Abstract
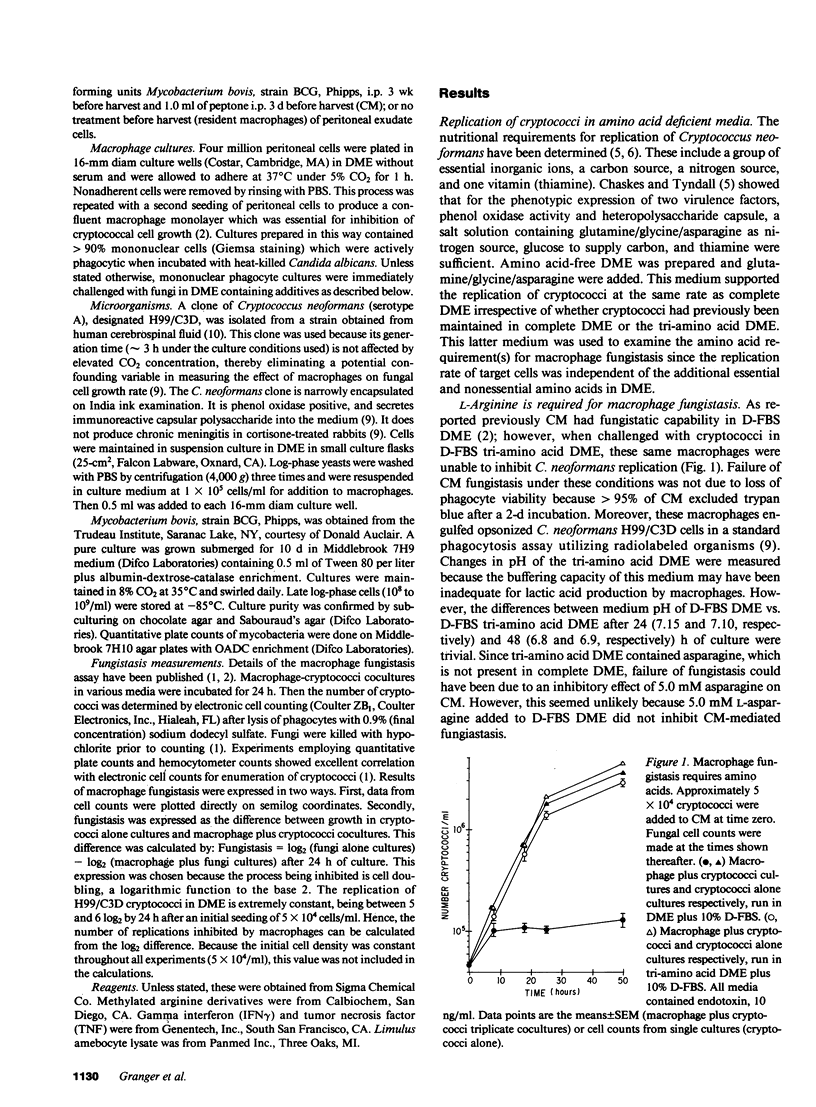
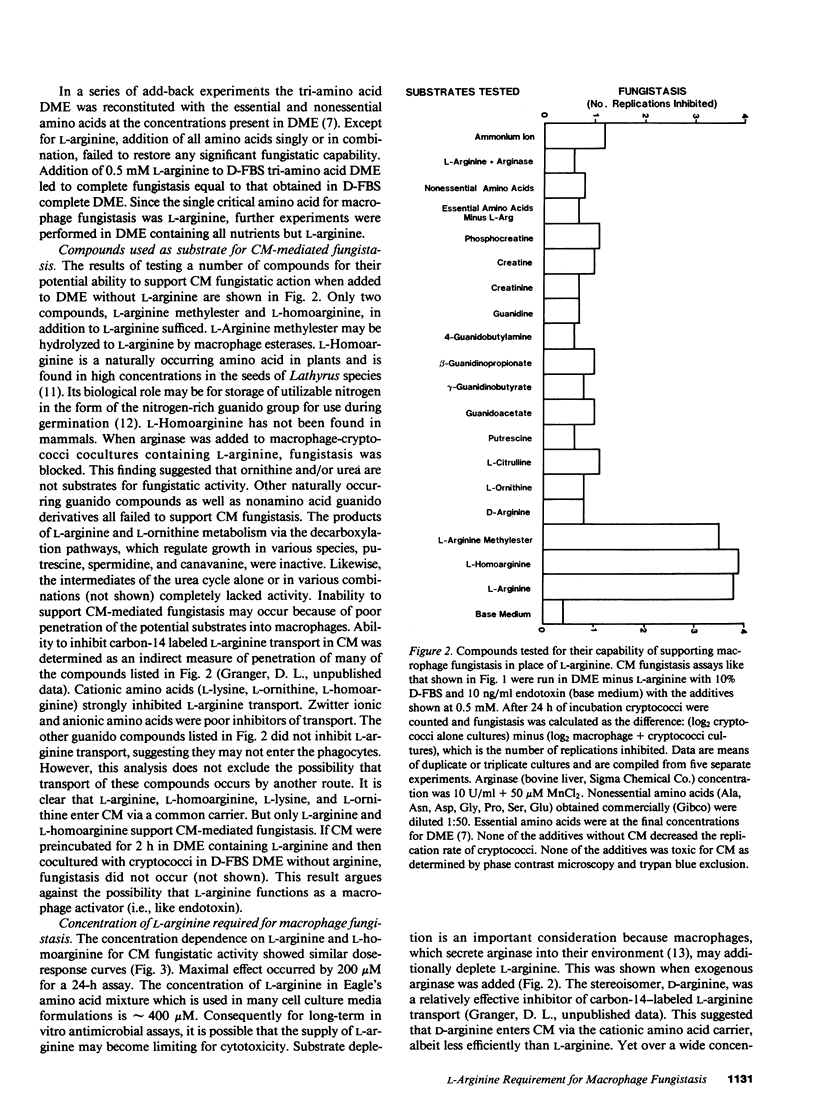
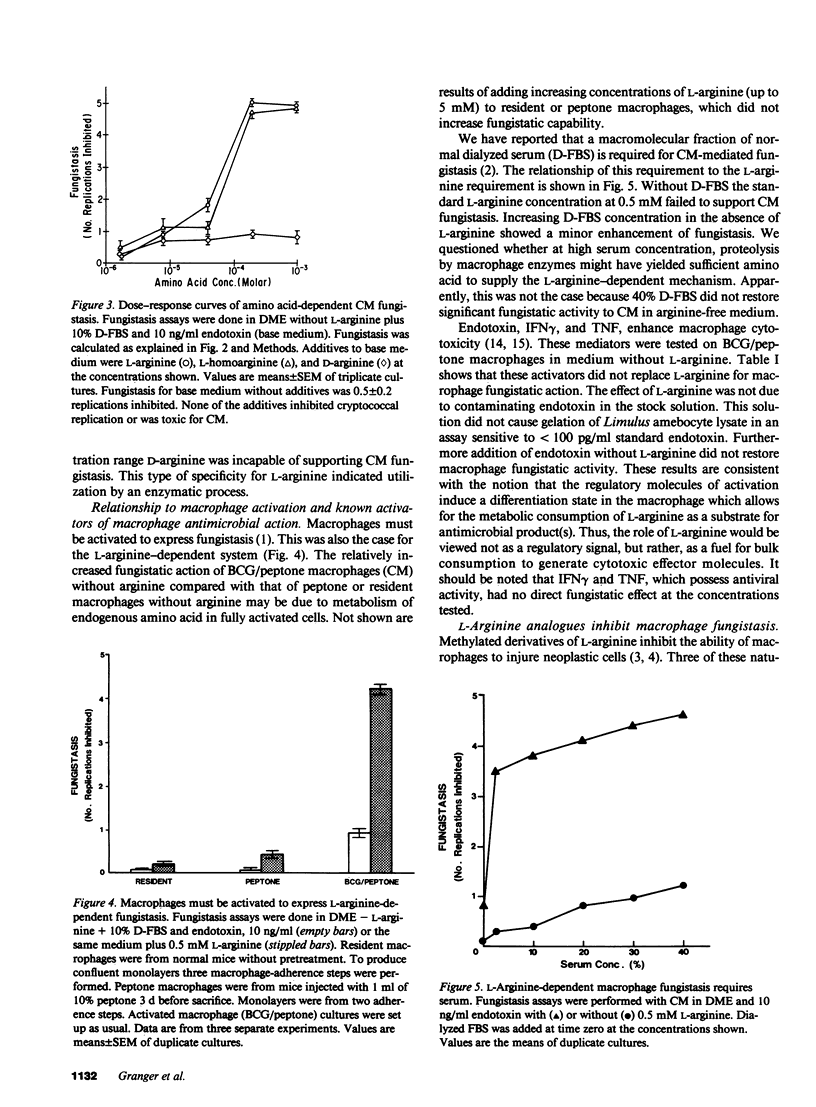
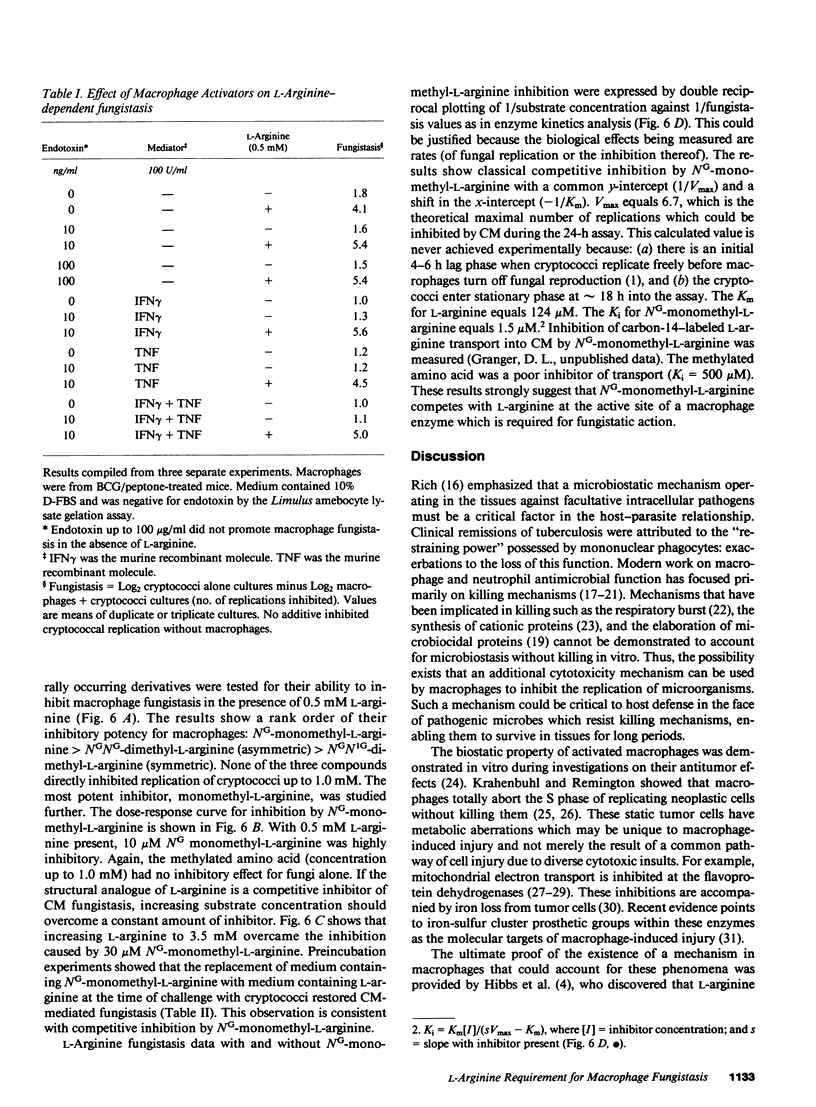
The microbiostatic action of macrophages was studied in vitro employing peritoneal cytotoxic macrophages (CM) from mice acting against Cryptococcus neoformans cultured in Dulbecco's medium with 10% dialyzed fetal bovine serum. Fungistasis was measured using electronic particle counting after lysis of macrophages with detergent. Macrophage fungistasis failed in medium lacking only L-arginine. Complete fungistasis was restored by L-arginine; restoration was concentration dependent, maximal at 200 microM. Deletion of all other essential amino acids did not abrogate fungistasis provided that L-arginine was present. Of twenty guanido compounds, including D-arginine, only three (L-arginine, L-homoarginine, and L-arginine methylester) supported fungistasis. Known activators or mediators of macrophage cytotoxicity (endotoxin, interferon gamma, tumor necrosis factor) did not replace L-arginine for CM-mediated fungistasis. The guanido analogue NG-monomethyl-L-arginine was a potent competitive inhibitor of CM-mediated fungistasis giving 50% inhibition at an inhibitor/L-arginine ratio of 1:27. Although CM completely blocked fungal reproduction via an L-arginine-dependent mechanism, the majority of the dormant fungi remained viable. Thus, this mechanism is viewed as a microbiostatic process similar or identical to the tumoristatic effect of macrophages. This suggests the production of a broad spectrum biostatic metabolite(s) upon consumption of L-arginine by cytotoxic macrophages.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL E. A. Associations of ninhydrin-reacting compounds in the seeds of 49 species of Lathyrus. Biochem J. 1962 May;83:225–229. doi: 10.1042/bj0830225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast R. C., Jr, Cleveland R. P., Littman B. H., Zbar B., Rapp H. J. Acquired cellular immunity: extracellular killing of Listeria monocytogenes by a product of immunologically activated macrophages. Cell Immunol. 1974 Feb;10(2):248–259. doi: 10.1016/0008-8749(74)90116-6. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Magnitude of the host nutritional responses to infection. Am J Clin Nutr. 1977 Aug;30(8):1236–1247. doi: 10.1093/ajcn/30.8.1236. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaskes S., Tyndall R. L. Pigment production by Cryptococcus neoformans from para- and ortho-Diphenols: effect of the nitrogen source. J Clin Microbiol. 1975 Jun;1(6):509–514. doi: 10.1128/jcm.1.6.509-514.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull B. J., Hotchkiss J. H. Activated oxygen and mammalian nitrate biosynthesis. Carcinogenesis. 1984 Sep;5(9):1161–1164. doi: 10.1093/carcin/5.9.1161. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. A reevaluation of the roles of the O2-dependent and O2-independent microbicidal systems of phagocytes. Rev Infect Dis. 1983 Sep-Oct;5(5):843–853. doi: 10.1093/clinids/5.5.843. [DOI] [PubMed] [Google Scholar]
- Gabay J. E., Heiple J. M., Cohn Z. A., Nathan C. F. Subcellular location and properties of bactericidal factors from human neutrophils. J Exp Med. 1986 Nov 1;164(5):1407–1421. doi: 10.1084/jem.164.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986 Jan;136(2):672–680. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis: requirement for a macromolecular component in serum. J Immunol. 1986 Jul 15;137(2):693–701. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985 Aug;76(2):508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Taintor R. R., Cook J. L., Hibbs J. B., Jr Injury of neoplastic cells by murine macrophages leads to inhibition of mitochondrial respiration. J Clin Invest. 1980 Feb;65(2):357–370. doi: 10.1172/JCI109679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Tannenbaum S. R., Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981 Apr 3;212(4490):56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem Biophys Res Commun. 1984 Sep 17;123(2):716–723. doi: 10.1016/0006-291x(84)90288-2. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn R. G., Klostergaard J., Lopez-Berestein G. Activated macrophages secrete a soluble factor that inhibits mitochondrial respiration of tumor cells. J Immunol. 1984 Nov;133(5):2577–2581. [PubMed] [Google Scholar]
- Krahenbuhl J. L. Effects of activated macrophages on tumor target cells in discrete phases of the cell cycle. Cancer Res. 1980 Dec;40(12):4622–4627. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. Cytostatic effects of activated macrophages on tumor target cells: inhibition of cytotoxic action of ARA-C. J Immunopharmacol. 1980;2(3):325–348. doi: 10.3109/08923978009046465. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ferrari L. G., Patterson-Delafield J., Sorrell T. Fungicidal activity of rabbit alveolar and peritoneal macrophages against Candida albicans. Infect Immun. 1980 Jun;28(3):1001–1008. doi: 10.1128/iai.28.3.1001-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Perfect J. R., Lang S. D., Durack D. T. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol. 1980 Oct;101(1):177–194. [PMC free article] [PubMed] [Google Scholar]
- Saul R. L., Archer M. C. Oxidation of ammonia and hydroxylamine to nitrate in the rat and in vitro. Carcinogenesis. 1984 Jan;5(1):77–81. doi: 10.1093/carcin/5.1.77. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. A., Young V. R., Tannenbaum S. R. Mammalian nitrate biosynthesis: incorporation of 15NH3 into nitrate is enhanced by endotoxin treatment. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4518–4521. doi: 10.1073/pnas.80.14.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. D., Moldawer L. L., Sakamoto A., Keenan R. A., Matthews D. E., Young V. R., Wannemacher R. W., Jr, Blackburn G. L., Bistrian B. R. Leukocyte endogenous mediator alters protein dynamics in rats. Metabolism. 1983 Jul;32(7):654–660. doi: 10.1016/0026-0495(83)90120-8. [DOI] [PubMed] [Google Scholar]



