Abstract
Stress urinary incontinence (SUI) has a significant impact on the quality of life for many women. Most women do not seek medical attention for this condition. Treatment for this problem includes initial conservative therapies and then surgery is an option. More than 200 surgical procedures have been described in the literature for the treatment of stress incontinence. The gold-standard surgical treatment of SUI in patients with a mobile bladder neck and normally functioning urethra has been accomplished through a retropubic approach using either a Burch or Marshall-Marchetti-Krantz procedure. By the absolute success of Trans obturator tape (TOT) application in treatment of SUI and the niche it has created for itself in the maze of treatment modalities available for SUI, there seems to be little doubt that TOT is all set to become the new Gold Standard for treatment of SUI in times to come. It is difficult to imagine any further improvements in the midurethral sling procedures or surgeries for SUI. However 10 years ago, no one could have imagined the progress and development that has been seen over these few short years in the treatment of SUI. The future may hold promise in technologies such as stem cells that may be injected in or around the urethral support structures and provide regeneration of the lacking support structures. What so ever, it's definitely time to provide millions of women with knowledge that empowers them to make lifestyle changes to decrease their risk of SUI and to understand the reality that they are not alone if they have SUI.
Keywords: Burch, female, incontinence, Kegel's exercises, stress urinary incontinence, trans obturator tape
INTRODUCTION
One out every three women will experience stress urinary incontinence (SUI) at some point in their life. Too many of them “just live with” the condition, too embarrassed to seek help or thinking that it is a “normal” part of aging and having children. As males consider discussing the subject of erectile dysfunction embarrassing, SUI is still not openly and publicly discussed by women; not even among some healthcare providers. We think it's high time to talk about SUI! We can’t leave the women wet for wrong reasons! In this review, we shall try to cover the spectrum of SUI from what it is, why it happens, how it happens, what to do once it happens, and a call upon all stakeholders in women's health care to be awake to the problem of incontinent women.
Issues involving women have always been surrounded with myths and mysteries. Physiological processes such as menstruation, which now seems so simple and obvious, were surrounded by superstitious beliefs and myths throughout recorded history. Just around two centuries ago, distinguished gynecologists and psychiatrists supported the practice of ovariotomy: the surgical removal of normal ovaries, for the treatment of “menstrual madness,” which equates with today's premenstrual dysphoric disorder.[1] Indeed, attitudes and ideas about female physiology have changed slowly. There are many myths about SUI. Examples of these include but are not limited to: “SUI is a normal part of being a woman.” “SUI is a normal, inevitable part of aging – it only happens to older, not younger women.” “Surgery is the only way to treat SUI.” “SUI can’t be treated.” “SUI surgery is not permanent and will only last a few years.” “My mother had SUI, so I have it – it's hereditary.” It is imperative on us, the women health care physicians, to remove these myths from the minds of women.
SUI has a significant impact on the quality of life for many women, although estimates of prevalence vary widely due to inconsistencies in the definitions of SUI and differences in populations studied.[2] Hampel et al., in a large meta-analysis in 1997[3] and then in 2004[4] reported an estimated prevalence for urinary incontinence of 30% in women aged 30–60 years, with approximately half of the cases attributed to SUI. Treatment for this problem include initial conservative therapies (i.e., life style interventions, pelvic floor muscle training, and bladder training), and then surgery is an option for women whose quality of life is still impaired after a diagnosis of genuine stress incontinence has been confirmed. Advances in surgical techniques have led to availability of a number of different procedures to treat stress incontinence.
SUI is defined by the International Continence Society as a condition defined by urodynamic observations associated with characteristic signs or symptoms.[5] Simply, it is involuntary loss of urine that occurs when physical forces on the bladder are increased during physical movement of the body. Urodynamic stress incontinence is defined as the involuntary leakage of urine during increased abdominal pressure in the absence of a detrusor contraction. Under the category of lower urinary tract symptoms, SUI is a storage disorder for which the characteristic symptom is the involuntary leakage of urine on effort or exertion, or on sneezing or coughing. The sign of SUI is the observation of involuntary leakage from the urethra synchronous with exertion or effort, such as sneezing or coughing. Current terminology refers to the condition described by both symptoms and urodynamic findings.
Stress incontinence has been divided into hypermobile stress incontinence, caused by anatomic defects, and intrinsic sphincter deficiency, with incontinence resulting from a poorly functioning urethra. This separation has become less distinct with time. SUI may include a wide spectrum of varying degrees of disruption of normal anatomy causing hypermobility or, somewhat paradoxically, scarring and fixation of these same tissues. Most experts in the field are of the opinion that there is a contribution of each kind of dysfunction in most patients.
Most women do not seek medical attention for this condition.[6] It is estimated that only one in four women will seek medical advice for incontinence due to embarrassment, limited access to health care, or poor screening by health care providers.[7] Urinary incontinence can significantly impair the quality of life, leading to disrupted social relationships, psychological distress from embarrassment and frustration, hospitalizations due to skin breakdown and urinary tract infection, and hospital admissions. SUI can affect intimate relationships and may limit sexual interaction.[8] An incontinent elderly woman is 2.5 times more likely to be admitted to a hospital than a continent one.[9]
RISKS FOR URINARY INCONTINENCE
Age
The prevalence gradually increases with age with a broad peak at middle age, which steadily increases after age 65.[7] The type of incontinence may differ by age; some studies suggest a higher prevalence of stress incontinence in women younger than 60 years and urge incontinence in older women.[7]
Race
Traditionally, caucasian women are believed to have higher rates of urinary incontinence than women of other races.[10] It is not yet clear whether these differences are biologic, related to health care access, or affected by cultural expectations and symptom tolerance thresholds. Since most of the studies have been conducted on caucasian population, further studies of non-caucasian populations are needed. The authors urge women health care physicians around the globe to come forward in this direction.
Obesity
Increased body mass index (BMI) is a significant and independent risk factor for SUI.[11] Evidence suggests that the prevalence of both urge and stress incontinence increases proportionately with BMI.[12] Theoretically, the increase in intra-abdominal pressure that coincides with an increased BMI results in a proportionally higher intravesical pressure, which overcomes urethral closing pressure and leads to incontinence.[13] Deitel reported a decline in the prevalence of SUI, from 61 to 11%, in 138 morbidly obese women following weight loss.[14]
Menopause
Few studies suggest an increase in urinary dysfunction after menopause.[15] However in these, separating hypoestrogenic effects from the effects of aging is difficult. High-affinity estrogen receptors have been identified in the urethra, pubococcygeal muscle, and bladder trigone.[16] Hypoestrogenic collagen changes and reductions in urethral vascularity and volume of skeletal muscle collectively may contribute to impaired urethral function via a decreased resting urethral pressure.[17]
Childbirth and pregnancy
SUI is more in parous compared with nulliparous women. The effects of childbirth on incontinence may result from direct injury to pelvic muscles and connective tissue attachments and also nerve damage from trauma or stretch.[18]
Smoking and chronic lung disease
Women older than 60 years with chronic obstructive pulmonary disease are at a high risk for SUI.[19,20] Similarly, cigarette smoking is also an independent risk.[21] With number of women smoking in India and other nations increasing, this is a cause of concern.
Hysterectomy
Hysterectomy has been shown inconsistently to be a risk factor for developing urinary incontinence.[15] However, evidence neither supports avoidance of clinically indicated hysterectomy nor performance of supracervical hysterectomy as measures to prevent urinary incontinence.[22,23]
PATHOPHYSIOLOGY
How continence is maintained
The bladder is a storage organ of urine with the capacity to accommodate large increases in urine volume with minimal increases in intravesical pressure. The ability to maintain urine storage with convenient and socially acceptable voluntary emptying is continence. It requires complex coordination of multiple components that include muscle contraction and relaxation, appropriate connective tissue support, and integrated innervation of and communication between these structures. Simplistically, during filling, urethral contraction is coordinated with bladder relaxation and urine is stored. In turn, during voiding, the urethra relaxes and the bladder contracts. These mechanisms can be challenged by uninhibited detrusor contractions, marked increases in intra-abdominal pressures, and changes to the various anatomic components of the continence mechanism.
Continence theories
Theories on continence are abundant and involve concepts relating to pressure transmission, anatomic support, and urethral integrity. Precisely dissecting the mechanism behind incontinence is difficult, thus artificial separation of etiology may provide little value to the general practitioner. Continence can be conceptualized in terms of urethral support and urethral integrity.
Pressure transmission
In an ideally supported urogenital tract, increases in intra-abdominal pressure are equally transmitted to the bladder, bladder base, and urethra. In women who are continent, increases in downward-directed pressure from cough, laugh, sneeze, and valsalva maneuver are countered by supportive tissue tone provided by the levator ani muscle and vaginal connective tissue. In those with a weakened supportive “backboard,” however, downward forces are not countered [Figure 1]. This leads to funneling of the urethrovesical junction, a patent urethra, and in turn, urine leakage.
Figure 1.
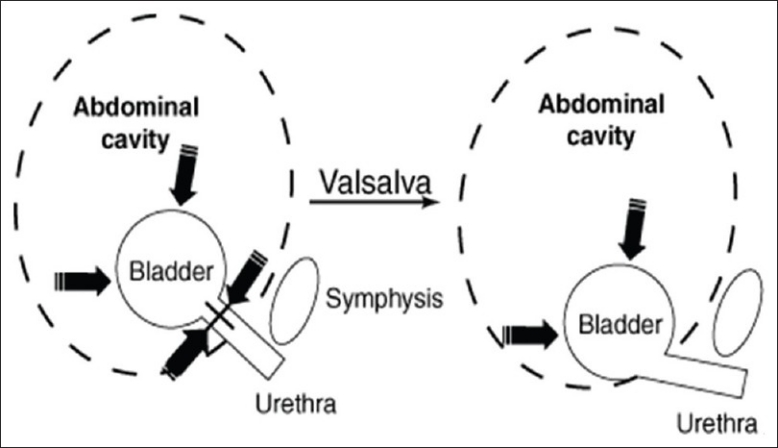
A weakened supportive “backboard” can not counter the downward forces due to raised intra-abdominal pressures
This mechanistic theory is the basis for surgical re-establishment of this support. Procedures such as burch colposuspension are used to recreate this support.
Urethral support
Urethral support is integral to continence. This support is derived from: (1) ligaments along the lateral aspects of the urethra, termed the pubourethral ligaments; (2) the vagina and its lateral fascial condensation; (3) the arcus tendinous fascia pelvic; and (4) levator ani muscles. Loss of urethral support results in reduced urethral closing pressures, inability to resist increases in bladder pressure and finally, incontinence.
MANAGEMENT OF SUI
Before considering the operative approaches to the treatment of stress incontinence, it is reasonable to discuss other means of management. The first-line therapies commence with lifestyle interventions, which include weight reduction, smoking cessation, and dietary and fluid modification. Estrogen deficiency requires treatment followed by reassessment. Supervised pelvic floor muscle training (PFMT) and bladder training are recommended. The key word here is supervised.[24] An appropriate duration of therapy is 8–12 weeks before reassessment for further treatment. Vaginal support devices can be included in the treatment options, depending on availability of the product, ability of the patient to manage the product, patient acceptance, and cost.
PFMT, which is directed toward the strengthening of the levator ani and pubococcygeal muscles, can be affected by isometric exercises as described by Kegel.[25] Although a number of modifications of these exercises exist, one useful application is to teach the patient to contract these muscles for the count of 10, 5–10 times, and to repeat this several times a day. Kegel originally suggested that the patient contract her pubococcygeal muscles 5 times on waking, 5 times on rising, and 5 times every half hour throughout the day. The patient can be instructed on how to contract these muscles by being told to attempt to stop the urinary stream while she is voiding. After she learns which muscles to contract, she may perform the exercises at any time without any relationship to voiding.
It is important to locate the correct muscles because exercising the wrong muscles will not help incontinence and can actually make matters worse. There are several ways for this. One way is to squeeze the muscles of the anus as to prevent passing gas will help to locate the pelvic floor muscles. Women should feel a “pulling” sensation at the anus when using this technique. In another technique, a woman can lie down and insert a finger into her vagina, trying to squeeze the muscles around her finger. She should be able to feel the sensation in her vagina and feel the pressure on her finger.
Biofeedback is another method of teaching pelvic floor muscle control that uses special instruments that measure what the muscles are doing. Electrical stimulation is still another way to teach women how to locate and control pelvic floor muscles. Usually, a small probe is put in the vagina and used to contract the muscles electrically. This helps her to find the right muscles and know how it feels when they are squeezing.
Role of surgery
More than 200 surgical procedures have been described in the literature for the treatment of stress incontinence. This reflects a combination of the alteration of techniques and approaches of established and effective procedures and the introduction of newer technologies and materials.
Surgical techniques do involve anterior repair started by Schultz in 1870, followed by Kelly in form of Kelly's plication in 1913 in which he used a wedge of tissue to support ureterovesical junction.[26] However, in today's surgical practice, it is well established that performing an anterior repair or Kelly plication for the treatment of SUI is substandard compared with more effective procedures, and is aoption for women who prefer to sacrifice some chance of becoming continent for a reduced chance of complication.[27] Surgical repair for SUI by placing a material under the urethra and suspending it to the abdominal tissues was introduced as early as 1907 when Giordano used gracilis muscle transposed beneath the bladder neck.[28] Zoedler was the first to use a synthetic sling in form of a gauze hammock in 1961.[29]
The fascial sling acts like a hammock under the bladder neck to both elevate the urethrovesical junction into an intraabdominal location and to provide partial compression of the urethra. These techniques differ from the modern tension-free procedures because the ends of the sling or suspending sutures are fixed to the rectus fascia. Variations of the sling procedure in which the ends of the sling are attached to an immovable tissue (Cooper's ligament or bone anchors in the pubic symphysis) do not allow upward displacement of the sling and urethra during straining. In these operations, the sling is thought to create a secure platform of urethral support. Increases in intraabdominal pressure press the urethra downward against the sling, thereby compressing the urethra from both above and below. It is this compression of the urethra that is believed to lead to increases in urethral resistance and a resolution of stress incontinence. However, the potential for excess compression of the urethra also contributes to the most common complications of the sling procedure: Voiding dysfunction.
In the maze of surgeries for treatment of SUI, paravaginal repair was originally described by White in 1909,[30] and renewed by Richardson almost a quarter century later in 1981.[31] However, in view of the authors, this procedure is for correction of lateral defect cystocele and not for treatment of SUI. Although, came in a big way, but because of significant recurrence rates at even 1 and 2 years of follow-up, long-needle procedures such as the Peyera, Stamey, or others are not recommended.[32,33]
The gold-standard surgical treatment of SUI has been a retropubic approach using either a Burch retropubic urethropexy[34] or Marshall-Marchetti-Krantz (MMK) procedure.[35] Vancaillie in 1991 performed laparoscopic Burch.[36] Various modifications to the original Burch procedure were described, including using only two sutures; substituting mesh; or using tacks, anchors, and other tools to elevate the bladder neck, which lowered cure rates as compared with traditional open urethropexy. Placement of four permanent sutures identical to an open procedure, though, has yielded similar cure rates as an open Burch.
Although retropubic urethropexy has been widely used and is very effective, the newer midurethral, tension-free sling procedures are very popular, effective, and easier to perform. Tension-free slings are surgical procedures using a polypropylene mesh to support the midurethra without tension, a technique first described by Ulmsten et al.[37] The original technique uses a retropubic approach, but the transobturator approach, as described by Delorme later in year 2001 is fast becoming the most common tension-free sling technique performed worldwide for primary SUI.[38]
The major impetus for use of periurethral bulking came in 1994 when the U.S. Food and Drug Administration approved the use of Contigen®.[39] The criteria for their usage include the presence of immobility of the bladder neck, as well as a leak point pressure less than 100 cm of water. The ideal patient is one who meets the above criteria with a fixed bladder neck (Q-tip straining angle 40 degrees or less), who is medically compromised, and in whom an operative intervention may offer too much risk.
Midurethral slings provide several advantages. First, these techniques are effective and short-term cure rates approximate 90 percent.[40] Of the two, retropubic and transobturator approaches appear to offer comparable short-term continence results.[41–43]
Ward and Hilton[44] demonstrated that Gynecare trans vaginal tape (TVT) superseded the previous gold standard intervention, Burch colposuspension, and thereby set a new benchmark. Nevertheless, it was substantial surgery that carried a 7% bladder perforation rate and therefore demanded cystoscopy.[45] Simple local analgesia was insufficient and an office procedure was not possible.
The next generation of slings placed a similar device under the urethra, but now exited much more laterally through the medial obturator foramen in the top of the leg. A new mechanism of curative action was suggested. A gentler subfascial hammock was created by transobturator tapes, rather than the creation of a pubourethral neoligament and restoration of intra-abdominal pressure transmission that was the hallmark of a retropubic sling. A near-zero bladder perforation rate obviated the need for check cystoscopy. Barber and colleagues recently suggested that transobturator tapes are “not inferior” to retropubic tapes at 18 months follow-up.[46]
Trans obturator tape
By the absolute success of trans obturator tape (TOT) application in treatment of SUI and the niche it has created for itself in the maze of treatment modalities available for SUI, there seems to be little doubt that TOT is all set to become the new Gold Standard for treatment of SUI in times to come. It was in the Netherlands in 1998 that Nickel et al., reported a successful sling procedure using a polyester ribbon passed through the obturator foramen and around the urethra for treatment of refractory urethral sphincter incompetence in female dogs.[47] In France in 2001, Delorme introduced the transobturator sling procedure in humans.[38] Dargent et al., in 2002 then performed the operation in 71 patients using a technique inspired by Delorme, and found the short-term results similar to those of the TVT.[48] The TOT has two original features: Its non-woven polypropylene structure is coated with silicone on the urethral surface in order to limit retraction of polypropylene and to establish a barrier to extension of periurethral fibrosis; and a transmuscular insertion, through the obturator and puborectalis muscles, reproduces the natural suspension fascia of the urethra while preserving the retropubic space.
TOT mimics normal anatomy
The transobturator sling forms a subfascial hammock of support under the urethra and mimics the normal position of the pubourethral ligament [Figure 2]. This ligament provides the backboard of support to help prevent urinary leakage with stress events such as coughing, laughing, sneezing, exercising, etc. When this ligament is damaged or stretched out, stress urinary leakage may ensue. The angle of the TOT sling is much less acute than the traditional pubovaginal sling procedures such as the TVT, therefore not only is this more anatomic and natural, it also makes sense that there is less problems with urinary dysfunction such as urinary obstruction.
Figure 2.
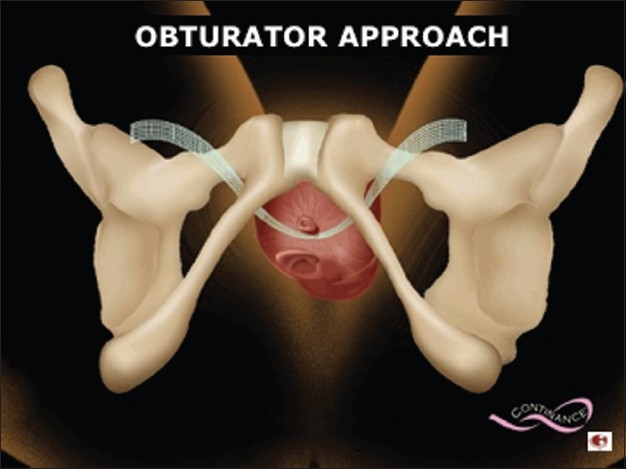
Transobturator tape forming a subfascial support under the urethra
One of the important and not well-recognized advantages of the TOT as compared to other mid urethral sling procedures is the lower rate of de novo urge incontinence. The lower incidence of de novo urgency may be explained by two factors. In the transobturator approach, the path of the tape, crossing the obturator foramen, muscle and fascia, reproduces the natural suburethral suspension by reinforcing the rotational pivot point, restoring continence while sparing the rertropubic space. Sparing the retropubic space may preserve any periurethral nerve fibers that may be associated with urethral function and stability. Second, the TOT is associated with a lower risk of urethral obstruction as compared to other mid urethral sling procedures.
The TVT mid urethral sling is associated with serious though rare complications including intestinal perforation, vascular injury, obturator nerve injury, and even death.[49] The transobturator sling procedure spares the retropubic space and thus eliminates these risks. As far as sexual activity is concerned, there is no significant change in patients’ sexual life as regards frequency of intercourse and pleasure, whereas there is significant decrease in coital incontinence.[50] In a big study conducted by Magon and Chopra on Indian population with the lead author of this review being the principal investigator, TOT application was successful in 93.2 percent cases with 86.4% of patients completely satisfied with the surgical outcome.[51] The mean age of the patients operated for SUI under this study was 46.2 years, which is so very close to the age of menopause of Indian women.
FUTURE POTENTIAL
Treatment for female SUI has seen revolutionary changes in the last ten years, with new minimally invasive techniques that have been proven safe and effective. The latest in the logical progression of synthetic slings used in the minimally invasive treatment of SUI was mini-sling. Barring the rare complication of groin pain, the risk of transobturator sling complications seemed to be very low. However, since the quest for advancement and for getting better than the best comes naturally to humans, the next step toward a less invasive, tension-free, mid-urethral sling was to develop a system that could be placed through one small vaginal incision without having to pass needles through the abdomen or groin. Because of the relatively new market introductions of the mini-slings (TVT-Secur™ in 2006 and MiniArc™ in 2007), there are limited published data available for these. Overall, short-term results with the TVT-Secur™ have not been very encouraging and have not been shown as effective as either the retropubic or transobturator sling approach. Cure rates have been reported in the range 69% to 83% in short-term follow-up, with a significant learning curve reported to be required for maximal results.[52] Technologies for the treatment of female SUI will certainly not stop with this. Anecdotal and early scientific reports of positive outcomes with short-term follow-up seem to reinforce the idea that the mini-sling concept may be the next generation of pubovaginal slings for female SUI. It may well be that this new technology is the next obvious step in the “smaller-is-better” concept.
The next step beyond the needleless, single small vaginal incision technique could perhaps be the total elimination of any skin incision at all. Although treatment of female SUI without surgical intervention may be heresy to surgeons, patients would be eternally grateful to avoid the knife, regardless of how small the incision has become. Recent developments in radiofrequency technology have created opportunities in controlled scarring of paraurethral tissue in an effort to create support for the hypermobile urethra.[53] The Renessa™ (Novasys Medical, Newark, CA) device uses radiofrequency to heat the inside of the urethra to treat mild SUI in an office setting. This procedure may provide “gap” coverage for women who want to delay definitive treatment of SUI that requires surgical intervention, or for women with just mild SUI, as the current results do not support its use in more severe SUI. The future may hold promise in technologies such as stem cells that may be injected in or around the urethral support structures and provide regeneration of the lacking support structures. It is difficult to imagine any further improvements in the midurethral sling procedures or surgeries for SUI. However ten years ago, no one could have imagined the progress and development that has been seen over these few short years in the treatment of SUI.
CONCLUSION
Understanding the past not only helps us appreciate how far we have come, but may also point out that change comes with a price, metaphorically and realistically. Advances in surgical treatment for SUI via pubovaginal slings have provided physicians and patients with many opportunities and advantages: decreased surgical time, decreased patient morbidity and shortened recovery time, improved outcomes including quality of life, and limited or acceptable complications. Treatment for female SUI has seen revolutionary changes in the last 10 years, with new minimally invasive techniques that have been proven safe and effective. The TVT sling was first developed and then the TOT sling followed, which provided a safer means to place a tension-free mesh tape sling with seemingly equivalent cure rates and lower rates of voiding dysfunction. Undoubtedly, even with the advent of single-incision mini-slings, the search for newer procedures and technologies will and shall continue. But as we have seen with the advancements that have evolved over the last decade, there can sometimes be confounding side effects and unforeseen consequences caused by the changes created by new and improved technologies. Retropubic slings clearly offered distinct advantages over the earlier retropubic urethropexy-type procedures in minimizing the surgical incisions and decreasing postoperative morbidity. But the retropubic sling procedure sometimes produced complications by perforating unwanted tissues like urinary bladder during the procedure. Likewise, the TOT sling has not been cent percent risk-free as groin pain has been reported in some approach. It is too early to comment on the mini-slings as to any possible unforeseen complications that may occur when the product is implanted in a volume of patients, and thus further study via random controlled trials are indicated. However, there seems to be one remaining fact—that female SUI will be an ongoing pathology in search of improved treatment options and more efficacious outcomes with minimal patient morbidity.
Whatsoever treatment is given should suffice an individual patient's needs and aspirations for cure. Women who seek medical advice should feel comfortable and equipped to discuss their condition, as well as management and treatment options. This effort shall be a major step toward removing the unnecessary stigma associated with SUI and to better serve our patients. It's time to talk about SUI! It's time to not let the women wet for the wrong reasons! It's time for all to take action! It's time to enlighten the general public about SUI. It's time to educate healthcare professionals so that they can improve the quality of care provided to patients. It's time to provide millions of women with knowledge that empowers them to make lifestyle changes to decrease their risk of SUI and to understand the reality that they are not alone if they have SUI.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Magon N. Gonadotropin releasing hormone agonists: Expanding vistas. Indian J Endocrinol Metab. 2011;15:261–7. doi: 10.4103/2230-8210.85575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luber KM. The definition, prevalence, and risk factors for stress urinary incontinence. Rev Urol. 2004;6(Suppl 3):S3–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Hampel C, Wienhold D, Benken N, Eggersmann C, Thüroff JW. Definition of overactive bladder and epidemiology of urinary incontinence. Urology. 1997;50(6A Suppl):4–14. doi: 10.1016/s0090-4295(97)00578-5. discussion 15-7. [DOI] [PubMed] [Google Scholar]
- 4.Hampel C, Artibani W, Espuña Pons M, Haab F, Jackson S, Romero J, et al. Understanding the burden of stress urinary incontinence in Europe: A qualitative review of the literature. Eur Urol. 2004;46:15–27. doi: 10.1016/j.eururo.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–26. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 6.Hunskaar S, Arnold EP, Burgio K, Diokno AC, Herzog AR, Mallett VT. Epidemiology and natural history of urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:301–19. doi: 10.1007/s001920070021. [DOI] [PubMed] [Google Scholar]
- 7.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: The Norwegian EPINCONT study.Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150–7. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 8.Mallett VT, Brubaker L, Stoddard AM, Borello-France D, Tennstedt S, Hall L, et al. The expectations of patients who undergo surgery for stress incontinence. Am J Obstet Gynecol. 2008;198:308.e1–6. doi: 10.1016/j.ajog.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Langa KM, Fultz NH, Saint S, Kabeto MU, Herzog AR. Informal caregiving time and costs for urinary incontinence in older individuals in the United States. J Am Geriatr Soc. 2002;50:733–7. doi: 10.1046/j.1532-5415.2002.50170.x. [DOI] [PubMed] [Google Scholar]
- 10.Bump RC. Racial comparisons and contrasts in urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 1993;81:421–5. [PubMed] [Google Scholar]
- 11.Parazzini F, Colli E, Origgi G, Surace M, Bianchi M, Benzi G, et al. Risk factors for urinary incontinence in women. Eur Urol. 2000;37:637–43. doi: 10.1159/000020231. [DOI] [PubMed] [Google Scholar]
- 12.Hannestad YS, Rortveit G, Daltveit AK, Hunskaar S. Are smoking and other lifestyle factors associated with female urinary incontinence.The Norwegian EPINCONT Study? BJOG. 2003;110:247–54. [PubMed] [Google Scholar]
- 13.Bai SW, Kang JY, Rha KH, Lee MS, Kim JY, Park KH. Relationship of urodynamic parameters and obesity in women with stress urinary incontinence. J Reprod Med. 2002;47:559–63. [PubMed] [Google Scholar]
- 14.Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr. 1988;7:147–53. doi: 10.1080/07315724.1988.10720232. [DOI] [PubMed] [Google Scholar]
- 15.Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998;25:723–46. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 16.Iosif CS, Batra S, Ek A, Astedt B. Estrogen receptors in the human female lower urinary tract. Am J Obstet Gynecol. 1981;141:817–20. doi: 10.1016/0002-9378(81)90710-9. [DOI] [PubMed] [Google Scholar]
- 17.Carlile A, Davies I, Rigby A, Brocklehurst JC. Age changes in the human female urethra: A morphometric study. J Urol. 1988;139:532–5. doi: 10.1016/s0022-5347(17)42512-2. [DOI] [PubMed] [Google Scholar]
- 18.Snooks SJ, Swash M, Henry MM, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. Int J Colorectal Dis. 1986;1:20–4. doi: 10.1007/BF01648831. [DOI] [PubMed] [Google Scholar]
- 19.Brown JS, Seeley DG, Fong J, Black DM, Ensrud KE, Grady D. Urinary incontinence in older women: Who is at risk? Study of osteoporotic fractures research group. Obstet Gynecol. 1996;87:715–21. doi: 10.1016/0029-7844(96)00013-0. [DOI] [PubMed] [Google Scholar]
- 20.Diokno AC, Brock BM, Herzog AR, Bromberg J. Medical correlates of urinary incontinence in the elderly. Urology. 1990;36:129–38. doi: 10.1016/0090-4295(90)80211-5. [DOI] [PubMed] [Google Scholar]
- 21.Bump RC, McClish DK. Cigarette smoking and urinary incontinence in women. Am J Obstet Gynecol. 1992;167:1213–8. doi: 10.1016/s0002-9378(11)91691-3. [DOI] [PubMed] [Google Scholar]
- 22.Vervest HA, van Venrooij GE, Barents JW, Haspels AA, Debruyne FM. Non-radical hysterectomy and the function of the lower urinary tract.II: Urodynamic quantification of changes in evacuation function. Acta Obstet Gynecol Scand. 1989;68:231–5. doi: 10.3109/00016348909020994. [DOI] [PubMed] [Google Scholar]
- 23.Wake CR. The immediate effect of abdominal hysterectomy on intravesical pressure and detrusor activity. Br J Obstet Gynaecol. 1980;87:901–2. doi: 10.1111/j.1471-0528.1980.tb04445.x. [DOI] [PubMed] [Google Scholar]
- 24.Bump RC, Hurt WG, Fantl JA, Wyman JF. Assessment of Kegel pelvic floor muscle exercise performance after brief verbal instruction. Am J Obstet Gynecol. 1991;165:322–7. doi: 10.1016/0002-9378(91)90085-6. discussion 327-9. [DOI] [PubMed] [Google Scholar]
- 25.Kegel AH. Stress incontinence of urine in women: Physiologic treatment. J Int Coll Surg. 1956;25:487–99. [PubMed] [Google Scholar]
- 26.Kelly HA. Incontinence of urine in women. Urol Cutaneous Rev. 1913;17:291. [Google Scholar]
- 27.ACOG Practice Bulletin Number 63. Urinary Incontinence in Women. 2005 [Google Scholar]
- 28.Von Giordano D. 20 ieme Congres Franc de Chirug. 1907:506. [Google Scholar]
- 29.Zoedler D. On surgical treatment of stress incontinence in women. Z Urol. 1961;54:355–8. [PubMed] [Google Scholar]
- 30.White GR. Cystocele–a radical cure by suturing lateral sulci of the vagina to the white line of pelvic fascia. 1909. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:288–92. doi: 10.1007/BF02765486. [DOI] [PubMed] [Google Scholar]
- 31.Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357–62. [PubMed] [Google Scholar]
- 32.Pereyra AJ. A simplified surgical procedure for the correction of stress incontinence in women. West J Surg Obstet Gynecol. 1959;67:223–6. [PubMed] [Google Scholar]
- 33.Stamey TA. Endoscopic suspension of the vesical neck for urinary incontinence. Surg Gynecol Obstet. 1973;136:547–54. [PubMed] [Google Scholar]
- 34.Burch JC. Cooper's ligament urethrovesical suspension for stress incontinence. Am J Obstet Gynecol. 1968;100:764–74. doi: 10.1016/s0002-9378(15)33576-6. [DOI] [PubMed] [Google Scholar]
- 35.Marshall VF, Marchetti AA, Krantz KE. The correction of stress incontinence by simple vesicourethral suspension. Surg Gynecol Obstet. 1949;88:509–18. [PubMed] [Google Scholar]
- 36.Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169–73. doi: 10.1089/lps.1991.1.169. [DOI] [PubMed] [Google Scholar]
- 37.Ulmsten M, Henriksson L, Johnson P, Varghos G. An ambulatory surgical procedure under local anaesthesia for treatment of female urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:81–5. doi: 10.1007/BF01902378. discussion 85-6. [DOI] [PubMed] [Google Scholar]
- 38.Delorme E. Transobturator urethral suspension: Mini-invasive procedure in the treatment of stress urinary incontinence in women. Prog Urol. 2001;11:1306–13. [PubMed] [Google Scholar]
- 39.McGuire EJ, Appell RA. Transurethral collagen injection for urinary incontinence. Urology. 1994;43:413–5. doi: 10.1016/0090-4295(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 40.Lim JL, Cornish A, Carey MP. Clinical and quality-of-life outcomes in women treated by the TVT-O procedure. BJOG. 2006;113:1315–20. doi: 10.1111/j.1471-0528.2006.01095.x. [DOI] [PubMed] [Google Scholar]
- 41.deTayrac R, Deffieux X, Droupy S, Chauveaud-Lambling A, Calvanèse-Benamour L, Fernandez H. A prospective randomized trial comparing tension-free vaginal tape and transobturator suburethral tape for surgical treatment of stress urinary incontinence. Am J Obstet Gynecol. 2004;190:602–8. doi: 10.1016/j.ajog.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 42.Sung VW, Schleinitz MD, Rardin CR, Ward RM, Myers DL. Comparison of retropubic vs transobturator approach to midurethral slings: A systematic review and meta-analysis. Am J Obstet Gynecol. 2007;197:3–11. doi: 10.1016/j.ajog.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laurikainen E, Valpas A, Kivelä A, Kalliola T, Rinne K, Takala T, et al. Retropubic compared with transobturator tape placement in treatment of urinary incontinence: A randomized controlled trial. Obstet Gynecol. 2007;109:4–11. doi: 10.1097/01.AOG.0000249607.82768.a1. [DOI] [PubMed] [Google Scholar]
- 44.Ward K, Hilton P. United Kingdom and Ireland Tension. free Vaginal Tape Trial Group. Prospective multicentre randomised trial of tension.free vaginal tape and colposuspension as a primary treatment for stress incontinence. BMJ. 2002;325:67. doi: 10.1136/bmj.325.7355.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monga A, Duval B, Espuna M, Haab F, Quaas L. Presented at: International Continence Society 33rd Annual Meeting. Florence, Italy: 2003. Oct 5-9, The SPARC procedure for urodynamic stress incontinence: A multicentre prospective study with 1 year follow up. [Google Scholar]
- 46.Barber MD, Kleeman S, Karram MM, Paraiso MF, Walters MD, Vasavada S, et al. Transobturator tape compared with tension-free vaginal tape for the treatment of stress urinary incontinence: A randomized controlled trial. Obstet Gynecol. 2008;111:611–21. doi: 10.1097/AOG.0b013e318162f22e. [DOI] [PubMed] [Google Scholar]
- 47.Nickel RF, Wiegand U, van den Brom WE. Evaluation of a transpelvic sling procedure with and without colposuspension for treatment of female dogs with refractory urethral sphincter mechanism incompetence. Vet Surg. 1998:94–104. doi: 10.1111/j.1532-950x.1998.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 48.Dargent D, Bretones S, George P, Mellier G. Insertion of a sub-urethral sling through the obturating membrane for treatment of female urinary incontinence. Gynecol Obstet Fertil. 2002;30:576–82. doi: 10.1016/s1297-9589(02)00389-2. [DOI] [PubMed] [Google Scholar]
- 49.Leboeuf L, Tellez CA, Ead D, Gousse AE. Complication of bowel perforation during insertion of tension-free vaginal tape. J Urol. 2003;170:1310. doi: 10.1097/01.ju.0000087616.63244.28. discussion 1310-1. [DOI] [PubMed] [Google Scholar]
- 50.Abdel-fattah M, Ramsay I, Pringle S, Bjornsson S, Hardwick C, Tierney J, et al. Transobturator suburethral tapes in the management of urinary incontinence: Success, safety and impact on sexual life. Gynecol Surg. 2007;4:267–73. [Google Scholar]
- 51.Magon N, Chopra S. Trans obturator tape in treatment of stress urinary incontinence: Is it time for a new gold standard. doi: 10.4103/1947-2714.95905. (Unpublished data, submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karram M, Lucente V, Kandwhala S, Nilsson C, Artibani W, Dmochowski R. An evaluation of the Gynecare Secur System (tension-free support for incontinence) for the treatment of stress urinary incontinence. Int Urogynecol J. 2007;18(Suppl 1):S3. [Google Scholar]
- 53.Appell RA, Singh G, Klimberg IW, Graham C, Juma S, Wells WG, et al. Nonsurgical, radiofrequency collagen denaturation for stress urinary incontinence: Retrospective 3-year evaluation. Expert Rev Med Devices. 2007;4:455–61. doi: 10.1586/17434440.4.4.455. [DOI] [PubMed] [Google Scholar]


