Abstract
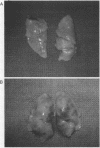
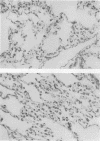
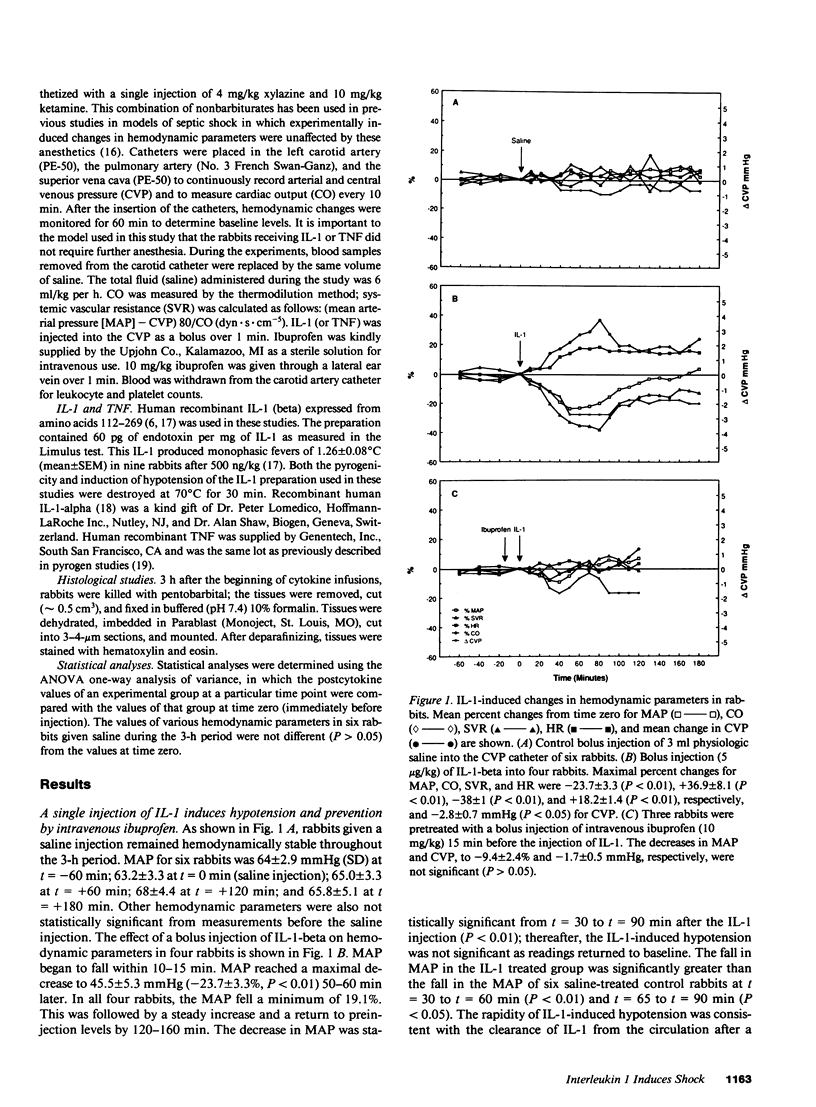
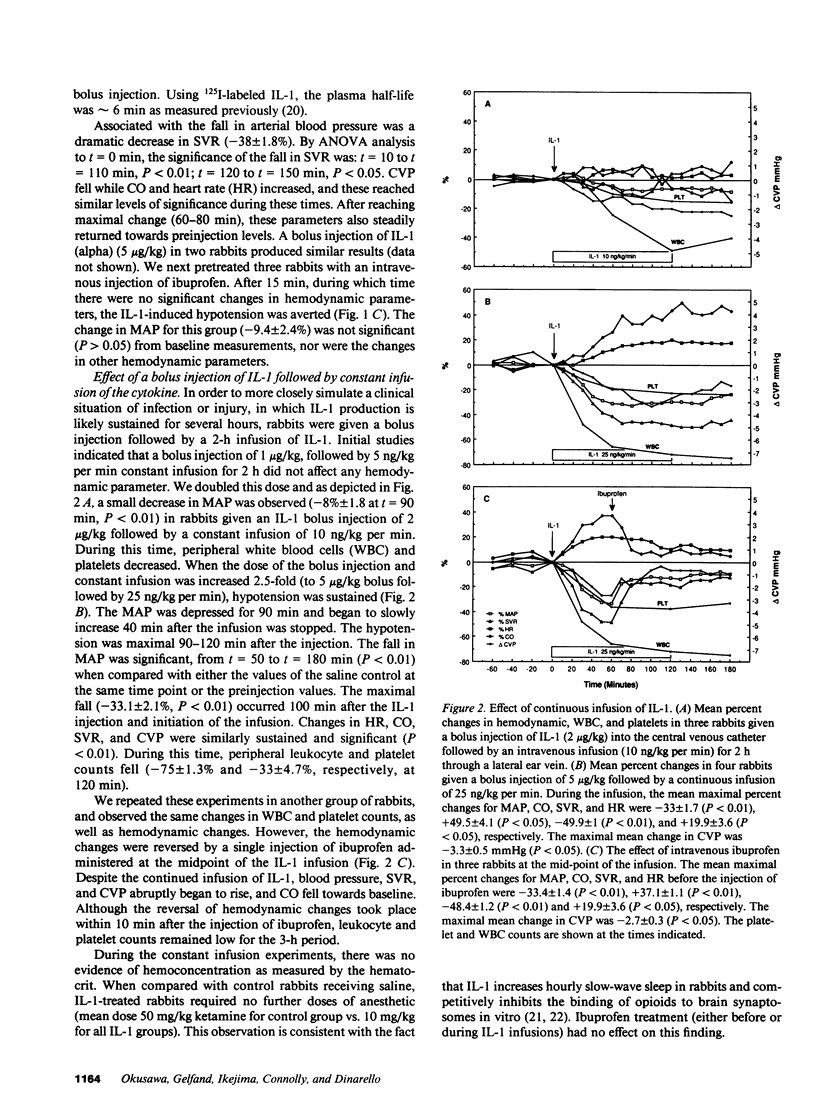
In addition to activating T and B lymphocytes, interleukin 1 (IL-1) induces several hematologic and metabolic changes typical of host responses to infection and injury. We now report a new biological property, namely, the induction of hypotension. Rabbits given a single intravenous injection of recombinant human IL-1-beta (5 micrograms/kg) rapidly developed decreased systemic arterial pressure, which reached the lowest levels after 50-60 min and slowly returned to pre-IL-1 values after 3 h. Associated with the hypotension, systemic vascular resistance and central venous pressure fell, while cardiac output and heart rate increased. These responses were prevented by ibuprofen given 15 min before the IL-1. A bolus injection of IL-1 followed by a 2-h infusion sustained the hypotension and was associated with leukopenia and thrombocytopenia. Ibuprofen given at the mid-point of the infusion reversed the changes in all hemodynamic parameters, but had no effect on the leukopenia or thrombocytopenia. Tumor necrosis factor (TNF) also induced a shock-like state in rabbits. When the dose of IL-1 or TNF was reduced to 1 microgram/kg, no hemodynamic changes were observed; however, the combination of these low doses of both cytokines resulted in a profound shock-like state including histological evidence of severe pulmonary edema and hemorrhage. Pretreatment with ibuprofen prevented the hemodynamic, leukocyte, and platelet changes induced by the low-dose cytokine combination, and ameliorated the pulmonary tissue damage. These results demonstrate that IL-1, like TNF, possesses the ability to induce hemodynamic and hematological changes typical of septic shock, and that the combination of IL-1 and TNF is more potent than either agent alone. These effects seem to require cyclooxygenase products, and suggest that intravenous cyclooxygenase inhibitors may be of therapeutic value in patients with IL-1/TNF-mediated shock.
Full text
PDF
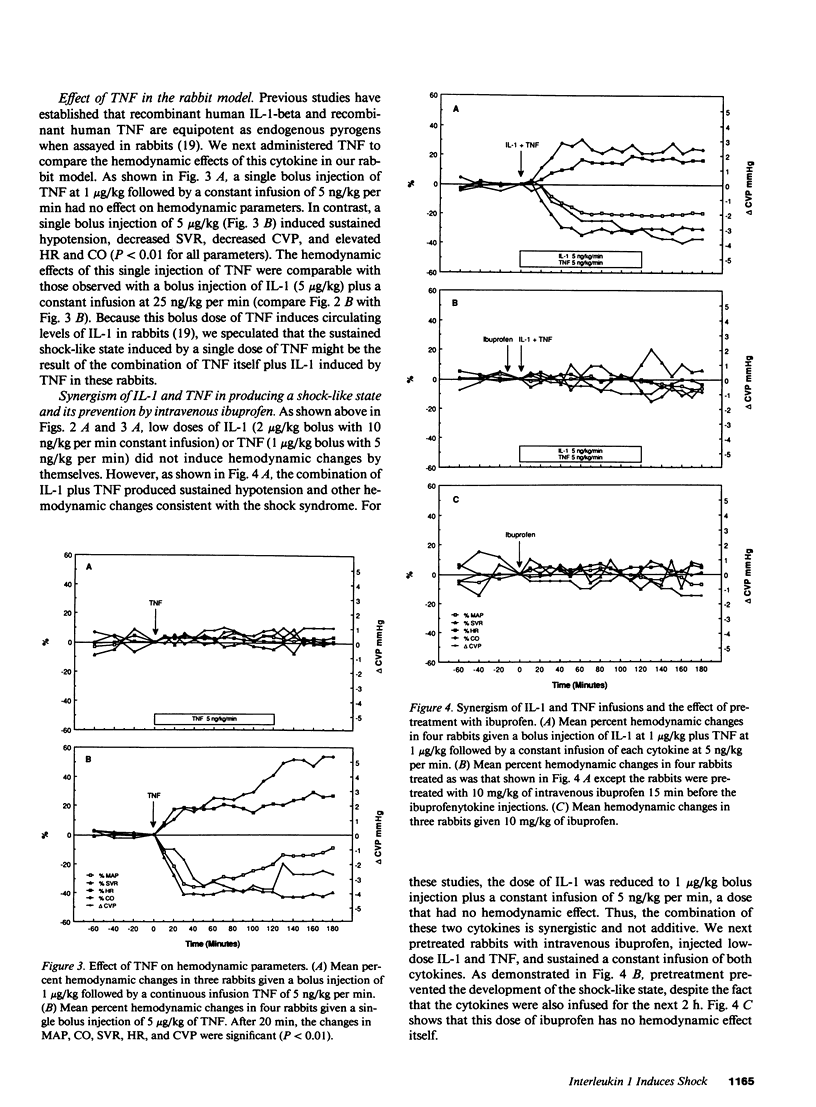
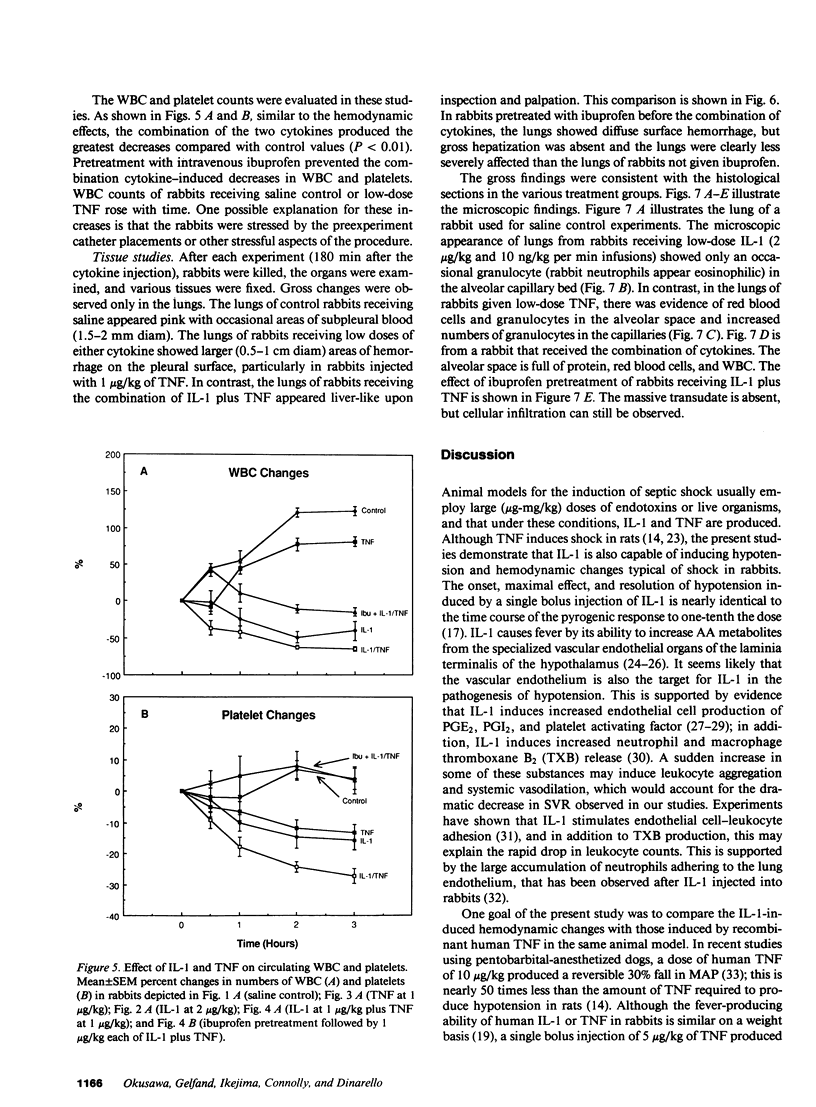
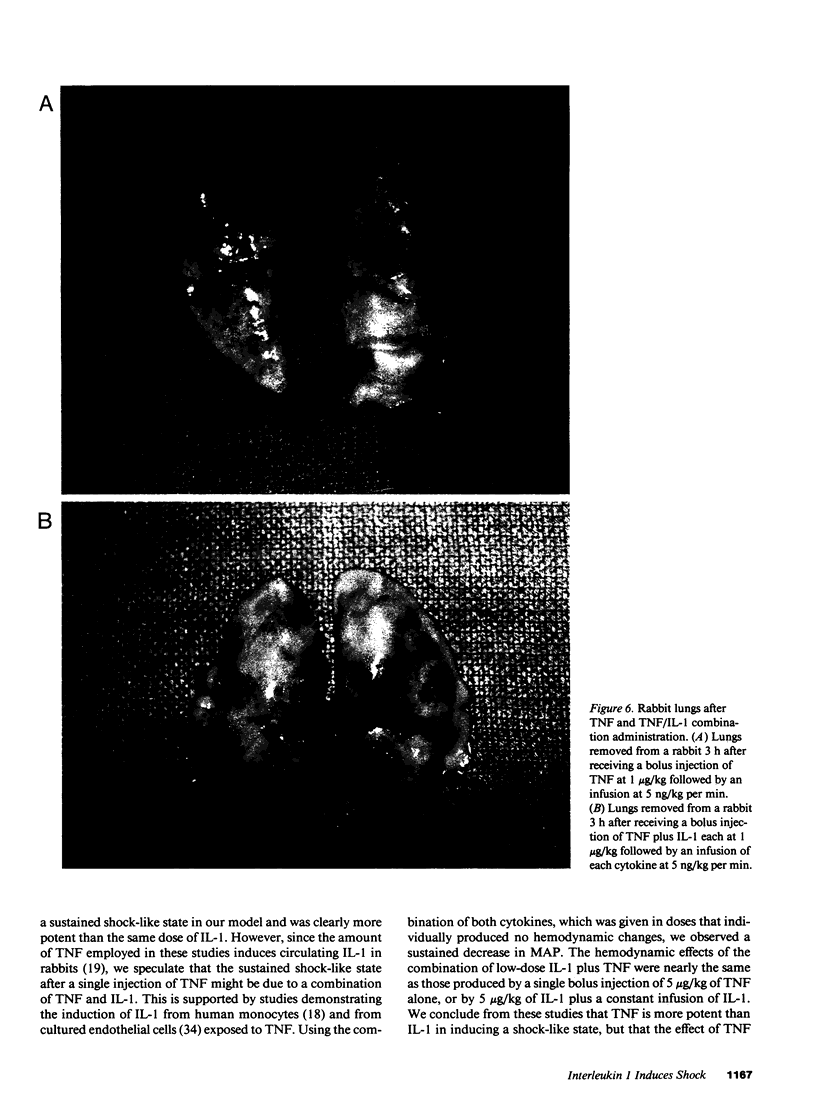


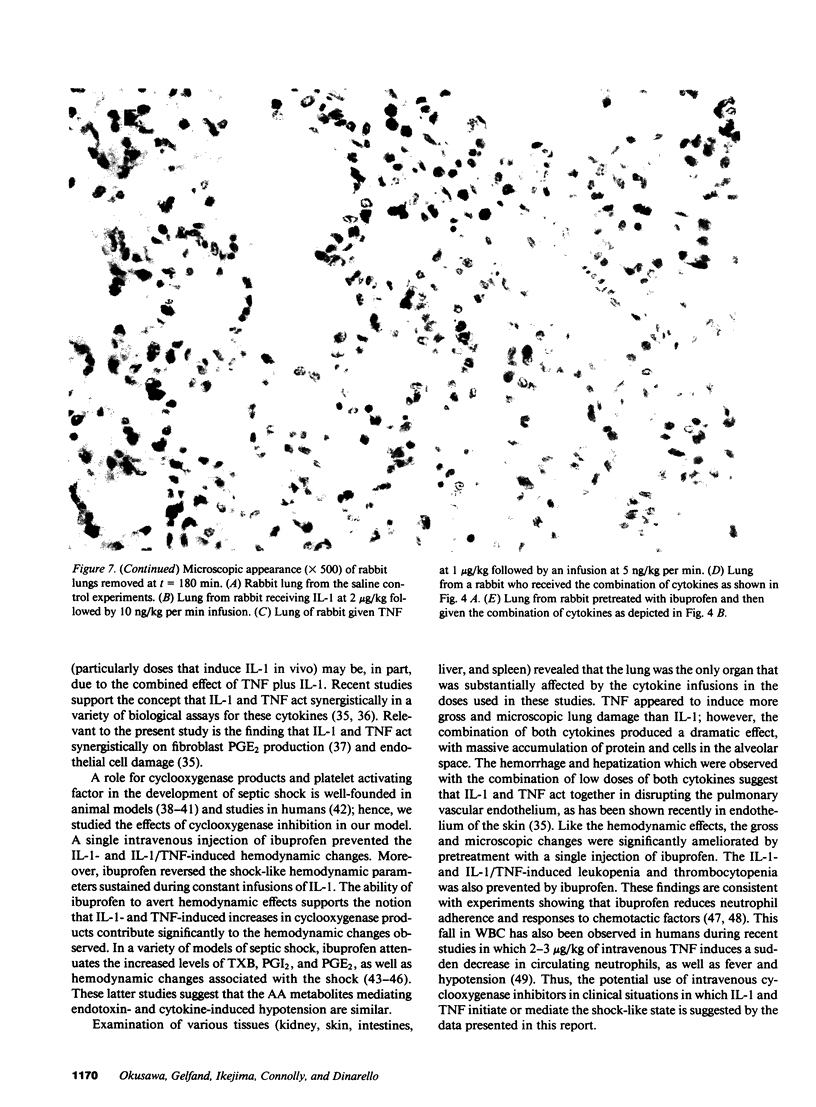


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M. S., Llanos-Q J., Dinarello C. A., Blatteis C. M. Interleukin 1 reduces opioid binding in guinea pig brain. Peptides. 1985 Nov-Dec;6(6):1149–1154. doi: 10.1016/0196-9781(85)90442-5. [DOI] [PubMed] [Google Scholar]
- Albrightson C. R., Baenziger N. L., Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [PubMed] [Google Scholar]
- Almqvist P. M., Kuenzig M., Schwartz S. I. Treatment of experimental canine endotoxin shock with ibuprofen, a cyclooxygenase inhibitor. Circ Shock. 1984;13(3):227–232. [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatteis C. M., Bealer S. L., Hunter W. S., Llanos-Q J., Ahokas R. A., Mashburn T. A., Jr Suppression of fever after lesions of the anteroventral third ventricle in guinea pigs. Brain Res Bull. 1983 Nov;11(5):519–526. doi: 10.1016/0361-9230(83)90124-7. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Dinarello C. A. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985 Mar 8;227(4691):1247–1249. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Kluger M. J. Endogenous pyrogen activity in human plasma after exercise. Science. 1983 May 6;220(4597):617–619. doi: 10.1126/science.6836306. [DOI] [PubMed] [Google Scholar]
- Chang S. W., Feddersen C. O., Henson P. M., Voelkel N. F. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987 May;79(5):1498–1509. doi: 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P. B., Lester T. J., Casper E. S., Gabrilove J. L., Wong G. Y., Kempin S. J., Gold P. J., Welt S., Warren R. S., Starnes H. F. Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J Clin Oncol. 1987 Dec;5(12):1942–1951. doi: 10.1200/JCO.1987.5.12.1942. [DOI] [PubMed] [Google Scholar]
- Coceani F., Bishai I., Lees J., Sirko S. Prostaglandin E2 and fever: a continuing debate. Yale J Biol Med. 1986 Mar-Apr;59(2):169–174. [PMC free article] [PubMed] [Google Scholar]
- Conti P., Cifone M. G., Alesse E., Reale M., Fieschi C., Dinarello C. A. In vitro enhanced thromboxane B2 release by polymorphonuclear leukocytes and macrophages after treatment with human recombinant interleukin 1. Prostaglandins. 1986 Jul;32(1):111–115. doi: 10.1016/0090-6980(86)90151-6. [DOI] [PubMed] [Google Scholar]
- Dejana E., Breviario F., Erroi A., Bussolino F., Mussoni L., Gramse M., Pintucci G., Casali B., Dinarello C. A., Van Damme J. Modulation of endothelial cell functions by different molecular species of interleukin 1. Blood. 1987 Feb;69(2):695–699. [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1: amino acid sequences, multiple biological activities and comparison with tumor necrosis factor (cachectin). Year Immunol. 1986;2:68–89. [PubMed] [Google Scholar]
- Duff G. W., Atkins E. The detection of endotoxin by in vitro production of endogenous pyrogen: comparison with limulus amebocyte lysate gelation. J Immunol Methods. 1982 Aug 13;52(3):323–331. doi: 10.1016/0022-1759(82)90004-7. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Baeder W., Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987 Jun 1;138(11):3812–3816. [PubMed] [Google Scholar]
- Fink M. P., MacVittie T. J., Casey L. C. Inhibition of prostaglandin synthesis restores normal hemodynamics in canine hyperdynamic sepsis. Ann Surg. 1984 Nov;200(5):619–626. doi: 10.1097/00000658-198411000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum S. E., Cohen D. A., Gillespie M. N., McClain C. J. Interleukin-1-induced granulocytopenia and pulmonary leukostasis in rabbits. J Appl Physiol (1985) 1987 Jan;62(1):122–128. doi: 10.1152/jappl.1987.62.1.122. [DOI] [PubMed] [Google Scholar]
- Gubler U., Chua A. O., Stern A. S., Hellmann C. P., Vitek M. P., DeChiara T. M., Benjamin W. R., Collier K. J., Dukovich M., Familletti P. C. Recombinant human interleukin 1 alpha: purification and biological characterization. J Immunol. 1986 Apr 1;136(7):2492–2497. [PubMed] [Google Scholar]
- Halushka P. V., Cook J. A., Wise W. C. Beneficial effects of UK 37248, a thromboxane synthetase inhibitor, in experimental endotoxic shock in the rat. Br J Clin Pharmacol. 1983;15 (Suppl 1):133S–139S. doi: 10.1111/j.1365-2125.1983.tb02124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikejima T., Dinarello C. A., Gill D. M., Wolff S. M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E. R., Soulsby M. E., Bone R. C., Wilson F. J., Jr, Hiller F. C. Ibuprofen in canine endotoxin shock. J Clin Invest. 1982 Sep;70(3):536–541. doi: 10.1172/JCI110645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampschmidt R. F. Infection, inflammation, and interleukin 1 (IL-1). Lymphokine Res. 1983;2(3):97–102. [PubMed] [Google Scholar]
- Krueger J. M., Walter J., Dinarello C. A., Wolff S. M., Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Physiol. 1984 Jun;246(6 Pt 2):R994–R999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Lonnemann G., Bingel M., Koch K. M., Shaldon S., Dinarello C. A. Plasma interleukin-1 activity in humans undergoing hemodialysis with regenerated cellulosic membranes. Lymphokine Res. 1987 Spring;6(2):63–70. [PubMed] [Google Scholar]
- Maderazo E. G., Breaux S. P., Woronick C. L. Protective effects of ibuprofen and methylprednisolone on chemotactic factor-induced transcutaneous hypoxia. J Pharmacol Exp Ther. 1986 Aug;238(2):453–456. [PubMed] [Google Scholar]
- Okusawa S., Dinarello C. A., Yancey K. B., Endres S., Lawley T. J., Frank M. M., Burke J. F., Gelfand J. A. C5a induction of human interleukin 1. Synergistic effect with endotoxin or interferon-gamma. J Immunol. 1987 Oct 15;139(8):2635–2640. [PubMed] [Google Scholar]
- Simon G. L., Gelfand J. A., Connolly R. A., O'Donnell T. F., Jr, Gorbach S. L. Experimental Bacteroides fragilis bacteremia in a primate model: evidence that Bacteroides fragilis does not promote the septic shock syndrome. J Trauma. 1985 Dec;25(12):1156–1162. [PubMed] [Google Scholar]
- Slotman G. J., Burchard K. W., Williams J. J., D'Arezzo A., Yellin S. A. Interaction of prostaglandins, activated complement, and granulocytes in clinical sepsis and hypotension. Surgery. 1986 Jun;99(6):744–751. [PubMed] [Google Scholar]
- Slotman G. J., Quinn J. V., Burchard K. W., Gann D. S. Thromboxane, prostacyclin, and the hemodynamic effects of graded bacteremic shock. Circ Shock. 1985;16(4):395–404. [PubMed] [Google Scholar]
- Stitt J. T. Evidence for the involvement of the organum vasculosum laminae terminalis in the febrile response of rabbits and rats. J Physiol. 1985 Nov;368:501–511. doi: 10.1113/jphysiol.1985.sp015872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P. D., Hamburger S. A., Judy W. V. The effects of vasoactive mediator antagonists on endotoxic shock in dogs. I. Circ Shock. 1984;12(4):277–286. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Fahey T. J., 3rd, Albert J. D., Fong Y., Hesse D., Beutler B., Manogue K. R., Calvano S., Wei H. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed] [Google Scholar]
- Venezio F. R., DiVincenzo C., Pearlman F., Phair J. P. Effects of the newer nonsteroidal anti-inflammatory agents, ibuprofen, fenoprofen, and sulindac, on neutrophil adherence. J Infect Dis. 1985 Oct;152(4):690–694. doi: 10.1093/infdis/152.4.690. [DOI] [PubMed] [Google Scholar]
- Wise W. C., Cook J. A., Halushka P. V. Implications for thromboxane A2 in the pathogenesis of endotoxic shock. Adv Shock Res. 1981;6:83–91. [PubMed] [Google Scholar]
- Wolff S. M. Biological effects of bacterial endotoxins in man. J Infect Dis. 1973 Jul;128(Suppl):259–264. doi: 10.1093/infdis/128.supplement_1.s259. [DOI] [PubMed] [Google Scholar]