Abstract
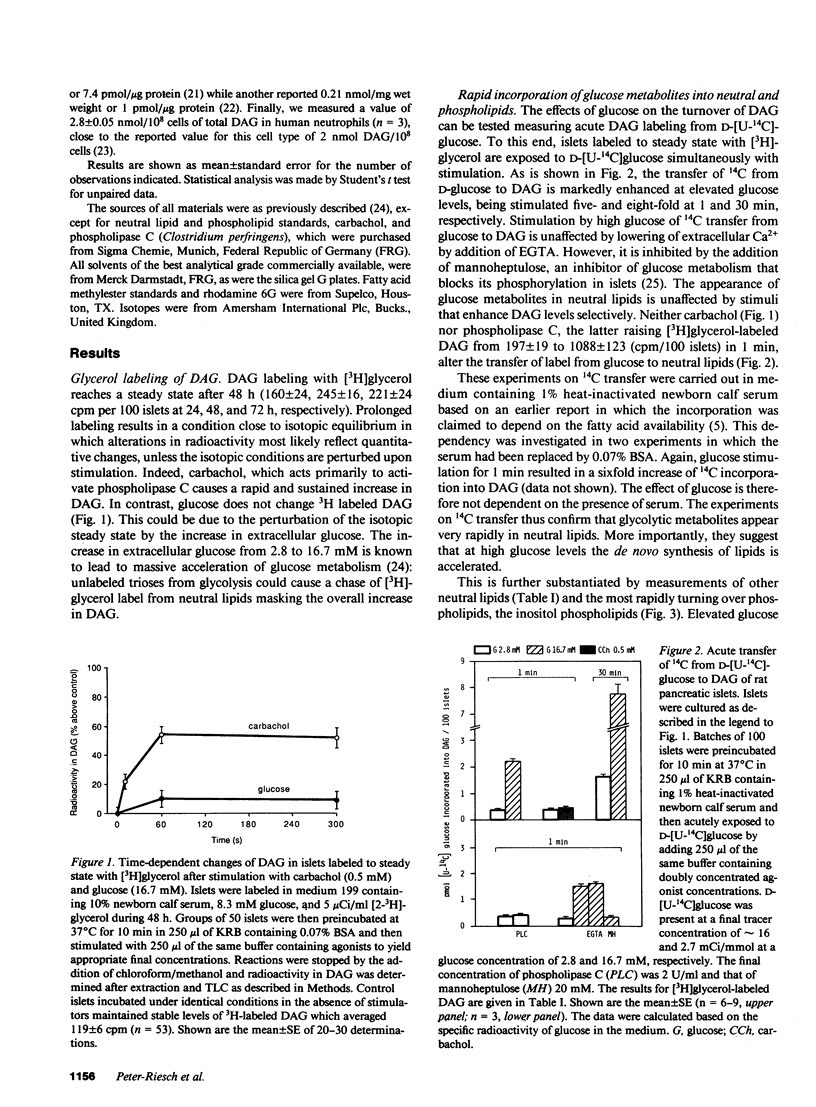
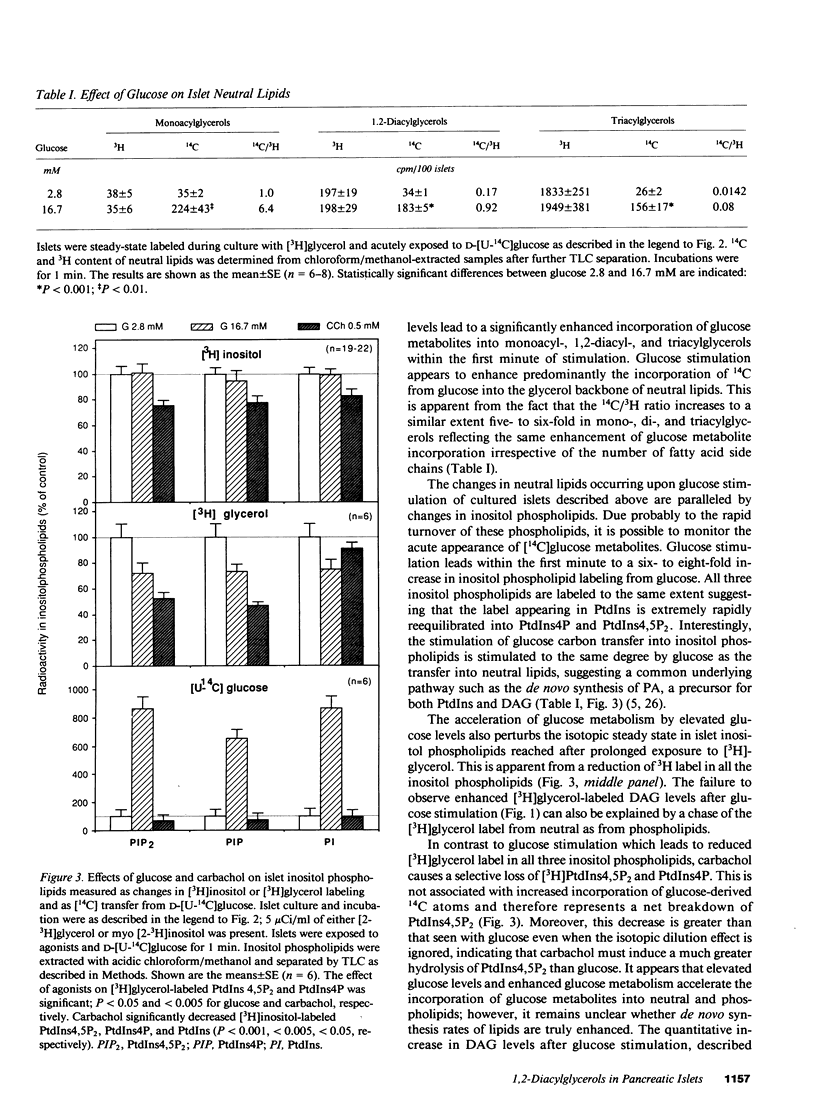
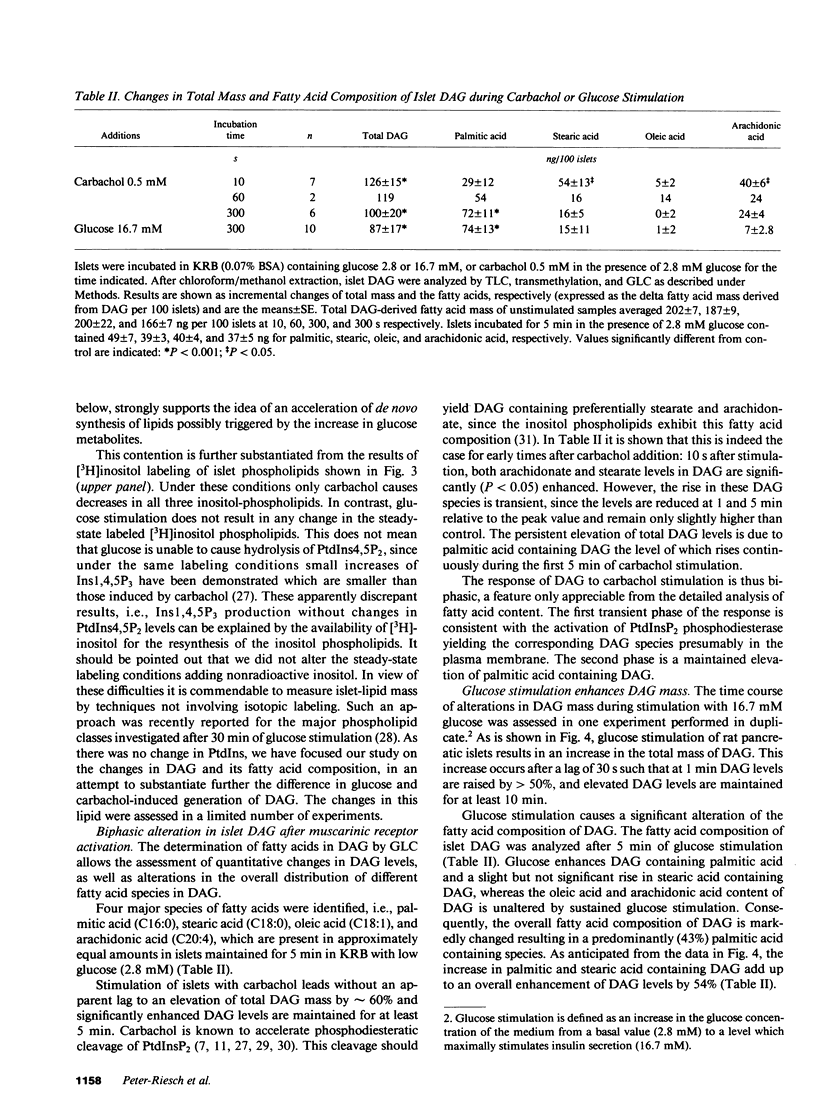
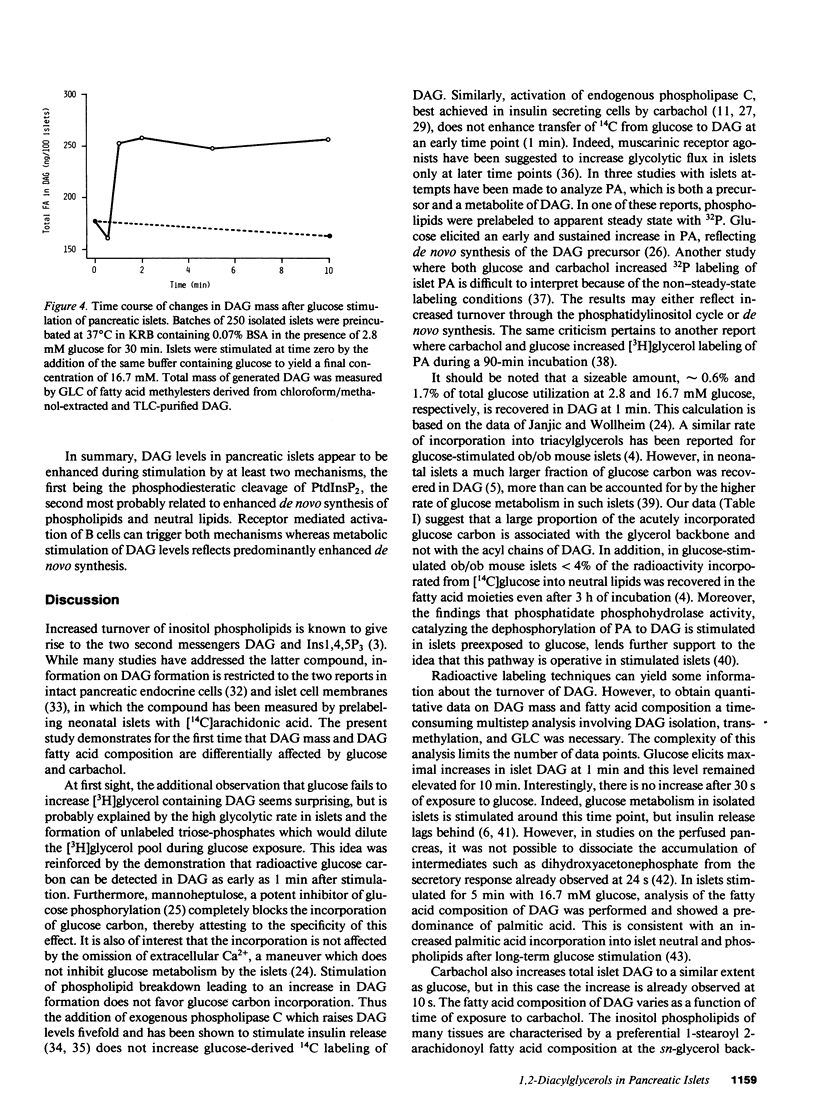
Diacylglycerols (DAG) modulate secretory responses by the activation of protein kinase C. Early changes in DAG formation induced by the muscarinic receptor agonist carbachol were compared to those caused by the nutrient secretagogue glucose in pancreatic islets. Turnover rates of DAG were investigated in radiolabeling experiments, whereas changes in total mass and fatty acid composition of DAG were assessed by gas-liquid chromatography. When islet lipids were labeled to steady state in tissue culture with [3H]glycerol, carbachol induced a rapid (10 s) and sustained increase of [3H]DAG generation. In contrast, glucose stimulation failed to increase [3H]glycerol containing DAG, and this was probably due to the isotopic dilution of the label secondary to enhanced glycolysis. This was substantiated by following the transfer of 14C from glucose into DAG. Within 1 min of acute exposure of islets to D-[U-14C]-glucose at stimulatory concentrations, DAG labeling increased fivefold representing up to 2% of total glucose usage. Similar stimulation of 14C incorporation into other neutral lipids and inositol phospholipids was observed, suggesting the enhanced de novo synthesis of phosphatidic acid, the common precursor for DAG, and inositol phospholipids from glycolytic intermediates. Transfer of 14C from glucose was not stimulated by agents such as carbachol and exogenous phospholipase C that act primarily on inositol phospholipid breakdown. The total mass of islet DAG was increased by 60% after both carbachol and glucose stimulation. However, analysis of the fatty acid composition of carbachol-generated DAG revealed at the early time point (10 s) a prevalent stearoyl-arachidonoyl configuration similar to that reported for inositol phospholipids. This pattern shifted to a DAG enriched in palmitic acid at a later time point. Glucose-stimulated islets displayed a predominance of palmitic acid containing DAG, indicating increased de novo synthesis of the putative second messenger rather than its formation by inositol phospholipid hydrolysis. Indeed, steady-state labeling of these phospholipids with [3H]inositol confirmed this idea since only carbachol caused detectable inositol phospholipid hydrolysis. Thus, although protein kinase C may be activated by both carbachol and glucose, the two secretagogues generate diacylglycerols through different mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Hedeskov C. J., Randle P. J. Glucose metabolism in mouse pancreatic islets. Biochem J. 1970 Jun;118(1):143–154. doi: 10.1042/bj1180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund K., Hellerström C. Glucose metabolism of pancreatic islets isolated from neonatal rats. Horm Metab Res. 1972 May;4(3):159–163. doi: 10.1055/s-0028-1094091. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Banschbach M. W., Geison R. L., Hokin-Neaverson M. Effects of cholinergic stimulation on levels and fatty acid composition of diacylglycerols in mouse pancreas. Biochim Biophys Acta. 1981 Jan 26;663(1):34–45. doi: 10.1016/0005-2760(81)90192-2. [DOI] [PubMed] [Google Scholar]
- Banschbach M. W., Geison R. L., O'Brien J. F. Use of (1-14C) aectic anhydride to quantitate diglycerides: a new analytical procedure. Anal Biochem. 1974 Jun;59(2):617–627. doi: 10.1016/0003-2697(74)90315-7. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Berne C. The metabolism of lipids in mouse pancreatic islets. The biosynthesis of triacylglycerols and phospholipids. Biochem J. 1975 Dec;152(3):667–673. doi: 10.1042/bj1520667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Best L., Malaisse W. J. Enhanced de novo synthesis of phosphatidic acid and phosphatidylinositol in rat pancreatic islets exposed to nutrient or neurotransmitter stimuli. Arch Biochem Biophys. 1984 Oct;234(1):253–257. doi: 10.1016/0003-9861(84)90347-3. [DOI] [PubMed] [Google Scholar]
- Best L., Malaisse W. J. Nutrient and hormone-neurotransmitter stimuli induce hydrolysis of polyphosphoinositides in rat pancreatic islets. Endocrinology. 1984 Nov;115(5):1814–1820. doi: 10.1210/endo-115-5-1814. [DOI] [PubMed] [Google Scholar]
- Best L., Tomlinson S., Hawkins P. T., Downes C. P. Production of inositol trisphosphates and inositol tetrakisphosphate in stimulated pancreatic islets. Biochim Biophys Acta. 1987 Jan 19;927(1):112–116. doi: 10.1016/0167-4889(87)90073-5. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Peter-Riesch B., Schlegel W., Wollheim C. B. Ca2+-mediated generation of inositol 1,4,5-triphosphate and inositol 1,3,4,5-tetrakisphosphate in pancreatic islets. Studies with K+, glucose, and carbamylcholine. J Biol Chem. 1987 Mar 15;262(8):3567–3571. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Exton J. H. Stimulation of 1,2-diacylglycerol accumulation in hepatocytes by vasopressin, epinephrine, and angiotensin II. J Biol Chem. 1985 Nov 15;260(26):14201–14207. [PubMed] [Google Scholar]
- Cockcroft S., Allan D. The fatty acid composition of phosphatidylinositol, phosphatidate and 1,2-diacylglycerol in stimulated human neutrophils. Biochem J. 1984 Sep 1;222(2):557–559. doi: 10.1042/bj2220557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Activity of endogenous phospholipase C and phospholipase A2 in glucose stimulated pancreatic islets. Biochem Biophys Res Commun. 1984 May 16;120(3):820–827. doi: 10.1016/s0006-291x(84)80180-1. [DOI] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Glucose-induced phospholipid-dependent protein phosphorylation in neonatal rat islets. Arch Biochem Biophys. 1986 Aug 1;248(2):562–569. doi: 10.1016/0003-9861(86)90509-6. [DOI] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Muscarinic-agonist and guanine nucleotide activation of polyphosphoinositide phosphodiesterase in isolated islet-cell membranes. Biochem J. 1986 Dec 15;240(3):731–737. doi: 10.1042/bj2400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. Pancreatic islets synthesize phospholipids de novo from glucose via acyl-dihydroxyacetone phosphate. Biochem Biophys Res Commun. 1985 Oct 30;132(2):467–473. doi: 10.1016/0006-291x(85)91157-x. [DOI] [PubMed] [Google Scholar]
- Dunlop M. E., Larkins R. G. The role of calcium in phospholipid turnover following glucose stimulation in neonatal rat cultured islets. J Biol Chem. 1984 Jul 10;259(13):8407–8411. [PubMed] [Google Scholar]
- Dunlop M. E., Malaisse W. J. Phosphoinositide phosphorylation and hydrolysis in pancreatic islet cell membrane. Arch Biochem Biophys. 1986 Feb 1;244(2):421–429. doi: 10.1016/0003-9861(86)90609-0. [DOI] [PubMed] [Google Scholar]
- Dunlop M., Larkins R. G. Presence of membrane-associated phosphatidate phosphohydrolase activity in cultured islets and its stimulation by glucose. FEBS Lett. 1985 Dec 2;193(2):231–235. doi: 10.1016/0014-5793(85)80158-7. [DOI] [PubMed] [Google Scholar]
- Farese R. V., DiMarco P. E., Barnes D. E., Sabir M. A., Larson R. E., Davis J. S., Morrison A. D. Rapid glucose-dependent increases in phosphatidic acid and phosphoinositides in rat pancreatic islets. Endocrinology. 1986 Apr;118(4):1498–1503. doi: 10.1210/endo-118-4-1498. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in pancreatic B-cells. Experientia. 1984 Oct 15;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- Holub B. J., Kuksis A. Metabolism of molecular species of diacylglycerophospholipids. Adv Lipid Res. 1978;16:1–125. doi: 10.1016/b978-0-12-024916-9.50007-x. [DOI] [PubMed] [Google Scholar]
- Hughes B. P., Rye K. A., Pickford L. B., Barritt G. J., Chalmers A. H. A transient increase in diacylglycerols is associated with the action of vasopressin on hepatocytes. Biochem J. 1984 Sep 1;222(2):535–540. doi: 10.1042/bj2220535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Peshavaria M., Brocklehurst K. W. Phorbol ester stimulation of insulin release and secretory-granule protein phosphorylation in a transplantable rat insulinoma. Biochem J. 1984 Dec 1;224(2):483–490. doi: 10.1042/bj2240483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjic D., Wollheim C. B. Interactions of Ca2+, Mg2+, and Na+ in regulation of insulin release from rat islets. Am J Physiol. 1983 Mar;244(3):E222–E229. doi: 10.1152/ajpendo.1983.244.3.E222. [DOI] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., Dekker A., Wirtz K. W., Gispen W. H. Corticotropin-(1--24)-tetracosapeptide affects protein phosphorylation and polyphosphoinositide metabolism in rat brain. Biochem J. 1981 Jan 15;194(1):283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laychock S. G. Identification and metabolism of polyphosphoinositides in isolated islets of Langerhans. Biochem J. 1983 Oct 15;216(1):101–106. doi: 10.1042/bj2160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Carpinelli A. R., Sener A. Stimulus-secretion coupling of glucose-induced insulin release. Timing of early metabolic, ionic, and secretory events. Metabolism. 1981 May;30(5):527–532. doi: 10.1016/0026-0495(81)90191-8. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Dunlop M. E., Mathias P. C., Malaisse-Lagae F., Sener A. Stimulation of protein kinase C and insulin release by 1-oleoyl-2-acetyl-glycerol. Eur J Biochem. 1985 May 15;149(1):23–27. doi: 10.1111/j.1432-1033.1985.tb08887.x. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ghosh A. K., Meglasson M. D., Prentki M., June V., von Allman D. Metabolic concomitants in pure, pancreatic beta cells during glucose-stimulated insulin secretion. J Biol Chem. 1986 Oct 25;261(30):14057–14061. [PubMed] [Google Scholar]
- Morgan N. G., Rumford G. M., Montague W. Studies on the role of inositol trisphosphate in the regulation of insulin secretion from isolated rat islets of Langerhans. Biochem J. 1985 Jun 15;228(3):713–718. doi: 10.1042/bj2280713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tanigawa K., Kuzuya H., Imura H., Taniguchi H., Baba S., Takai Y., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase in rat pancreas islets of langerhans. Its possible role in glucose-induced insulin release. FEBS Lett. 1982 Feb 22;138(2):183–186. doi: 10.1016/0014-5793(82)80436-5. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Marks J. S., Coll K. E., Williamson J. R. Quantitation and early kinetics of inositol lipid changes induced by vasopressin in isolated and cultured hepatocytes. J Biol Chem. 1983 May 10;258(9):5716–5725. [PubMed] [Google Scholar]
- Trimble E. R., Bruzzone R., Biden T. J., Farese R. V. Secretin induces rapid increases in inositol trisphosphate, cytosolic Ca2+ and diacylglycerol as well as cyclic AMP in rat pancreatic acini. Biochem J. 1986 Oct 15;239(2):257–261. doi: 10.1042/bj2390257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus M. D., Hintz C. S., Weinstein J. B., Williams A. D., Pagliara A. S., Matschinsky F. M. A comparison of the effects of glucose and acetylcholine on insulin release and intermediary metabolism in rat pancreatic islets. J Biol Chem. 1979 May 25;254(10):3921–3929. [PubMed] [Google Scholar]
- Turk J., Wolf B. A., Lefkowith J. B., Stump W. T., McDaniel M. L. Glucose-induced phospholipid hydrolysis in isolated pancreatic islets: quantitative effects on the phospholipid content of arachidonate and other fatty acids. Biochim Biophys Acta. 1986 Dec 5;879(3):399–409. doi: 10.1016/0005-2760(86)90232-8. [DOI] [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Vara E., Tamarit-Rodriguez J. Glucose stimulation of insulin secretion in islets of fed and starved rats and its dependence on lipid metabolism. Metabolism. 1986 Mar;35(3):266–271. doi: 10.1016/0026-0495(86)90212-x. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Second messenger function of inositol 1,4,5-trisphosphate. Early changes in inositol phosphates, cytosolic Ca2+, and insulin release in carbamylcholine-stimulated RINm5F cells. J Biol Chem. 1986 Jun 25;261(18):8314–8319. [PubMed] [Google Scholar]
- Wollheim C. B., Biden T. J. Signal transduction in insulin secretion: comparison between fuel stimuli and receptor agonists. Ann N Y Acad Sci. 1986;488:317–333. doi: 10.1111/j.1749-6632.1986.tb46568.x. [DOI] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Nakaki T., Nakadate T., Kato R. Insulinotropic effects of exogenous phospholipase A2 and C in isolated pancreatic islets. Eur J Pharmacol. 1982 Dec 17;86(1):121–124. doi: 10.1016/0014-2999(82)90409-5. [DOI] [PubMed] [Google Scholar]
- Zawalich W., Brown C., Rasmussen H. Insulin secretion: combined effects of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Dec 16;117(2):448–455. doi: 10.1016/0006-291x(83)91221-4. [DOI] [PubMed] [Google Scholar]



