Abstract
Tooth enamel is formed by epithelially-derived cells called ameloblasts, while the pulp dentin complex is formed by the dental mesenchyme. These tissues differentiate with reciprocal signaling interactions to form a mature tooth. In this study we have characterized ameloblast differentiation in human developing incisors, and have further investigated the role of extracellular matrix proteins on ameloblast differentiation. Histological and immunohistochemical analyses showed that in the human tooth, the basement membrane separating the early developing dental epithelium and mesenchyme was lost shortly before dentin deposition was initiated, prior to enamel matrix secretion. Presecretary ameloblasts elongated as they came into contact with the dentin matrix, and then shortened to become secretory ameloblasts. In situ hybridization showed that at the presecretory stage of odontoblasts expressed type I collagen mRNA, and also briefly expressed amelogenin mRNA. This was followed by upregulation of amelogenin mRNA expression in secretory ameloblasts. In vitro, amelogenin expression was up-regulated in ameloblast lineage cells cultured in Matrigel, and was further up-regulated when these cells/Matrigel were co-cultured with dental pulp cells. Co-culture also up-regulated type I collagen expression by the dental pulp cells. Type I collagen coated culture dishes promoted a more elongated ameloblast lineage cell morphology and enhanced cell adhesion via integrin α2β1. Taken together, these results suggest that the basement membrane proteins and signals from underlying mesenchymal cells coordinate to initiate differentiation of preameloblasts and regulate type I collagen expression by odontoblasts. Type I collagen in the dentin matrix then anchors the presecretary ameloblasts as they further differentiate to secretory cells. These studies show the critical roles of the extracellular matrix proteins in ameloblast differentiation.
Keywords: basement membrane proteins, type I collagen, integrin, dental epithelial cells, dental pulp cells, ameloblast lineage cells
1. Introduction
Enamel is the outer layer of the tooth and the hardest tissue in human body. Enamel is formed by differentiated dental epithelial cells known as ameloblasts. It is well known that ameloblast differentiation is regulated by reciprocal signaling interactions between the dental epithelium and mesenchyme (Jernvall and Thesleff, 2000; Mitsiadis et al., 2008; Morotomi et al., 2005). The proliferating dental epithelial cells first differentiate into inner enamel epithelium, and subsequently differentiate into preameloblasts, presecretory ameloblasts then secretory ameloblasts that synthesize and secrete enamel matrix proteins. The enamel matrix proteins self-assemble to form a matrix, which mineralizes as the ameloblasts continue to differentiate (Robinson et al., 1998).
A basement membrane separates the dental epithelium and mesenchyme during the early stages of dental epithelial cell differentiation. Laminin, type IV collagen, fibronectin, perlecan and nidogen are the major components of the basement membrane (Timpl, 1996). The basement membrane is considered to be involved in the proliferation and differentiation of preameloblasts (Adams and Watt, 1993; Fukumoto and Yamada, 2005; Smith et al., 1989; Thesleff and Hurmerinta, 1981; Thesleff et al., 1981), and fibronectin and laminin may provide attachment anchorage for preameloblasts (Fukumoto et al., 2006; Salmivirta et al., 1997; Tabata et al., 2004).
In rodents, when the basement membrane is lost, ameloblasts begin to secrete matrix proteins, including ameloblastin, which likely serves as an adhesion molecule to anchor and regulate the differentiation of these cells (Nanci et al., 1998). In ameloblastin-null mice, differentiated maturation stage ameloblasts lose cell polarity, detach from the enamel matrix, and resume proliferating ability while forming multiple cells layers (Fukumoto et al., 2004). In the maturation stage, a basal lamina reforms. Studies by Yuasa and co-workers have shown laminin α2 to be important for adhesion of maturation stage ameloblasts to the enamel surface (Yuasa et al., 2004).
Ameloblasts commit to apoptosis before a tooth erupts. Therefore, in humans, there is a limited availability of these cells to ascertain the molecular mechanisms driving the differentiation of dental epithelium to ameloblasts. Most studies of dental epithelial cell differentiation have therefore been done using rodents, primarily rat and mouse incisors (Fukumoto et al., 2004; Nanci et al., 1998; Salmivirta et al., 1997).
In this report, we have characterized developing human tooth buds to show the unique morphology of these cells in relation to the underlying extracellular matrix proteins at the presecretory stage of differentiation. In vitro studies were done using ameloblast lineage cells isolated from human fetal tooth buds (DenBesten et al., 2005; Le et al., 2007; Yan et al., 2006; Zhang et al., 2007). These ameloblast lineage cells have been shown to differentiate and up-regulate amelogenin expression in response to increased concentrations of calcium (Chen et al., 2009; DenBesten et al., 2005) or the leucine rich amelogenin peptide (LRAP) (Le et al., 2007). These cells are considered to represent an early stage of ameloblast differentiation. Further identification of cell/matrix interactions is important in understanding the fate decisions of ameloblast lineage cells as they differentiate to form a mature enamel matrix.
2. Results
2.1 Presecretary ameloblasts in the human tooth dramatically elongate as secretion of the dentin matrix is initiated, and subsequently shorten at the initiation of enamel matrix secretion
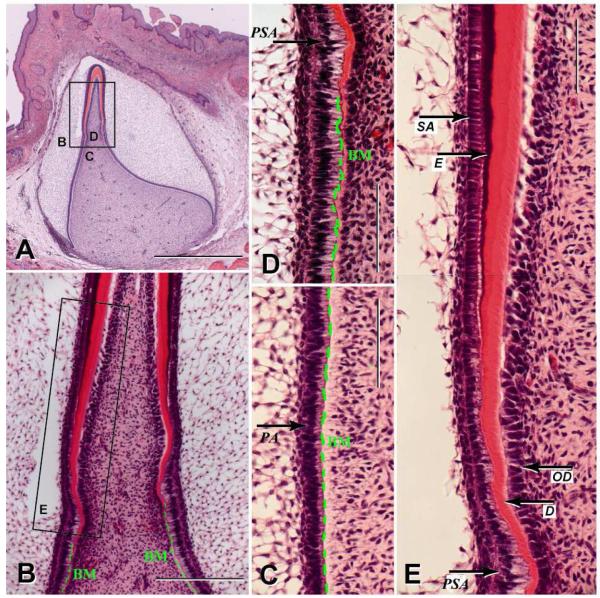
We examined human incisors from a total of eight 20 to 24 week old fetuses. Analyses of H&E stained sections of a bell stage human fetal incisor showed differentiation from preameloblasts to presecretory and secretory stage ameloblasts (Fig. 1A&B). During the early stages of differentiation, preameloblasts were separated from odontoblasts by a layer of basement membrane (Fig. 1B,C&D). As the odontoblasts condensed, began to polarize and deposit dentin matrix, the presecretory ameloblasts dramatically elongated with an apical to basal length of approximately 40μm (Fig. 1D&E). As differentiation went on, the odontoblasts continued to polarize and secreted matrix proteins to rapidly increase the width of the dentin matrix. At the same time the ameloblasts shortened to an apical/basal length of 25μm. Here vesicles formed in ameloblasts and enamel matrix secretion began as the cells formed Tomes’ process and appeared as the classically described secretory ameloblasts. The progressive differentiation of human ameloblasts is summarized in the cartoon illustrated in Fig. 2, and is compared to this same process as it occurs in rodent incisors.
Fig. 1.
H&E staining of a human developing bell stage incisor. (A) Sagittal section of dental primordium in the stage of hard tissue deposition (250 mm CRL, 26th week) was stained with H&E. Dentin is stained pink and enamel is stained purple. The box shows the location of Fig. 1B, 1C and 1D. Scale bar: 1000μm. (B) Magnification of box B in Fig. 1A. Prior to dentin formation, the preameloblasts obtain an elongated form, which was maintained during initial dentin deposition. Prior to enamel matrix secretion, these cells reduced their length. Basement membrane (BM) is labeled with a green dash line. Scale bar: 200 μm. (C) The inner enamel epithelial cells obtain a cuboidal outline and differentiate to preameloblasts (PA) with a columnar shape. The preameloblasts are separated from the dental mesenchyme by a BM, indicated with green dash line. Scale bar: 100 μm. (D) Preameloblasts elongate and differentiate into presecretary ameloblasts (PSA). At this stage the basement membrane is lost and the cells are in direct contact with the dentin matrix. Scale bar: 100μm. (E) Magnified section of box E from Fig. 1B. The transition of ameloblasts from tall columnar presecretary ameloblasts (PSA) to short columnar secretory ameloblasts (SA), which produce enamel matrix (E) as indicated by arrows. Dentin (D) and odontoblasts (OD) are also indicated. Scale bar: 100 μm.
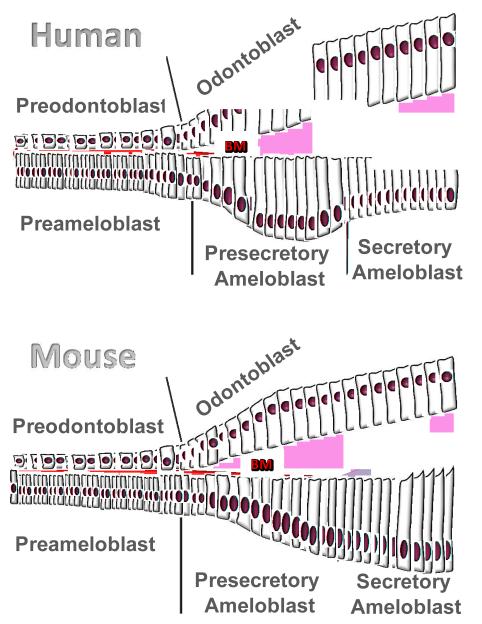
Fig. 2.
Diagrams of human and mouse ameloblasts differentiation. Human presecretory ameloblasts polarize and dramatically elongate as basement membrane (labeled as BM in red) disappears and the dentin matrix directly contacts the presecretory ameloblast cells. As compared to ameloblast differentiation in human, mouse ameloblasts gradually elongate and the basement membrane stays in place until enamel matrix synthesis is initiated, with the result that secretory stage rodent ameloblasts are longer than human secretory stage ameloblasts.
2.2 Dentin matrix formation and expression of type I collagen were correlated with a loss of basement membrane proteins
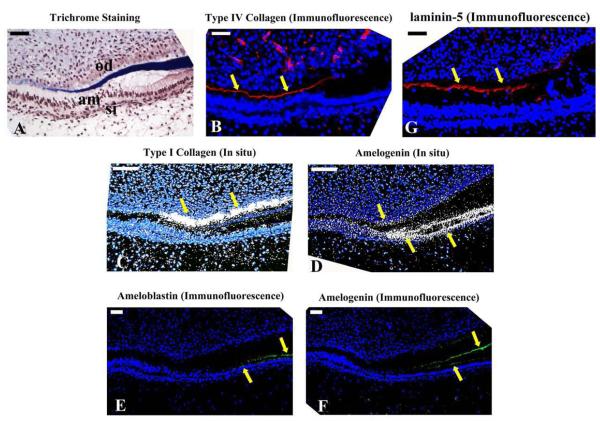
Trichrome staining of frozen sections of a human developing incisor (Fig. 3A) showed that secretion of dentin matrix was initiated just before the odontoblasts elongated and differentiated, and was correlated with upregulation of type I collagen mRNA expression (Fig. 3C). This upregulation of type I collagen occurred when type IV collagen (Fig. 3B) and laminin 5 (Fig. 3G) immunostaining was lost, and presecretory ameloblasts elongated.
Fig. 3.
Changes in ECM at the transition from presecretory to secretory ameloblasts in the human primary tooth incisor. (A) Trichrome staining showed that dentin was stained blue and the developing enamel matrix staining dark red. The presecretary ameloblasts easily pulled away from the dentin matrix after the loss of the basement membrane, resulting in sectioning artifacts. am: ameloblasts; od: odontoblasts; si: stratum intermedium. (B) In a serial section, type IV collagen (red fluorescent immunostaining) was localized between the epithelium and mesenchyme as indicated by arrows, and then disappeared at the presecretory stage of ameloblast differentiation. (C) Laminin 5α, another major component of the basement membrane was immunolocalized with a pattern similar to that of type IV collagen. (D) In situ hybridization with a type I collagen probe showed a rapid up-regulation in the secretory stage of odontoblasts (arrows). (E) In situ hybridization with amelogenin probe showed abundant expression in secretary ameloblasts (double arrow), accompanied with a brief upregulation of amelogenin in the odontoblasts (single arrow). (F) Ameloblastin was detected in the secretary ameloblasts. (G) Amelogenin immunoreactivity was detected in the enamel matrix and secretory ameloblasts. Weak signals were also detectable in the odontoblasts. Positive signals are indicated by arrowheads. Scale bar: 50 μm.
In situ hybridization of amelogenin mRNA (Fig. 3D) showed that odontoblasts first briefly expressed low amounts of amelogenin mRNA, followed by a dramatic upregulation of amelogenin by ameloblasts. This suggests that amelogenin expressed by odontoblasts at the presecretory stage of enamel formation may have a role in signaling presecretary ameloblasts to further differentiate to secretory stage cells. Immunostaining for amelogenin (Fig. 3F), then ameloblastin (Fig. 3E) occurred at the beginning of the secretory stage when the ameloblasts shortened and began to secrete matrix proteins.
2.3 Amelogenin expressed by ameloblast lineage cells was upregulated by Matrigel (basement membrane proteins), and co-culture with dental pulp cells further enhanced amelogenin expression
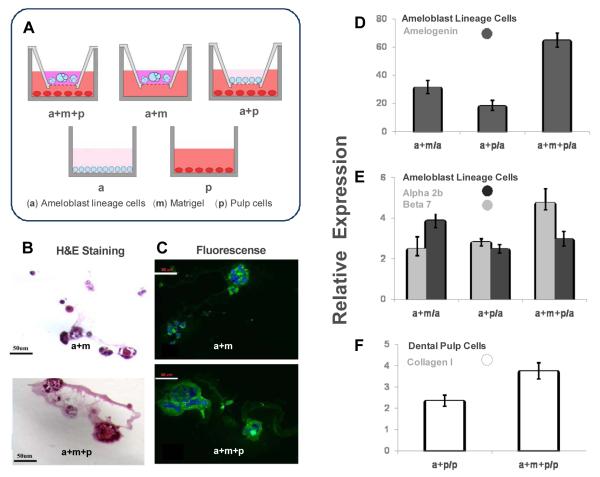
A diagram illustrating the various cell and Matrigel combinations is shown in Fig. 4A. Ameloblast lineage cells grown in the Matrigel formed spherical, acinar structures (Fig. 4B). When these same cells were grown in Matrigel with dental pulp cells cultured in the lower chamber of a transwell culture plate, the size of acini was greatly increased (Fig. 4B). Immunohistochemical staining showed that Matrigel enhanced amelogenin protein synthesis (Fig. 4C), and that amelogenin expression was further upregulated by ameloblast lineage cells in co-culture with pulp cells (Fig. 4C).
Fig. 4.
The effects of basement membrane matrix and signaling from dental pulp cells on the cultured ameloblast lineage cells grown in 3 dimensional (3-D) Matrigel. The conditions used for co-culture, 3D culture and monolayer culture were illustrated in Fig.4A. a: ameloblast lineage cells (in blue); m: Matrigel (in purple); p: pulp cells (in red). (B) H&E staining of the cross sections of 3-D culture showed ameloblast lineage cells grown in Matrigel co-cultured with pulp cells (a+m+p) formed larger acini than the acini formed by ameloblast lineage cells in Matrigel (a+m) without signaling from pulp cells. The larger acini formed by ameloblast lineage cells co-cultured with both Matrigel and pulp (a+m+p) displayed more amelogenin immunoreactivity than ameloblast lineage cells grown in Matrigel alone (a+m) (Fig.4C). qPCR results showed that Matrigel and signaling from pulp cells highly upregulated the expression of amelogenin in ameloblast lineage cells (P = 0.0045) (Fig.4D). (E) The expression level of integrin α2 was significantly up-regulated by Matrigel (a+m) and co-cultured pulp cells (a+m+p) as compared with the expression level of ameloblast lineage cells in monolayer culture (a) (P = 0.0039). The presence of Matrigel significantly increased the expression of integrin beta 7 (P = 0.0046). (F) Either pulp cells co-cultured with ameloblast lineage cells (a+p) or co-cultured with ameloblast lineage cells (a+m+p) in Matrigel up-regulated expression of type I collagen as compared to the expression level in the monolayer cultured pulp cells (p) (P = 0.01216). One-way ANOVA analysis, n=3.
After 7 days of co-culture, qPCR assay showed that expression of amelogenin in ameloblast lineage cells grown in Matrigel alone was upregulated 31 fold as compared to cells grown in monolayer (Fig. 4D). Amelogenin expression in ameloblast lineage cells in co-cultured with dental pulp was increased 18 fold as compared to the cells grown in monolayer, and amelogenin expression was 65 fold increased when ameloblast lineage cells were grown in both Matrigel and co-cultured with pulp cells (Fig. 4D). One-way ANOVA analysis showed P value was 0.0045.
An increase in integrin α2 expression (receptor for type I collagen) by approximately 4.5 fold was detected in the ameloblast lineage cells grown either with Matrigel or pulp cells as compared to ameloblast lineage cells grown in monolayer (P = 0.0039). Similarly integrin β7 (receptor for E-cadherin) was upregulated 2.5 fold in ameloblast lineage cells co-cultured with Matrigel and dental pulp cells (P = 0.0046) (Fig.4E). Pulp cells co-cultured with ameloblast lineage cells had a 2.4 fold upregulation of type I collagen expression, and a 3.6 fold upregulation of type I collagen expression when co-cultured with ameloblast lineage cells in Matrigel in comparison with the pulp cells in monolayer culture (P = 0.01216) (Fig. 4F).
2.4 Type I collagen enhanced the adhesion of ameloblast lineage cells through integrin receptor α2β1
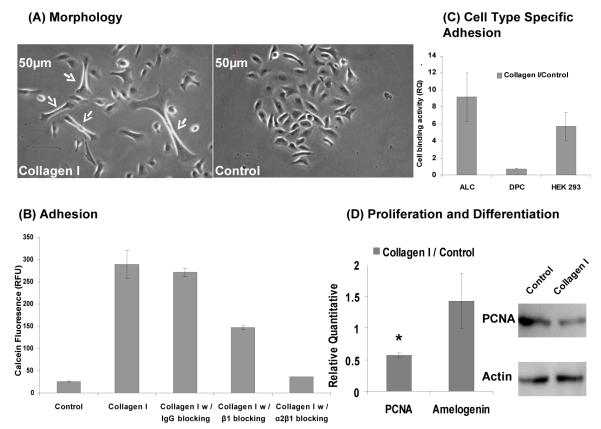
Ameloblast lineage cells grown on type I collagen coated dishes showed a dramatically elongated morphology (Fig. 5A). These same cells had significantly greater adhesion to collagen coated dishes as compared to uncoated dishes, measured by the fluorescent dye calcein acetoxymethyl (AM) ester ((P = 0.0001) (Fig. 5B). Similar experiments with fibronectin coated dishes also showed enhanced effect on cell adhesion, while amelogenin coated dishes appeared to inhibit ameloblast lineage cells adhesion (data not shown).
Fig. 5.
The effects of type I collagen on cultured ameloblast lineage cells. (A) Type I collagen promoted a more elongated cell morphology (arrows) as observed through a phase contrast image. (B) Ameloblast lineage cells bound to type I collagen via α2β1. Type I collagen significantly promoted adhesion of ameloblast lineage cells (p = 0.0001), however, the binding of ameloblast lineage cells to type I collagen was almost completely blocked by integrin α2β1 antibody (P = 0.0002), and partially blocked by integrin β1 antibody (P = 0.0015). Mouse IgG, used as a control, did not have an effect on type I collagen binding (P = 0.3952). (C) Cell type-specific binding activity of type I collagen. Ameloblast lineage cells, dental pulp cells and HEK 293 cells were plated on dishes coated with type I collagen. Type I collagen dramatically increased adhesion of ameloblast lineage cells (P = 0.0003) and HEK 293 cells (P = 0.0321), however, slightly decreased adhesion of dental pulp cells to cell culture plates (P = 0.006). (D) Type I collagen significantly inhibited the expression of PCNA (P = 0.0014). However, no significant differences were observed in the expression of amelogenin with or without the presence of type I collagen (P = 0.2143). Student t-test, n=3.
Antibody blocking experiments showed that when cells were pre-incubated with integrin α2β1 antibody, adhesion of ameloblast lineage cells was almost reduced to the level of the control (non-coated) dishes (P = 0.0002) (Fig. 5B). Integrin β1 antibody only partially blocked the adhesion to ameloblast lineage cells (P = 0.0015) (Fig. 5B). A cell attachment assay showed that type I collagen promoted cell type specific adhesion to epithelially derived cells including ameloblast lineage cells (P = 0.0003), and human embryonic kidney (HEK) 293 cells (P = 0.0321), but slightly decrease the adhesion to dental pulp cells (P = 0.006) (Fig. 5C).
Type I collagen significantly down-regulated the expression of proliferating cell nuclear antigen (PCNA) in ameloblast lineage cells (P = 0.0014), assayed by quantitative PCR and western blot analysis (Fig. 5D). However, amelogenin expression in cells grown on type I collagen coated dishes as compared to uncoated dishes was not significantly different (P = 0.2143) (Fig. 5D).
3. Discussion
In this study we used human developing teeth to study differentiation of ameloblasts, focusing on differentiation from preameloblasts, to presecretary ameloblasts, to secretory ameloblasts. We found that the cell morphology of forming human ameloblasts differed from that of rodent ameloblast morphology. In rodents, ameloblasts gradually elongated from preameloblasts to secretory ameloblasts. However, in humans, presecretory ameloblasts were longer than secretory stage ameloblasts, with the result that human secretory ameloblasts were shorter than rodent secretory ameloblasts.
In humans, the cells of the inner enamel epithelium gradually elongated, and formed preameloblasts of about 20μm in length. As dentin formation began, marked by deposition of type I collagen, the presecretory ameloblasts dramatically elongated to about 40μm in length, followed by a loss of the basement membrane, as indicated by a cessation of type IV collagen secretion. At this transition from presecretory ameloblasts to secretory ameloblasts, the cells appeared to come into physical contact with dentin matrix proteins. The ameloblasts then shortened to about 25 M and began to secrete enamel matrix proteins.
These morphological changes in human developing primary teeth are different from those in rodents, where the basement membrane disappears in the secretory stage rather than pre-secretory stage, and pre-secretory ameloblasts are shorter than secretory ameloblasts (Fukumoto et al, 2004; Fukumoto and Yamada, 2005; Smith et al., 1998). The observation of dramatic morphological changes in human ameloblasts accompanied by changes in the opposing (mesenchymal) cells and extracellular matrix led us to investigate the roles of the extracellular matrix proteins as well as the dental mesenchyme on human ameloblast differentiation.
The presence or absence of a basement membrane seemed to be linked to changes in differentiation of both ameloblasts and odontoblasts. We investigated the role of the basement membrane on ameloblast differentiation by growing ameloblast lineage cells, isolated from the developing incisor tooth buds, in Matrigel. Matrigel consists primarily of laminin, including laminin alpha5, which has been to be important for dental epithelial growth, polarization and the development of tooth bud shape (Fukumoto et al., 2006).
We found that ameloblast lineage cells grown in Matrigel formed spherical cells structures which had up-regulated expression of amelogenin, integrin α2 and integrin β7. Integrin β7 is a known receptor for E-cadherin (Shaw and Brenner, 1995), an early marker of epithelial cell differentiation. These results are consistent with our laser capture analyses of preameloblast cells, from which amelogenin mRNA can be amplified (data not shown). This suggests that that the basement membrane proteins may promote the cellular interactions and polarization, that could initiate differentiation of ameloblast lineage cells.
Integrin β7 is a member of the large family of heterodimeric trans-membrane integrin receptors, comprised of α and β subunits that link the extracellular matrix (ECM) to the cellular cytoskeleton (Gullberg et al., 1992; Hynes, 2002). It has been reported that other integrin β subunits are also likely to have a role in basement membrane mediated cell differentiation. Chen and co-workers demonstrated that deficiency in integrin β1 integrin caused impaired amelogenesis (Chen et al., 2009), further pointing to the important role of the basement membrane in ameloblast differentiation.
Our in vitro studies showed that not only basement membrane proteins, promoted ameloblast differentiation, but also soluble signals from dental pulp cells greatly enhanced amelogenin expression. These results suggest that diffusible signals from the dental mesenchyme can cross the basement membrane to promote ameloblast differentiation. The pulp cells used for these co-culture studies were the passage 1 adult dental pulp cells. At passage 1, a small percentage of the pulp cells grown in culture are Stro-1 positive (Gronthos et al., 2002; Shi and Gronthos, 2003) and some of cells express dentin matrix proteins including type I collagen, dentin sialoprotein (DSP), matrix extracellular phosphoglycoprotein (MEPE) and alkaline phosphatase (Liu et al., 2004; Liu et al., 2005), suggesting a mixed odontogenic cell population. Presumably some of these cells contained signals similar to those from the early differentiating dental mesenchyme, responsible for signaling ameloblast differentiation. The soluble factors secreted by pulp cells that promoted dental epithelial cell differentiation, may include secretory matrix proteins, or other factors.
Our in situ hybridization results suggested a brief up-regulation of amelogenin mRNA expression in the dental mesenchyme underlying the presecretory ameloblasts (Fig. 3F). The small alternatively spliced leucine rich amelogenin peptide, LRAP, is known to regulate the development of mesenchymal-derived cells (Karg et al., 1997; Nagano et al., 2003; Papagerakis et al., 2003), and we have shown that LRAP promotes differentiation of ameloblast lineage cells (Le et al., 2007).
Our in vivo analyses suggest that when the basement membrane is lost the presecretory ameloblasts adhere directly to the dentin matrix. As a major organic component of dentin, the primary role of type I collagen is to provide scaffold for dentin (Mizuno et al., 2003; Reznikoff et al., 1987; Rocha et al., 1985). However, our cell adhesion assay demonstrated that type I collagen also serves as a cell adhesion molecule to anchor ameloblast lineage cells.
Ameloblast lineage cells grown on type I collagen had an elongated shape, a decreased rate of proliferation, but no alteration of amelogenin expression. In vitro, the presence of dental pulp cells in co-culture significantly up-regulated expression of integrin α2 in ameloblast lineage cells. It is possible that this up-regulated integrin α subunit noncovalently binds to integrin β1 and mediates (presecretory ameloblast) cell adhesion to type I collagen (Gullberg et al., 1992). We found that the binding activity of type I collagen was almost completely blocked by integrin α2β1 antibody and partially blocked by integrin β1, further indicating that type I collagen mediates adhesion to ameloblast lineage cells via integrin α2β1. Type I collagen mediated cell adhesion did not promote adhesion of dental pulp cells, indicating that it was cell type specific.
Collectively, this data suggests that type I collagen, contained within the dentin matrix, functions as an anchor for the differentiating presecretory ameloblasts after the basement membrane is discontinued. The loss of basement membrane appears to allow direct contact between the dentin matrix and the presecretory ameloblats, with resulting interactions between type 1 collagen and ameloblasts promoting ameloblast elongation. This interaction is supported by the results from our in vitro studies, showing that type I collagen caused elongation of ameloblast lineage cells in the culture. Some studies have shown that integrins can link extracellular ligands to the cytoskeleton, providing strong attachment to enable cell-shape change and tissue integrity. This connection is made possible by an intracellular complex of proteins, which links to actin filaments and controls signaling cascades that regulate cytoskeletal rearrangements (Delon and Brown, 2009; Hynes, 2002; Zaidel-Bar et al., 2007). Taken together, this suggests that type I collagen may contribute to elongation of ameloblasts in the pre-secretory stage via integrin-cytoskeleton link that regulate cytoskeletal reorganization.
In summary, we propose that the dentin matrix regulates differentiation of early stage human dental epithelial cells. Soluble signals from the odontoblast/pulp complex, which may include matrix proteins, promote ameloblast differentiation while type I collagen anchors the differentiated presecretory ameloblasts at the newly forming dental enamel junction. As soon as the enamel matrix is secreted, this direct physical contact between ameloblasts and dentin is disrupted, and other matrix proteins such as ameloblastin may then take over the anchorage of ameloblasts to the underlying matrix.
In this study we have defined morphological differences between human and rodent incisors at the critical transition from presecretory to secretory ameloblasts, and identified roles of both extracellular matrix and dental mesenchyme on human ameloblast differentiation. These studies are important as in the future we seek to promote epithelial precursors to differentiate into dental lineage in vitro, toward the ultimate goal of engineering tooth structures.
4. Materials and methods
4.1 Human developing incisor morphological analysis
The heads of eight human fetuses ranging from 240 – 275 mm crown-rump-length (CRL), approximately 26th week, were fixed in Bouin’s solution according to standard histological procedures. The specimens were dehydrated in alcohol with increasing concentrations up to 100% following by decalcification using 10% EDTA for 30 days. The specimens were embedded in paraffin and sectioned in sagittal at 10μm thickness using a microtome (Leica, Reichert-Jung, RM 2065, Nussloch, Germany). The sections were stained with hematoxylin-eosin (H&E). The dental primordia were viewed and measured under the light microscope (Zeiss, Oberkochen, Germany). The human specimens were obtained from legal abortions according to German law.
4.2 In situ hybridization
In situ hybridization was done using human developing tooth incisors dissected from 20-24-wk-old fetal cadaver tissue under approved guidelines set by the University of California at San Francisco. The dissected mandibles were fixed with 4% paraformaldehyde, dehydrated with xylene and a graded ethanol series and then embedded in paraffin. Mandibles were sectioned at 10μm thickness and sections were mounted on RNase free glass slides. Probes were prepared by using PCR to amplify the corresponding gene sequences from a cDNA library derived from human developing tooth organs, and cloned into either PCR-II TOPO TA (type I collagen) or PCR-blunt II TOPO (amelogenin) cloning vectors (Invitrogen, Carlsbad, CA, USA). The sequences of the probes were confirmed by automated DNA sequencing. Sense and antisense riboprobes were labeled with 35S and in situ hybridization was done as previously described (Albrecht et al., 1997). Following in situ hybridization, the sections were counter-stained with a nuclear stain (Hoechst Stain; Sigma Aldrich, St. Louis, MO, USA). Hybridization signals were detected by darkfield optics, and the nuclear stain was visualized by epifluorescence.
4.3 Immunohistochemistry
Mandibles from human fetuses were embedded either in O.C.T. compound (Tissue-Tek, Hatfield, PA, USA) and sectioned at 10μm thickness. Masson’s trichrome staining was used for morphological analyses. For the immunofluorescences, the sections were incubated in blocking solution containing 0.1% bovine serum albumin, 3% goat serum and 0.1% Triton-X in phosphate-buffered saline (PBS) for 1h at room temperature after fixing. Antibodies were diluted with the same blocking solution. The primary antibodies including monoclonal mouse anti-type IV collagen (1:500) (Sigma-Aldrich), rabbit anti-recombinant ameloblastin (1:500) (Santa Cruz Biotechnology Inc., Santa Cruz, CA), rabbit anti-recombinant amelogenin (1: 500, purified by our laboratory), mouse anti-laminin 5 (1:500, Sigma-Aldrich) were incubated with sections overnight at 4°C. The primary antibody was detected by TRITC conjugated anti-mouse IgG secondary antibody (1:200) (Sigma Aldrich), or FITC conjugated anti-rabbit secondary antibody (1:160) (Sigma Aldrich). Nuclei were counterstained with 0.5ug/mL Hoechst 33342 (Invitrogen, Carlsbad, CA, USA) in the dark for 5 min. After mounting, the tissue sections were photographed with the Nikon Eclipse 300 fluorescence microscope (Compix Inc, Sewickley, PA, USA).
4.4 In vitro culture of ameloblast lineage cells and HEK 293 cells
Primary ameloblast lineage cells were isolated from 18 to 23-week-old human fetal tooth organs, as previously described (DenBesten, 2005; Yan et al., 2006; Zhang et al., 2007). Briefly, the tissue mass from these tooth organs was dispersed by the addition of 2mg/mL collagenase/dispase mixture dissolved in PBS at 37°C for 2 hr. The tissue mass was further digested with 0.05% trypsin with EDTA for 5 min at 37°C. Cells were selectively grown in supplemented keratinocyte growth medium (KGM-2) (Lonza, Walkersville, MD, USA) with 0.05 mM calcium, 1% penicillin and streptomycin on BD Primaria Tissue Culture Dishes (BD, Franklin Lakes, NJ, USA). HEK 293 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM), plus 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin. Cell morphology was observed with phase contrast microscopy and photographed by camera connected to microscopy. Passage one primary ameloblast lineage cells were used for all in vitro studies.
4.5 Three-dimensional culture of ameloblast lineage cells in Matrigel and co-culture with dental pulp cells
Matrigel™ Basement Membrane Matrix was purchased from BD Biosciences (BD Biosciences, San Jose, CA, USA). After the Matrigel was thawed on ice, the primary human ameloblast lineage cells were detached and mixed with Matrigel, then cultured on the top of the transwell membrane. Ameloblast lineage cells alone were cultured on the top of the transwell membrane as a control.
Pulp cells were isolated from extracted human adult molars as previous reported (Gronthos et al., 2002) and placed in the lower chamber of the transwell culture ware. Growth factor supplemented keratinocyte growth medium (KGM-2) was added to the cells on the top of transwell membrane. Adult pulp cells were maintained in the lower chamber in Dulbecco’s modified Eagle’s medium low glucose supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
4.6 Cell adhesion assay
Triplicate wells in a BD Falcon Primaria 96-well culture plate were coated with 100 μg/ml type I collagen (Sigma Aldrich) for 4°C overnight. Primary ameloblast lineage cells, dental pulp cells and HEK 293 cells were detached by 0.05% trypsin-EDTA and re-suspended to a concentration of 1×106 cells/ml. A Vybrant Cell Adhesion Assay Kit (Invitrogen) was used for the cell adhesion assay by following the manufacturer’s instruction. Briefly, ameloblast lineage cells at a concentration 1×106 cells/ml were labeled with a final concentration of 5 mol/L calcein AM, for 30 min at 37°C. Labeled cells were washed with pre-warmed culture media and re-suspended to 1×105 cells/ml. Then 1 × 104 cells/well were plated to the coated wells of a 96-well plate. Cells were incubated for 1 hr at 37°C to allow adhesion. Following this incubation, non-adherent calcein labeled cells were removed by careful washing. The total fluorescence per well was measured at EX/EM = 494/517 nm with a SpectraMax Gemini fluorescence plate reader (Molecular Devices, Sunnyvale, CA, USA).
To characterize the integrin receptors of type I collagen, an adhesion assay was conducted with the same protocol following a 45-min, 4°C pre-incubation of the 1×105 cells/ml calcein labeled ameloblast lineage cells with a final concentration of 10 g/ml of mouse anti-human α2β1 blocking antibody (Millipore, Billerica, MA, USA), mouse anti-human β1 (Santa Cruz Biotechnology Inc.), or normal mouse control IgG (Santa Cruz Biotechnology Inc.). Cell morphology was observed over 24 hours using phase contrast microscope and recorded by attached camera.
4.7 qPCR analysis of gene expression
Type I collagen at 100 g/ml was placed on a 6-cm culture dishes overnight to coat the surface of the culture ware. Excess protein was removed by rinsing the plates with PBS three times prior to plating cells. Ameloblast lineage cells (1.0 × 106 cells) were placed on the type I collagen coated or uncoated control dishes. After 24 hr incubation, ameloblast lineage cells were detached from the plates with incubation in 0.05% trypsin/EDTA for 5 min at 37°C. Cells grown in Matrigel were recovered from the gel by using Cell Recovery Solution following the manufacturer’s instruction (BD Biosciences). Total RNA was isolated from harvested cells using Qiagen’s RNAeasy Kit (Qiagen, Valencia, CA, USA). Purified RNA was quantified by UV spectroscopy on a Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA) and cDNA was synthesized using a Superscript III kit (Invitrogen). mRNA expression levels of target genes was determined by quantitative PCR. Reactions were performed with FastStart TaqMan Probe Master (ROX) (Roche Diagnostics, Indianapolis, IN, USA), and TaqMan® Gene Expression Assays (Applied Biosystems Inc., Foster City, CA, USA) using an ABI 7500 Real-Time PCR machine (Applied Biosystems Inc.). Amelogenin was used as an ameloblast differentiation marker, and PCNA was used as a marker for cell proliferation. GAPDH was used as an endogenous control. Conditions for PCR were 5 min at 94°C as an initial denaturing step, followed by 40 amplification cycles of 15 sec at 94°C and 1 min at 60°C.
To quantitate the relative expression levels of the target genes, the comparative CT (threshold cycle) method was used. The corresponding arithmetic formulas used are the following: ΔCT = CTtarget gene - CTGAPDH; and CTLinear =2-(ΔCTtreated-ΔCTcontrol). CTLinear represents the fold change in mRNA expression between the control and treated groups, with assuming a doubling of amplified product with each PCR cycle. All data were analyzed by either One-way ANOVA analysis or student t-test by using Prism software (GraphPad Software Inc, San Diego, CA, USA).
4.8 Western Blot
Ameloblast lineage cells grown on type 1 collagen coated and uncoated plates were harvested and lysed in RIPA lysis buffer (Millipore) containing a protease inhibitor cocktail (Sigma-Aldrich). Equal amounts of protein from each sample were loaded on 12% SDS-PAGE and then transferred to nitrocellulose membrane (GE Healthcare, Piscataway, NJ, USA). After 1 h of blocking with 5% non-fat milk, the membranes were probed with primary antibodies overnight at 4 °C. A control blot incubated with rabbit anti-human actin antibody (Santa Cruz Biotechnology Inc.) was used to normalize the amount of protein loaded in each sample. Primary antibodies used in this study were mouse anti-PCNA 1:100 (Santa Cruz Biotechnology Inc.) and rabbit anti-actin 1:400 (Santa Cruz Biotechnology Inc.). The membrane was washed and then probed with a species-specific horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature, followed by Amersham ECL Western Blotting Detection System (GE Healthcare). The intensities of the immunoreactive bands were scanned and measured with NIH Image, version 1.30.
Acknowledgments
This study was supported by NIH/NIDCR grant 1R21DE017910-01 to P.D.B., and NIDCR R01 DE016402 and NIAMS R21 AR052513 to R.A.S.. We thank Joseph Mendoza his assistance in illustrating figures 2 and 4A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Albrecht BE, Breitenbach U, Stühmer T, Harvey RJ, Darlison MG. In situ hybridization and reverse transcription--polymerase chain reaction studies on the expression of the GABA(C) receptor rho1- and rho2-subunit genes in avian and rat brain. Eur. J. Neurosci. 1997;9:2414–2422. doi: 10.1111/j.1460-9568.1997.tb01658.x. [DOI] [PubMed] [Google Scholar]
- Chen B, Goodman E, Lu Z, Bandyopadhyay A, Magraw C, He T, Raghavan S. Function of beta1 integrin in oral epithelia and tooth bud morphogenesis. J. Dent. Res. 2009;88:539–544. doi: 10.1177/0022034509338008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang Y, Mendoza J, DenBesten P. Calcium-mediated differentiation of ameloblast lineage cells in vitro. J. Exp. Zoolog. B. Mol. Dev. Evol. 2009;312:458–464. doi: 10.1002/jez.b.21279. [DOI] [PubMed] [Google Scholar]
- Delon I, Brown NH. The integrin adhesion complex changes its composition and function during morphogenesis of an epithelium. J. Cell Sci. 2009;122:4363–4374. doi: 10.1242/jcs.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DenBesten PK, Machule D, Zhang Y, Yan Q, Li W. Characterization of human primary enamel organ epithelial cells in vitro. Arch. Oral. Biol. 2005;50:689–694. doi: 10.1016/j.archoralbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, Krebsbach PH, Nanci A, Kulkarni AB, Yamada Y. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J. Cell. Biol. 2004;167:973–983. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Miner JH, Ida H, Fukumoto E, Yuasa K, Miyazaki H, Hoffman MP, Yamada Y. Laminin alpha5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J. Biol. Chem. 2006;281:5008–5016. doi: 10.1074/jbc.M509295200. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Yamada Y. Review: extracellular matrix regulates tooth morphogenesis. Connect. Tissue. Res. 2005;46:220–226. doi: 10.1080/03008200500344017. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Gehlsen KR, Turner DC, Ahlén K, Zijenah LS, Barnes MJ, Rubin K. Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1 integrins in cell--collagen interactions: identification of conformation dependent alpha 1 beta 1 binding sites in collagen type I. EMBO. J. 1992;11:3865–3873. doi: 10.1002/j.1460-2075.1992.tb05479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Karg HA, Burger EH, Lyaruu DM, Wöltgens JH, Bronckers AL. Gene expression and immunolocalisation of amelogenins in developing embryonic and neonatal hamster teeth. Cell Tissue Res. 1997;288:545–55. doi: 10.1007/s004410050840. [DOI] [PubMed] [Google Scholar]
- Le TQ, Zhang Y, Li W, Denbesten PK. The effect of LRAP on enamel organ epithelial cell differentiation. J. Dent. Res. 2007;86:1095–1099. doi: 10.1177/154405910708601114. [DOI] [PubMed] [Google Scholar]
- Liu H, Li W, Gao C, Kumagai Y, Blacher RW, DenBesten PK. Dentonin, a fragment of MEPE, enhanced dental pulp stem cell proliferation. J. Dent. Res. 2004;83:496–499. doi: 10.1177/154405910408300612. [DOI] [PubMed] [Google Scholar]
- Liu H, Li W, Shi S, Habelitz S, Gao C, Denbesten P. MEPE is downregulated as dental pulp stem cells differentiate. Arch. Oral. Biol. 2005;50:923–928. doi: 10.1016/j.archoralbio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Tucker AS, De Bari C, Cobourne MT, Rice DP. A regulatory relationship between Tbx1 and FGF signaling during tooth morphogenesis and ameloblast lineage determination. Dev. Biol. 2008;320:39–48. doi: 10.1016/j.ydbio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Miyamoto T, Wada K, Watatani S, Zhang GX. Type I collagen regulated dentin matrix protein-1 (Dmp-1) and osteocalcin (OCN) gene expression of rat dental pulp cells. J. Cell. Biochem. 2003;88:1112–1119. doi: 10.1002/jcb.10466. [DOI] [PubMed] [Google Scholar]
- Morotomi T, Kawano S, Toyono T, Kitamura C, Terashita M, Uchida T, Toyoshima K, Harada H. In vitro differentiation of dental epithelial progenitor cells through epithelial-mesenchymal interactions. Arch. Oral. Biol. 2005;50:695–705. doi: 10.1016/j.archoralbio.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Nanci A, Zalzal S, Lavoie P, Kunikata M, Chen W, Krebsbach PH, Yamada Y, Hammarstrom L, Simmer JP, Fincham AG, Snead ML, Smith CE. Comparative immunochemical analyses of the developmental expression and distribution of ameloblastin and amelogenin in rat incisors. J. Histochem. Cytochem. 1998;46:911–934. doi: 10.1177/002215549804600806. [DOI] [PubMed] [Google Scholar]
- Nagano T, Oida S, Ando H, Gomi K, Arai T, Fukae M. Relative levels of mRNA encoding enamel proteins in enamel organ epithelia and odontoblasts. J. Dent. Res. 2003;82:982–986. doi: 10.1177/154405910308201209. [DOI] [PubMed] [Google Scholar]
- Papagerakis P, MacDougall M, Hotton D, Bailleul-Forestier I, Oboeuf M, Berdal A. Expression of amelogenin in odontoblasts. Bone. 2003;32:228–240. doi: 10.1016/s8756-3282(02)00978-x. [DOI] [PubMed] [Google Scholar]
- Reznikoff CA, Loretz LJ, Pesciotta DM, Oberley TD, Ignjatovic MM. Growth kinetics and differentiation in vitro of normal human uroepithelial cells on collagen gel substrates in defined medium. J. Cell. Physiol. 1987;131:285–301. doi: 10.1002/jcp.1041310302. [DOI] [PubMed] [Google Scholar]
- Robinson C, Brookes SJ, Shore RC, Kirkham J. The developing enamel matrix: nature and function. Eur. J. Oral. Sci. 1998;106:282–291. doi: 10.1111/j.1600-0722.1998.tb02188.x. [DOI] [PubMed] [Google Scholar]
- Rocha V, Ringo DL, Read DB. Casein production during differentiation of mammary epithelial cells in collagen gel culture. Exp. Cell. Res. 1985;159:201–210. doi: 10.1016/s0014-4827(85)80049-5. [DOI] [PubMed] [Google Scholar]
- Salmivirta K, Sorokin LM, Ekblom P. Differential expression of laminin alpha chains during murine tooth development. Dev. Dyn. 1997;210:206–215. doi: 10.1002/(SICI)1097-0177(199711)210:3<206::AID-AJA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Shaw SK, Brenner MB. The beta 7 integrins in mucosal homing and retention. Semin Immunol. 1995;7:335–342. doi: 10.1016/1044-5323(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone. Miner. Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Smith CE. Cellular and chemical events during enamel maturation. Crit. Rev. Oral. Biol. Med. 1998;9:128–161. doi: 10.1177/10454411980090020101. [DOI] [PubMed] [Google Scholar]
- Smith CE, Pompura JR, Borenstein S, Fazel A, Nanci A. Degradation and loss of matrix proteins from developing enamel. Anat. Rec. 1989;224:292–316. doi: 10.1002/ar.1092240219. [DOI] [PubMed] [Google Scholar]
- Tabata MJ, Matsumura T, Fujii T, Abe M, Kurisu K. Fibronectin accelerates the growth and differentiation of ameloblast lineage cells in vitro. J. Histochem. Cytochem. 2004;51:1673–1679. doi: 10.1177/002215540305101211. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Barrach HJ, Foidart JM, Vaheri A, Pratt RM, Martin GR. Changes in the distribution of type IV collagen, laminin, proteoglycan, and fibronectin during mouse tooth development. Dev. Biol. 1981;81:182–192. doi: 10.1016/0012-1606(81)90361-4. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Hurmerinta K. Tissue interactions in tooth development. Differentiation. 1981;18:75–88. doi: 10.1111/j.1432-0436.1981.tb01107.x. [DOI] [PubMed] [Google Scholar]
- Timpl R. Macromolecular organization of basement membranes. Curr. Opin. Cell. Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- Yan Q, Zhang Y, Li W, DenBesten PK. Differentiation of human ameloblast-lineage cells in vitro. Eur. J. Oral. Sci. 2006;114:154–158. doi: 10.1111/j.1600-0722.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Fukumoto S, Kamasaki Y, Yamada A, Fukumoto E, Kanaoka K, Saito K, Harada H, Arikawa-Hirasawa E, Miyagoe-Suzuki Y, Takeda S, Okamoto K, Kato Y, Fujiwara T. Laminin alpha2 is essential for odontoblast differentiation regulating dentin sialoprotein expression. J. Biol. Chem. 2004;279:10286–10292. doi: 10.1074/jbc.M310013200. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat. Cell. Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li W, Chi HS, Chen J, Denbesten PK. JNK/c-Jun signaling pathway mediates the fluoride-induced down-regulation of MMP-20 in vitro. Matrix. Biol. 2007;26:633–641. doi: 10.1016/j.matbio.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]