Abstract
Chronic compression (CCD) of the dorsal root ganglion (DRG) is a model of human radicular pain produced by intraforaminal stenosis and other disorders affecting the DRG, spinal nerve, or root. Previously, we examined electrophysiological changes in small-diameter lumbar level 3 (L3) and L4 DRG neurons treated with CCD; the present study extends these observations to medium-sized DRG neurons, which mediate additional sensory modalities, both nociceptive and non-nociceptive. Whole-cell patch-clamp recordings were obtained from medium-sized somata in the intact DRG in vitro. Compared with neurons from unoperated control animals, CCD neurons exhibited a decrease in the current threshold for action potential generation. In the CCD group, current densities of TTX-resistant and TTX-sensitive Na+ current were increased, whereas the density of delayed rectifier voltage-dependent K+ current was decreased. No change was observed in the transient or “A” current after CCD. We conclude that CCD in the mouse produces hyperexcitability in medium-sized DRG neurons, and the hyperexcitability is associated with an increased density of Na+ current and a decreased density of delayed rectifier voltage-dependent K+ current.
Keywords: neuropathic pain, ion channels, whole-cell recordings
an experimental, chronic compression (CCD) of the dorsal root ganglion (DRG) is a model of radicular pain that is produced in humans by an intraforaminal stenosis or other disorders affecting the DRG, spinal nerve, or root. In a previous study, we found that CCD elicited pain behavior in the mouse and increased the excitability of small-diameter DRG neurons, in part due to an increased expression of voltage-gated Na+ currents (Fan et al. 2011). In this study, we consider the effects of CCD on medium-sized neurons of mice. Medium-sized neurons have thinly myelinated axons and include different types of peripheral nociceptors and also low-threshold mechanoreceptors (Ma et al. 2003). The low-threshold type might not normally contribute to pain but might contribute to tactile allodynia if the central neurons to which they project become sensitized, for example, after peripheral inflammation or after injury of the peripheral nerve (Ma and Woolf 1996; Neumann et al. 1996; Noguchi et al. 1995).
Previously, we investigated the effects of CCD on the excitability of medium-sized neurons that innervated the skin (Tan et al. 2006). However, these experiments were performed on dissociated neuronal cell bodies in culture. Not only are these neurons acutely injured and deprived of their axon, which is sometimes a source of abnormal, spontaneous discharge after CCD (Ma and LaMotte 2007), but they are also prevented from chemically interacting with their satellite glia (Zhang et al. 2009) and with other neurons and cell types in the DRG. In an effort to minimize these problems, the present study investigated the effects of CCD on voltage-gated Na+ and K+ currents in medium-sized neurons of the intact DRG in vitro using techniques previously developed in our laboratory (Fan et al. 2011).
METHODS
CCD surgery.
CCD surgery was performed as described previously (Fan et al. 2011). All protocols were approved by Yale University Institutional Animal Care and Use Committee and were in accordance with guidelines provided by the National Institutes of Health and the International Association for the Study of Pain. In brief, the mouse was anesthetized with isoflurane inhalation anesthesia. Intervertebral foramina of lumbar 3-4 (L3-4) and L4-5 were exposed; a stainless L-shaped steel rod, 2 mm in length and 0.3 mm in diameter, was implanted into each intervertebral foramen to compress the L4 and L3 DRGs. The incision was closed in layers. Another group of mice received no surgery and was used as controls. Sham surgeries were not used, because neither pain behavior nor electrophysiological changes were observed in sham-operated groups in previous studies (Song et al. 1999; Zhang et al. 1999).
Unlike our previous CCD study in the mouse (Fan et al. 2011), the present one does not include behavioral tests to confirm the presence of tactile allodynia. However, we believe it unlikely that the mice in the present study would have exhibited any differences in behavior from those tested in our companion study. First, in the former study, the CCD surgery produced, in each mouse, a consistent behavioral effect of tactile allodynia on the ipsilateral (but not contralateral) foot. Second, the same experimenter, as in our former study, performed the identical surgical procedure in the same strain of mouse under the same conditions and within a consecutive period of several months. Thus factors that have been cited as accounting for differences in allodynia when different laboratories used the same model of neuropathic pain in rodents, such as variations in surgical technique, strain of animal, or diet (Djouhri et al. 2006; Lee et al. 1997; Shir et al. 2001), are not operative in the present case.
DRG preparation.
Intact DRGs were prepared as described previously (Fan et al. 2011). In brief, at postoperative days 5–7, control mice or CCD-treated mice were anesthetized with pentobarbital sodium (Nembutal, 50 mg/kg ip), and the L4 and L3 DRGs, with their corresponding dorsal roots, spinal nerves, and sciatic nerve, were dissected out. The DRGs were placed in cold, oxygenated artificial cerebrospinal fluid (ACSF), consisting of (in mM) 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 10 dextrose, 1.2 MgCl2, and 1.2 CaCl2 (pH 7.3). Under a dissecting microscope, the epineurium and perineurium, which form the capsule of each ganglion, were removed. The DRGs were placed in a 10-ml vial with ACSF with collagenase P (1 mg/ml; Roche, Mannheim, Germany) and protease (0.2 mg/ml; Protease Type XIV, Sigma-Aldrich, St. Louis, MO) for 30 min at 37°C (Zhang et al. 1998).
Electrophysiological recordings.
Fire-polished electrodes (1–2 MΩ) were fabricated from 1.2-mm borosilicate capillary glass (Sutter Instrument, Novato, CA) using a P-97 puller (Sutter Instrument). A single DRG was placed in a recording chamber, which was mounted on the stage of an inverted microscope (Diaphot, Nikon, Tokyo, Japan). The ganglion was fixed to the bottom of the chamber by gentle pressure, applied by a pipette held by a manipulator (Zhang et al. 1998). The DRG was superfused continuously at 2 ml/min with oxygenated ACSF. Recordings were obtained at room temperature using a patch-clamp amplifier (HEKA EPC 10, HEKA Instruments, Bellmore, NY) under computer control (HEKA Patchmaster, HEKA Instruments). The techniques used to get access of neurons from the intact ganglion were the same as described previously (Fan et al. 2011). Recordings were obtained from medium-sized neurons with diameters of 30–45 μm and with cell capacitances of 30–65 pF (Lopez-Santiago et al. 2006). The liquid junction potential for recording the Na+ current was calculated as 11.2 mV. Data were not corrected to account for liquid junction potential, since it was constant across experimental groups.
Measurements of excitability were obtained under current clamp mode. Neurons that had a stable membrane potential, which was more negative than −40 mV, were accepted. After measurement in the current clamp configuration, the amplifier was switched to the whole-cell, voltage-clamp mode to measure K+ currents. Excitability measurements included the current threshold (rheobase), action potential (AP) threshold, resting membrane potential, repetitive discharge, and input resistance. The definition and measurements of each of these parameters were the same as described previously (Fan et al. 2011). Patch-clamp recordings were filtered at 10 kHz and digitized at 50 kHz.
Solutions for electrophysiological recording.
For recordings under current clamp mode, the bath solution contained (in mM) 145 NaCl, 3 KCl, 1 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose. The pH was adjusted to 7.4 with NaOH and the osmolarity adjusted using glucose to 300–310 mOsm. The pipette solution contained (in mM) 120 K-gluconate, 20 KCl, 1 CaCl2, 2 MgCl2, 11 EGTA, 10 HEPES-K, and 2 Mg-ATP, pH 7.2, with an osmolarity of 290–300 mOsm.
The same pipette solution was used when switched to voltage clamp mode to record K+ currents, but the bath solution was changed to one that contained (in mM) 140 choline Cl, 3 KCl, 1 CaCl2, 1 MgCl2, 0.1 CdCl2, 10 HEPES, and 10 glucose. The pH was adjusted to 7.4 with Tris-base and the osmolarity adjusted to 300–310 mOsm.
In experiments in which the Na+ current was recorded, the bath solution contained (in mM) 35 NaCl, 10 HEPES, 105 choline Cl, 10 glucose, 1 CaCl2, 1 MgCl2, 0.1 CdCl2, and 20 tetraethylammonium-Cl, pH adjusted to 7.4 with NaOH. The pipette solution contained (in mM) 140 CsF, 10 NaCl, 2 MgCl2, 0.1 CaCl2, 1 EGTA, and 10 HEPES, pH adjusted to 7.2 with CsOH.
Data analysis and statistics.
The electrophysiological data were analyzed using Fitmaster (HEKA Elektronic, Germany) and Origin 8 (OriginLab, Northampton, MA). The data are expressed as means ± SE. Statistical differences between CCD and control neurons were analyzed by Student's unpaired t-test. In the case of repeated measurements, the data were analyzed initially by ANOVA with repeated measures. Post hoc testing was by Tukey's multiple comparison test or Student's t-test with a Holm-Bonferroni correction to the critical P value. Population percentages were compared using Fisher's exact test. Significance was set at P < 0.05.
The analysis of steady-state activation and inactivation of Na+ currents was as described (Fan et al. 2011). The protocol used for steady-state inactivation of TTX-resistant (TTX-R) and TTX-sensitive (TTX-S) Na+ currents consisted of a 500-ms prepulse potential over the range of −130 mV to −10 mV in 5-mV increments, followed by a 30-ms depolarization to a command potential of −10 mV. Inactivation of the TTX-R current was assumed to occur positive to −50 mV.
RESULTS
The effects of CCD on neuronal excitability.
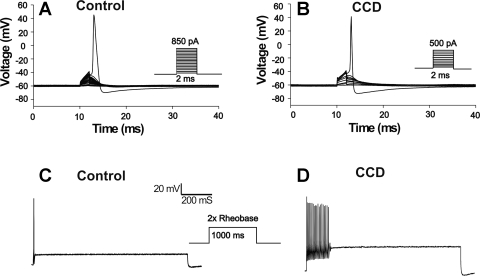
Passive membrane properties and AP characteristics were recorded under current clamp mode (Table 1) in nine control neurons (from seven mice) and eight CCD neurons (from five mice). Compared with control neurons, CCD neurons exhibited a significantly lower rheobase (P < 0.05, Student's t-test) (Fig. 1) and a trend to a more negative voltage threshold, although this did not reach a significant P value. The proportion of cells firing multiple (≥ 2) APs at 2× rheobase was not significantly higher in CCD than in control neurons (3/8 vs. 0/9; P = 0.082, Fisher's exact test; Fig. 1). There were no significant differences between CCD and control neurons in cell capacitance, resting membrane potential, input resistance, AP amplitude, and AP duration.
Table 1.
Membrane properties of control and CCD neurons measured in whole-cell patch-clamp experiments
| n | Diameter, μm | Cm, pF | Vm, mV | Rin, MΩ | AP Rheobase, pA | AP VTH, mV | AP Amplitude, mV | AP Duration, ms | MS/non-MS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 9 | 38.9 ± 2.1 | 48.9 ± 5.3 | −61.2 ± 2.0 | 108.9 ± 19.9 | 627.8 ± 43.4 | −26.2 ± 6.7 | 115.4 ± 7.7 | 1.5 ± 0.3 | 0/9 |
| CCD | 8 | 40.8 ± 1.4 | 52.7 ± 3.5 | −58.9 ± 1.7 | 125.9 ± 19.6 | 456.3 ± 78.7* | −35.1 ± 3.2 | 109.1 ± 7.8 | 1.2 ± 0.2 | 3/5 |
Values are mean ± SE. CCD, chronic compression; n, number of cells; Cm, cell capacitance; Vm, membrane potential; Rin, input resistance; AP, action potential; AP Rheobase, current threshold of AP; VTH, voltage threshold; MS, number of cells firing multiple (≥2) spikes to current step at 2× rheobase; non-MS, number of cells discharging 1 spike.
P < 0.05 (Student's t-tests, significant differences between control and CCD).
Fig. 1.
Properties of action potentials (APs) recorded under current clamp in a chronic compression (CCD) and a control neuron. A: the current threshold, obtained in response to a series of current steps injected in increments of 50 pA, was lower for the CCD than for the control neuron. B: there was a loss of accommodation in the CCD neuron in response to injection of current of 2× rheobase (1-s duration). In general, control neurons responded with 1 AP, whereas CCD neurons were more likely to discharge with multiple APs.
Effects of CCD on voltage-gated Na+ currents.
TTX-S and TTX-R Na+ currents were quantified using a voltage protocol (Fig. 2). The total Na+ current was elicited by a series of test pulses, each 80 ms in duration, from −100 mV to +40 mV in 10-mV steps, preceded by a 500-ms prepulse to −100 mV. The interpulse interval was 1 s. The TTX-R component was evoked using the same command test pulses but using a 500-ms prepulse to −50 mV, which inactivated TTX-S currents (Lopez-Santiago et al. 2006). By digitally subtracting the TTX-R component from the total Na+ current, the TTX-S current was obtained.
Fig. 2.
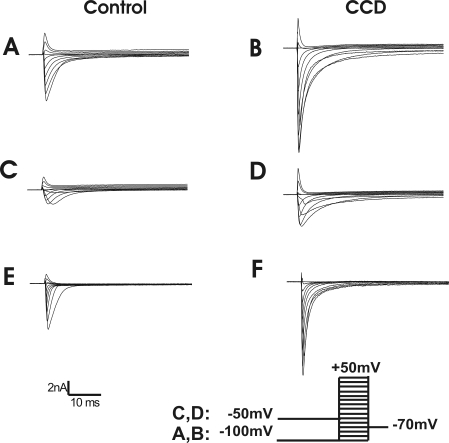
Representative traces of the voltage-gated Na+ currents for a control and a CCD neuron. A and B: total Na+ currents were obtained using a series of 80-ms test pulses in 10-mV steps from −100 to 50 mV, preceded by a 500-ms prepulse of −100 mV. C and D: TTX-resistant (TTX-R) currents were recorded in response to the same series of test pulses but preceded by a 500-ms prepulse of −50 mV. E and F: TTX-sensitive (TTX-S) currents were obtained by subtraction of C and D from A and B.
Twenty-three medium-sized neurons, including 12 from seven control mice and 11 from six CCD mice, were recorded and analyzed. Both TTX-R- and TTX-S-type currents were observed in all of the neurons analyzed. Some neurons expressed little TTX-R current; two neurons from CCD and one neuron from control were excluded for analysis, where the ratio of peak TTX-R to TTX-S currents was <10% (Tan et al. 2006).
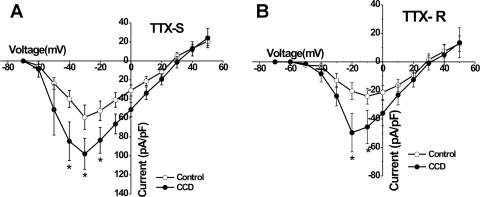
The activation thresholds for TTX-S currents were approximately −50 mV for both CCD and for control neurons and reached their peak amplitudes at about −30 mV (Fig. 3A). Compared with control neurons, the mean current density of the TTX-S Na+ current was significantly greater for CCD at −40, −30, and −20 mV (P < 0.05, Student's t-tests; Fig. 3A).
Fig. 3.
Comparison of mean voltage-current relationships for voltage-gated Na+ currents in control and CCD neurons. Mean peak current density of TTX-S (A) and TTX-R (B) obtained from control neurons (open circles) and CCD neurons (closed circles), as shown in Fig. 2. Voltages, where CCD neurons exhibited significantly greater current density than control, are indicated with an asterisk (*P < 0.05, Student's t-test; CCD vs. control).
For TTX-R currents, activation occurred at approximately −40 mV for CCD and control neurons and reached maximal levels at −20 and −10 mV, respectively (Fig. 3B). Mean current density of the TTX-R Na+ current was significantly greater in CCD compared with control neurons at −20 and −10 mV (P < 0.05 Student's t-tests; Fig. 3B).
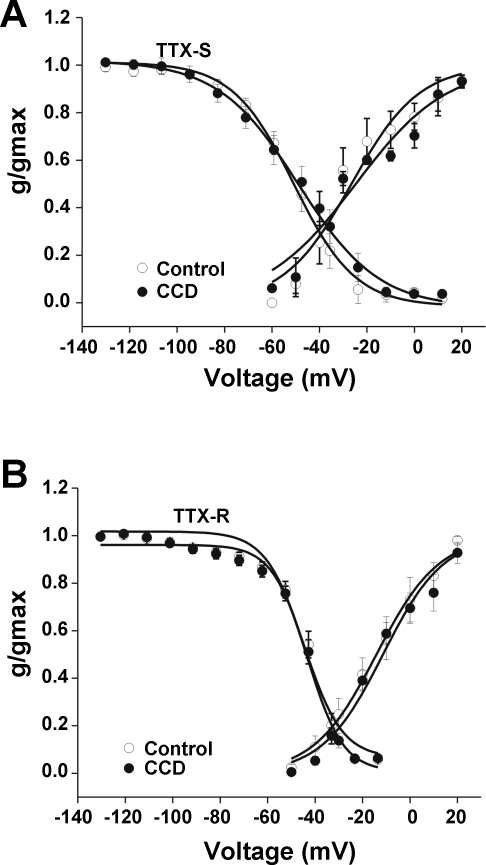
No significant difference was observed in the voltage dependence of activation of TTX-S or TTX-R currents between CCD and control neurons. For TTX-S currents, the mean midpoint of activation (V1/2) was −26.5 ± 2.4 mV for control and −24.7 ± 2.8 mV for CCD. The mean slope factor for activation was 15.2 ± 2.4 mV for control neurons (n = 8) and 19.2 ± 2.9 mV for CCD neurons (n = 11) (Fig. 4A). For TTX-R currents, the mean V1/2 was −14.2 ± 1.3 for control neurons vs. −11.4 ± 1.6 mV for CCD. The mean slope factor for activation for control neurons was 13.2 ± 1.2 mV (n = 9) vs. 12.8 ± 1.5 for CCD (n = 8; P > 0.05, Student's t-tests; Fig. 4B).
Fig. 4.
Activation and steady-state inactivation characteristics of TTX-S and TTX-R Na+ currents in control and CCD neurons. The mean relative peak conductance of TTX-S and TTX-R Na+ current is normalized to the maximal conductance (g/gmax) and plotted against voltage. A: steady-state activation and inactivation characteristics of TTX-S currents. B: steady-state activation and inactivation characteristics of TTX-R currents. No significant differences in levels of activation or inactivation for TTX-S and TTX-R currents were observed between CCD and control neurons (closed and open circles, respectively; see main text for further details).
The midpoint potential for steady-state inactivation and slope factor for inactivation of the TTX-S and TTX-R Na+ current were not changed significantly following CCD. The mean V1/2 of inactivation for the TTX-S Na+ current was −62.8 ± 0.6 mV for control (n = 10) and −61.3 ± 1.2 mV for CCD (n = 9; Fig. 4A). The mean slope factor for TTX-S inactivation was 14.3 ± 1.2 for CCD vs. 11.0 ± 0.5 mV for control (P > 0.05, Student's t-test; Fig. 4A). The mean V1/2 inactivation potential of TTX-R was −40.4 ± 1.1 mV for CCD (n = 7) and −42.0 ± 0.7 mV for control (n = 7; Fig. 4B). The mean slope factor for TTX-R inactivation for CCD and control neurons was 7.7 ± 0.9 and 8.3 ± 0.7 mV, respectively.
Effects of CCD on voltage-gated K+ currents.
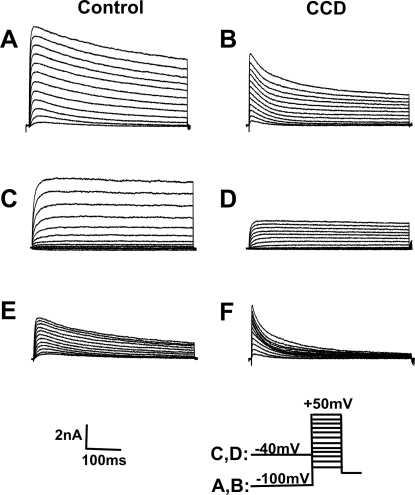
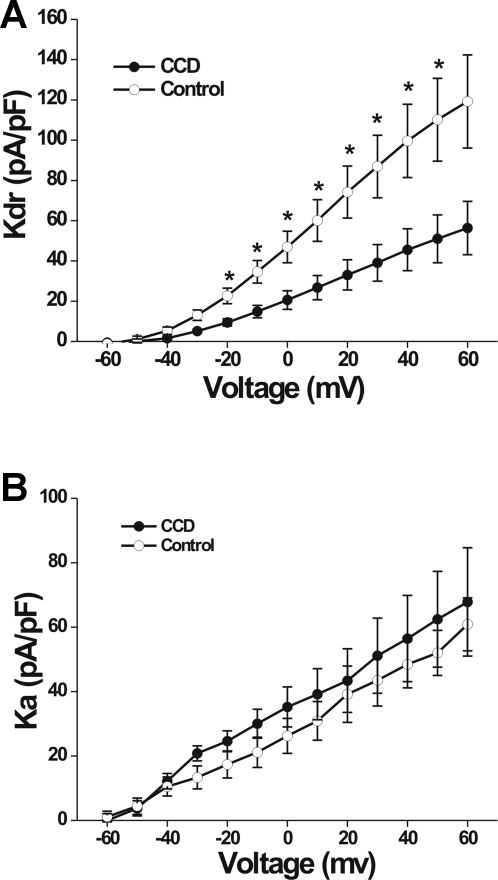
K+ currents were elicited by a series of 500-ms test pulses ranging from −60 mV to +50 mV in 10-mV increments, preceded by either a holding potential of −100 mV or −40 mV (Fan et al. 2011). The command potential following a −100-mV holding potential evoked both a transient (Ka) and sustained (Kdr) outward current (Fig. 5, A and B). The Ka was inactivated at the −40-mV holding potential, and only a Kdr current was observed (Fig. 5, C and D). Subtraction of the Kdr current from the total K+ current yielded the Ka (Fig. 5, E and F). The density of the Kdr current for CCD neurons (n = 9; four mice) was significantly smaller than that for control neurons (n = 15, 10 mice; repeated measures ANOVA; P = 0.014; Fig. 6A). Post hoc comparisons at each voltage revealed that the magnitude of the Kdr current was smaller in CCD neurons at each potential above −20 mV (Fig. 6A). No significant difference was observed in the density of the Ka current between CCD and control neurons (repeated measures ANOVA; Fig. 6B).
Fig. 5.
Representative waveforms of the voltage-gated K+ currents for a control neuron and a CCD neuron. A series of 500-ms test pulses, ranging from −60 to +50 mV in 10-mV steps, was used to elicit K+ currents. A and B: total K+ currents. The neuron was held at −100 mV before each test pulse. C and D: delayed rectifier K+ currents. The neuron was held at −40 mV before each test pulse. E and F: transient K+ currents (Ka) obtained by subtracting currents in C and D from those in A and B, respectively.
Fig. 6.
Mean current-voltage relationships for voltage-gated K+ currents in control and CCD neurons. A: the mean density of sustained K+ current (Kdr), plotted for control and CCD neurons (open and closed circles, respectively), was significantly smaller for CCD neurons. B: the mean current-voltage relationships for the fast-inactivating Ka current did not differ significantly for control and CCD neurons.
DISCUSSION
We previously demonstrated that CCD in the mouse produces neuropathic pain behavior that is characterized by tactile allodynia and hyperexcitability of small-diameter neurons (Fan et al. 2011). In this companion study, we found that CCD produced an increase in excitability of medium-sized neurons, as indicated by a reduction in rheobase. Many medium-sized sensory neurons have peripheral nociceptors that are responsive to thermal and/or mechanical noxious stimuli. Others have low-threshold mechanoreceptors (Boada and Woodbury 2007). An increase in the excitability of the cell bodies of either nociceptive or non-nociceptive neurons (and possibly their peripheral terminals) may cause an increased number of APs to be generated in response to painful or innocuous stimuli causing unpleasant paresthesias. If occurring in nociceptive neurons, spontaneous discharges originating from the compressed DRG might cause a chronic sensitization of nociceptive neurons in the spinal dorsal horn and as a consequence, allodynia or hyperalgesia. Paresthesias, allodynia, and hyperalgesia could similarly occur as a consequence of the increased excitability of small-diameter neurons (Fan et al. 2011), which also include both nociceptive and non-nociceptive modalities.
Na+ currents.
Similar to the effects on small-diameter neurons (Fan et al. 2011), CCD was associated with an increase in both TTX-S and TTX-R currents in medium-sized neurons in the mouse. Tan et al. (2006), using the CCD model in the rat but with ganglion dissociation prior to recording, also found an increase in the TTX-R current. However, unlike the present results, theirs included no increase in the magnitude of TTX-S currents. Whether these differences are in some way related to the effects of dissociation or differences between rats and mice awaits further study.
CCD produced changes in the expression of Na+ currents in DRG neurons, consistent with those accompanying peripheral inflammation. A subcutaneous injection of carrageenan or complete Freund's adjuvant (Black et al. 2004; Gould et al. 1998; Tanaka et al. 1998; Wang et al. 2007) or a local inflammation of the DRG by the application of zymosan (Wang et al. 2007) produces an increase in both TTX-S and TTX-R Na+ currents in small-sized DRG neurons. CCD injury may cause the release of inflammatory mediators, such as PGs, which contribute to the increase in TTX-S and TTX-R Na+ currents in medium- as well as small-sized DRG neurons. For example, PGD2 has also been demonstrated to increase the TTX-R current in small- to medium-sized DRG neurons (Ebersberger et al. 2011), and PGE2 upregulates TTX-S currents in medium-sized DRG neurons (Tripathi et al. 2011).
K+ currents.
After peripheral inflammation or nerve injury, voltage-gated K+ currents are altered in the cell bodies of DRG neurons. This has been observed in several models of visceral inflammation and in the axotomy model of nerve injury. After sciatic nerve transection, voltage-gated K+ currents are downregulated in small, medium, and large DRG neurons (Abdulla and Smith 2001; Everill and Kocsis 1999). Ka or “A-type” K+ current in sensory ganglion neurons is reduced after gastric ulcer or pancreatitis (Stewart et al. 2003; Xu et al. 2006). A-type K+ current decreased in small trigeminal ganglion neurons after temporomandibular joint inflammation (Takeda et al. 2006). In some of these models, the Kdr current was also reduced (Abdulla and Smith 2001; Harriott et al. 2006; Stewart et al. 2003; Yang et al. 2004; Yoshimura and de Groat 1999). However, alterations in channel expression are not uniform across neuronal populations in response to different types of injury, inflammation, and other pathologies. For example, the densities of total voltage-gated K+ current, A-type Ka current, and Kdr delayed currents were markedly reduced in medium- and large- but not in small-diameter DRG neurons in painful neuropathic diabetic rats associated with the activation of brain-derived neurotrophic factor (Cao et al. 2010). The in vivo application of an inflammatory agent, zymosan, to the DRG resulted in an increase in both Kdr and Ka currents in dissociated, small-sized neurons (Wang et al. 2007). Similarly, we previously found an increase of Kdr currents after CCD in small-diameter neurons (Fan et al. 2011) but now find a decrease in Kdr current in medium-sized neurons. Tan et al. (2006), with the use of a rat CCD model but with ganglion dissociation prior to recording, found, in contrast to our present findings, a decrease in Ka current and no significant change in Kdr delayed currents. Whether these discrepancies are species dependent or possibly due to other factors, such as the process of cell dissociation, is presently unknown.
In conclusion, in accordance with other studies of neuropathic pain, CCD produced an increase in excitability, demonstrated by the lower rheobase in sensory neurons. An increase in Na+ current and a decrease in Kdr current likely contributed to the increase in neuronal excitability and may contribute to the pain and sensory dysesthesias that can accompany a compression injury of the DRG.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-014624.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.H.L. conception and design of research; N.F. and D.F.D. performed experiments; N.F. and D.F.D. analyzed data; N.F. and R.H.L. interpreted results of experiments; N.F. prepared figures; N.F. drafted manuscript; N.F., D.F.D., and R.H.L. edited and revised manuscript; N.F., D.F.D., and R.H.L. approved final version of manuscript.
REFERENCES
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol 85: 644–658, 2001 [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 108: 237–247, 2004 [DOI] [PubMed] [Google Scholar]
- Boada MD, Woodbury CJ. Physiological properties of mouse skin sensory neurons recorded intracellularly in vivo: temperature effects on somal membrane properties. J Neurophysiol 98: 668–680, 2007 [DOI] [PubMed] [Google Scholar]
- Cao XH, Byun HS, Chen SR, Cai YQ, Pan HL. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem 114: 1460–1475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 26: 1281–1292, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersberger A, Natura G, Eitner A, Halbhuber KJ, Rost R, Schaible HG. Effects of prostaglandin D2 on tetrodotoxin-resistant Na+ currents in DRG neurons of adult rat. Pain 152: 1114–1126, 2011 [DOI] [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol 82: 700–708, 1999 [DOI] [PubMed] [Google Scholar]
- Fan N, Sikand P, Donnelly DF, Ma C, Lamotte RH. Increased Na+ and K+ currents in small mouse dorsal root ganglion neurons after ganglion compression. J Neurophysiol 106: 211–218, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, 3rd, England JD, Liu ZP, Levinson SR. Rapid sodium channel augmentation in response to inflammation induced by complete Freund's adjuvant. Brain Res 802: 69–74, 1998 [DOI] [PubMed] [Google Scholar]
- Harriott AM, Dessem D, Gold MS. Inflammation increases the excitability of masseter muscle afferents. Neuroscience 141: 433–442, 2006 [DOI] [PubMed] [Google Scholar]
- Lee DH, Chung K, Chung JM. Strain differences in adrenergic sensitivity of neuropathic pain behaviors in an experimental rat model. Neuroreport 8: 3453–3456, 1997 [DOI] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Pertin M, Morisod X, Chen C, Hong S, Wiley J, Decosterd I, Isom LL. Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J Neurosci 26: 7984–7994, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. J Neurosci 27: 14059–14068, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol 89: 1588–1602, 2003 [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain 67: 97–106, 1996 [DOI] [PubMed] [Google Scholar]
- Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384: 360–364, 1996 [DOI] [PubMed] [Google Scholar]
- Noguchi K, Kawai Y, Fukuoka T, Senba E, Miki K. Substance P induced by peripheral nerve injury in primary afferent sensory neurons and its effect on dorsal column nucleus neurons. J Neurosci 15: 7633–7643, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shir Y, Sheth R, Campbell JN, Raja SN, Seltzer Z. Soy-containing diet suppresses chronic neuropathic sensory disorders in rats. Anesth Analg 92: 1029–1034, 2001 [DOI] [PubMed] [Google Scholar]
- Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol 82: 3347–3358, 1999 [DOI] [PubMed] [Google Scholar]
- Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol 552: 797–807, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ikeda M, Nasu M, Kadoi J, Yoshida S, Matsumoto S. Enhanced excitability of rat trigeminal root ganglion neurons via decrease in A-type potassium currents following temporomandibular joint inflammation. Neuroscience 138: 621–630, 2006 [DOI] [PubMed] [Google Scholar]
- Tan ZY, Donnelly DF, LaMotte RH. Effects of a chronic compression of the dorsal root ganglion on voltage-gated Na+ and K+ currents in cutaneous afferent neurons. J Neurophysiol 95: 1115–1123, 2006 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport 9: 967–972, 1998 [DOI] [PubMed] [Google Scholar]
- Tripathi PK, Cardenas CG, Cardenas CA, Scroggs RS. Up-regulation of tetrodotoxin-sensitive sodium currents by prostaglandin E in type-4 rat dorsal root ganglion cells. Neuroscience 185: 14–26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JG, Strong JA, Xie W, Zhang JM. Local inflammation in rat dorsal root ganglion alters excitability and ion currents in small-diameter sensory neurons. Anesthesiology 107: 322–332, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pasricha PJ. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 291: G424–G431, 2006 [DOI] [PubMed] [Google Scholar]
- Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N. Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience 123: 867–874, 2004 [DOI] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Mei X, Zhang P, Ma C, White FA, Donnelly DF, Lamotte RH. Altered functional properties of satellite glial cells in compressed spinal ganglia. Glia 57: 1588–1599, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Donnelly DF, LaMotte RH. Patch clamp recording from the intact dorsal root ganglion. J Neurosci Methods 79: 97–103, 1998 [DOI] [PubMed] [Google Scholar]
- Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol 82: 3359–3366, 1999 [DOI] [PubMed] [Google Scholar]