotoxin, TEA failed to stimulate nerve activity in normoxia and tended to enhance the response at lower levels of oxygen.
3.2 Afferent nerve response to sham drug treatment
In order to determine the effect of multiple exposures to hypoxia on the O2 response curve, a similar protocol was undertaken with sham drug treatment. In general, sham drug treatment had no major effect, but the responses to subsequent hypoxic challenges were slightly decreased from the original (Fig 2A). Since AP frequency at given level of PO2 was variable among units, likely due to variations in sensor depth, the differences in AP frequencies were calculated as differences between hypoxia trials for each PO2 level, 150 torr, 140 torr…80 torr, and analyzed as repeated measures. For young rats, the difference between trials was not different across PO2 levels (p=0.64, N=8) and the level regression slope was not different than zero (p=0.32). For older rats with sham drug treatment, the change in AP rate differed across O2 levels (p<0.001) and was different than zero (p=0.007, N=9). For instance, the decrease in rate at 100 torr with sham drug treatment was −2.03±.47 Hz (N=9).
3.3 Afferent nerve response to TEA and increased charybdotoxin concentration
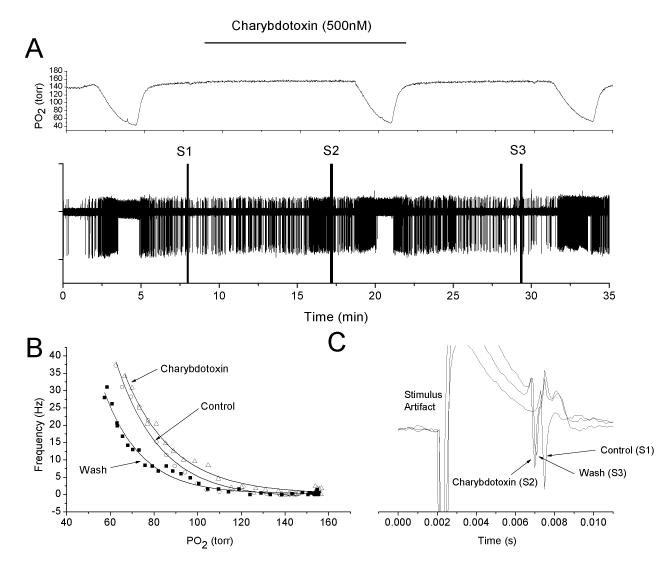
The relatively small response to charybdotoxin may be due to permeation difficulties within the carotid body tissue. This was experimentally addressed in two ways. In two experiments the charybdotoxin concentration was increased to 500nM which is expected to increase the flux by the same factor (5X). However, the response at this higher concentration was comparable to that obtained with the lower concentration (Fig 3). Secondly, two experiments were undertaken in which charybdotoxin was replaced by TEA (5mM), a blocker of voltage-dependent K+ channels in addition to MaxiK channels. TEA has a molecular weight of 165, compared to 4295 for charybdotoxin. As with charybdotoxin, TEA caused an upward shift in the O2 response curve, but only marginal changes in nerve activity during normoxia (Fig 4).
Fig 3.
Charybdotoxin at 500nM also fails to alter, in a major way, the chemoreceptor response to hypoxia. Panels are the same as in figure 1. Note relatively similar shifts in the O2 response curve, compared to figure 1, despite the 5-fold increase in charybdotoxin concentration.
3.4 Glomus cell calcium responses to MaxiK channel blockade
In glomus cells from immature rats (n=57), calcium levels rapidly increased during perfusion with increased K+ BSS and with hypoxia (Fig 5). In the presence of charybdotoxin, the peak [Ca2+]i response to hypoxia was significantly increased (p=0.000) and remained so during the third hypoxia challenge (Fig 5). However, the magnitude of increase was relatively small, representing a ~35 nM difference between the first hypoxia challenge and the 2nd hypoxia challenge with charybdotoxin (Fig 5).
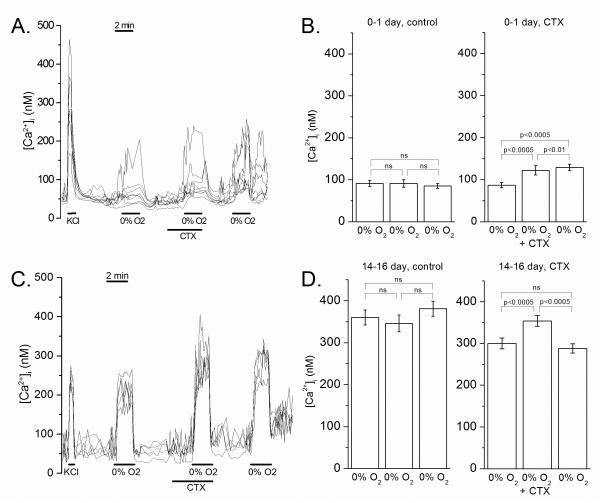
Fig 5.
Intracellular calcium response of newborn and mature glomus cells to hypoxia and KCl in the absence and presence of charybdotoxin. A: Example of an intracellular calcium experiment on glomus cells from 0-1 day old rats. Each line reflects intracellular calcium of a single glomus cell, estimated from the 340/380 nM FURA-2 ratio. B: Averaged peak [Ca2+]i response to three hypoxia challenges in sham and charybdotoxin experiments at 0-1 day. Charybdotoxin caused a slight, but significant, increase in the response to hypoxia. C: Example of an intracellular calcium experiment on glomus cells from ~14 day old rats. Each line reflects intracellular calcium of a single glomus cell. D: Averaged peak [Ca2+]i response to three hypoxia challenges in sham and charybdotoxin experiments at 14-16 day. As with the immature, charybdotoxin caused a slight, but significant, increase in the response to hypoxia.
Glomus cells from mature rats also responded to an increase in extracellular K+ concentration and hypoxia by an increase in intracellular calcium (Fig 5). The magnitude of response in the mature was greater than the newborn, as previously described (Wasicko et al., 2006; Wasicko et al., 1999). Charybdotoxin increased the peak [Ca2+]i response to hypoxia by ~ 18% compared to the first hypoxia challenge without charybdotoxin (p=0.000) (Fig 5). After washout of the drug, the average peak [Ca2+]i level during the third hypoxia challenge was not significantly different from the peak [Ca2+]i level during the first challenge (Fig 5).
3.5 Calcium responses to hypoxia following sham-drug treatment
Control experiments using three consecutive hypoxia challenges were undertaken, without drug, to determine the variability of [Ca2+]i responses to hypoxia over time. In the newborn control group (n=33), peak [Ca2+]i response during the first, second and third hypoxia challenges did not differ significantly (p=0.618) (Fig 5). Similarly, in mature controls (n=66), peak [Ca2+]i values during the three hypoxia challenges did not differ significantly (p=0.12) (Fig 5).
4. DISCUSSION
The major conclusion from the present work is that MaxiK channels play little or no role in control of rat carotid body nerve activity or glomus cell calcium during normoxia and during moderate hypoxia for both the newborn and mature age groups. This extends previous studies by demonstrating little/no role for MaxiK channels over a range of oxygen levels and demonstrating that MaxiK channels are not a major factor in carotid body maturation.
4.1 Role of MaxiK channels in oxygen sensing of the mature carotid body
The initial argument for a role for MaxiK channels in chemoreceptor hypoxia sensing was based on patch-clamp recordings of dissociated rat carotid body cells. In support of a function role, both hypoxia and charybdotoxin caused depolarization of the glomus cell (Wyatt et al., 1995), suggesting that MaxiK channels were active during normoxia and their inhibition by hypoxia mediates depolarization. However, later experiments did not confirm these findings and showed little effect of MaxiK inhibition on resting membrane potential, baseline calcium levels or the increase in calcium observed during exposure to strong hypoxic solutions (Buckler, 1997). Hypoxia-induced release of catecholamine, as measured by voltammetry (Doyle and Donnelly, 1994) or 3H-labelled tyrosine (Gomez-Nino et al., 2009) was unchanged by administration of charybdotoxin or low doses of TEA. Similarly, blockade of MaxiK channels were observed to have little effect on chemoreceptor nerve activity during normoxia although the drug-induced inhibition should have emulated severe hypoxia and produced strong nerve excitation if MaxiK channel inhibition played a major role in mediating organ function (Donnelly, 1995). Furthermore, the carotid body continued to respond normally to strong hypoxia in the presence of the MaxiK channel blockade (Cheng and Donnelly, 1995; Lahiri et al., 1998; Osanai et al., 1997). Our present results are consistent with these previous observations showing little effect of MaxiK block on calcium levels or nerve activities during normoxia and severe hypoxia.
Given the unequivocal evidence that MaxiK channels are present in glomus cells, what is their role in modulating glomus cell function if blocking these channels appears to have such little effect? Gomez-Nino (Gomez-Nino et al., 2009) offered an explanation: during normoxia, MaxiK channels are largely inactive since membrane potential is relatively hyperpolarized and the calcium levels are low. Thus, pharmacologic inhibition of the channels would produce little effect during normoxia. During strong hypoxia, the channels are directly inhibited by hypoxia so inhibition would produce little further effect. Thus, if there is a physiologic role for the MaxiK channel, it must occur at moderate hypoxic levels where hypoxia-mediated channel inhibition is smaller. This is the specific point addressed in the present study.
The results, however, were inconsistent with the postulate: charybdotoxin was without significant effect on nerve activity across a range of O2 values, although there appeared to be trend to increased nerve activity in the presence of charybdotoxin (Fig 1, 3). Charybdotoxin also increased conduction velocity. These results suggest that any modulatory action of MaxiK current on organ function is relatively minor and falls below the resolution afforded by single-unit recordings. Although charybdotoxin significantly enhanced the calcium response to hypoxia (Fig 6), the magnitude of increase was about 18%. A similar increase in nerve activity is not necessarily expected. For instance, endothelin-1 enhances the calcium response to hypoxia by 87% but enhances the nerve response by only 25% (Chen et al., 2000). Thus, an 18% increase in calcium might translate to less than a 5% change in nerve activity, which likely falls below our limits of resolution.
These observations are generally consistent with a role for voltage-sensitive K+ channels in damping the voltage or calcium changes during stimulation with various agents. Gomez-Nino demonstrated that chemical hypoxia produced by exposure to dinitrophenol (DNP), a depolarizing agent that does not inhibit MaxiK channels, elevates glomus cell [Ca2+]i and stimulates carotid body catecholamine release, a response enhanced by MaxiK channel blockade. Similarly, we previously reported that TEA and 4-aminopyridine (4-AP), drugs which block not only the MaxiK currents but other voltage-activated K+ currents, had no effect on normoxic [Ca2+]i levels, but enhanced the [Ca2+]i response to hypoxia in acutely dissociated glomus cells from two week old rats (Wasicko et al., 2006). Thus, the voltage-activated K+ channels may, in general, serve to moderate the responsiveness of glomus cells to various stimulating agents and the role of MaxiK channels may be limited to non-hypoxic stimulating factors. This is similar to the established role of MaxiK channels in participating in the repolarization of neurons following an action potential (Faber and Sah, 2002).
The present results are, however, in contrast with results obtained on cultured slices of carotid body and cultured glomus cells. In carotid body slices, TEA and iberiotoxin, a MaxiK channel blocker, produced catecholamine release under normoxic conditions (Pardal et al., 2000), consistent with the original postulate that MaxiK channels are important in oxygen sensing. Similar results were obtained on cultured glomus cells which released catecholamine in response to iberiotoxin (Jackson and Nurse, 1997). These results, obtained after long-term culture (2-12 days), are opposite to that obtained on acute preparations and raise the possibility that the culture conditions significantly alter the cellular response characteristics. This has been observed with other stimuli. For instance, carotid body cells in long term culture respond to low glucose by increasing catecholamine release and increasing synaptic activity of co-cultures (glomus cells co-cultured with petrosal neurons), suggesting that carotid body cells act as glucose sensors in addition to acting as hypoxia sensors (Pardal and Lopez-Barneo, 2002; Zhang et al., 2007). However, in freshly harvested tissue, low glucose fails to stimulate nerve activity or catecholamine release despite being applied for prolonged periods of time (Bin-Jaliah et al., 2004; Conde et al., 2007). This suggests that cultured cells respond in a fundamentally different way compared to intact cells or freshly harvested cells. Alternatively, as initially suggested by Pardal (Pardal et al., 2000) and reiterated by Peers (Peers and Wyatt, 2007), poorly lipid soluble channel blockers may poorly penetrate the carotid body and, thus, fail to stimulate nerve activity. We attempted to address this issue by increasing the concentration of charybdotoxin 5-fold, but the response to the drug was unaltered. Furthermore, we used TEA, a small molecule of molecular weight of 165 compared to charybdotoxin at 4295. Like charybdotoxin, TEA tended to shift the O2 response curve upward but failed to significantly enhance nerve activity during normoxia. In contrast, we and others previously demonstrated that suramin (MW 1429) and A317491 (MW 565), both water soluble, readily penetrate the CB parenchyma and inhibit afferent nerve activity (Niane et al., 2011). Thus, it seems unlikely that the carotid body excludes a small water-soluble molecule (TEA) while permitting diffusion of a much larger water-soluble molecule (suramin). Consistent with this speculation, TEA has been used previously to block nicotinic acetylcholine receptors within the carotid body parenchema and was found effective in blocking the excitatory action of exogenous acetylcholine (Moe et al., 1948).
4.2 Developmental changes in the role of carotid body MaxiK channels
Hatton and colleagues studied the postnatal development of MaxiK currents in carotid body glomus cells from 4 day old, 10 day old and adult rats (Hatton et al., 1997). At a membrane potential of −10 mV, the relative contribution of MaxiK current to glomus cell whole-cell K+ current was ~ 18% at 4 days, ~ 31% at 10 days and ~ 28% in adults. Although the functional expression of MaxiK currents was lower in glomus cells from 4 day old rats, the percentage inhibition of whole-cell K+ currents (at −10 mV) by hypoxia averaged only ~6% for 4 day olds compared to ~35 and ~32% for cells from 10 day old and adults respectively (Hatton et al., 1997). These authors concluded that, not only was MaxiK current expression lower in newborn glomus cells, but that it was also substantially less inhibited by hypoxia compared to mature glomus cells.
MaxiK channels have a large single-channel conductance compared to other K+ selective channels and especially compared to TASK-like K+ channels, which are believed to play a role in driving hypoxia-induced glomus cell depolarization. We hypothesized that, in immature glomus cells, if MaxiK channels are less inhibited by hypoxia, the effect would be to dampen hypoxia-induced depolarization, which could contribute to the low responsiveness of the newborn carotid body to hypoxia. The results of the present study, however, show that nerve responses to graded hypoxia were unaffected by charybdotoxin, suggesting that MaxiK currents are not causing major damping of the hypoxia response.
Despite the lack of effect of charybdotoxin on the nerve response, glomus cells from newborns showed a small enhancement of the [Ca2+]i response to hypoxia when charybdotoxin was present. This result is generally consistent with the findings of Hatton, that MaxiK current expression levels are low and their contribution to glomus cell O2-sensitive K+ current is small (Hatton et al., 1997). Although this enhancement was statistically significant, it was quite small and apparently not of sufficient magnitude to affect nerve activity.
Taken together, the results are consistent in demonstrating little or no role for MaxiK current in carotid body functional maturation at the level of nerve activity or glomus cell calcium. Since carotid bodies show a several fold developmental increase in nerve activity and glomus cell [Ca2+]i responses to hypoxia, the mechanism must be mediated by changes in channels other than MaxiK type or other elements of the transduction pathway.
Acknowledgements
This work was funded by NIH grant HL054621.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bamford OS, Sterni LM, Wasicko MJ, Montrose MH, Carroll JL. Postnatal maturation of carotid body and type I cell chemoreception in the rat. Am. J. Physiol. 1999;276:L875–L884. doi: 10.1152/ajplung.1999.276.5.L875. [DOI] [PubMed] [Google Scholar]
- Bin-Jaliah I, Maskell PD, Kumar P. Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat. J Physiol. 2004;556:255–266. doi: 10.1113/jphysiol.2003.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J. Physiol. (London) 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J. Physiol. (London) 1994;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525(Pt 1):135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, He L, Dinger B, Fidone S. Stimulus-specific signaling pathways in rabbit carotid body chemoreceptors. Neuroscience. 2000;95:283–291. doi: 10.1016/s0306-4522(99)00399-1. [DOI] [PubMed] [Google Scholar]
- Cheng PM, Donnelly DF. Relationship between changes of glomus cell current and neural response of rat carotid body. J Neurophysiol. 1995;74:2077–2086. doi: 10.1152/jn.1995.74.5.2077. [DOI] [PubMed] [Google Scholar]
- Conde SV, Obeso A, Gonzalez C. Low glucose effects on rat carotid body chemoreceptor cells’ secretory responses and action potential frequency in the carotid sinus nerve. J Physiol. 2007;585:721–730. doi: 10.1113/jphysiol.2007.144261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF. Modulation of glomus cell membrane currents of intact rat carotid body. J. Physiol. (London) 1995;489:677–688. doi: 10.1113/jphysiol.1995.sp021082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DF, Rigual R. Single-unit recordings of arterial chemoreceptors from mouse petrosal ganglia in vitro. J. Appl. Physiol. 2000;88:1489–1495. doi: 10.1152/jappl.2000.88.4.1489. [DOI] [PubMed] [Google Scholar]
- Doyle TP, Donnelly DF. Effect of Na+ and K+ channel blockade on baseline and anoxia-induced catecholamine release from rat carotid body. J Appl Physiol. 1994;77:2606–2611. doi: 10.1152/jappl.1994.77.6.2606. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C, Monti-Bloch L, Baron M, Hayashida Y, Woodbury JW. Changes in glomus cell membrane properties in response to stimulants and depressants of carotid nerve discharge. Brain Res. 1989;477:265–279. doi: 10.1016/0006-8993(89)91414-5. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nino A, Obeso A, Baranda JA, Santo-Domingo J, Lopez-Lopez JR, Gonzalez C. MaxiK potassium channels in the function of chemoreceptor cells of the rat carotid body. Am J Physiol Cell Physiol. 2009;297:C715–722. doi: 10.1152/ajpcell.00507.2008. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol. Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Carpenter E, Pepper DR, Kumar P, Peers C. Developmental changes in isolated rat type I carotid body K+ currents and their modulation by hypoxia. J. Physiol. (London) 1997;501:49–58. doi: 10.1111/j.1469-7793.1997.049bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Nurse C. Dopaminergic properties of cultured rat carotid body chemoreceptors grown in normoxic and hypoxic environments. J Neurochem. 1997;69:645–654. doi: 10.1046/j.1471-4159.1997.69020645.x. [DOI] [PubMed] [Google Scholar]
- Kholwadwala D, Donnelly DF. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J. Physiol. (London) 1992;453:461–473. doi: 10.1113/jphysiol.1992.sp019239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Roy A, Rozanov C, Mokashi A. K+-current modulated by PO2 in type I cells in rat carotid body is not a chemosensor. Brain Res. 1998;794:162–165. doi: 10.1016/s0006-8993(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K current modulated by pO2 in type I chemoreceptor cells. Science. 1988;241:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez J, Gonzalez C, Urena J, Lopez-Barneo J. Low PO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J. Gen Physiol. 1989;93:1001–1014. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez JR, DeLuis DA, Gonzalez C. Properties of a transient K+ current in chemoreceptor cells of rabbit carotid body. J. Physiol. (London) 1993;460:15–32. doi: 10.1113/jphysiol.1993.sp019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe GK, Capo LR, Peralta B. Action of tetraethylammonium on chemoreceptor and stretch receptor mechanisms. Am. J. Physiol. 1948;153:601–605. doi: 10.1152/ajplegacy.1948.153.3.601. [DOI] [PubMed] [Google Scholar]
- Niane LM, Donnelly DF, Joseph V, Bairam A. Ventilatory and carotid body chemoreceptor responses to purinergic P2X receptor antagonists in newborn rats. J Appl Physiol. 2011;110:83–94. doi: 10.1152/japplphysiol.00871.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai S, Buerk DG, Modashi A, Chugh DK, Lahiri S. Cat carotid body chemosensory discharge (in vitro) is insensitive to charybdotoxin. Brain Res. 1997;747:324–327. doi: 10.1016/s0006-8993(96)01313-3. [DOI] [PubMed] [Google Scholar]
- Pardal R, Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- Pardal R, Ludewig U, Garcia-Hirschfeld J, Lopez-Barneo J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc Natl Acad Sci U S A. 2000;97:2361–2366. doi: 10.1073/pnas.030522297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2+-activated K+ current. Neurosci. Lett. 1990;119:253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Peers C, O’Donnell J. Potassium currents recorded in type I carotid body cells from the neonatal rat and their modulation by chemoexcitatory agents. Brain Res. 1990;522:259–266. doi: 10.1016/0006-8993(90)91470-2. [DOI] [PubMed] [Google Scholar]
- Peers C, Wyatt CN. The role of maxiK channels in carotid body chemotransduction. Respir Physiol Neurobiol. 2007;157:75–82. doi: 10.1016/j.resp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Ross FA, Rafferty JN, Dallas ML, Ogunbayo O, Ikematsu N, McClafferty H, Tian L, Widmer H, Rowe IC, Wyatt CN, Shipston MJ, Peers C, Hardie DG, Evans AM. Selective expression in carotid body type I cells of a single splice variant of the large conductance calcium- and voltage-activated potassium channel confers regulation by AMP-activated protein kinase. J Biol Chem. 2011 doi: 10.1074/jbc.M110.189779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasicko MJ, Breitwieser GE, Kim I, Carroll JL. Postnatal development of carotid body glomus cell response to hypoxia. Respir Physiol Neurobiol. 2006;154:356–371. doi: 10.1016/j.resp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Wasicko MJ, Sterni LM, Bamford OS, Monrose MH, Carroll JL. Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells. J. Physiol. (London) 1999;514:493–503. doi: 10.1111/j.1469-7793.1999.493ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA, Buckler KJ. Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type 1 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L221–230. doi: 10.1152/ajplung.00010.2003. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Wright C, Bee D, Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic transduction. Proc. Natl. Acad. Sci. 1995;92:295–299. doi: 10.1073/pnas.92.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Buttigieg J, Nurse CA. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J Physiol. 2007;578:735–750. doi: 10.1113/jphysiol.2006.121871. [DOI] [PMC free article] [PubMed] [Google Scholar]